Abstract
Understanding the distribution, function, and lineage relationship of CD8+ T-cell subpopulations is of fundamental value for the monitoring of the immune system in several experimental and clinical situations. However, the available data concerning the description of effector and memory CD8+ subsets in humans remain rather fragmentary because different studies favored the usage of distinct and restricted sets of cell surface markers and functional parameters. We associated multiple markers to subdivide CD8+ T cells into 14 different cell types, several of which were not described previously, and evaluated the coexpression of 18 genes simultaneously in individual cells from each subset. Our results show that each subset has a defined pattern of gene expression. Moreover, effector gene expression of CCR7− cells correlated only with CD27 expression levels and CD27/CD28 coexpression but not with CD45RA/R0 phenotypes. Our findings thus describe new CD8+ cell subsets, allow the identification of relatively homogeneous CD8+ subpopulations, provide a predictable and precise correlation between particular cell surface markers and CD8+ T-cell functional properties, and identify effector cells present in both CCR7−CD45RA+ and CCR7−CD45R0+ compartments. The results also indicate that activated cells might modulate the expression of CD45RA/R0 asynchronously rather than CCR7−CD45RA+ cells always issuing from CD45RA− precursors.
Introduction
CD8+ T lymphocytes play a key role in defense against cytosolic pathogens and tumors. Understanding the mechanisms through which the immune system controls such pathological situations to avoid disease depends upon the thorough characterization of all CD8+ T subpopulations and differentiation stages, from naive precursors to fully mature effectors. For that purpose, the CD8+ T-cell compartment was subdivided into several different subsets with distinct properties. In humans, it has been established that expression of the lymph node homing receptor CCR7 can be used to separate both CD4+ and CD8+ CD45RA− T cells into 2 functionally distinct subsets: CCR7+CD45RA− and CCR7−CD45RA−, named “central memory” (TCM) and “effector memory” (TEM), respectively. Unlike CD4+ T cells, an additional CCR7− subset that expresses CD45RA (TEMRA) can also be found in the CD8+ compartment. Because this population harbors cells expressing high perforin levels, it was suggested that the TEMRA subset should correspond to a population of terminally differentiated CD27− effector cells previously described by Hamann and collaborators.1 These cells display a Vβ repertoire significantly different from naive cells, containing oligoclonal expansions of particular T-cell receptor (TCR) Vβ elements and also having shorter telomeres, suggesting that CD27−CD45RA+ cells have been selected in vivo through antigen stimulation and evolved through extensive rounds of division.2 The same authors had proposed an alternative classification for CD8+ T lymphocytes in which CD45RA and CD27 expression was used to identify naive (CD27+CD45RA+), memory (CD27+CD45RA−), and effector (CD45RA+CD27−) CD8+ T cells in humans.1 This classification, however, underestimates the complexity of the memory CD8+ T-cell subset revealed by the expression of CCR7. For instance, memory cells defined by the CD45RA−CD27+ phenotype would include both TCM and TEM, which were shown to enclose distinct functional specializations.3,4 Furthermore, virus-specific CD27+CD8+ T cells may also express CD45RA.5 Heterogeneity of the effector/memory compartments was shown to be further extended to CD28 differential expression. CD8+ T cells specific for several persistent human viruses were extensively characterized regarding their surface phenotype, perforin, and granzyme A (GZMA) expression and ex vivo cytotoxic capacity. Based on these data, it was shown that coexpression of CD27 and CD28 could be used to distinguish 3 functionally different subsets of CD8+ T cells according to the progressive expression of effector functions: early (CD27+CD28+), intermediate (CD27+CD28−), and late (CD27−CD28−) differentiated cells.5 This classification reflects, however, the differentiation status of antigen-experienced CD8+ T cells rather than discriminating effector and memory cells. Moreover, CD27 and CD28 expression does not allow distinguishing TCM from TEM or TEM from TEMRA.
The prevailing data concerning the description of naive, effector, and memory CD8+ T-cell populations in humans remain, thus, rather fragmentary. Manifestly, analysis of CD8+ T cells including solely 2 or 3 parameters is not sufficient to reveal the whole heterogeneity of the antigen-experienced CD8+ lymphoid compartment. The compound subsets are not clearly established, especially within the TEM and TEMRA compartments, and the correspondent differential roles and lineage relationships remain undisclosed. Interestingly, in different human chronic viral infections, such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), hepatitis C virus (HCV), and HIV-1, virus-specific CD8+ T cells display distinct predominant phenotypes.5-9 However, it is not clear whether the lack of certain CD8+ T subpopulations results from a specific virus-induced blockage in differentiation or, instead, is a consequence of different clinical settings.4,10 Thus, understanding the distribution, function, and relationship of the CD8+ T-cell subpopulations is of fundamental value for the monitoring of the immune system in several experimental and clinical situations.
The present study aims to describe thoroughly the heterogeneity of the human CD8+ T-cell compartment by establishing an accurate correlation between cell surface phenotype and functional properties of each subset. In particular, this study characterizes the circulating human CD8+ T-cell populations based on the simultaneous association of CCR7, CD45RA, CD27, CD28, CD11a, and CD62L cell surface markers. The association of these particular markers allowed the identification of 14 different CD8+ T-cell subsets, some of which were never previously described. We isolated individual cells of each subset and studied in each cell the expression of 18 different mRNAs coding for cytokines, chemokines, cytotoxic molecules, and several receptors. This work describes new CD8+ T-cell subsets, identifies homogeneous CD8+ T-cell subpopulations, and allows a predictable correlation between cell surface phenotype and in vivo function. Moreover, it reports the presence of effector cells both in TEM and TEMRA subsets and suggests an asynchronous modulation of CD45RA/R0 expression after priming rather than CCR7−CD45RA+ cells always issuing from CD45RA− precursors.
Materials and methods
Isolation of peripheral blood cells
Heparinized venous blood was obtained from healthy volunteers of both sexes with ages ranging from 22 to 56 years at Etablissement Francais du Sang (EFS) after informed consent following French ethical recommendations (French Code for Public Health: Titre II, Livre II, Première Partie, articles L.1221-1 to L.1221-13, D.1221-1 to D.1221-15, and R.1221-16 to R.1221-42). Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Histopaque-1077 Hybri-Max density gradient (Sigma, Lyon, France) and CD8+ T lymphocytes obtained using the Dynal (Paisley, United Kingdom) CD8 Negative Isolation Kit, which includes anti-CD4, anti-CD14, anti-CD16 (a and b), anti-CD19, anti-CD36, anti-CD56, anti-CDw123, and anti-glycophorin A depleting antibodies. CCR7 depletion was performed by removing CCR7-labeled cells (R&D Systems, Lille, France) with anti-IgG Dynabeads (Dynal). Resulting CCR7−CD8+ T cells were more than 98% pure.
Antibodies and reagents
Antihuman antibodies used were fluorescein isothiocyanate (FITC)–labeled anti-CCR7 (R&D Systems); phycoerythrin (PE)–labeled anti-CD27, allophycocyanin–cyanin 7 (APC-Cy7)–labeled anti-CD8α, biotinylated anti-CD27 and anti-CD28–PE-Cy7 (eBioscience, San Diego, CA); FITC-labeled anti-CD3 and CD8β-PE (Caltag); streptavidin-PE-Cy7, peridinin-chlorophyll-protein complex (PerCP)–Cy5.5-labeled streptavidin, anti–CD62L-FITC, anti–αβ-TCR–FITC, anti–CD45R0-FITC, anti–CD11a-PE, anti-CD4–biotin, anti–CD28-biotin, anti–γδ-TCR–biotin, anti–CD3–PerCP-Cy5.5, and anti–CD45RA-APC (PharMingen, Erembodegem, Belgium).
Cell sorting and flow cytometry
Cells were double sorted using a FACS Vantage upgraded to DiVa configuration and equipped with an automatic cell deposition unit (Becton Dickinson, Le Pont de Claix, France). Single cells were collected as described.11 Cytofluorometric analysis was performed in a BD-LSR I flow cytometer.
Primers and quantitative multiplex reverse transcriptase–polymerase chain reaction
Procedures, primers, and quantitative analysis for the simultaneous amplification of multiple genes in single cells were performed as described.11 The efficiency of amplifications for each gene and for each set of primers was calculated and proven to be maximal and uniform for all the genes. Competition was assessed, and no interference was detected between the different primers and/or amplicons during multigene amplification.11
Results
Phenotypic characterization of CD8+ T-cell subsets from human peripheral blood
We isolated αβ-TCR CD8+ peripheral blood lymphocytes (PBLs) after depletion of other minor cellular sets that can express CD8. The use of anti-CD56 and anti-CD16 in a depletion step should remove natural killer (NK) cells. We further ensured that our purified population did not contain NK cells by assessing the expression of CD3ϵ mRNA in all individual cells we studied (see “Heterogeneity of CD8+ T-cell subpopulation evaluated at a single-cell level”). Our depletion strategy should also remove most NKT cells because CD56− NKT cells represent less than 0.09% of lymphocytes.12,13 We found that our purified CD8+ subset contained more than 99.9% αβ-TCR cells, whereas only up to 0.2% stained with anti-γδ-TCR (data not shown).
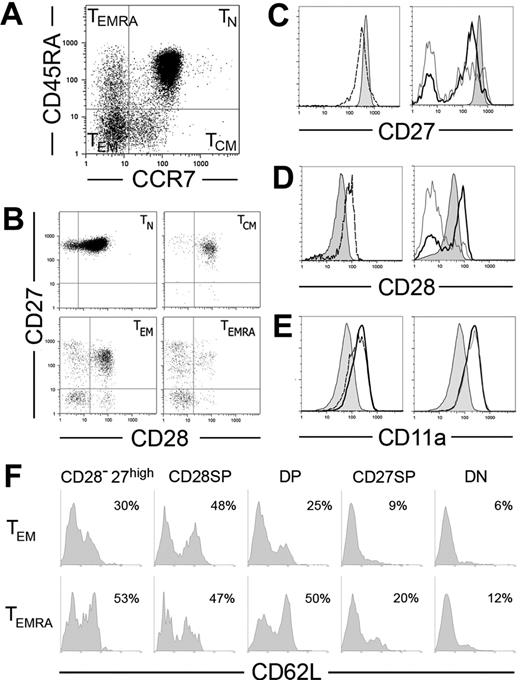
We subdivided CD8+ T cells into 4 major subpopulations based on the expression of CCR7 and CD45RA, as reported3,14 (Figure 1A). Because CD45RA/R0 phenotypes are mutually exclusive, the populations labeled with CD45RA were the mirror image of CD45R0− sets and vice versa (not shown). The coexpression of CD27, CD28, CD11a (the alpha chain of LFA-1), CD8β, and CD8α was further evaluated within each subset. Both TN (CD45RA+CCR7+) and TCM (CD45RA−CCR7+) were quite homogeneous. Most coexpressed all these additional markers (Figure 1B) as well as CD8α and CD8β chains (not shown). In contrast, CCR7− subpopulations were very heterogeneous. Both TEM (CD45RA−CCR7−) and TEMRA (CD45RA+CCR7−) compartments contained T cells coexpressing CD28 and CD27 (double positive [DP]), expressing either one of these markers (single positive [SP]), or expressing none (double negative [DN]). Rare CD8α+β− cells could be found within the CCR7− compartment, but they did not show preferential distribution among CD27/CD28 subsets (not shown). All these subsets were present in every donor and had the same characteristics in different donors (see last subsection of “Results”), but their frequency varied between individuals (Table 1).
Expression of CD27, CD28, CD11a, and CD62L in CD8+ T PBLs. (A-B) CD8-enriched PBLs were simultaneously stained for CD8, CCR7, CD45RA, CD27, and CD28. CD8high cells were then subdivided using CCR7 and CD45RA expression in naive (TN), central memory (TCM), effector memory (TEM), and effector memory CD45RA+ (TEMRA) cell subpopulations (A), and CD27/CD28 coexpression was evaluated in each of these cell sets (B). (C-E) Comparison of CD27 (C), CD28 (D), and CD11a (E) expression levels on TN cells (shaded histograms) with TCM (dashed), TEM (thick line), and TEMRA (gray line). (F) CD8+ cells depleted for CCR7 were simultaneously stained for CD8, CD62L, CD45RA, CD27, and CD28. TEM and TEMRA subpopulations gated on CD8high were subdivided according to their expression of CD27 and CD28 into CD27high, CD28+CD27− (CD28-SP), CD28+CD27+ (DP), CD28−CD27+ (CD27-SP), and CD28−CD27− (DN) cell sets. Results show CD62L expression in each of these sets in 1 representative donor.
Expression of CD27, CD28, CD11a, and CD62L in CD8+ T PBLs. (A-B) CD8-enriched PBLs were simultaneously stained for CD8, CCR7, CD45RA, CD27, and CD28. CD8high cells were then subdivided using CCR7 and CD45RA expression in naive (TN), central memory (TCM), effector memory (TEM), and effector memory CD45RA+ (TEMRA) cell subpopulations (A), and CD27/CD28 coexpression was evaluated in each of these cell sets (B). (C-E) Comparison of CD27 (C), CD28 (D), and CD11a (E) expression levels on TN cells (shaded histograms) with TCM (dashed), TEM (thick line), and TEMRA (gray line). (F) CD8+ cells depleted for CCR7 were simultaneously stained for CD8, CD62L, CD45RA, CD27, and CD28. TEM and TEMRA subpopulations gated on CD8high were subdivided according to their expression of CD27 and CD28 into CD27high, CD28+CD27− (CD28-SP), CD28+CD27+ (DP), CD28−CD27+ (CD27-SP), and CD28−CD27− (DN) cell sets. Results show CD62L expression in each of these sets in 1 representative donor.
CD27 and CD28 coexpression in CD8+CCR7− T cells
| . | CD27+CD28 DP . | CD27+ SP . | CD28+ SP . | CD27−CD28 DN . | CD27high DP . | CD27high SP . |
|---|---|---|---|---|---|---|
| Mean CD45RA−, % (range) | 50 (19-74) | 19 (2-34) | 6 (2-15) | 15 (2-46) | 6 (0.6-19) | 4 (1-13) |
| Mean CD45RA+, % (range) | 17 (2-44) | 32 (7-58) | 3 (0.5-12) | 40 (7-74) | 2 (0.3-8) | 6 (0.4-17) |
| . | CD27+CD28 DP . | CD27+ SP . | CD28+ SP . | CD27−CD28 DN . | CD27high DP . | CD27high SP . |
|---|---|---|---|---|---|---|
| Mean CD45RA−, % (range) | 50 (19-74) | 19 (2-34) | 6 (2-15) | 15 (2-46) | 6 (0.6-19) | 4 (1-13) |
| Mean CD45RA+, % (range) | 17 (2-44) | 32 (7-58) | 3 (0.5-12) | 40 (7-74) | 2 (0.3-8) | 6 (0.4-17) |
Results represent the distribution of different subpopulations expressing CD27 and/or CD28 within TEM and TEMRA compartments. They show the mean percentage of cellular subsets in 18 donors.
DP indicates double positive; SP, single positive; and DN, double negative.
In vitro activation of CD8+ T cells induces the down-regulation of CD27 and CCR7 3,15,16 and up-regulation of CD28 and CD11a.17,18 Accordingly, TN cells had the highest levels of CD27 and CCR7 (Figure 1A,C), as compared with TCM, while TEM and TEMRA CD27-SP expressed even lower levels of CD27 than TCM. CD28 and CD11a followed the opposite trend: CD28 expression was lower in TN than in all subsets of primed cells expressing this molecule, and CD11a was progressively up-regulated from TN to TCM, TEM and TEMRA (Figure 1D-E; Table 2). However, within the TEM and TEMRA, cells expressing CD27 and/or CD28 (DP or SP) displayed similar expression levels of these markers as well as identical high levels of CD11a (Figure 1). Therefore, CD27, CD28, and CD11a expression levels confirm the putative differentiation hierarchy TN to TCM and from these to CCR7−CD8+ T cells but do not allow further discrimination within the complex CCR7− compartment.
Expression levels of different markers in CD8+ T cells
| . | CD27 . | CD28 . | CD11a . |
|---|---|---|---|
| TN | 120 | 17 | 67 |
| TCM | 109 | 31 | 195 |
| TEM | 59 | 33 | 225 |
| TEMRA | 50 | 22 | 237 |
| CD27high | 147 | 37 | 240 |
| . | CD27 . | CD28 . | CD11a . |
|---|---|---|---|
| TN | 120 | 17 | 67 |
| TCM | 109 | 31 | 195 |
| TEM | 59 | 33 | 225 |
| TEMRA | 50 | 22 | 237 |
| CD27high | 147 | 37 | 240 |
Results show mean fluorescence intensity (MFI) of stainings for different cell surface markers gated on positive populations in PBLs of 1 donor. Similar relationships were found in 10 other donors.
Besides these major subsets, we also identified minor CCR7−CD8+ T-cell subsets expressing high levels of CD27 (CD27high) (Figure 1B; Table 1). These subsets were present in both TEM and TEMRA compartments and included mostly CD27-SP cells but could also harbor DP cells (Figure 1B). Independently of their additional CD28 or CD45RA phenotypes, the CD27high cells expressed CD11a at higher levels than naive or TCM cells (Table 2). In striking contrast to the other CD8+ subpopulations, the presence of CD27high cells in the blood appeared to be transitory, because the representation of these subsets in the same donor varied significantly with time. It was previously reported that CD27 could be transiently up-regulated shortly after in vitro activation.16 The high CD11a expression and their transitory presence in the blood suggest CD27high cells may be recently activated CD8+ T cells.
Correlation of CD62L expression with the CD27/CD28 phenotype
CD62L plays a fundamental role in the migration of lymphocytes to secondary lymphoid organs. While TN and TCM CD8+ T cells are consistently CD62L+, only some CCR7− cells express this adhesion molecule.3 We have further investigated if CD62L expression was related to peculiar CCR7−CD8+ T-cell subtypes (Figure 1F). We found a correlation with CD27/CD28 expression but no differences between TEM/TEMRA subsets. Thus, independently of their CD45RA phenotype, CD27high, DP, and CD28-SP cells contained abundant CD62L+ cells. CD27-SP populations usually had low levels of CD62L, and in DN cells CD62L expression was even lower. It is unlikely that such low expression as found in DN cells might be sufficient to ensure migration to the lymph nodes, because we could never detect DN CD8+ T populations in lymph node cells (M.M., unpublished data, November 2005).
Altogether, these observations revealed that TN and TCM are homogeneous populations with respect to all additional markers, whereas TEM and TEMRA are significantly heterogeneous, containing multiple subpopulations that likely cross a large spectrum of effector differentiation.
Heterogeneity of CD8+ T-cell subpopulations evaluated at a single-cell level
The further addition of CD27 and CD28 to previously established CD45RA and CCR7 cell surface markers subdivided the CD8+ T-cell compartment into 14 different subsets: TN and TCM (Figure 1) and 12 distinct CCR7− subsets (Table 1). Whether this subdivision is sufficient to fully identify homogeneous populations with similar functional properties or if each of the individual CD8+ T-cell subsets can be yet heterogeneous, harboring cells with multiple functional potentialities is not known. To evaluate the homogeneity of CD8+ T-cell populations, we envisaged isolating individual cells from each subpopulation and studied in each cell the simultaneous expression of genes coding for inflammatory chemokines, cytokines, cytotoxic molecules, and several receptors described to be involved in CD8+ T-cell responses. To ensure the accuracy of single-cell sorting eliminating the possibility of including other cellular contaminants in the study, we purified αβ-TCR CD8+ T lymphocytes prior to sorting and further assessed in each cell the presence of the mRNA coding for CD3ϵ. Thus, the data presented here correspond exclusively to CD8+ T cells expressing the CD3ϵ mRNA.
We found that some genes (coding for IL-2, IL-10 or MIP-1α/CCL3) were expressed at such low frequencies (less than 5%) that their impact on functional profiles could not be analyzed at the single-cell level. Also, some of these subpopulations were so rare (less than 0.05% of CD8 cell sets) that they could not be sorted reliably. For this reason, we failed to characterize CD28-SP of the TEMRA compartment and CD27high-DP cells. In addition, we could only collect TEM CD28-SP cells from 1 single donor. For the remaining 11 populations we have characterized 15 parameters in each individual cell in 3 independent donors. A representative donor is shown in Figures 2–3.
Single-cell gene expression profiles of the less activated CD8+ T-cell subpopulations. αβ-TCR+ CD8+ PBLs were simultaneously stained for CD8, CCR7, CD45RA, CD27, and CD28. CD8high T cells identified as (A) naive, (B) CD45RA− (left) or CD45RA+ (right) CCR7−CD27high, and (C) TCM were single-cell sorted, and the expression of multiple genes was determined simultaneously in each individual cell. The accuracy of cell sorting was evaluated by the expression of CD3ϵ mRNA, with only cells positive for this molecule being depicted. Each horizontal row depicts the same individual cell, which is numbered. Each vertical row represents a different gene. Results show the profiles from a single representative donor of the 3 we studied. The genes, ccl5, ccl4, tgfb1, tnf, lta, ifng, prf1, gzma, gzmb, faslg, tgfbr1, tgfbr2, ifngr2, and il10ra, code, respectively, for RANTES, MIP-1β, TGF-β1, TNF-α, TNF-β, IFN-γ, perforin, GZMA, GZMB, Fas-L, TGF-βR1, TGF-βR2, IFN-γR2, and IL-10Rα.
Single-cell gene expression profiles of the less activated CD8+ T-cell subpopulations. αβ-TCR+ CD8+ PBLs were simultaneously stained for CD8, CCR7, CD45RA, CD27, and CD28. CD8high T cells identified as (A) naive, (B) CD45RA− (left) or CD45RA+ (right) CCR7−CD27high, and (C) TCM were single-cell sorted, and the expression of multiple genes was determined simultaneously in each individual cell. The accuracy of cell sorting was evaluated by the expression of CD3ϵ mRNA, with only cells positive for this molecule being depicted. Each horizontal row depicts the same individual cell, which is numbered. Each vertical row represents a different gene. Results show the profiles from a single representative donor of the 3 we studied. The genes, ccl5, ccl4, tgfb1, tnf, lta, ifng, prf1, gzma, gzmb, faslg, tgfbr1, tgfbr2, ifngr2, and il10ra, code, respectively, for RANTES, MIP-1β, TGF-β1, TNF-α, TNF-β, IFN-γ, perforin, GZMA, GZMB, Fas-L, TGF-βR1, TGF-βR2, IFN-γR2, and IL-10Rα.
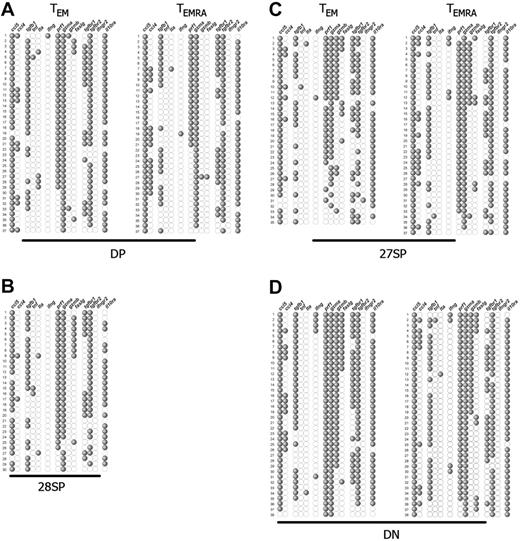
Single-cell expression profiles of the most differentiated CD8+ T-cell subpopulations. αβ-TCR+ CD8+ PBLs were subdivided by their expression of CCR7, CD45RA, CD27, and CD28, and individual cells from each subset were sorted and studied as described in Figure 2. Each single cell corresponds to 1 horizontal row, whereas the expression of the several genes studied is depicted vertically. The representative gene expression patterns presented correspond to (A) DP, (B) CD28-SP, (C) CD27-SP, and (D) DN CCR7−CD8+ T subsets. Each profile is from 1 representative donor of 3, with the exception of the minority CD28-SP set, which we could isolate only from this donor. The genes, ccl5, ccl4, tgfb1, tnf, lta, ifng, prf1, gzma, gzmb, faslg, tgfbr1, tgfbr2, ifngr2, and il10ra, code, respectively, for RANTES, MIP-1β, TGF-β1, TNF-α, TNF-β, IFN-γ, perforin, GZMA, GZMB, Fas-L, TGF-βR1, TGF-βR2, IFN-γR2, and IL-10Rα.
Single-cell expression profiles of the most differentiated CD8+ T-cell subpopulations. αβ-TCR+ CD8+ PBLs were subdivided by their expression of CCR7, CD45RA, CD27, and CD28, and individual cells from each subset were sorted and studied as described in Figure 2. Each single cell corresponds to 1 horizontal row, whereas the expression of the several genes studied is depicted vertically. The representative gene expression patterns presented correspond to (A) DP, (B) CD28-SP, (C) CD27-SP, and (D) DN CCR7−CD8+ T subsets. Each profile is from 1 representative donor of 3, with the exception of the minority CD28-SP set, which we could isolate only from this donor. The genes, ccl5, ccl4, tgfb1, tnf, lta, ifng, prf1, gzma, gzmb, faslg, tgfbr1, tgfbr2, ifngr2, and il10ra, code, respectively, for RANTES, MIP-1β, TGF-β1, TNF-α, TNF-β, IFN-γ, perforin, GZMA, GZMB, Fas-L, TGF-βR1, TGF-βR2, IFN-γR2, and IL-10Rα.
TN cells lack effector functions but express several receptor types
As expected, the less activated CD8+ T-cell set was TN (Figure 2A). These cells did not express mRNAs coding for chemokines, cytotoxic molecules, or effector cytokines, such as TNF-α or IFN-γ. However, some cells (less than 15%) generally expressed TNF-β (coded by the lTA gene). In addition, about 40% to 50% of the cells expressed TGF-β1, and most expressed TGF-β receptor 2 (TGF-βR2). In addition to TGF-βR2, TGF-β receptor 1 (TGF-βR1) expression is also required for TGF-β1–induced signaling to occur. Coexpression of TGF-βR1 and TGF-βR2 was detected in more than 30% of the naive cells. The TN population also contained the highest frequency of cells expressing IFN-γ receptor 2 (IFN-γR2), which determines the responsiveness to exogenous IFN-γ, and approximately half of the cells expressed IL-10 receptor α (IL-10Rα) (Figure 2A).
Cells expressing CD27high display the gene expression pattern more closely related to TN cells
Surprisingly, the CD8+ subpopulations most resembling TN were the TEM CD27high and TEMRA CD27high subsets (Figure 2B). These populations expressed TGF-β1 at the same frequency as naive cells, and TNF-β was expressed to a slightly lesser extent. In contrast, IFN-γR2 was no longer expressed, a finding we observed in all CCR7−CD8+ T-cell subsets. IL-10Rα frequencies increased up to 75%, and perforin and granzymes were expressed on average by 20% and 10% of the cells, respectively. However, expression of perforin and either granzyme A (GZMA) or granzyme B (GZMB) was detected in separate cells, suggesting that cells expressing CD27high are not cytotoxic. Remarkably, the mRNA coding for the inflammatory chemokine RANTES (also known as CCL5) was detected in 45% to 70% of CD27high cells. Importantly, TEM and TEMRA CD27high subsets had undistinguishable gene expression profiles, suggesting they might play similar functional roles in vivo.
TCM: the memory subset expressing the fewest effector functions
TCM cells, although expressing CCR7, displayed higher frequency of effector genes than CCR7−CD27high subpopulations (Figure 2C). Three evident differences were noticed. First, the mRNA coding for GZMA was up-regulated because it was expressed in up to 40% of the cells. However, few TCM cells (less than 15%) coexpressed this molecule along with perforin, indicating that only a small fraction of these cells can be cytotoxic. Secondly, expression of RANTES was also up-regulated and could be detected in more than 70% of the cells. Finally, expression frequency of IFN-γR2 was down-regulated, being detected solely in a small fraction of cells. Notably, only TN and TCM subsets express IFN-γR2 mRNA, with all other sets of activated T cells lacking this molecule.
TEM and TEMRA harbor 3 hierarchically differentiated subsets
The gene expression pattern of all CCR7− subpopulations, with the exception of the previously described CD27high subsets, shows a degree of functional differentiation significantly higher than that of TCM cells (Figure 3). This was revealed by increased expression of RANTES, which is consistently expressed by more than 90% of the cells, and perforin, GZMA, and IL-10Rα together with further expression of additional molecules.
Within CCR7− cells, the DP cellular subsets were those more closely resembling the TCM subset. As compared with TCM cells, virtually all TEM and TEMRA DPs now expressed RANTES, GZMA, and IL-10Rα, and perforin was expressed in much higher frequencies ranging from 50% to 80% (Figure 3A). In a fraction of these cells, we detected for the first time expression of Fas-L (6% to 20%) and macrophage inflammatory protein-1β (MIP-1β), also named CCL4 (14% to 40%). Only very rare cells could score positive for IFN-γ or GZMB. Strikingly, the patterns of gene expression of TEM and TEMRA DP populations were nearly overlapping, the sole difference concerning slightly increased frequencies of perforin and FAS-L expression in TEM DPs. We were able to isolate CD28-SP cells from a single donor and solely of the TEM compartment. Interestingly, this subset displayed a pattern of gene expression very similar to the DP subpopulations (Figure 3B), suggesting a close relationship.
The TEM and TEMRA CD27-SP subsets were more differentiated than the DP subsets (Figure 3C). Indeed, in addition to the genes already expressed at high frequency by DP cells, GZMB expression was up-regulated (10% to 50%), while the frequency of Fas-L expression shows a less striking increase (15% to 25%). Once again, we were surprised to notice an evident overlap between the expression patterns of TEM and TEMRA CD27-SP subsets that was extended to all the molecules we have studied.
TEM and TEMRA DN CD8+ T cells displayed the most differentiated gene expression profile (Figure 3D). Now, nearly all cells expressed perforin and GZMA, GZMB was expressed by most cells, and Fas-L expression frequency increased. In some donors, IFN-γ expression was also up-regulated, being detected in up to 30% of the cells. Again, we found no difference in gene expression profiles between TEM and TEMRA DN CD8+ T-cell sets.
Thus, the results obtained by single-cell multiplex reverse transcriptase–polymerase chain reaction (RT-PCR) clearly depict a hierarchy of T-cell differentiation status in antigen-experienced cells. Importantly, this hierarchy is defined by CCR7 expression, CD27 expression levels, and CD27/CD28 coexpression but does not correlate with expression of CD45RA.
Gene expression of TEM and TEMRA compounding subsets is similar at the quantitative level
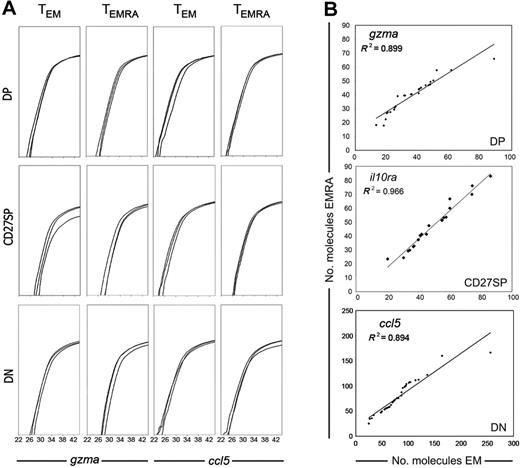
Because the gene expression profiles of all CCR7− T-cell subsets correlated with CD27/CD28 expression but not with CD45RA+/CD45RA− phenotype, we next investigated if we could distinguish TEM and TEMRA cell sets by a different amount of expressed mRNA molecules. This approach was possible because in the methodology used here the efficiency of reverse transcription was evaluated.11 Therefore, we were able to directly quantify the number of mRNA molecules coding for distinct genes expressed by each individual cell. Moreover, our methodology also uses PCR reactions of identical efficiency for all genes, allowing the comparison of different gene expression levels.11 We have thus quantified the expression of all genes in individual cells from different populations. As expected, not all the genes were expressed at the same level. Perforin and MIP-1β had the lowest number of mRNA molecules per cell, while RANTES and the receptors for TGF-β and IL-10R displayed the highest expression levels (not shown). Nevertheless, each gene was expressed quite similarly in all CCR7− CD27/CD28 cell subsets. This is shown for 3 different cells from each population and for 2 of the genes in Figure 4A. Moreover, gene expression levels were equivalent in TEM and TEMRA, because no significant differences for any of the genes were found when comparing the number of gene-specific mRNA molecules expressed per cell in TEM versus TEMRA subpopulations (Mann-Whitney test, P > .05). In addition, in all genes we found an important correlation between the number of mRNA molecules per cell in TEM and TEMRA compounding subsets, which clearly illustrates that the mRNA expression levels of TEM and TEMRA cells have the same range and the same distribution (Figure 4B and data not shown). We thus conclude that CD45RA expression cannot discriminate CCR7−CD8+ T-cell subtypes either at a qualitative or at a quantitative level.
Quantitative assessment of mRNA expression in single cells. Single cells of each CD8+ T-cell subset were sorted, and cells expressing each gene identified, as shown in Figures 2–3. In all cells that scored positive for the expression of any particular gene, the mRNA expression levels of that gene were further quantified. (A) Each graph shows 3 cells from each population. We show GZMA and RANTES amplification because these genes can be found in all cell sets, and thus their expression levels can be directly compared. (B) Correlation between the number of mRNA molecules expressed by every single cell of TEM and TEMRA compounding subsets. Individual cells expressing GZMA, IL-10Rα, and RANTES from either TEM or TEMRA were ordered according to the expression level of that gene and attributed an ordinal point. The number of mRNA molecules associated with each ordinal point from TEM subsets was plotted against the correspondent ordinal point values of the equivalent TEMRA subset. The dots in the graphs represent the intersection of the values derived from the number of mRNA molecules expressed by 1 TEM cell (horizontal axis) and 1 TEMRA cell (vertical axis). Data are from 1 of 3 donors.
Quantitative assessment of mRNA expression in single cells. Single cells of each CD8+ T-cell subset were sorted, and cells expressing each gene identified, as shown in Figures 2–3. In all cells that scored positive for the expression of any particular gene, the mRNA expression levels of that gene were further quantified. (A) Each graph shows 3 cells from each population. We show GZMA and RANTES amplification because these genes can be found in all cell sets, and thus their expression levels can be directly compared. (B) Correlation between the number of mRNA molecules expressed by every single cell of TEM and TEMRA compounding subsets. Individual cells expressing GZMA, IL-10Rα, and RANTES from either TEM or TEMRA were ordered according to the expression level of that gene and attributed an ordinal point. The number of mRNA molecules associated with each ordinal point from TEM subsets was plotted against the correspondent ordinal point values of the equivalent TEMRA subset. The dots in the graphs represent the intersection of the values derived from the number of mRNA molecules expressed by 1 TEM cell (horizontal axis) and 1 TEMRA cell (vertical axis). Data are from 1 of 3 donors.
T-cell populations of the same phenotype have the same characteristics in different individuals
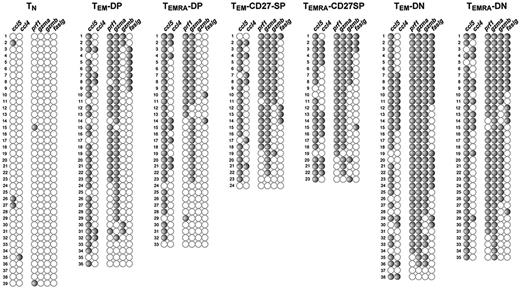
To evaluate if cell surface phenotypes always correlated with peculiar functional profiles, we compared the gene expression profiles of 3 individuals. We found that within each phenotype gene expression profiles were remarkably similar between all donors. Due to space limitations, we show in Figure 5 the pattern of gene expression from a single additional donor, focusing on the cytotoxic molecules and chemokines, because these molecules better define the signature of each CD8+ subtype. Comparison of the expression profiles of different donors (Figures 3,Figure 4–5) shows that each subpopulation has the same characteristics. Again, the profile of gene expression of CCR7− subpopulations correlates only with CD27/CD28 phenotype, with TEM or TEMRA cells of the same CD27/CD28 phenotype displaying the same characteristics. In conclusion, each cellular subset has equivalent characteristics in different donors.
The profile of gene expression of each CD8 subpopulation is the same in different donors. Results represent the profiles of gene expression of various CD8 subsets from a different donor than the one shown in Figures 2–3. Cell sets were isolated, and individual cells and genes were disposed as described in Figures 2–3. To fit into a single figure, we show only major represented populations and cytotoxic and chemokine mRNAs, because these genes by themselves clearly define the properties of each CD8 subtype.
The profile of gene expression of each CD8 subpopulation is the same in different donors. Results represent the profiles of gene expression of various CD8 subsets from a different donor than the one shown in Figures 2–3. Cell sets were isolated, and individual cells and genes were disposed as described in Figures 2–3. To fit into a single figure, we show only major represented populations and cytotoxic and chemokine mRNAs, because these genes by themselves clearly define the properties of each CD8 subtype.
Discussion
The human CD8+ T-cell compartment encloses several subpopulations with multiple functionalities, including naive, effector, and memory subsets. The prevailing data describing these subsets in the peripheral blood are unclear in several aspects mainly due to multiple analyses of CD8+ T-cell subpopulations using different and limited sets of surface markers and functional properties. The present study aims to elucidate the ambiguous and missing data concerning the heterogeneity of the human CD8+ T-cell compartment. For this purpose, we used 2 approaches. First, we performed a detailed characterization of the cell surface phenotype of the circulating CD8+ T-cell populations ex vivo based on the simultaneous association of the most common and relevant cell surface markers described in the literature, namely CCR7, CD45RA, CD27, CD28, CD62L, and CD11a. These molecules are widely used to identify CD8+ T-cell subsets, but they are usually only partially associated.1,3-5,14 The concurrent association of all these cell surface markers allowed the identification of multiple CD8+ T-cell subsets, several of which were never described previously. Importantly, we could directly compare the primed subsets within the CD45RA+ and CD45RA− compartments, whose precise differential functions remained unclear. To ensure that the single cells we studied corresponded to classical αβ-TCR lymphocytes, only cells expressing the CD3ϵ mRNA were integrated in this study. A previous depletion step using a cocktail of antibodies that included CD4, CD16, CD19, and CD56 issued a population consisting of more than 99.9% αβ-TCR+ cells, where most NKT cells should also be absent. The possibility remains, however, that very rare CD56− NKT cells expressing CD8αα were still present in our subsets. Although we found some T cells expressing CD8αα in the CCR7− subsets of some donors, they were distributed similarly among the different subsets. Moreover, CD56− NKT cells were described to constitute less than 0.09% of all lymphocytes.13 Considering we studied populations by characterizing individual cells, such rare cells could not have an impact on the general gene expression profiles we define here.
The second strategy concerned an approach to evaluate the heterogeneity of each one of these cellular subsets. We studied individual cells in each population, and each cell was characterized for the expression of 18 different mRNAs involved in T-cell functions. Single-cell gene expression analysis allowed the assessment of functional heterogeneity inside each cellular subset and gave important insight concerning the differential function and differentiation of the various subpopulations. Furthermore, we also found that each one of these particular phenotypes corresponded to specific patterns of gene expression, because each one displayed reproducible gene expression patterns in all the donors studied. Hence, the phenotypes we describe here apparently can be used to predict defined characteristics in CD8+ subpopulations in healthy individuals.
Our results show that the combination of CCR7, CD45RA, and CD27 expression levels and CD28 permits us to discriminate 14 CD8+ T-cell subsets. With the exception of CD27high cells, which display characteristics of recently activated populations, all remaining subsets could be found in all donors, albeit with different representations.
At the single-cell level, each cellular subset displayed a characteristic pattern of gene expression. In CCR7− cells, this pattern strongly correlated with expression of both CD27 and CD28 following a hierarchy of differentiation in which CD27high, DP, CD28-SP, CD27-SP, and DN cells display progressively higher degrees of differentiation. Surprisingly within each of these subsets, TEM subpopulations showed the same gene expression patterns at both the qualitative and quantitative level as their TEMRA counterparts. These findings contradict the paradigm that T-cell differentiation necessarily leads to CD45RA loss and that further maturation induces CD45RA reexpression in such way that effector cells should be present only in the TEMRA compartment. They rather suggest that, instead of TEMRA cells forcedly differentiating from TEM precursors, CD8+ T cells may or may not modulate CD45RA/R0 expression after activation and/or may transit from a CD45RA to CD4R5R0 phenotype and vice versa. Indeed, we clearly identified fully differentiated cells coexpressing multiple “killer” genes in both CD45RA+ and CD45RA− populations. Our results concerning the CD27high subpopulations also support that the CD45RA isoform can be maintained after T-cell activation. It has been previously reported that CD27 is transiently up-regulated after T-cell activation in vitro, the peak expression level occurring by 24 hours.19,20 Our results strongly support that CD27 is also up-regulated following in vivo activation, because CD27high cells exhibit characteristics of recently activated cells: they were hardly detectable in some donors, and their frequency in the same donor was not stable, suggesting that they may disappear with time. Furthermore, the gene expression profile of these CD27high subpopulations was very close to that of naive cells. As a major difference, an important fraction of CD27high cells expressed RANTES, a gene reported to be induced relatively early following in vitro activation and before cytotoxic genes. It was described to be already up-regulated 3 to 5 days after T-cell activation.21-23 However, “recently activated” CD27high cells with the same characteristics could be found in both TEM and TEMRA populations, indicating that primed populations may maintain CD45RA expression after activation. Other independent evidence suggests TEMRA populations can derive directly from naive cells, because their replication history may approach that of naive T cells.14 It is possible that besides CD45RA/R0 modulation, activated T cells may alternatively maintain or lose CD28/CD27. We could detect recently activated CD27high cells that coexpressed both molecules as well as primed cells that were CD28-SP cells.
Because our results argue against the model of a mandatory origin of TEMRA from TEM cells, it is appropriate to review the experimental evidences leading to this notion. The CD45R0 phenotype was believed to be characteristic of primed cells, but the detection of CD45RA+CD8+cells with all characteristics of effector cells forced us to review this issue. In vivo analysis of antigen-specific cells for persistent human viruses, commonly human CMV (HCMV) and EBV, showed that at early time points of acute infections epitope-specific CD8+ T cells were prevalent in the CD45R0+ subset, but in the chronic phase both CD45RA+ and CD45R0+ CD8+ T-cell subsets contain significant frequencies of cells with the same specificity.24,25 It was therefore assumed that after the primary response some of the clonally expanded CD45R0+ virus-specific cytotoxic T lymphocytes (CTLs) revert into a memory CD45RA+ phenotype.25 Nevertheless, this hypothesis was never fully confirmed. In the primary immune response to EBV infection, a frequency of 5% to 14% of EBV-specific cells yet expresses the CD45RA isoform early after infection, and the CD45RA/R0 distribution of individual clones was not investigated.24 Wills and collaborators analyzed the distribution of a single CMV-specific clone in only 2 donors and always found the same clone in both CD45RA/R0+ subsets, albeit at different frequencies.25 However, a more extensive study investigating CD45 isotype expression and the TCR-Vβ usage showed that the dominance of the CD45 phenotype was extremely variable between individuals, because in some cases the immunodominant clone was predominantly CD45RA+ and in others CD45R0+.26 Finally, the replication history of the CD45RA+ CD8+ T-cell subpopulations supports the idea that those cells can differentiate directly from the naive pool and, thus, a CD45R0+ stage is not necessary.14 Furthermore, recent in vitro studies failed to induce CD45RA reexpression in TEM cells, while TCM cells reexpressed CD45RA exclusively under cytokine influence but never after T-cell triggering. It is therefore clear that more thorough ex vivo studies are required to determine the lineage relationships between these cell types, possibly through the evaluation of the distribution of multiple clones in several independent donors.
The possibility that CCR7−CD45RA+CD8+ T lymphocytes can issue directly from naive cells14 is in apparent contradiction with other data suggesting that TEMRA corresponds to a terminal differentiation stage, in contrast to TEM, given the highest perforin content and reduced division capacity.3,4 Notably, though, in these later studies CD45RA+ and CD45RA− cells were not subdivided based on CD27 and CD28 expression. Our results clarify these apparent contradictory data. We show that TEM and TEMRA have different distribution of the compounding subsets DP, CD27-SP, CD28-SP, and DN. DN cells, the most differentiated CD8+ T-cell subset, are enriched in the CD45RA+ compartment, explaining why CD45RA+ cells appeared to harbor more differentiated cells in previous studies. These results emphasize the importance of the additional characterization of CCR7− cells through assessment of CD27 and CD28 coexpression.
We also show that each subset within TEM and TEMRA CD8+ T-cell subpopulations is characterized by the acquisition of a particular effector function. Interestingly, this phenomenon appears to occur sequentially in such a way that along the hierarchy of differentiation the expression of each gene once induced is never lost in the subsequent differentiation stages. Hence, the DP subsets had high frequencies of cells expressing RANTES and GZMA and relatively low perforin expression, whereas CD27-SP cells maintained high frequencies of RANTES and GZMA but now up-regulated perforin, with some cells expressing GZMB. Subsequently, DN cells coexpressed high frequencies of RANTES, GZMA, and perforin but also up-regulated GZMB and Fas-L at a greater extent. These results demonstrate that cellular differentiation leads to a progressive coexpression of multiple “killer” mRNAs by the same cell. Because each of these molecules mediates killing by a different mechanism, their coexpression may occur to improve the killer efficiency of individual cells. Indeed, association of granzymes and perforin is fundamental for perforin-mediated cytotoxicity,27 and Fas-L cytolysis alone is not very efficient.28 In humans, it was shown that different viral infections selectively induce a preferential differentiation of cells to distinct phenotypes.5 Generally, EBV induces DP cells, HIV induces CD27-SPs, and CMV generates DN types. These differential phenotypes were attributed to a different capacity of lymphocytes to become fully activated. Nevertheless, all these infections induce major expansions of CD8+ T lymphocytes in the acute phase, and infection by EBV is well controlled by DP cells. Thus, an alternative possibility is that the immune response to each virus requires the generation of particular effector subsets. Actually, because all TEM and TEMRA populations (with the exception of CD27high subsets) coexpress perforin and granzymes and are able to mediate cytotoxicity, it is possible that the panel of molecules expressed by each one CD8+ T-cell subpopulation confers particular advantages for the control of each type of infection. Therefore, the predominance of a given phenotype among virus-specific CD8+ T cells can result from the selection of the most advantageous CD8+ T-cell subset in the control of each type of infection.
Authorship
Contribution: M.M., C.E., and B.R. designed research, analyzed data, and wrote the paper; M.M, C.E., and A.L. performed research; A.N. contributed vital analytical tools; and M.M. collected data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marta Monteiro, INSERM Unit 591, Necker Institute, René-Descartes Medical School, 156 rue de Vaugirard - 75730 PARIS cedex 15, France; e-mail: marta.monteiro@necker.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Bill & Melinda Gates Foundation (M.M.) and a fellowship from the Science and Technology Foundation (Portugal) (C.E., M.M.). C.E. is a PGDB (Gulbenkian PhD Program in Biomedicine) student. B.R. held a patent deposed by the Necker Institute describing the single-cell multiplex RT-PCR method (patent no. 0208593). We thank to S. Dias for stimulating discussions and revision of this manuscript, C. Cordier and J. Mégret for cell sorting, Dr J. P. Viard for access to blood donors, P. Almeida for precious assistance in programming for data analysis, and M. Netter for illustrations.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal