Abstract
In T cells anergy may be evoked by an unbalanced stimulation of the T-cell receptor in the absence of costimulation. Anergic T cells are unresponsive to new antigen receptor engagement and do not produce interleukin 2. We present evidence that anergizing stimuli induce changes in histone acetylation, which mediates transcriptional repression of interleukin 2 expression. In response to calcium signaling, anergic T cells up-regulate the expression of Ikaros, a zinc finger transcription factor essential for lymphoid lineage determination. Ikaros binds to the interleukin 2 promoter where it induces histone deacetylation. Confirming the role of Ikaros in the induction of T-cell anergy, cells with reduced Ikaros activity show defective inactivation in response to an anergizing stimulus. We propose a model in which tolerizing stimuli induce epigenetic changes on the interleukin 2 locus that are responsible for the stable inhibition of the expression of this cytokine in anergic T cells.
Introduction
Random generation of antigen receptors allows the adaptive immune system to initiate specific and effective responses against a vast array of invading pathogens. Randomly rearranged receptors, however, also have the potential to recognize self-antigens. To prevent the catastrophic consequences of an immune attack against the body's own constituents, several mechanisms are in place to control the activity of self-reactive lymphocytes. Most T cells that carry self-reactive T-cell receptors (TCRs) are clonally eliminated in the thymus; however, many of these cells survive this process and must be inactivated in the periphery. One of the mechanisms that contributes to the establishment of peripheral T-cell tolerance is anergy: an intrinsic process that leads to the inactivation of self-reactive T cells. In anergic T cells, the ability to proliferate and synthesize interleukin 2 (IL-2) upon antigen encounter is profoundly reduced, whereas the expression of other cytokines is affected to different degrees.
Signals transmitted through the TCR are able to implement 2 opposite programs: activation and anergy. The presence or absence of costimulatory and/or negative signals will determine the outcome. Thus, TCR engagement together with signals transmitted by CD28 induces T-cell activation, whereas in the absence of such costimulation, TCR engagement will lead to anergy.1-4 Negative signals derived from receptors such as CTLA-4 also contribute to the establishment of T-cell anergy in vivo.5,6
One of the consequences of TCR engagement in the absence of costimulation is a sustained increase in intracellular calcium, which leads to the activation of the calmodulin-dependent phosphatase calcineurin (Cn).7 Activated Cn dephosphorylates and induces the nuclear translocation of nuclear factor of activated T cells (NFAT) proteins, a family of transcription factors that play crucial roles in T-cell development and function.8,9 Members of the AP-1 family of transcription factors are the main transcriptional copartners of NFAT in T cells and require costimulation to be fully activated.10 In the absence of AP-1 cooperation, NFAT proteins induce the expression of a set of genes that are specifically up-regulated in T cells in response to anergizing stimuli.7 The mechanisms that induce unresponsiveness in anergic T cells are currently being elucidated. Recently, several ubiquitin ligases, whose expression is induced by calcium/Cn, have been shown to participate in the blocking of TCR signaling in anergic T cells by modifying or targeting components of the TCR signalosome for degradation.11-14 The analysis of specific genes activated in anergic T cells supports the existence of different mechanisms of tolerance induction in T lymphocytes, including not only protein degradation but also interference with signaling pathways coupled to antigen receptors, direct control of cell-cycle progression, and transcriptional modulation.7
Covalent modifications of the chromatin in specific regions of the genome can regulate the interactions of different proteins with the DNA, controlling processes such as recombination, replication, or gene transcription. Active chromatin generally associates with hyperacetylated histones, whereas silenced or inactive chromatin is usually associated with deacetylated histones. These modifications are the result of the activity of histone acetyl-transferases and histone deacetylases (HDACs). Targeting of these enzymes to a specific locus is likely mediated by transcriptional activators or repressors that recognize specific DNA motifs and are able to recruit them.15,16 The expression of Ikaros, the founding member of a family of transcription factors with essential roles in lymphoid lineage development,17-19 is up-regulated during the induction of T-cell anergy.7 Ikaros has a well-characterized transcriptional repressor activity mediated by its ability to recruit chromatin remodeling complexes containing HDACs.20
In this study we intended to determine whether epigenetic changes might regulate the expression of IL-2 in anergic cells. Our results indicate that during anergy induction, calcium signaling induces histone deacetylation of the il2 promoter in T-helper 1 (Th1) cells. We show that anergizing stimuli induce up-regulation of the expression of Ikaros, which binds to the il2 promoter and induces deacetylation of histone 4 (H4) and silencing of il2 expression. Furthermore, we present evidence that this direct mechanism of transcriptional repression may be crucial to achieve a complete inhibition of il2 expression in anergic T cells.
Therefore, we propose a model in which signals transmitted by calcium in response to anergic stimuli induce the expression of transcriptional repressors that cause stable epigenetic changes that lead to silencing of the il2 gene. These mechanisms play a critical role in the maintenance of anergic T-cell unresponsiveness.
Materials and methods
Mice
Wild-type C57BL6/J or DO11.10 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were maintained in pathogen-free conditions. All animal work was performed according to the guidelines set by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
Cell culture
CD4+ T cells were isolated from lymph nodes and spleens from 4- to 6-week-old mice using Dynabeads-CD4 magnetic beads (Dynal, Lake Success, NY). CD4+ T cells were stimulated with plate-bound anti–mouse CD3ϵ and CD28 (BD, San Jose, CA) at 0.5 μg/mL. Th1 cells were differentiated for 7 days in the presence of mouse IL-12 (10 ng/mL), anti–mouse IL-4 (10 μg/mL), and mouse IL-2 (10 units/mL; BD). T cells were cultured in RPMI. Jurkat and Phoenix Ecotropic (a gift from G. P. Nolan) cells were cultured in DMEM media supplemented with 10% FBS, 2 mM L-glutamine, and 50 μM β-mercaptoethanol.
Oral tolerance
A single dose of 25 mg of ovalbumin (Sigma, St Louis, MO; 100 mg/mL in PBS) was orally administered to DO11.10 mice. Seven days later, CD4+ T cells were isolated from these mice and from age- and sex-matched PBS-placebo–fed controls.
Induction of anergy
Th1 cells were treated with 1 μM ionomycin (Calbiochem, San Diego, CA) for 16 hours. In some experiments, trichostatin A (TSA; Upstate Biotechnology, Lake Placid, NY) at 10 nM was also added during the ionomycin treatment. Cells were then washed and rested for 2 to 4 hours in fresh medium before stimulation. Alternatively, cells were stimulated for 16 to 20 hours with 1 to 2 μg/mL plate-bound anti-CD3. Cells were then detached from the wells, washed, and allowed to rest for 72 hours before analysis.
Intracellular IL-2 staining
Cells were stimulated for 4 hours with anti-CD3 and anti-CD28. For the last 2 hours, Brefeldin A (Sigma-Aldrich, St Louis, MO) was added to the culture at 10 μg/mL. Cells were then fixed in 4% paraformaldehyde, permeabilized in 0.5% saponin, and incubated in with 10 μg/mL phycoerythrin (PE)–conjugated anti–mouse IL-2 antibody (BD) for 30 minutes. Washed cells were analyzed on a FACSCAN analyzer (Becton Dickinson, San Jose, CA).
ELISA
Culture supernatants were collected 24 hours following T-cell stimulation and IL-2 levels were measured in a sandwich enzyme-linked immunosorbent assay (ELISA; BD) following the manufacturer's recommendations.
Proliferation assay
Primary Th1 cells were stimulated with anti-CD3 and anti-CD28 for 48 hours and then labeled with 5-bromo-2′-deoxy-uridine for 12 hours. Incorporation of 5-bromo-2′-deoxy-uridine was determined using a BrdU labeling and detection kit (Roche, Indianapolis, IN) according to the manufacturer's instructions.
Transfections and reporter assays
Jurkat cells were transfected by electroporation in serum-free medium with pulses of 250 V and 960 μF. Plasmids used include pIL-2 (−585)–Luc (5 μg per transfection), containing the murine il2 promoter (kindly provided by D. J. McKean), and the Ikaros1-expressing plasmid pCDM8-Ik-1 (a gift from K. Georgopoulos). Twenty-four hours after transfection, cells were stimulated with 500 nM ionomycin and 20 nM phorbol-12-myristate-13-acetate (PMA; Calbiochem) or plate-bound anti–human CD3 and CD28 (BD) at 1 μg/mL. Eight hours after stimulation, cells were lysed and assayed for luciferase activity using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Cotransfection of a Renilla-luciferase plasmid was performed to normalize assays.
Retroviral infection of primary Th1 cells
The retroviral vector RV-IRES-GFP has been previously described.7 The RV-HA-Ikaros1-IRES-GFP contains a cDNA encoding murine Ikaros1 HA-tagged at its 5′ end. Phoenix Ecotropic packaging cells were transfected using a calcium/phosphate method with retroviral vectors. Retroviral supernatants were collected 48 hours after transfection, supplemented with polybrene (8 μg/mL), and used to infect CD4+ T cells 24 and 48 hours after stimulation. Infected cells were sorted for GFP expression. Ikaros expression was assessed by Western blot using anti-Ikaros (Santa Cruz Biotechnology, Santa Cruz, CA; or a gift from S. T. Smale) or anti-HA (Zymed, South San Francisco, CA) antibodies.
Chromatin immunoprecipitation (ChIP) assays
Histone acetylation status and Ikaros binding were assayed using a ChIP assay kit (Upstate Biotechnology), following the manufacturer's protocol. Nuclear lysates from 2 × 106 to 10 × 106 cells were subjected to immunoprecipitation overnight at 4°C with antiacetylated H4, anti-H4 (Upstate Biotechnology), anti-Ikaros, or anti-HA antibodies. Specific primer pairs were designed to amplify the il2 promoter (5′-TTAAAACAACAACGACAA AATAG/5′-GTGGTGGACAAACGCAAGAG), the CD3ϵ promoter (5′-CATTTCCAAGTGACGTGG/5′-AACACACTGGCTGCATGC), and the murine β-actin gene (5′-AAGGACTCCTATGTGGGTGACGA/5′-ATCTTCTCCATGTCGTCCCAGTTC). Polymerase chain reaction (PCR) products were visualized on an agarose gel stained with ethidium bromide. Band intensities were quantified using Image J software (National Institutes of Health [NIH], Bethesda, MD). For quantification, the intensity of any given band was corrected by the amount of input, and background or isotype control intensities were subtracted.
Quantitative real-time PCR (qPCR)
Reactions were performed in a SmartCycler (Cepheid, Sunnyvale, CA) using SYBR Green PCR mix (Abgene, Rochester, NY) and primers for mouse Ikaros (5′-GCTGGCTCTCAAGGAGGAG, 5′-CGCACTTGTACACCTTCAGC). A threshold was set in the linear range of the amplification curve (fluorescence = f[cycle]), and the number of cycles needed to reach it (Ct) was calculated for every sample. Fold induction was calculated as 2−ΔΔCt, using primers for actin as control. Melting curves and agarose gel electrophoresis established the specificity of the amplified band.
RNA interference assays
Ik-19i, an Ikaros-specific siRNA (5′-AAGACCTGTGCAAGATAGGAT-3′), was introduced into the pSUPER RNAi (Oligoengine, Seattle, WA). Control and pSUPER-IK-19i constructs were used together with a GFP plasmid to transfect Th1 cells by electroporation using the Mouse T-cell Nucleofector kit (AMAXA, Koeln, Germany) according to the manufacturer's protocol. Thirty-six hours after transfection, cells were sorted for GFP expression and then anergized with ionomycin. IL-2 production was assayed by ELISA following stimulation.
Results
T-cell activation induces histone hyperacetylation on the il2 promoter
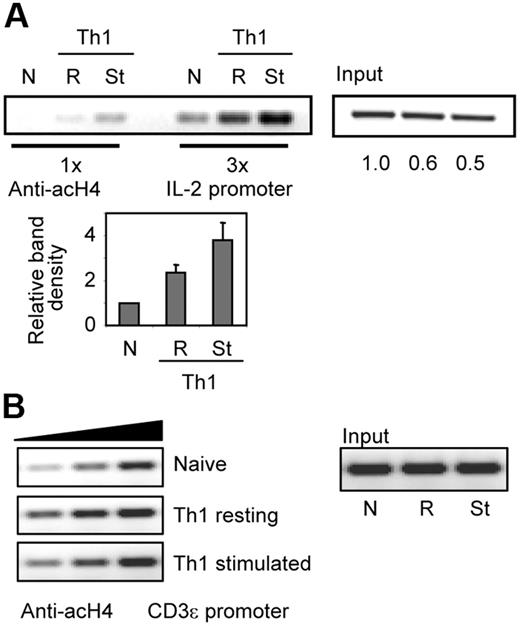
In order to determine how histone acetylation changes correlated with T-cell activation and IL-2 expression, we compared H4 acetylation status in naive and in resting or activated Th1 cells. In accordance with previous reports,21 naive CD4+ T cells showed a low level of H4 acetylation, which correlates with their limited ability to express IL-2. Upon differentiation, and having therefore gained increased capacity to transcribe the il2 promoter, Th1 cells showed enhanced H4 acetylation that was further increased after stimulation (Figure 1A). To determine whether this effect was specific for the IL-2 locus, we used primers to amplify the CD3ϵ promoter in samples obtained from naive and from resting and stimulated Th1 cells. A slight increase of H4 acetylation was observed in Th1 cells compared with naive cells. Nevertheless, stimulation did not induce further increase of H4 acetylation on the CD3ϵ promoter (Figure 1B).
T-cell stimulation induces hyperacetylation of the il2 promoter. ChIP assays were performed using antiacetylated H4 antibodies in naive (N) and in Th1 cells resting (R) or stimulated (St) for 6 hours with anti-CD3 and anti-CD28. PCR amplifications with primers for the (A) il2 or the (B) CD3ϵ promoters were done with different amounts of sample to confirm linearity of the amplification. One experiment of 2 with similar results is shown. Numbers below input bands indicate relative (to naive cells) amount quantified using qPCR. Graph shows relative (to naive cells) intensity of amplified complexes from all experiments (mean + SEM).
T-cell stimulation induces hyperacetylation of the il2 promoter. ChIP assays were performed using antiacetylated H4 antibodies in naive (N) and in Th1 cells resting (R) or stimulated (St) for 6 hours with anti-CD3 and anti-CD28. PCR amplifications with primers for the (A) il2 or the (B) CD3ϵ promoters were done with different amounts of sample to confirm linearity of the amplification. One experiment of 2 with similar results is shown. Numbers below input bands indicate relative (to naive cells) amount quantified using qPCR. Graph shows relative (to naive cells) intensity of amplified complexes from all experiments (mean + SEM).
The il2 promoter undergoes histone deacetylation in anergic T cells
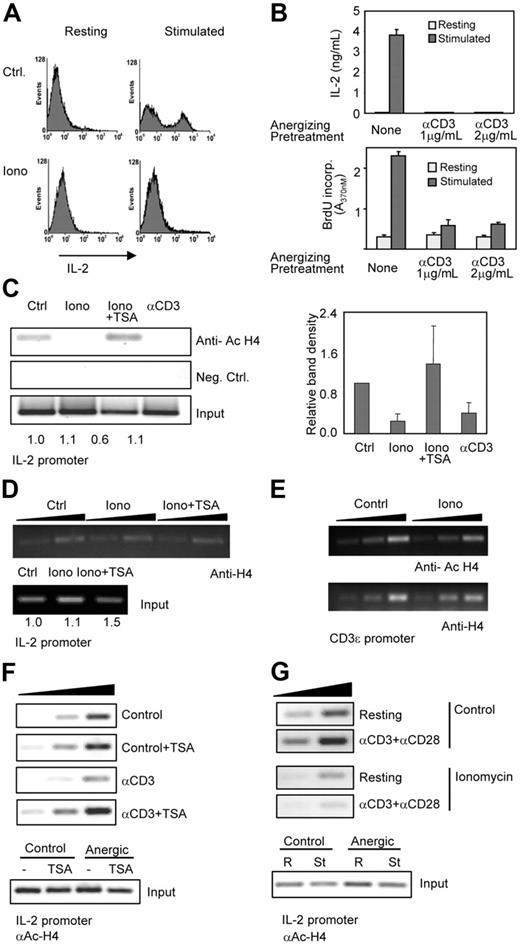
Anergic T cells are characterized by their inability to proliferate and produce IL-2 in response to TCR stimulation. We had previously shown that calcium-mediated signals were necessary for the induction of clonal anergy in Th1 cells.7 To determine whether unopposed calcium signaling could not only result in defects in TCR signaling but also exert a direct effect on the regulation of IL-2 expression, we used a model of clonal anergy in mouse Th1 cells by inducing a sustained increase in intracellular calcium with ionomycin. Ionomycin-treated Th1 cells were unresponsive and unable to produce IL-2 when restimulated (Figure 2A). A different model of clonal anergy induction was also analyzed by stimulating Th1 cells with anti-CD3 (signal1) in the absence of costimulation (signal 2). Unresponsiveness to TCR engagement in these cells was also assessed by failure to proliferate or produce IL-2 in response to full stimulation (Figure 2B). In anergic T cells, these defects reflect an inability to activate il2 transcription.7 To determine whether direct chromatin modifications of the il2 promoter could underlie and contribute to this defect in IL-2 expression, we analyzed H4 acetylation by ChIP using antiacetylated H4 antibodies. As shown in Figure 1, in Th1 cells, which had been primed 1 week before analysis and had gained the capacity to express IL-2, H4 molecules on the il2 promoter were clearly acetylated (Figure 1A and Figure 2C, Ctrl lane). However, when those same cells were subjected to an anergizing stimulus, a drastic loss of H4 acetylation was clearly detected (Figure 2C). H4 acetylation was reduced to levels even below those detected in naive T cells. Histone deacetylation was prevented when TSA was added during anergy induction to inhibit HDAC activity (Figure 2C). To rule out the possibility that the absence of detectable amplified ChIP fragments in the anergic samples could be the result of nucleosome mobilization, we performed ChIPs using an antibody that recognized H4 independently of its acetylation status. Similar amounts of chromatin from the il2 promoter were precipitated in control and anergic cells with this antibody (Figure 2D). The specificity of this effect was determined using primers for the CD3ϵ promoter, whose expression is not affected in anergic T cells. No differences in H4 acetylation were detected between control and anergized cells on this locus (Figure 2E). Anergic T cells present a long-lasting status of unresponsiveness to antigen stimulation. Epigenetic changes in the il2 promoter could account for the imposition of a stable closed inactive status on this locus. To test this possibility, Th1 cells were anergized by stimulation with anti-CD3 and left resting for 3 days before performing ChIP assays to assess the level of H4 acetylation. These experiments showed that loss of H4 acetylation at the il2 promoter was stable in anergic Th1 cells (Figure 2F). As shown in the experiments performed with ionomycin, TSA prevented anti-CD3–induced H4 deacetylation of the il2 promoter but had no effect on control untreated Th1 cells (Figure 2F). We had previously seen that increased IL-2 expression upon stimulation was associated with increased H4 acetylation of the IL-2 promoter (Figures 1A, 2G). We wanted to determine not only whether the il2 promoter in anergic T cells underwent H4 active histone deacetylation but also whether the inability to synthesize IL-2 in response to TCR engagement also correlated with an inability to increase histone acetylation at this locus. ChIP assays using antiacetylated H4 were carried out in previously anergized Th1 cells restimulated with anti-CD3 and anti-CD28. As opposed to control cells, anergic T cells could not induce enhanced histone acetylation of the IL-2 promoter upon stimulation (Figure 2G).
Anergizing stimuli induce H4 deacetylation of the il2 promoter in anergic Th1 cells. (A) Th1 cells were treated with 1 μM ionomycin (Iono) for 16 hours and analyzed by intracellular IL-2 staining following stimulation with anti-CD3 and anti-CD28. (B) Th1 cells were anergized with plate-bound anti-CD3 and, after a 3-day resting period, stimulated with anti-CD3 and anti-CD28. IL-2 expression (ELISA) and BrdU incorporation were measured. Error bars show mean ± SEM of 3 experiments. (C) ChIP assays were performed with antiacetylated H4 antibodies to assess acetylation of the il2 promoter. Control cells were analyzed and compared with cells anergized using either ionomycin (Iono) or anti-CD3. In some cells, TSA (10 nM) was added together with ionomycin to inhibit the activity of HDACs. Experiments were also performed with a control IgG. PCR products are shown for precipitated fractions and sample inputs. One experiment of 4 is shown. Numbers below input bands indicate relative amount (to control cells) quantified using qPCR. Graph shows relative intensity of immunoprecipitated complexes from all experiments (mean + SEM). (D) ChIP experiments were performed in control (Ctrl) and anergized (Iono) cells (± TSA) using anti-H4 antibodies. PCR reactions were carried out with different amounts of sample (2-fold increases) to confirm linearity of the amplification. (E) Samples from panels C and D were also analyzed using primers for the CD3ϵ promoter. (F) Th1 cells were anergized by stimulation with anti-CD3 for 16 hours in the presence or the absence of TSA, washed, and left resting for 3 days. After that, ChIP assays were performed using antiacetylated H4 antibodies and primers specific for the IL-2 promoter. The gels show PCR amplifications of 3 different amounts of chromatin (2-fold increases) for the anti-CD3–treated and control cells. PCR amplifications of the inputs from the different samples are also shown. (G) Control cells or cells anergized with ionomycin were restimulated with anti-CD3 and anti-CD28 for 6 hours and then used to immunoprecipitate chromatin complexes using an antiacetylated H4 antibody. Amplified products from different amounts of each sample (2-fold increases) and from the different inputs are shown.
Anergizing stimuli induce H4 deacetylation of the il2 promoter in anergic Th1 cells. (A) Th1 cells were treated with 1 μM ionomycin (Iono) for 16 hours and analyzed by intracellular IL-2 staining following stimulation with anti-CD3 and anti-CD28. (B) Th1 cells were anergized with plate-bound anti-CD3 and, after a 3-day resting period, stimulated with anti-CD3 and anti-CD28. IL-2 expression (ELISA) and BrdU incorporation were measured. Error bars show mean ± SEM of 3 experiments. (C) ChIP assays were performed with antiacetylated H4 antibodies to assess acetylation of the il2 promoter. Control cells were analyzed and compared with cells anergized using either ionomycin (Iono) or anti-CD3. In some cells, TSA (10 nM) was added together with ionomycin to inhibit the activity of HDACs. Experiments were also performed with a control IgG. PCR products are shown for precipitated fractions and sample inputs. One experiment of 4 is shown. Numbers below input bands indicate relative amount (to control cells) quantified using qPCR. Graph shows relative intensity of immunoprecipitated complexes from all experiments (mean + SEM). (D) ChIP experiments were performed in control (Ctrl) and anergized (Iono) cells (± TSA) using anti-H4 antibodies. PCR reactions were carried out with different amounts of sample (2-fold increases) to confirm linearity of the amplification. (E) Samples from panels C and D were also analyzed using primers for the CD3ϵ promoter. (F) Th1 cells were anergized by stimulation with anti-CD3 for 16 hours in the presence or the absence of TSA, washed, and left resting for 3 days. After that, ChIP assays were performed using antiacetylated H4 antibodies and primers specific for the IL-2 promoter. The gels show PCR amplifications of 3 different amounts of chromatin (2-fold increases) for the anti-CD3–treated and control cells. PCR amplifications of the inputs from the different samples are also shown. (G) Control cells or cells anergized with ionomycin were restimulated with anti-CD3 and anti-CD28 for 6 hours and then used to immunoprecipitate chromatin complexes using an antiacetylated H4 antibody. Amplified products from different amounts of each sample (2-fold increases) and from the different inputs are shown.
Histone deacetylation contributes to the inhibition of IL-2 expression in anergic Th1 cells
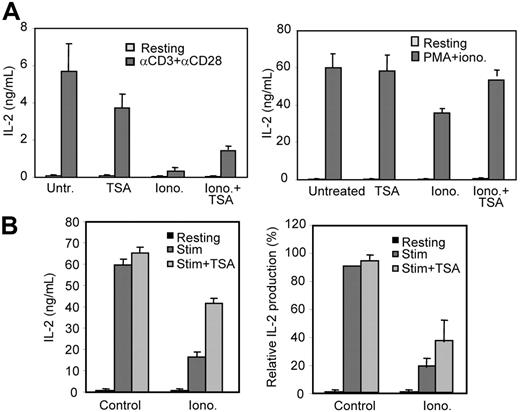
To evaluate the contribution of anergy-induced histone deacetylation to the overall defect in IL-2 synthesis in anergic T cells, we inhibited anergy-induced HDAC activity using TSA. Th1 cells were treated with ionomycin in the presence or absence of TSA, and IL-2 production in response to TCR stimulation was analyzed. As expected, ionomycin induced an unresponsive status that was characterized by a profound defect in IL-2 production following stimulation (Figure 3A). TSA had no effect on the ability of control nonanergic T cells to make IL-2; however, TSA partially reversed the anergizing effect of ionomycin (Figure 3A left panel). Cells anergized in the presence of TSA were able to produce 3 times as much IL-2 as control anergic cells (P < .05 when comparing means with a t test for paired samples). These results suggested that TCR signaling blockade, although playing an important role in preventing IL-2 expression in anergic T cells, needed complementary mechanisms of active silencing to completely shut down IL-2 production. In order to bypass proximal blocks on TCR signaling in anergic T cells, we evaluated the response of these cells to stimulation with PMA and ionomycin. As previously shown,22,23 cells pretreated with ionomycin showed only partial recovery when activated with PMA plus ionomycin, which suggested the presence of other blocks downstream of protein kinase C. Recovery was, however, almost complete when TSA was added during anergy induction (Figure 3A right panel). Inhibition of HDAC activity with TSA in anergic cells during a secondary stimulation was also able to partially restore IL-2 expression (Figure 3B), suggesting that mechanisms to prevent reacetylation—most likely by inducing further deacetylation of histones—were engaged in anergic T cells when restimulated. The presence of TSA during restimulation had, however, no effect on control cells or cells anergized in the presence of TSA (Figure 3B; data not shown).
HDAC inhibition interferes with IL-2 expression blockade in anergic Th1 cells. (A) Th1 cells were treated with ionomycin in the presence or absence of TSA. Untreated and TSA-treated cells were used as controls. After a 16-hour treatment, cells were allowed to rest for 4 hours and then restimulated with anti-CD3 and anti-CD28 (right) or PMA and ionomycin (left). IL-2 production was determined by ELISA. Bars show mean + SEM of 3 experiments. (B) After a 16-hour treatment with ionomycin, Th1 cells were allowed to rest for 4 hours and then restimulated with anti-CD3 and anti-CD28 in the presence or absence of TSA. IL-2 production was measured by ELISA. Graphs show either IL-2 values from one representative experiment (mean + SEM from 3 different samples per condition; left) or IL-2 production relative to control stimulated Th1 cells from 4 independent experiments (mean + SEM; right).
HDAC inhibition interferes with IL-2 expression blockade in anergic Th1 cells. (A) Th1 cells were treated with ionomycin in the presence or absence of TSA. Untreated and TSA-treated cells were used as controls. After a 16-hour treatment, cells were allowed to rest for 4 hours and then restimulated with anti-CD3 and anti-CD28 (right) or PMA and ionomycin (left). IL-2 production was determined by ELISA. Bars show mean + SEM of 3 experiments. (B) After a 16-hour treatment with ionomycin, Th1 cells were allowed to rest for 4 hours and then restimulated with anti-CD3 and anti-CD28 in the presence or absence of TSA. IL-2 production was measured by ELISA. Graphs show either IL-2 values from one representative experiment (mean + SEM from 3 different samples per condition; left) or IL-2 production relative to control stimulated Th1 cells from 4 independent experiments (mean + SEM; right).
Ikaros binds the il2 promoter in anergic Th1 cells
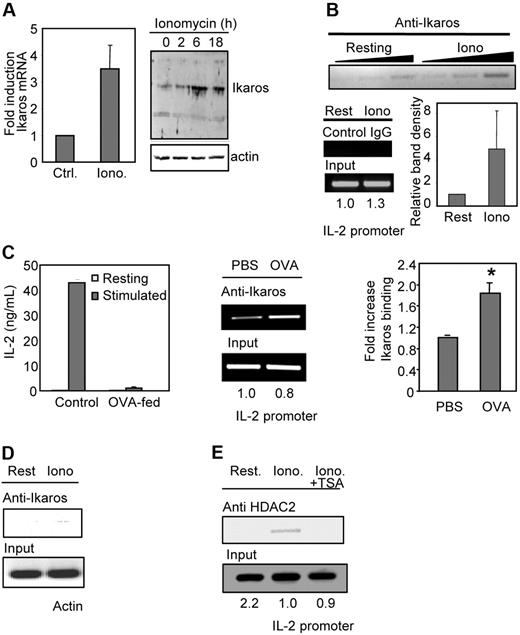
We had previously shown that the expression of a specific set of genes was up-regulated during anergy induction in T cells. Supporting a possible role of Ikaros in T-cell anergy, we were able to detect a clear up-regulation of Ikaros mRNA expression in Th1 cells in response to calcium signals (3- to 4-fold) that translated into an increased accumulation of Ikaros protein in those cells (Figure 4A).
Calcium signaling induces increased expression and binding of Ikaros to the il2 promoter in anergic Th1 cells. (A) Th1 cells were treated with ionomycin (Iono) for 6 hours and the expression of Ikaros mRNA was measured by qPCR compared with resting cells (Ctrl). Graph shows mean + SEM of 3 independent experiments. Ikaros expression was also determined by Western blot. Anti–mouse β-actin antibodies were used to control loading. (B) In vivo binding of Ikaros to the il2 promoter in anergic Th1 cells was determined by ChIP using anti-Ikaros antibodies and primers for the il2 promoter on ionomycin-treated (Iono) and control resting (Rest) Th1 cells. ChIPs were also performed with a control IgG. PCR products are shown for precipitated fractions and sample inputs. One experiment of 4 with similar results is shown. Numbers below input bands represent relative amount (to control cells) quantified using qPCR. Reactions were carried out with 3 different amounts of sample (2-fold increases) to confirm linearity of the amplification. Graph shows relative band (to control cells) intensity of immunoprecipitated complexes from all experiments (mean + SEM). (C) CD4+ T cells from DO11.10 mice were isolated from OVA-fed mice and age- and sex-matched PBS-fed controls. Anergy was assessed by ELISA following stimulation with anti-CD3 and anti-CD28. Bars show mean + SEM of 3 different experiments. Chromatin complexes were immunoprecipitated using an anti-Ikaros antibody. PCR products using primers for the il2 promoter are shown for the precipitated fraction and the sample inputs. Gel shows one experiment of 4 with similar results. Numbers below input bands represent relative amount (to the value in PBS-fed mice) quantified using qPCR. Reactions were carried out with different amounts of immunoprecipitated sample (2-fold increases) to confirm linearity of the amplification. Graph shows relative intensity of immunoprecipitated complexes from 4 different sets of mice (mean + SEM). *P < .02 when comparing band intensities from PBS- and OVA-fed mice using a t test. (D) Samples from panel B were analyzed using primers for mouse actin. (E) ChIP assays were performed using anti–mouse HDAC2 antibodies on samples prepared from resting (Rest) Th1 cells or cells treated with ionomycin (Iono) ± TSA. Samples were amplified with primers for the il2 promoter. Gel shows 1 of 3 experiments. Numbers below input bands represent relative amount (to the value in resting cells) quantified using qPCR.
Calcium signaling induces increased expression and binding of Ikaros to the il2 promoter in anergic Th1 cells. (A) Th1 cells were treated with ionomycin (Iono) for 6 hours and the expression of Ikaros mRNA was measured by qPCR compared with resting cells (Ctrl). Graph shows mean + SEM of 3 independent experiments. Ikaros expression was also determined by Western blot. Anti–mouse β-actin antibodies were used to control loading. (B) In vivo binding of Ikaros to the il2 promoter in anergic Th1 cells was determined by ChIP using anti-Ikaros antibodies and primers for the il2 promoter on ionomycin-treated (Iono) and control resting (Rest) Th1 cells. ChIPs were also performed with a control IgG. PCR products are shown for precipitated fractions and sample inputs. One experiment of 4 with similar results is shown. Numbers below input bands represent relative amount (to control cells) quantified using qPCR. Reactions were carried out with 3 different amounts of sample (2-fold increases) to confirm linearity of the amplification. Graph shows relative band (to control cells) intensity of immunoprecipitated complexes from all experiments (mean + SEM). (C) CD4+ T cells from DO11.10 mice were isolated from OVA-fed mice and age- and sex-matched PBS-fed controls. Anergy was assessed by ELISA following stimulation with anti-CD3 and anti-CD28. Bars show mean + SEM of 3 different experiments. Chromatin complexes were immunoprecipitated using an anti-Ikaros antibody. PCR products using primers for the il2 promoter are shown for the precipitated fraction and the sample inputs. Gel shows one experiment of 4 with similar results. Numbers below input bands represent relative amount (to the value in PBS-fed mice) quantified using qPCR. Reactions were carried out with different amounts of immunoprecipitated sample (2-fold increases) to confirm linearity of the amplification. Graph shows relative intensity of immunoprecipitated complexes from 4 different sets of mice (mean + SEM). *P < .02 when comparing band intensities from PBS- and OVA-fed mice using a t test. (D) Samples from panel B were analyzed using primers for mouse actin. (E) ChIP assays were performed using anti–mouse HDAC2 antibodies on samples prepared from resting (Rest) Th1 cells or cells treated with ionomycin (Iono) ± TSA. Samples were amplified with primers for the il2 promoter. Gel shows 1 of 3 experiments. Numbers below input bands represent relative amount (to the value in resting cells) quantified using qPCR.
Sequence analysis had revealed that the il2 promoter might contain several binding sites for Ikaros.24 To determine whether Ikaros could bind the il2 promoter in anergic T cells, we performed ChIP assays using anti-Ikaros antibodies. These experiments showed direct Ikaros binding to the il2 promoter in anergic Th1 cells (Figure 4B). We were also able to detect similar Ikaros binding to the IL-2 promoter in an in vivo model of T-cell tolerance. Oral administration of antigens has been shown to induce peripheral T-cell tolerance by 2 different mechanisms: deletion/anergy or induction of regulatory T cells. The specific outcome is determined by the administration regimen. High doses, like the one used in our experiments, preferentially induce anergy, whereas low doses induce a new population of T cells identified as regulatory T cells.25 We analyzed CD4+ T cells from DO11.10 mice, which express a transgenic TCR that recognizes ovalbumin323-339, fed with high doses of ovalbumin and confirmed they were anergic (Figure 4C). We had previously seen that these T cells up-regulated the expression of several potential anergy-inducing genes, including Ikaros.7 When ChIPs were performed on these cells using an antibody against Ikaros, a clear increase in Ikaros binding to the il2 promoter (2-fold) was detected in cells from orally tolerized mice compared with the control PBS-fed mice (Figure 4C).
Binding of Ikaros to the il2 promoter proved to be specific, as no amplified fragments could be detected when primers for mouse actin were used under the same conditions in which clear binding was detected using il2 promoter primers (compare Figure 4B and 4D). To determine whether HDAC activity was also being recruited together with Ikaros to the il2 promoter in response to unopposed calcium signaling, ChIP assays were performed using an anti-HDAC2 antibody. HDAC2 recruitment to the il2 promoter could only be detected in Th1 cells anergized with ionomycin but not in control untreated Th1 cells or cells anergized in the presence of the HDAC inhibitor TSA (Figure 4E).
Ikaros inhibits il2 expression and induces histone deacetylation on the il2 promoter in T cells
In order to determine whether Ikaros could repress il2 expression, we transfected Jurkat cells with a luciferase reporter plasmid containing the murine il2 promoter and increasing amounts of an Ikaros-expressing plasmid. Under these conditions, transcription of the il2 promoter was inhibited by Ikaros in a dose-dependent manner (Figure 5A). Using retroviral vectors allowed us to carry out our studies in primary T cells and avoid problems associated with overexpression. Levels of Ikaros expression attained in retrovirally infected Th1 cells (RV-HA-Ikaros-IRES-GFP virus) were very similar to those detected in response to calcium stimulation (Figure 5B). In transduced cells, hemagglutinin (HA)–tagged Ikaros was readily detectable using anti-HA antibodies and localized to the nucleus (data not shown). As seen in Jurkat cells, synthesis of IL-2 in stimulated Th1 cells was markedly reduced in cells transduced with an Ikaros-expressing retroviral vector (Figure 5B). ChIP assays were also performed in infected cells using an anti-HA antibody. Ikaros binding to the il2 promoter was detected in RV-Ikaros-IRES-GFP–transduced cells compared with control cells (ie, RV-IRES-GFP–infected cells; Figure 5C). ChIP experiments using antibodies against acetylated H4 in Ikaros-expressing Th1 cells were performed to determine whether Ikaros binding could induce histone deacetylation on the il2 promoter. Cells expressing HA-Ikaros showed a profound loss of H4 acetylation on the il2 promoter compared with control cells (Figure 5D). The specificity of this effect was confirmed using primers for the CD3ϵ promoter. Ikaros expression did not affect the H4 acetylation of the CD3ϵ promoter (Figure 5D). To confirm that repression of IL-2 expression by Ikaros was in fact mediated through its capacity to recruit HDACs, the effect of inhibiting HDAC activity with TSA was determined in Th1 cells infected with a retrovirus directing the expression of Ikaros. TSA treatment prevented Ikaros-mediated inhibition of IL-2 expression and restored its production to control levels (Figure 5E).
Binding of Ikaros to the il2 promoter induces H4 deacetylation and inhibits il2 expression. (A) Jurkat cells were transfected with an il2–luciferase reporter plasmid and increasing amounts of an Ikaros expression plasmid. Twenty-four hours after transfection, cells were stimulated with PMA and ionomycin, and luciferase activity was determined. (B) Western blot using an anti-Ikaros antibody was performed with samples from RV-GFP–infected control resting (C) and ionomycin-treated (Io) cells and cells infected with RV-Ikaros-IRES-GFP (Ik). Infected cells were stimulated with anti-CD3 and anti-CD28 for 24 hours and IL-2 expression determined by ELISA. Bars show mean + SEM of 3 independent experiments. (C) Th1 cells transduced with virus expressing HA-Ikaros (Ik) and GFP or control GFP-virus were sorted and ChIPs performed using an anti-HA antibody. PCR products amplified with primers for the il2 promoter are shown for immunoprecipitated complexes and inputs. Gel shows one of 3 experiment with similar results. Numbers below input bands represent relative amount (to the value in RV-GFP–infected cells) quantified using qPCR. Graph shows relative band intensity (to the intensity in GFP-infected control cells) of immunoprecipitated complexes from all experiments (mean + SEM). (D) ChIP assays were performed using an antiacetylated H4 antibody on samples from transduced Th1 cells expressing GFP or HA-Ikaros and GFP (Ik). PCR products amplified with primers for the il2 promoter are shown for immunoprecipitated complexes and inputs. One experiment of 3 with similar results is shown. Numbers below input bands represent relative amount (to the value in RV-GFP–infected cells) quantified using qPCR. Graph shows relative band intensity of amplified immunoprecipitated complexes from all experiments (mean + SEM). Samples were also amplified using primers for the CD3ϵ promoter. (E) Infected Th1 cells with retroviruses expressing either GFP (control) or Ikaros and GFP (Ikaros) were treated with TSA from day 2 after infection. Four days later, cells were stimulated with anti-CD3 and anti-CD28 for 24 hours and IL-2 expression was determined by ELISA. Bars show mean + SEM of 3 different samples.
Binding of Ikaros to the il2 promoter induces H4 deacetylation and inhibits il2 expression. (A) Jurkat cells were transfected with an il2–luciferase reporter plasmid and increasing amounts of an Ikaros expression plasmid. Twenty-four hours after transfection, cells were stimulated with PMA and ionomycin, and luciferase activity was determined. (B) Western blot using an anti-Ikaros antibody was performed with samples from RV-GFP–infected control resting (C) and ionomycin-treated (Io) cells and cells infected with RV-Ikaros-IRES-GFP (Ik). Infected cells were stimulated with anti-CD3 and anti-CD28 for 24 hours and IL-2 expression determined by ELISA. Bars show mean + SEM of 3 independent experiments. (C) Th1 cells transduced with virus expressing HA-Ikaros (Ik) and GFP or control GFP-virus were sorted and ChIPs performed using an anti-HA antibody. PCR products amplified with primers for the il2 promoter are shown for immunoprecipitated complexes and inputs. Gel shows one of 3 experiment with similar results. Numbers below input bands represent relative amount (to the value in RV-GFP–infected cells) quantified using qPCR. Graph shows relative band intensity (to the intensity in GFP-infected control cells) of immunoprecipitated complexes from all experiments (mean + SEM). (D) ChIP assays were performed using an antiacetylated H4 antibody on samples from transduced Th1 cells expressing GFP or HA-Ikaros and GFP (Ik). PCR products amplified with primers for the il2 promoter are shown for immunoprecipitated complexes and inputs. One experiment of 3 with similar results is shown. Numbers below input bands represent relative amount (to the value in RV-GFP–infected cells) quantified using qPCR. Graph shows relative band intensity of amplified immunoprecipitated complexes from all experiments (mean + SEM). Samples were also amplified using primers for the CD3ϵ promoter. (E) Infected Th1 cells with retroviruses expressing either GFP (control) or Ikaros and GFP (Ikaros) were treated with TSA from day 2 after infection. Four days later, cells were stimulated with anti-CD3 and anti-CD28 for 24 hours and IL-2 expression was determined by ELISA. Bars show mean + SEM of 3 different samples.
T cells with reduced Ikaros expression are more resistant to anergy induction
In order to determine the contribution of Ikaros-mediated il2 repression to T-cell anergy, we analyzed the effect on anergy of blocking Ikaros expression in Th1 cells. To avoid problems resulting from abnormal T-cell development in Ikaros-null mice,19 wild-type mouse Th1 cells were transfected with a plasmid expressing an siRNA specific for Ikaros mRNA. siRNA-transfected cells were treated with ionomycin to induce unresponsiveness. Reduction of Ikaros expression was determined by Western blot and varied from 40% to 60% (Figure 6A). The defect in IL-2 production in anergic cells was 3-fold smaller in siRNA-transfected cells than the one seen in control transfected cells (P <.01 when comparing means of fold reduction values with a t test for paired samples; Figure 6A). Cells were also retrovirally transduced with a virus expressing Ikaros 6 (Ik6), an isoform of Ikaros that lacks DNA binding activity but is able to dimerize with other Ikaros proteins and therefore block their activity. While anergic control cells underwent deacetylation of the il2 locus, in Ik6-expressing cells, defective induction of anergy correlated with deficient H4 deacetylation. This effect was il2 promoter specific, as no differences were detected when control primers for the CD3ϵ promoter were used (Figure 6B).
Inhibition of Ikaros activity results in defective anergy induction and il2 promoter deacetylation in Th1 cells. (A) Th1 cells were transfected with the pSUPER-IK-19i vector (Ik-siRNA) or empty pSUPER (Control) and pGFP. After 36 hours, GFP+ sorted cells were treated with or without ionomycin (Iono) and stimulated with anti-CD3 and anti-CD28. IL-2 production was determined by EILSA. Bars show mean + SEM of 3 different experiments. Following ionomycin treatment, Ikaros levels from both cell populations were measure by Western blot. Blots were also probed with antiactin antibody to control loading. (B) ChIP assays were performed on Th1 cells transduced with virus expressing GFP or Ikaros-6 and GFP (Ik6) and sorted for GFP expression. Cells were rested or treated with ionomycin (Io). Samples precipitated with an antiacetylated H4 antibody were amplified with primers for the il2 promoter. Products are shown for precipitated complexes and inputs. One experiment of 2 is shown. Numbers below bands represent relative intensity (to RV-GFP–infected cells). Precipitated complexes were also amplified using primers for the CD3ϵ promoter. Anergy induction in sorted cells was also analyzed by ELISA following stimulation with anti-CD3 and anti-CD28. Bars show mean + SEM of the anergy index value (ratio of IL-2 produced by control cells and anergized cells) of 2 different experiments performed in triplicate.
Inhibition of Ikaros activity results in defective anergy induction and il2 promoter deacetylation in Th1 cells. (A) Th1 cells were transfected with the pSUPER-IK-19i vector (Ik-siRNA) or empty pSUPER (Control) and pGFP. After 36 hours, GFP+ sorted cells were treated with or without ionomycin (Iono) and stimulated with anti-CD3 and anti-CD28. IL-2 production was determined by EILSA. Bars show mean + SEM of 3 different experiments. Following ionomycin treatment, Ikaros levels from both cell populations were measure by Western blot. Blots were also probed with antiactin antibody to control loading. (B) ChIP assays were performed on Th1 cells transduced with virus expressing GFP or Ikaros-6 and GFP (Ik6) and sorted for GFP expression. Cells were rested or treated with ionomycin (Io). Samples precipitated with an antiacetylated H4 antibody were amplified with primers for the il2 promoter. Products are shown for precipitated complexes and inputs. One experiment of 2 is shown. Numbers below bands represent relative intensity (to RV-GFP–infected cells). Precipitated complexes were also amplified using primers for the CD3ϵ promoter. Anergy induction in sorted cells was also analyzed by ELISA following stimulation with anti-CD3 and anti-CD28. Bars show mean + SEM of the anergy index value (ratio of IL-2 produced by control cells and anergized cells) of 2 different experiments performed in triplicate.
Discussion
Functional inactivation of self-reactive T cells is necessary to ensure proper maintenance of immune tolerance. The molecular mechanisms responsible for the establishment and maintenance of T-cell anergy are only recently beginning to be elucidated. In T cells, clonal anergy may be induced by partial or suboptimal stimulation. Anergizing stimuli activate calcium/Cn/NFAT signals that lead to the induction of a specific set of genes.7 Evidence supports that proteins encoded by some of those genes are responsible for the inhibition of T-cell function during anergy. In anergic T cells, signaling pathways activated by TCR engagement may be affected at different levels, leading to inhibition of cell activation, clonal proliferation, or cytokine expression.26-30 Our results show that an active mechanism of transcriptional repression is engaged in anergic Th1 cells to inhibit IL-2 expression. Anergic T cells induce Ikaros expression that targets the il2 promoter, where it binds and recruits HDACs, inducing epigenetic changes that result in inhibition of il2 expression.
Changes in chromatin structure have been shown to regulate il2 expression in activated T cells. The distal region of the il2 promoter has a partially open conformation in naive T cells, which are already able to synthesize IL-2 upon stimulation.21 Increased susceptibility to nucleases of the il2 promoter locus follows T-cell stimulation, which correlates with increased ability to activate il2 transcription.21,31 Consistent with these data, our results show that in Th1 cells, which have already undergone primary TCR engagement, core H4 acetylation on the il2 promoter is enhanced compared with naive cells. This may ensure a permissive il2 locus able to sustain increased expression following secondary stimulation. Similar regulation of locus accessibility has been described for other cytokines, like IFNγ and IL-4, during T-helper differentiation.32,33 In both cases, changes in histone acetylation of those genes ultimately determine whether they become active or silent in Th1 or Th2 subpopulations.33 Partial stimulation of the TCR in the absence of costimulation should not induce further histone acetylation and chromatin remodeling of the il2 locus, as signals activated by CD28 engagement seem to be required for this process.34 We show that epigenetic changes are also established in T cells in response to anergizing stimuli. Active histone deacetylation of the il2 promoter occurs in Th1 cells following partial stimulation, causing stable epigenetic changes in the il2 locus. As in other differentiation processes, epigenetic changes may, thus, contribute to induce a permanent inactive status on the il2 locus and to make inhibition of the il2 gene expression stable in anergic T cells.
We had previously shown that calcium signals activated during anergy induction are responsible for the up-regulation of Ikaros expression in a Cn/NFAT-dependent manner.7 Ikaros has been identified as a key regulator of lymphoid lineage development.17-19 During T-cell development, Ikaros contributes to gene silencing by facilitating the assembly of silent chromatin at specific loci.18,35,36 Several mechanisms have been proposed for Ikaros' transcriptional repressor activity,20,37,38 including the recruitment of chromatin remodeling complexes containing HDACs.20,35,39 The ability of Ikaros to recruit HDACs plays a crucial role in its function as a repressor of gene expression.20 In lymphoid cells, Ikaros associates with NurD and mSIN3 complexes, which contain HDACs, contributing to the control of gene expression during lymphopoiesis.35 Our results indicate that Ikaros may also control mature T-cell function through similar mechanisms. In anergic T cells, Ikaros binds to the il2 promoter and recruits HDAC activity (Figures 4–5). Activity of HDACs seems to be necessary for Ikaros-mediated il2 transcriptional repression because inhibition of HDACs with TSA partially blocks suppression of IL-2 production (Figure 3). Activation of il2 transcription has also been found to be associated with active demethylation of the il2 promoter.40 In activated T cells, Ikaros can colocalize with DNA methyltransferases during the S phase of the cell cycle,41 therefore we cannot rule out the possibility that additional mechanisms might also be involved in silencing il2 gene expression in anergic T cells.
Several mechanisms have been proposed to explain the Ras/mitogen-activated protein kinase (MAPK) pathways block that causes defects in JNK and ERK activation in anergic T cells.22,42 Hyperactivated Rap1 may compete with Ras for Raf1 binding, preventing activation of MAPKs.43 The involvement of several E3 ubiquitin ligases, including Itch, Nedd4, Grail, and Cbl-b, has also been proposed to explain TCR signaling blockade in anergic T cells. These proteins would add ubiquitin tags to selected proteins involved in TCR signaling, targeting them for degradation, recycling, or inactivation.11-14 Recently, inactivation of dyacylglycerol-mediated signaling through activation of dyacylglycerol kinase has also been shown to contribute to Ras activity block in anergic T cells.28,29 All of these mechanism would result in severe signaling defects ranging from altered immune synapse formation to protein lipase C γ1 (PLC-γ1) degradation and Ras inactivation. Stimulation of anergic T cells with PMA and ionomycin has been shown to restore IL-2 production and proliferation. Direct activation of PKC by PMA should overcome proximal blocks. Nevertheless, restoration of T-cell activation in anergic T cells with PMA and ionomycin is only partial.22,23 It is clear, thus, that other mechanisms, likely downstream of PKC activation, must also contribute to the unresponsive state of anergic T cells.23 Our results clearly reveal the existence of such a mechanism. Ikaros would induce changes in histone acetylation at the il2 locus. Binding of Ikaros could account, at least in part, for the disparate effects that anergy has on the expression of different cytokines. While IL-2 expression is completely blocked, the expression of IL-4 or IFNγ is only slightly affected27 and IL-10 expression may even be enhanced.7 The imposition of a new stable pattern of histone acetylation in the il2 promoter would be responsible for its silencing and, in cooperation with other mechanisms, completely inhibit IL-2 synthesis.
Preventing IL-2 expression is critical to maintain anergy, as signals transmitted by the IL-2 receptor are able to reverse anergy in T cells.3,44 Binding of CREB/CREM complexes to the −180 AP-1 site of the il2 promoter and il2 repression by Smad3 binding to negative regulatory elements on the il2 promoter have also been proposed to regulate IL-2 expression in anergic T cells, supporting the existence of transcriptional mechanisms that can inhibit il2 expression in anergic T cells.45,46 Binding of these factors might also be facilitated by changes in histone acetylation in this locus.
Regulation of chromatin accessibility to the il2 promoter may not be restricted only to anergic Th1 cells. It has recently been reported that in CD4+CD25+ regulatory T cells, restoring defective JNK activation with a constitutively active kinase did not overcome deficient IL-2 expression. Marked differences in chromatin accessibility at the il2 promoter were detected, though, between CD4+CD25− and CD4+CD25+ T cells, which might be able to account for the lack of il2 transcription in regulatory T cells.47 It would also be interesting to determine whether other members of the Ikaros family might play similar roles in controlling self-reactivity in T cells or other lymphocytes. We have not detected up-regulation of any other Ikaros family member in anergic Th1 cells, but Aiolos-deficient mice develop an autoimmune phenotype characterized by hyperactivated B cells that spontaneously form germinal centers, increased antinuclear antibody production, and development of a systemic lupus–like syndrome.48,49
The importance of keeping self-reactive T cells inactivated to prevent autoimmune reactions may underscore the existence of multiple complementary mechanisms designed to inhibit self-reactive T-cell activation. We have shown that Ikaros induces histone deacetylation of the il2 promoter and inhibits il2 expression in anergic T cells. We propose, thus, that epigenetic modifications can play a crucial role in the establishment and maintenance of T-cell anergy.
Authorship
Contribution: F.M. and S.B. designed research; S.B., M.D., N.S.-N., and I.P. performed research; S.B. and M.P. contributed new reagents/analytic tools; S.B. and F.M. analyzed data; and F.M. wrote the paper.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Fernando Macian, Albert Einstein College of Medicine, Department of Pathology, 1300 Morris Park Avenue, Bronx, NY 10461; e-mail: fmacianj@aecom.yu.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health training grants GM007491 (N.S.-N.) and GM007288 (M.P.), the Irene Diamond Fund, and a National Institutes of Health grant AI059738 (F.M.).
We thank members of our laboratory for helpful discussions and A. Jordan and A. M. Cuervo for critical reading of the manuscript. We would like to thank K. Georgopoulos, D. J. McKean, G. P. Nolan, and S. T. Smale for the generous gift of reagents used in this work. Monoclonal antibody against murine IL-4 was obtained form the Biological Resources Branch preclinical repository.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal