Abstract
Well-characterized mouse models of alloimmune antibody-mediated hemolysis would provide a valuable approach for gaining greater insight into the pathophysiology of hemolytic transfusion reactions. To this end, mouse red blood cells (mRBCs) from human glycophorin A transgenic (hGPA-Tg) donor mice were transfused into non-Tg recipients that had been passively immunized with IgG or IgM hGPA-specific monoclonal antibodies (mAbs). In this novel murine “blood group system,” mRBCs from hGPA-Tg mice are “antigen positive” and mRBCs from non-Tg mice are “antigen negative.” Passive immunization of non-Tg mice with the IgG1 10F7 and IgG3 NaM10-2H12 anti-hGPA mAbs each induced rapid clearance of incompatible transfused hGPA-Tg-mRBCs in a dose-response manner. Using various knockout mice as transfusion recipients, both the complement system and activating Fcγ receptors were found to be important in the clearance of incompatible mRBCs by each of these IgG mAbs. In addition, the IgM E4 anti-hGPA mAb induced complement-dependent intravascular hemolysis of transfused incompatible hGPA-Tg-mRBCs accompanied by gross hemoglobinuria. These initial studies validate the relevance of these new mouse models for addressing important questions in the field of transfusion medicine.
Introduction
Hemolytic transfusion reactions (HTRs) are dangerous complications of blood transfusions. IgM-mediated HTRs (IgM-HTRs) occur acutely, are usually due to ABO incompatibility, typically cause intravascular hemolysis, and can lead to shock, renal failure, coagulopathy, and death.1 Although infrequent, IgM-HTRs have a high mortality rate. IgG-mediated HTRs (IgG-HTRs) are more common, but usually less severe, than IgM-HTRs; they can occur acutely or be delayed, typically cause extravascular hemolysis, and occasionally result in renal failure, coagulopathy, and death.2 Symptomatic immune-mediated red blood cell (RBC) destruction also occurs in autoimmune hemolytic anemia (AIHA),3 blood group incompatible transplantation,4 and immune thrombocytopenic purpura (ITP) treated with Rh-immune globulin.5 Although various different modalities are used to treat HTRs, there is no definitive evidence regarding efficacy. Given the sporadic nature of HTRs, designing human trials to evaluate treatment options is difficult. Therefore, to study the mechanisms underlying HTRs, to evaluate the efficacy of existing treatments, and to develop new treatments, a relevant, inexpensive, and tractable animal model is required.6 A mouse model would be ideal for this purpose,7 given the abundance of reagents and relevant knockout (KO) and transgenic (Tg) animals. Although mouse blood group polymorphisms exist,8 and transfused incompatible mouse RBC (mRBCs) are rapidly cleared,9,10 no one has exploited these findings to develop a model of HTRs. The availability of a Tg mouse expressing human glycophorin A (hGPA; gene symbol designation, GYPA) on mRBCs11 offers an approach using a well-described glycoprotein target and well-characterized reagents.
We describe initial studies validating mouse models of IgG- and IgM-mediated alloimmune hemolysis. Thus, mRBCs from hGPA-Tg donors were transfused into non-Tg recipients that were passively immunized with IgG or IgM hGPA-specific monoclonal antibodies (mAbs). The clearance of the transfused mRBCs was quantified and the roles of complement and Fcγ receptors were evaluated.
Materials and methods
Antibodies
Purified mAbs NaM10-2H12 (IgG3), NaM26-3F4 (IgG1), and NaM10-6G4 (IgG2a), which recognize peptide epitopes on the M and N forms of hGPA,12 were purchased from EFS (Nantes, France). Hybridomas producing mAb 6A7, an IgG1 specific for a sialic acid-dependent epitope on M-type hGPA,13-15 10F7, an IgG1 recognizing a nonpolymorphic epitope on hGPA,13,14 and J11d, a rat IgM recognizing CD24 on mRBCs,16,17 were purchased from the American Type Culture Collection (Manassas, VA). The hybridoma producing mAb N92, an IgG2a anti-N mAb,18 was provided by Elwira Lisowska (Ludwik Hirszfeld Institute, Wroclaw, Poland). The hybridoma producing mAb E4, an IgM that recognizes a nonpolymorphic epitope present on hGPA,19 was provided by Marilyn Telen (Duke University, Durham, NC).
Throughout culture and mAb purification, endotoxin contamination was minimized. IgG mAbs were purified by immunoaffinity chromatography. Thus, 5 mL goat anti–mouse IgG agarose (Sigma, St Louis, MO) in a disposable 10-mL chromatography column (Pierce, Rockford, IL) was equilibrated with 50 mL column wash buffer (CWB; 50 mM Tris, pH 8.0, prepared using endotoxin-free water and 1.0 M Tris-HCl, pH 8.0 [Mediatech, Herndon, VA]). A peristaltic pump and pyrogen-free tubing (VWR, West Chester, PA) were used to perfuse the column with 100 mL conditioned medium or ascites at 1 mL/min overnight at 4°C. The column was then washed with 50 mL CWB and eluted with Immunopure IgG elution buffer (Pierce) in 4-mL fractions into tubes containing 1 mL 1.0 M Tris-HCl in endotoxin-free water, pH. 8.0. Peak fractions (ie, A280nm > 0.050) were pooled, changed into endotoxin-free Tris-buffered saline (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl [Teknova, Hollister, CA]), and concentrated to 1 mg/mL (determined by the bicinchoninic acid [BCA] assay [Pierce]) using Centriprep YM-10 centrifugal filters (Millipore, Billerica, MA). Similarly, the IgM J11d and E4 mAbs were purified using a protein L column (Pierce) and the mannose binding protein-based Immunopure IgM Purification Kit (Pierce), respectively.
All mAbs used in vivo were purified to electrophoretic homogeneity. Endotoxin levels were quantified using the LAL QCL-1000 kit (Cambrex, East Rutherford, NJ). The purified mAbs were clear solutions with no visible precipitates; however, they were not further evaluated to exclude the presence of higher oligomers or aggregates. To measure mAb activity, hemagglutination titers were determined (see “Mice”), using commercially available reagent-quality human RBC (Ortho-Clinical Diagnostics, Raritan, NJ). To evaluate IgM mAb function in vitro, hemolytic complement assays were performed. Thus, RBCs were incubated for 1 hour at room temperature (RT) in mAb E4 or J11d diluted in normal saline (NS; 0.154 M NaCl, pH 7.4). Washed RBCs (10 μL; see “Mice”) in NS (2 × 108 cells/mL) were added to 190 μL of an 18.75% (vol/vol) mixture of guinea pig serum (Sigma) in gelatin veronal buffer +2 (GVB+2; Sigma) in a microtiter plate and incubated at 37°C for 30 minutes. Following centrifugation for 5 minutes at 4°C, supernatant absorbance at 414 nm was determined. Nonopsonized human RBCs and mRBCs were negative controls. As positive controls, RBCs were completely hemolyzed using distilled water instead of GVB+2 buffer; after hemolysis, 10 μL guinea pig serum was added.
Mice
Wild-type C57BL/6 and BALB/C mice and Fcgr2b KO mice on the BALB/C background, were from Taconic Labs (Hudson, NY). FVB/NJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Fcgr KO,20 complement C3 KO,21 and Fcgr/C3 double-KO mice (all on the C57BL/6 background) were maintained by Dr Clynes; C3 KO breeding pairs were provided by Michael Carroll (Harvard University, Boston, MA). Animal protocols were approved by the Institutional Animal Care and Use Committee at the Columbia University. Narla Mohandas (New York Blood Center) provided breeding pairs of hGPA-Tg mice,11 which were constructed on the FVB/NJ background using human BAC clone 159F4 (catalog no. 982132; Invitrogen, Carlsbad, CA), which contains GYPA. FVB/NJ mice do not express functional complement C5 protein.22
A multiplex polymerase chain reaction (mPCR) assay identified hGPA-Tg mice, using gDNA extracted from tail biopsies to amplify a 291-bp GYPA sequence using forward (5′-TATTAGCTCAGAGCCTCACACATT-3′) and reverse (5′-GAAATTTCTGAAACTTCATGAGCTC-3′) primers; as a positive control, an 88-bp segment of the mouse glycophorin gene Gypa23 was amplified (forward: 5′-CTTTGTCATTCGGCTGATA-3′; reverse: 5′-TGATGATACTCATAATTTTGGG-3′). Fluorescence in situ hybridization (FISH) was performed by standard methods using the 159F4 BAC clone to probe bone marrow cells from femurs of wild-type and hGPA-Tg mice.
In humans, GYPA encodes the MN blood group antigens. The MN phenotype of hGPA-Tg-mRBCs was determined by standard microtiter plate hemagglutination assays using the 6A7 anti-M13,14 and N92 anti-N18 mAbs. Briefly, 25-μL aliquots of serial 2-fold dilutions of the mAbs were prepared in NS. Washed mRBCs, obtained from retro-orbital plexus blood, were resuspended to a 3% hematocrit in NS and 25-μL aliquots were added to each well. Following a 1-hour incubation at 37°C, agglutinates were identified macroscopically. Controls included reagent-quality M+N−, M+N+, and M−N+ human RBCs and wild-type FVB/NJ mRBCs.
Passive immunization
Prior to injection, mAbs were dialyzed against endotoxin-free NS (Phoenix Scientific, St Joseph, MO). Recipients received IgG mAbs by intraperitoneal injection 16 hours before transfusion; alternatively, the IgM mAb was infused into the right jugular vein 2 minutes before transfusion.
Preparation of 51Cr-labeled mRBCs
Donors were anesthetized, exsanguinated by cardiac puncture using 25-gauge needles and heparin-coated syringes, and humanely killed. For each transfusion, a 1-mL aliquot of a 3% suspension of washed mRBCs in a pyrogen-free 1.5-mL Microfuge tube was centrifuged at 10 000g for 3 seconds, supernatant was removed, and packed RBCs (pRBCs) were resuspended in 100 μL endotoxin-free NS and 20 μL 51Cr (1 mCi/mL [37 MBq]; GE Healthcare, Piscataway, NJ). Following 30 minutes at RT, the tube was centrifuged, supernatant removed, and the pRBCs resuspended in 100 μL NS.
Transfusion of 51Cr-labeled mRBCs
The recipients were anesthetized and, using sterile technique, a 1-cm vertical midline incision was made and the left jugular vein exposed by blunt dissection. The 51Cr-labeled mRBCs (∼30 μL pRBCs) in a 25-gauge butterfly was transfused over 5 seconds into the jugular vein. Following transfusion, hemostasis was achieved, and the wound was closed. This procedure was completed in less than 5 minutes. A zero time point (T = 0) sample of about 25 μL blood was then obtained from the retro-orbital plexus.
Survival of transfused RBCs
At designated times after transfusion, mice were anesthetized and an end point sample of about 25 μL retro-orbital plexus blood was obtained. Microhematocrit tubes containing the T = 0 and terminal blood samples were centrifuged for 1 minute; the height of the pRBC column was measured and the tubes analyzed for counts per minute (cpm) in a γ counter (Perkin-Elmer, Wellesley, MA). The percentage of radiolabeled mRBCs remaining at time “n” following transfusion (T = n) was calculated as: {(cpm/mm of pRBCs in the T = n sample)/(cpm/mm of pRBC in the T = 0 sample)} × 100
Following the terminal time point sample, recipients were exsanguinated and humanely killed.
Determination of hemoglobinuria
In some experiments, recipients were maintained in metabolic cages. Urine was collected from metabolic cages while mice were alive; bladder urine was also collected at autopsy. Urine absorbance was measured by wavelength scanning spectrophotometry at 300 to 700 nm using a spectrophotometer (Nanodrop Technologies, Wilmington, DE).
Results
Further characterization of hGPA-Tg mice
Animal husbandry.
There were no behavioral differences between hGPA-Tg and wild-type FVB/NJ mice. hGPA-Tg mice were healthy, bred well, bore litters of expected size (averaging ∼8 neonates/litter), and nurtured their offspring attentively.
Serologic characterization of hGPA-Tg-mRBCs.
In the initial study of hGPA-Tg mice, hGPA expression on mRBCs was characterized using only 2 anti-hGPA mAbs, which had no blood group MN specificity.11 We extended these studies using well-characterized anti-hGPA mAbs.12-15,18,19 Hemagglutination with anti-M and anti-N mAbs (6A7 and N92, respectively) revealed that hGPA-Tg-mRBC expressed the M allele (Table 1). These results are particularly interesting because 6A7 recognizes a sialic acid-dependent epitope,15 and mouse and human RBC sialic acids differ.24 In addition, the NaM16-1B10, NaM10-2H12, NaM26-3F4, and 10F7 mAbs, which recognize nonallelic hGPA peptide epitopes,12-14 agglutinated human RBCs and hGPA-Tg-mRBCs, but not wild-type mRBCs (Table 1). Similar results were obtained with the hGPA peptide-specific NaM10-6G4 and E4 mAbs (not shown). The variation in agglutination titer seen with 10F7 and NaM26-3F4 with different RBC isolates is intriguing but is not yet understood. Taken together, these results demonstrate that a broad array of anti-hGPA mAbs recognize a glycosylated recombinant form of hGPA on hGPA-Tg-mRBCs.
Serologic characterization of hGPA expressed on the surface of hGPA-Tg-mRBCs
| Source of RBCs . | RBC type . | mAb/specificity/isotype . | |||||
|---|---|---|---|---|---|---|---|
| 6A7/M/IgG1 . | N92/N/IgG2a . | NaM16-1B10/hGPA/IgM . | 10F7/hGPA/IgG1 . | NaM10-2H12/hGPA/IgG3 . | NaM26-3F4/hGPA/IgG1 . | ||
| Human no. 1 | M+N− | > 128 | 0 | 64 | 16 | 1600 | 64 |
| Human no. 2 | M−N+ | 0 | + | 64 | 16 | 1600 | 32 |
| Mouse no. 1 | hGPA-Tg | 128 | 0 | 64 | 64 | 1600 | 8 |
| Mouse no. 2 | hGPA-Tg | 128 | 0 | 64 | 128 | 1600 | 8 |
| Mouse no. 3 | hGPA-Tg | > 128 | 0 | 64 | 128 | 1600 | 8 |
| Mouse no. 4 | Wild-type | 0 | 0 | 0 | 0 | 0 | 0 |
| Source of RBCs . | RBC type . | mAb/specificity/isotype . | |||||
|---|---|---|---|---|---|---|---|
| 6A7/M/IgG1 . | N92/N/IgG2a . | NaM16-1B10/hGPA/IgM . | 10F7/hGPA/IgG1 . | NaM10-2H12/hGPA/IgG3 . | NaM26-3F4/hGPA/IgG1 . | ||
| Human no. 1 | M+N− | > 128 | 0 | 64 | 16 | 1600 | 64 |
| Human no. 2 | M−N+ | 0 | + | 64 | 16 | 1600 | 32 |
| Mouse no. 1 | hGPA-Tg | 128 | 0 | 64 | 64 | 1600 | 8 |
| Mouse no. 2 | hGPA-Tg | 128 | 0 | 64 | 128 | 1600 | 8 |
| Mouse no. 3 | hGPA-Tg | > 128 | 0 | 64 | 128 | 1600 | 8 |
| Mouse no. 4 | Wild-type | 0 | 0 | 0 | 0 | 0 | 0 |
A panel of mouse mAbs recognizing either a sialic acid-dependent M epitope on hGPA (ie, 6A7), a sialic-independent N epitope on hGPA (ie, N92), or peptide epitopes on both M- and N-type hGPA (ie, NaM16-1B10, 10F7, NaM10-2H12, and NaM26-3F4) was used to evaluate hGPA expression on hGPA-Tg-mRBCs. Human RBCs of defined MN type were positive controls. RBCs from a wild-type FVB/NJ mouse were used as a negative control. The inverse agglutination titers are provided in each case, except for the results of the N92 mAb with the RBCs obtained from human no. 2, in which strong agglutination was seen, but titers were not performed. As additional controls, wild-type and hGPA-Tg-mRBCs were agglutinated by a rabbit polyclonal anti–mRBC IgG antibody (not shown).
Genotyping and phenotyping hGPA-Tg mice.
We developed mPCR and hemagglutination assays to provide a definitive means of identifying hGPA-Tg mice. Thus, mPCR of gDNA yielded a 218-bp band when the GYPA Tg was present, and an 88-bp band corresponding to mouse glycophorin,23 as an internal standard (Figure 1). In contrast, mPCR results from mice lacking the GYPA Tg yielded only the 88-bp mouse glycophorin band (Figure 1). For older mice, hemagglutination assays using the 6A7 anti-hGPA mAb allowed phenotypic confirmation of the presence or absence of the Tg. Thus, mAb 6A7 agglutinated both M+N− human RBCs (positive control) and hGPA-Tg-mRBCs, but not wild-type mRBCs or M−N+ human RBCs (negative controls; not shown). These assays always produced concordant results.
Identification of hGPA-Tg mice by mPCR. The presence of the GYPA Tg was detected using mPCR of gDNA extracted from biopsied tail tissue with the primers described in “Materials and methods.” This yielded a 219-bp band if the GYPA Tg was present. Amplification of the endogenous mouse glycophorin gene was used as an internal standard and yielded an 88-bp band in each case.
Identification of hGPA-Tg mice by mPCR. The presence of the GYPA Tg was detected using mPCR of gDNA extracted from biopsied tail tissue with the primers described in “Materials and methods.” This yielded a 219-bp band if the GYPA Tg was present. Amplification of the endogenous mouse glycophorin gene was used as an internal standard and yielded an 88-bp band in each case.
Identification of the GYPA Tg integration site.
Initially, it was not clear whether the GYPA Tg integrated at one or multiple loci.11 FISH provides a definitive means of examining chromosomal integration sites. As a positive control, GYPA is a single-copy gene in human leukocytes and maps to chromosome 4q28-q31 (Figure 2A). Similarly, in hGPA-Tg mouse bone marrow cells, the GYPA Tg integrated at a single locus (Figure 2C–D). No hybridization was observed with wild-type FVB/NJ mouse bone marrow cells (Figure 2B).
Localizing the GYPA gene by FISH. FISH was performed using the 159F4 BAC probe to examine the chromosomal location of the GYPA gene, which encodes the hGPA protein. As a positive control, the probe hybridized to human chromosome 4 (A), and, as a negative control, the probe did not hybridize to any wild-type FVB/NJ mouse chromosome (B). However, the probe did hybridize to one chromosome from hemizygous hGPA-Tg mice (C-D). The locations of hybridization are indicated by white arrows.
Localizing the GYPA gene by FISH. FISH was performed using the 159F4 BAC probe to examine the chromosomal location of the GYPA gene, which encodes the hGPA protein. As a positive control, the probe hybridized to human chromosome 4 (A), and, as a negative control, the probe did not hybridize to any wild-type FVB/NJ mouse chromosome (B). However, the probe did hybridize to one chromosome from hemizygous hGPA-Tg mice (C-D). The locations of hybridization are indicated by white arrows.
RBC survival studies
IgG-mediated hemolysis in vivo of transfused incompatible mRBCs.
When transfused into wild-type FVB/NJ and C57BL/6 mice that had been passively immunized with varying amounts of the 10F7, NaM10-2H12, and NaM10-6G4 IgG mAbs, incompatible hGPA-Tg-mRBCs were promptly cleared from the circulation in amounts proportional to the amount of infused anti-hGPA mAb. For example, when immunized with increasing amounts of mAb 10F7, 24-hour RBC survival progressively decreased (Figure 3). Because of these results, 500 μg mAb 10F7 was used subsequently. Similar dose-response experiments with NaM10-2H12 and NaM10-6G4 (not shown) revealed that 50 μg of each of these mAbs would suffice for inducing dramatic RBC clearance (Table 2). As negative controls, no destruction of transfused, compatible, wild-type mRBCs was observed in mice immunized with any IgG anti-hGPA mAb (not shown). In addition, when an isotype-matched irrelevant IgG1 mAb was used as a control for 10F7, the survival of hGPA-Tg-mRBCs was normal at 24 hours (D.A.S, S.L.S., and James C. Zimring, unpublished data, October 2006). Similarly, no destruction of transfused hGPA-Tg-mRBCs was detected in unimmunized recipients (not shown and Table 3). Therefore, despite the H-2 difference between donors (FVB/NJ background) and recipients (ie, C57BL/6 and BALB/C), no “naturally occurring” anti–H-2 antibodies caused accelerated mRBC destruction.8,10
IgG1-mediated clearance of incompatible mRBCs. Wild-type FVB/NJ mice were passively immunized intraperitoneally with the indicated amounts of purified 10F7, an anti–hGPA IgG1 mAb; 16 hours later they were transfused with 51Cr-labeled wild-type (ie, (−) control) or hGPA-Tg-mRBCs, and 24-hour RBC survivals were quantified. Results are recorded as percentage of transfused RBCs remaining in the vasculature after 24 hours for groups of 2 to 5 mice (mean ± 1 SD). These results show that incompatible hGPA-Tg-mRBCs were cleared from the circulation of mice in proportion to the dose of purified 10F7 mAb that they received.
IgG1-mediated clearance of incompatible mRBCs. Wild-type FVB/NJ mice were passively immunized intraperitoneally with the indicated amounts of purified 10F7, an anti–hGPA IgG1 mAb; 16 hours later they were transfused with 51Cr-labeled wild-type (ie, (−) control) or hGPA-Tg-mRBCs, and 24-hour RBC survivals were quantified. Results are recorded as percentage of transfused RBCs remaining in the vasculature after 24 hours for groups of 2 to 5 mice (mean ± 1 SD). These results show that incompatible hGPA-Tg-mRBCs were cleared from the circulation of mice in proportion to the dose of purified 10F7 mAb that they received.
IgG anti-hGPA mAbs rapidly cleared hGPA-Tg-mRBC in vivo
| mAb . | IgG subclass . | No. of mice . | Percent 4-h RBC survival, mean ± 1 SD . |
|---|---|---|---|
| 10F7 | IgG1 | 5 | 59.9 ± 3.4 |
| NaM10-6G4 | IgG2a | 4 | 29.6 ± 9.2 |
| NaM10-2H12 | IgG3 | 10 | 17.0 ± 7.2 |
| mAb . | IgG subclass . | No. of mice . | Percent 4-h RBC survival, mean ± 1 SD . |
|---|---|---|---|
| 10F7 | IgG1 | 5 | 59.9 ± 3.4 |
| NaM10-6G4 | IgG2a | 4 | 29.6 ± 9.2 |
| NaM10-2H12 | IgG3 | 10 | 17.0 ± 7.2 |
Wild-type FVB/NJ recipient mice that had been passively immunized intraperitoneally with mAb 10F7 (500 μg), NaM10-6G4 (50 μg), or NaM10-2H12 (50 μg) were then transfused 16 hours later with 51Cr-labeled hGPA-Tg-mRBCs and the 4-hour RBC survivals were determined as described in “Materials and methods.”
Evaluation of the role of activating Fcγ receptors and complement component C3 in IgG-mediated clearance of incompatible hGPA-Tg-mRBCs
| Mice . | Passive immunization . | No. of mice . | Percent 4-h RBC survival, mean ± 1 SD . |
|---|---|---|---|
| Wild type | None | 6 | 98.2 ± 1.6 |
| Wild type | NaM10-2H12 | 6 | 26.8 ± 8.1 |
| Fcgr KO | NaM10-2H12 | 2 | 56.5 ± 0.4 |
| C3 KO | NaM10-2H12 | 6 | 81.7 ± 7.2 |
| Fcgr/C3 double-KO | NaM10-2H12 | 6 | 80.6 ± 9.1 |
| Wild type | None | 6 | 98.2 ± 1.6 |
| Wild type | 10F7 | 6 | 27.2 ± 14.5 |
| Fcgr KO | 10F7 | 6 | 85.7 ± 19.9 |
| C3 KO | 10F7 | 6 | 58.6 ± 4.5 |
| Fcgr/C3 double-KO | 10F7 | 4 | 80.2 ± 9.2 |
| Mice . | Passive immunization . | No. of mice . | Percent 4-h RBC survival, mean ± 1 SD . |
|---|---|---|---|
| Wild type | None | 6 | 98.2 ± 1.6 |
| Wild type | NaM10-2H12 | 6 | 26.8 ± 8.1 |
| Fcgr KO | NaM10-2H12 | 2 | 56.5 ± 0.4 |
| C3 KO | NaM10-2H12 | 6 | 81.7 ± 7.2 |
| Fcgr/C3 double-KO | NaM10-2H12 | 6 | 80.6 ± 9.1 |
| Wild type | None | 6 | 98.2 ± 1.6 |
| Wild type | 10F7 | 6 | 27.2 ± 14.5 |
| Fcgr KO | 10F7 | 6 | 85.7 ± 19.9 |
| C3 KO | 10F7 | 6 | 58.6 ± 4.5 |
| Fcgr/C3 double-KO | 10F7 | 4 | 80.2 ± 9.2 |
All mice were on the C57BL/6 background. Mice were passively immunized intraperitoneally with either 50 μg of the IgG3 NaM10-2H12 anti-hGPA mAb or 500 μg of the IgG1 10F7 anti-hGPA mAb 16 hours prior to transfusion. All mice were then transfused with 51Cr-labeled hGPA-Tg-mRBCs. In mice immunized with 10F7, clearance of incompatible mRBCs was highly dependent on the presence of activating Fcγ receptors and moderately dependent on C3. In contrast, clearance of incompatible RBCs in mice immunized with NaM10-2H12 was moderately dependent on activating Fcγ receptors and highly dependent on C3.
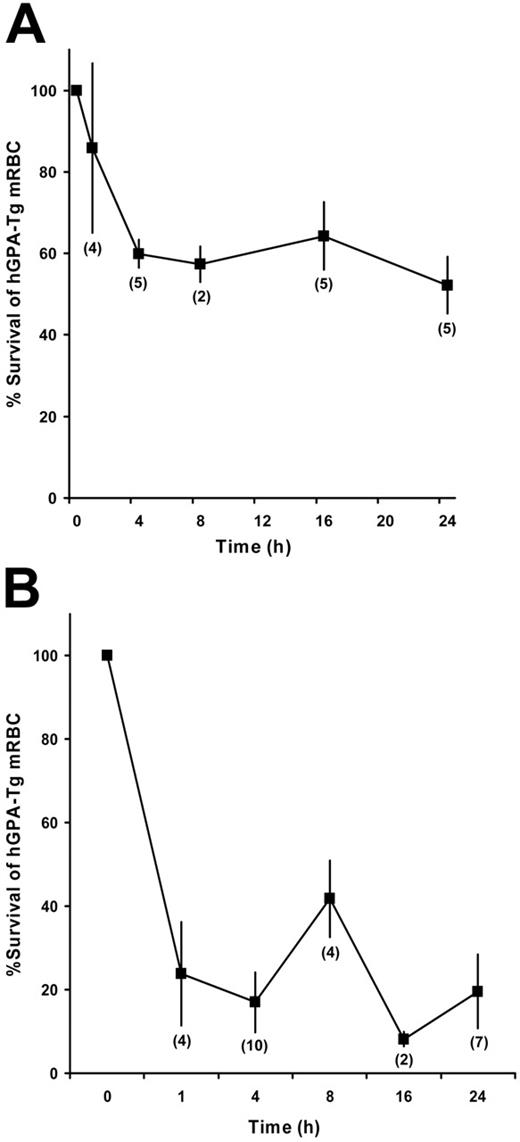
Transfused incompatible hGPA-Tg-mRBCs were rapidly cleared from passively immunized mice. For example, in mice immunized with mAb 10F7 or NaM10-2H12, incompatible hGPA-Tg-mRBC clearance was complete by 4 hours after transfusion (Figure 4).
IgG-mediated clearance of incompatible hGPA-Tg-mRBCs: time-course studies. Wild-type FVB/NJ mice were passively immunized intraperitoneally with 500 μg of the purified 10F7 anti–hGPA IgG1 mAb (A) or with 50 μg of the purified NaM10-2H12 anti–hGPA IgG3 mAb (B). Sixteen hours later they were transfused with 51Cr-labeled hGPA-Tg-mRBCs, and then RBC survival was quantified at the indicated time points. The number of mice evaluated at each time point is indicated in parentheses; the mean ± 1 SD is provided for each time point.
IgG-mediated clearance of incompatible hGPA-Tg-mRBCs: time-course studies. Wild-type FVB/NJ mice were passively immunized intraperitoneally with 500 μg of the purified 10F7 anti–hGPA IgG1 mAb (A) or with 50 μg of the purified NaM10-2H12 anti–hGPA IgG3 mAb (B). Sixteen hours later they were transfused with 51Cr-labeled hGPA-Tg-mRBCs, and then RBC survival was quantified at the indicated time points. The number of mice evaluated at each time point is indicated in parentheses; the mean ± 1 SD is provided for each time point.
To evaluate the roles of activating Fcγ receptors and the complement system in IgG-mediated clearance of incompatible mRBCs, Fcgr KO, C3 KO, and Fcgr/C3 double-KO mice, all on the C57BL/6 background, were passively immunized with IgG anti-hGPA mAbs and transfused with incompatible mRBCs. When coated by IgG mAbs of different subclasses, incompatible mRBCs were cleared by activating different pathways. For example, as compared with wild-type C57BL/6 mice, IgG1-mediated clearance of hGPA-Tg-mRBC by mAb 10F7 was markedly inhibited in Fcgr KO mice and only moderately inhibited in C3 KO mice (Table 3). Thus, although complement participates in IgG1-mediated clearance of hGPA-Tg-mRBCs, activating Fcγ receptors play a dominant role. In contrast, when immunized with the IgG3 NaM10-2H12 anti-hGPA mAb, hGPA-Tg-mRBC destruction was markedly inhibited in C3 KO mice and only moderately inhibited in Fcgr KO mice (Table 3); thus, complement is dominant, with some contribution by activating Fcγ receptors. With both of these mAbs, clearance of hGPA-Tg-mRBCs was markedly, but not completely, inhibited in Fcgr/C3 double-KO mice, suggesting that other mechanisms may also be involved in mRBC clearance.
Inhibitory Fcγ receptors were also investigated using Fcgr2b KO mice (BALB/C background). No difference in IgG3-mediated clearance of incompatible mRBCs by mAb NaM10-2H12 was observed when comparing Fcgr2b KO and wild-type BALB/C mice (not shown). This was not unexpected given the low affinity of FcγRII for IgG325 ; in addition, Kupffer cells, which appear to play a major role in mRBC destruction (D.A.S. and S.L.S., unpublished data, July 2005; and Azeredo da Silveira,26 Fossati-Jimack et al,27,28 and Schiller et al29 ), either lack FcγRIIb30 or have defective regulation of phagocytosis by FcγRIIb.29
To examine the role of terminal complement components in IgG-mediated mRBC clearance, we compared wild-type FVB/NJ mice, which lack complement component C5,22 with wild-type C57BL/6 mice. Clearance of hGPA-Tg-mRBCs in mAb 10F7-immunized FVB/NJ mice was markedly reduced compared with C5-replete C57BL/6 mice (Tables 2–3). This suggests that complement C5 plays a significant role, perhaps because C5a enhances activating Fcγ receptor function.31 In contrast, there was little difference between FVB/NJ and C57BL/6 mice in the clearance of incompatible mRBCs by the IgG3 NaM10-2H12 mAb (Tables 2–3). These differences may relate to the individual epitope specificities, affinities, or IgG subclasses of these 2 mAbs. In addition, it will be interesting to determine whether C5 is completely responsible for the findings with 10F7 by using congenic mouse strains that differ only at the C5 locus.32
Complement-mediated hemolysis in vitro of IgM-coated hGPA-Tg-mRBCs.
hGPA-Tg-mRBCs are susceptible to complement-mediated hemolysis in vitro in the presence of guinea pig serum. Thus, hGPA-Tg-mRBCs coated in vitro with either the IgM E4 anti-GPA mAb or the IgM J11d anti-CD24 mAb were hemolyzed following incubation in complement-containing serum, as were wild-type mRBCs coated with J11d (Table 4). As negative controls, wild-type mRBCs incubated with the E4 mAb, and uncoated hGPA-Tg-mRBCs, were not hemolyzed. Thus, the hGPA epitope density on hGPA-Tg-mRBCs and the avidity of mAb E4 were sufficient to activate complement, up to and including formation of functional C5b-9 membrane attack complexes.
Complement-mediated lysis in vitro of IgM-opsonized mRBCs
| Test RBCs . | mAb . | |||
|---|---|---|---|---|
| J11d, 2 μg/mL . | E4, 0.25 μg/mL . | E4, 0.1 μg/mL . | E4, 0.05 μg/mlL . | |
| Wild-type mRBCs | 91.8 | 0.0 | 0.0 | 1.9 |
| hGPA-Tg-mRBCs | 118.0 | 67.9 | 38.9 | 31.9 |
| Test RBCs . | mAb . | |||
|---|---|---|---|---|
| J11d, 2 μg/mL . | E4, 0.25 μg/mL . | E4, 0.1 μg/mL . | E4, 0.05 μg/mlL . | |
| Wild-type mRBCs | 91.8 | 0.0 | 0.0 | 1.9 |
| hGPA-Tg-mRBCs | 118.0 | 67.9 | 38.9 | 31.9 |
Wild-type and hGPA-Tg-mRBCs were incubated with distilled water as a positive control (defined as 100% hemolysis) or with guinea pig serum alone as a negative control (defined as background hemolysis). Test samples contained guinea pig serum as a source of complement and defined concentrations of purified mAbs. Hemolysis was determined by spectrophotometric evaluation of absorbance at 414 nm of the supernatant of each reaction and the results are presented as percent hemolysis compared to the distilled water control. All samples were tested in duplicate and the average results are presented. Binding of the mRBC-specific J11d anti–CD24 IgM mAb led to hemolysis of both types of mRBCs; the E4 anti–hGPA IgM mAb only hemolyzed hGPA-Tg-mRBCs.
IgM-mediated hemolysis in vivo of transfused incompatible hGPA-Tg-mRBCs.
Dose-response and time-course studies demonstrated that passive immunization intravenously of wild-type C57BL/6 mice with 100 μg of the IgM anti–hGPA E4 mAb caused dramatic and very rapid clearance of transfused incompatible hGPA-Tg-mRBCs (Table 5). For example, by comparing the counts per minute per millimeter of pRBCs at T = 0 of mAb E4-immunized recipients transfused with either wild-type or hGPA-Tg-mRBCs, approximately 50% of the hGPA-Tg-mRBCs were cleared in the short time between transfusion and drawing the T = 0 sample (ie, 2-5 minutes). In addition, by 2 hours after transfusion, hGPA-Tg-mRBC clearance in E4-immunized mice was virtually complete, with little change at the 24-hour time point (not shown). Finally, no significant RBC clearance occurred in mice immunized with mAb E4 and transfused with wild-type mRBCs (Table 5).
IgM-mediated clearance of incompatible hGPA-Tg-mRBCs
| Experiment no. . | mAb . | mRBCs transfused . | No. of mice . | Percent 2-h RBC survival, mean ± 1 SD . | Percent 24-h RBC survival, mean ± 1 SD . | Urine absorbance at 414 nm . |
|---|---|---|---|---|---|---|
| 1 | E4 | Wild-type | 4 | 91.3 ± 6.3 | ND | 0.04, 0.05, 0.10, 0.06 |
| 1 | E4 | hGPA-Tg | 4 | 19.1 ± 2.0 | ND | 0.28, 1.63, 0.74, 0.77 |
| 2 | None | hGPA-Tg | 3 | 99.6 ± 1.1 | 98.9 ± 3.9 | ND |
| 2 | E4 | hGPA-Tg | 5 | 29.4 ± 5.1 | 23.2 ± 3.2 | ND |
| Experiment no. . | mAb . | mRBCs transfused . | No. of mice . | Percent 2-h RBC survival, mean ± 1 SD . | Percent 24-h RBC survival, mean ± 1 SD . | Urine absorbance at 414 nm . |
|---|---|---|---|---|---|---|
| 1 | E4 | Wild-type | 4 | 91.3 ± 6.3 | ND | 0.04, 0.05, 0.10, 0.06 |
| 1 | E4 | hGPA-Tg | 4 | 19.1 ± 2.0 | ND | 0.28, 1.63, 0.74, 0.77 |
| 2 | None | hGPA-Tg | 3 | 99.6 ± 1.1 | 98.9 ± 3.9 | ND |
| 2 | E4 | hGPA-Tg | 5 | 29.4 ± 5.1 | 23.2 ± 3.2 | ND |
Wild-type C57BL/6 mice were passively immunized intravenously with 100 μg of the purified E4 IgM anti-hGPA mAb, or with the same volume of NS, 2 minutes prior to transfusion with 51Cr-labeled wild-type FVB/NJ mRBCs or hGPA-Tg-mRBCs. The RBC survival at 2 and 24 hours was then determined as described in “Materials and methods.” In addition, scanning wavelength spectrophotometry was used to identify hemoglobin (which has a characteristic absorption peak at 414 nm) in urine collected from the bladder at autopsy. Experiments 1 and 2 were performed on different days with different lots of mAb E4; the mice were humanely killed at 2 hours after transfusion in experiment 1 and at 24 hours after transfusion in experiment 2. Bladder urine obtained at autopsy in all mice in experiment 2 was clear yellow; therefore, the absorbance was not determined (ND).
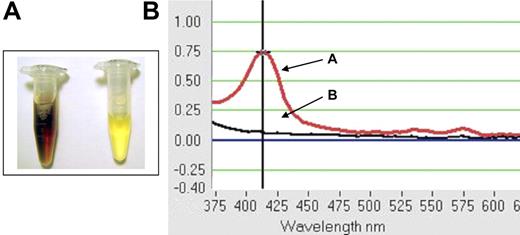
By 2 hours after transfusion, abundant, soluble, red-brown pigment was seen in the urine of all mAb E4-immunized wild-type C57BL/6 mice transfused with hGPA-Tg-mRBCs (Figure 5A). This pigment was not pelleted by centrifugation (not shown); thus, this color change was not due to gross hematuria. However, spectrophotometry confirmed that the pigment was free hemoglobin with a peak absorbance at 414 nm (Figure 5B); thus, the rapid clearance of transfused hGPA-Tg-mRBCs produced gross hemoglobinuria (Figure 5; Table 5). In contrast, no abnormal pigment was seen with mAb E4-immunized mice transfused with wild-type mRBCs. These results strongly suggest that the IgM E4 anti-hGPA mAb rapidly produced dramatic, intravascular, complement-mediated hemolysis of transfused incompatible hGPA-Tg-mRBCs.
Gross hemoglobinuria in wild-type C57BL/6 mice following passive immunization with the IgM E4 anti-hGPA mAb and transfusion with incompatible hGPA-Tg-mRBCs. Mice were passively immunized intravenously with 100 μg of the purified IgM E4 mAb. (A) Representative posttransfusion urine samples were obtained at autopsy from mice receiving incompatible hGPA-Tg-mRBCs (left sample) or receiving compatible wild-type mRBCs (right sample). (B) Urine samples from mice transfused with incompatible hGPA-Tg-mRBCs displayed absorbance peaks at 414 nm (eg, curve A); no absorbance peak at 414 nm was detectable in any urine sample obtained from mice transfused with compatible wild-type mRBC (eg, curve B).
Gross hemoglobinuria in wild-type C57BL/6 mice following passive immunization with the IgM E4 anti-hGPA mAb and transfusion with incompatible hGPA-Tg-mRBCs. Mice were passively immunized intravenously with 100 μg of the purified IgM E4 mAb. (A) Representative posttransfusion urine samples were obtained at autopsy from mice receiving incompatible hGPA-Tg-mRBCs (left sample) or receiving compatible wild-type mRBCs (right sample). (B) Urine samples from mice transfused with incompatible hGPA-Tg-mRBCs displayed absorbance peaks at 414 nm (eg, curve A); no absorbance peak at 414 nm was detectable in any urine sample obtained from mice transfused with compatible wild-type mRBC (eg, curve B).
To confirm the role of the membrane attack complex, FVB/NJ mice were used, which are C5 deficient.22 Interestingly, the survival of transfused hGPA-Tg-mRBCs in mAb E4-immunized FVB/NJ mice was only slightly less than that observed in complement-replete wild-type C57BL/6 mice (not shown). However, no hemoglobinuria was observed in FVB/NJ recipients. Thus, although complement-mediated intravascular hemolysis was strikingly evident in C57BL/6 mice, the predominant clearance mechanism was probably CR3-mediated phagocytosis of IgM/C3b-coated mRBCs. This will be evaluated further in future studies using C3 KO or CVF-treated, complement-deficient mice. The specific role of CR3, as opposed to CRIg,33 can also be evaluated using Cr3 KO mice34 and the M1/70 CR3-blocking mAb.35,36 Finally, the role of C5 can be explored further using congenic strains that only differ at this locus.32
Discussion
The need for mouse models to study immune-mediated hemolysis
Various animal models were used to study immune-mediated RBC destruction and its sequelae.7,37-40 However, these are not optimal for studying human HTRs due to their expense and the limited flexibility. Although mRBC blood group systems were described,8 and although antibody-mediated clearance of transfused, incompatible mRBCs occurs,9,10 these polymorphisms were not exploited to study HTRs. However, murine AIHA models, which clear mRBCs following infusion of monoclonal mRBC-specific autoantibodies,26-28,41-44 allowed evaluation of the roles of antibody isotype, IgG subclass, complement, and Fcγ receptors. Nonetheless, incomplete knowledge of target epitopes, the inability to evaluate early kinetics of mRBC destruction, and in vivo agglutination by IgM,41,45 make these less than ideal models for human HTRs. For example, autoantibody-induced agglutination in vivo almost never occurs in humans.46 Finally, transfusion of hen egg lysozyme (HEL) Tg mRBCs into wild-type mice actively immunized to HEL surprisingly caused removal of HEL from the mRBCs, with no mRBC destruction.47 Therefore, the current study describes novel mouse models of alloantibody-mediated intravascular and extravascular hemolysis, potentially suitable for studying HTR pathogenesis.
The relevance of Tg hGPA for immune-mediated hemolysis
Using hGPA-Tg-mRBCs to study antibody-induced mRBC clearance is highly relevant for the human situation. Thus, hGPA is the most abundant human RBC glycoprotein.48,49 Its primary importance results from carrying the MN antigens,48 glycopeptide antigens encoded by amino acid polymorphisms at positions 1 and 550,51 that also depend on O-glycosylation at positions 2-4.15,18,48,49,52,53 In addition, hGPA carries other types of blood group antigens.54,55 Antibodies to hGPA antigens cause HTRs,56 hemolytic disease of the newborn,57 and AIHA.55,58,59
The hGPA-Tg mouse provides a genetically inheritable, murine “blood group system.” Thus, wild-type mRBCs are “antigen-negative” and hGPA-Tg-mRBCs are “antigen positive.” Wild-type mice can be actively immunized with hGPA53 and many mouse mAbs are available.12-15,18,19,53,60-63 This type of blood group polymorphism (ie, the presence or absence of an entire protein) also occurs in humans: En(a−) RBCs lack hGPA, S−s−U− RBCs lack human glycophorin B, and “Rh-negative” RBCs lack the Rh(D) protein.
IgG-mediated hemolysis in passively immunized mice transfused with hGPA-Tg-mRBCs
This study provides useful insights into the pathogenesis of antibody-mediated hemolysis. For example, the kinetics of IgG1- and IgG3-mediated clearance of hGPA-Tg-mRBCs (Figure 4; Table 2) correlated well with that seen in human IgG-HTRs.64 In addition, although complement participates in IgG1-mediated clearance of hGPA-Tg-mRBCs, Fcγ receptors play a dominant role (Table 3). Interestingly, in a recent study, the IgG1 subclass caused minimal platelet destruction due to its strong binding to the inhibitory FcγRII25 ; the efficacy of the IgG1 10F7 mAb in hGPA-Tg-mRBC clearance is consistent with these findings due to the liver being the likely locus of RBC clearance in mice (Azeredo da Silveira,26 Fossati-Jimack et al,27,28 and Schiller et al29 ; D.A.S. and S.L.S., unpublished data, July 2005) and the absence of, or defective regulation by, FcγRII on mouse Kupffer cells.29,30 Indeed, when using IgG class-switch variants of a monoclonal mRBC-specific autoantibody to induce AIHA, the IgG1 variant was moderately pathogenic27,43 ; furthermore, this variant's pathogenicity resulted from its interaction with activating Fcγ receptors. Therefore, the efficacy 10F7 (Table 2; Figures 3–4) correlates well with what is known of IgG1s in murine immune-mediated hematologic disorders.
The difference between FVB/NJ and C57BL/6 mice in IgG1-mediated mRBC clearance (Tables 2–3) was particularly intriguing because FVB/NJ mice are deficient in C5. Therefore, C5a or the C5b-C9 membrane attack complex may be important in mRBC clearance by mAb 10F7. In contrast, in a mouse AIHA model (ie, comparing mRBC clearance in DBA/2 [C5 deficient] and BALB/C mice [C5 replete]), C5 was not involved in the anemia induced by several mRBC-specific mAbs, including an IgG1.41 Thus, our alloimmune IgG-HTR model provides novel information as compared to existing AIHA mouse models. Additional studies using congenic mice that only differ at the C5 locus32 will further clarify these findings.
Both complement and activating Fcγ receptors are important in IgG3-mediated clearance by mAb NaM10-2H12, with complement being dominant (Table 3). Although in the initial description murine IgG3 did not activate complement,65 later studies clearly demonstrated complement activation.26,66,67 Our results that activating Fcγ receptors contribute to IgG3-mediated clearance are particularly interesting in light of recent papers concerning murine IgG3 antibodies. Thus, it was originally proposed that a unique IgG3-specific Fcγ receptor facilitates phagocytosis of IgG3-coated mRBCs by murine macrophages.68 In addition, recent results suggest that no known murine Fcγ receptors function as IgG3 receptors because they do not bind IgG3 in a quantitative binding assay.25 Our results in vivo with Fcgr KO mice (Table 3) are consistent with this hypothesis; that is, there may be an additional, as yet undescribed, activating Fcγ receptor requiring the FcRγ chain. Finally, when human RBCs or hGPA-Tg-mRBCs are coated in vitro with NaM10-2H12 in serum-free medium, they are phagocytosed by RAW 264.769 mouse macrophages (D.A.S., S.L.S., and Sunny Seo, unpublished data, April 2004), also suggesting the presence of an activating Fcγ receptor.
In contrast, others suggested that FcγRI is the only IgG3 receptor, in that IgG3 binds to transfected cells expressing recombinant FcγRI and macrophages from Fcgr1 KO mice do not phagocytose IgG3-coated RBCs.70,71 Interestingly, although Fcgr KO mice express about 20% of normal levels of FcγRI, they cannot phagocytose IgG3-coated RBCs because phagocytosis presumably requires the ITAM function of FcRγ.70
Finally, in mouse ITP25 and AIHA models26,27 IgG3 antibodies are either nonpathogenic or slightly pathogenic, respectively. In addition, in the latter, only complement was involved for this mild effect. However, in our in vivo studies NaM10-2H12 led to rapid and pronounced clearance of incompatible mRBCs involving both complement and Fcγ receptors. These results are similar to those found with an IgG3 monoclonal anti-mRBC autoantibody,72 although the roles of complement and Fcγ receptors were not assessed. Thus, the mechanisms of mRBC clearance by IgG3 mAbs may depend on additional factors, such as antigen specificity and antigen affinity. Studies directly comparing these systems (eg, infusing anti-hGPA mAbs into hGPA-Tg mice as an AIHA model, and constructing Tg mice expressing hGPA on platelets as an ITP model) would provide additional insights.
Thus, this new model has characteristics that will deepen and extend the mechanistic understanding of IgG-mediated RBC destruction beyond that provided by existing models.
IgM-mediated hemolysis in passively immunized mice transfused with hGPA-Tg-mRBCs
The IgM E4 anti-hGPA mAb produced dramatic, intravascular, complement-mediated hemolysis of transfused hGPA-Tg-mRBCs (Table 5; Figure 5). To our knowledge, this represents the first time that an IgM has been effectively used to cause intravascular hemolysis in mice. Although previous studies used monoclonal IgM anti-RBC mAbs to induce AIHA, the anemia was due to intravascular agglutination.41 In contrast, given the small quantities of incompatible mRBCs transfused in the current study, their clearance could only be caused by complement-mediated hemolysis or phagocytosis. Furthermore, the rapid kinetics of this IgM-mediated clearance agrees well with that in human IgM-HTRs.64 Interestingly, despite the presence of the CD59 complement regulatory protein on hGPA-Tg-mRBCs, and despite the impression that mouse complement is “weak,”73 E4 caused complement-mediated intravascular hemolysis. Thus, this model will prove useful in unraveling and clarifying the pathogenic mechanisms of IgM-HTRs. IgM-mediated intravascular hemolysis could also be enhanced by constructing hGPA-Tg × Cd59 KO mice74 and using these mRBCs for transfusion.
When IgM-opsonized RBCs were previously transfused into guinea pigs,37,75 they were rapidly cleared by the liver and then released back into the circulation within 24 hours. The mechanism involved complement activation and adherence of IgM/C3b-coated RBCs to complement receptors (presumably CR3), but not phagocytosis. In contrast, passively infused IgM E4 mAb not only rapidly cleared hGPA-Tg-mRBCs, but the cleared mRBCs were also not released back into the circulation (Table 5). One explanation is that the CR3 integrins in our mice were already activated (even though the mice are maintained in a barrier facility and less than 0.5 μg endotoxin contaminated the infused E4 mAb). Alternatively, the newly described CRIg complement receptor,33 which does not require prior activation, may be responsible. Finally, unique aspects of the specificity or affinity of mAb E4 may be relevant.
These mouse studies provided a surprising result that may be relevant to the pathogenesis of human IgM-HTRs. Thus, complement-mediated intravascular hemolysis has long been thought to be primarily responsible for destroying transfused incompatible RBCs, as well as for the associated morbidity and mortality of human IgM-HTRs. Indeed, the signs of human IgM-HTRs, including hemoglobinemia and hemoglobinuria, provide solid evidence of intravascular hemolysis. However, without studying IgM-HTRs in a controlled setting, it has not been possible to determine the extent to which intravascular hemolysis clears transfused, incompatible RBCs. For example, the dramatic hemoglobinuria in mAb E4-immunized C57BL/6 recipients transfused with hGPA-Tg-mRBCs provides clear evidence of intravascular hemolysis (Figure 5). Coupled with its rapid kinetics, it initially seemed that most, if not all, of the incompatible mRBCs were destroyed intravascularly. Thus, the results of MAb E4-mediated mRBC clearance in C5-deficient FVB/NJ recipients were particularly interesting. As expected, no hemoglobinuria occurred in any FVB/NJ mice; however, these recipients cleared incompatible mRBCs with the same kinetics and almost the same efficiency as the complement-replete C57BL/6 recipients. This suggests that, in contrast to what is believed with human IgM-HTRs, although complement does cause intravascular hemolysis of transfused, IgM-coated RBCs, the IgM-mediated clearance of the incompatible RBCs may be predominantly due to complement-mediated extravascular hemolysis resulting from phagocytosis by hepatic Kupffer cells or splenic macrophages (or both). Studies evaluating complement receptors (eg, Cr3 KO mice), early components in the complement cascade (eg, C3 KO mice), and macrophage phagocytosis (eg, using liposomal clodronate) will provide more insight.
Future directions
Future studies will evaluate the relevance of this mouse model for human HTRs. In the current study, small amounts of radiolabeled incompatible mRBCs were transfused into anesthetized mice. Despite rapid clearance of transfused mRBCs, recipients did not display the clinical sequelae seen in human HTRs. Therefore, in future studies large quantities of incompatible mRBCs will be transfused and various physiologic parameters will be monitored.76
Authorship
Contribution: All authors participated in designing and performing the research; D.A.S. and S.L.S. controlled and analyzed the data; D.A.S. and S.L.S. wrote the paper; and all authors critiqued various drafts and checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven L. Spitalnik, Department of Pathology, 630 West 168th St, College of Physicians & Surgeons of Columbia University, New York, NY 10023; e-mail: ss2479@columbia.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Blood Foundation (S.L.S.) and the MetLife Foundation (G.R.H.).
This work was initially inspired by conversations with Dr Louis Fink. The authors wish to thank Drs Elwira Lisowska, Marilyn Telen, and Narla Mohandas for providing valuable reagents; Dr Murty Vundavalli for performing the FISH analysis; Drs Raymond Baggs and Carl Pinkert for advice regarding the design of mouse experiments; Mary Bolognino and Sunny Seo for technical assistance during the initial studies; Drs Robert Pierce and James Zimring for helpful discussions; and Dr Michael Shelanski for support and encouragement.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal