Abstract
Optimal dose and timing of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy for aggressive non-Hodgkin lymphoma (NHL) is still an unresolved issue. We assessed whether dose intensifications with cyclophosphamide and doxorubicin might improve outcome in younger patients with intermediate-risk aggressive NHL. Previously untreated patients were assigned to receive either 8 courses of standard CHOP (n = 239) or 6 courses of intensified (I)–CHOP (n = 238). Although there was a tendency in favor of I-CHOP for overall survival (OS), disease-free survival (DFS), and event-free survival (EFS), the differences were not significant. However, although these analyses were not planned, when the intermediate-risk group was divided into low-intermediate- and high-intermediate-risk patients according to the International Prognostic Index (IPI), low-intermediate-risk patients had improved 6-year OS (67% vs 52%; P = .05), DFS (58% vs 45%; P = .06), and EFS (41% vs 30%; P = .21) when they were treated with I-CHOP compared with standard CHOP. On the other hand, high-intermediate-risk patients seem to have no benefit from I-CHOP. Although clinically relevant side effects occurred more often in the I-CHOP arm, treatment-related mortality was similar. These data suggest that I-CHOP might be preferable to standard CHOP in younger patients with low-intermediate-risk aggressive NHL.
Introduction
Improvement of cure rates in patients with aggressive non-Hodgkin lymphoma (NHL) by optimizing up-front chemotherapeutic regimens has been the subject of many trials for almost 3 decades. Despite many efforts, however, the first-generation regimen CHOP-21 (cyclophosphamide, doxorubicin, vincristine, and prednisone, given every 3 weeks), developed in 1976, is still the best available chemotherapy regimen.1 Approximately 45% of all patients with advanced stages of aggressive NHL will be cured by CHOP-21 or by the other so-called “second- and third-generation regimens,”27,p140 which are characterized by adding more different cytotoxic drugs to the CHOP backbone.1,2 However, the addition of rituximab, the monoclonal antibody against the CD20 B-cell antigen, to CHOP-21 is the first strategy that recently has shown improved outcome of patients with aggressive B-cell NHL.3,4
Cyclophosphamide and doxorubicin are the most important cytotoxic drugs in the treatment of aggressive NHL.5,6 As the dose intensity of both cyclophosphamide and doxorubicin is similar, or even lower, in the second and third regimens compared with CHOP-21, failure to improve outcome in aggressive NHL with these regimens might solely have been the result of this property.
Therefore, the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) decided in 1994 to study whether doubling of the dose intensity of cyclophosphamide and doxorubicin in the CHOP-21 regimen without changing cumulative dose by reduction of treatment intervals from 3 to 2 weeks, and by increasing their fractional dose (the intensified [I]–CHOP regimen), might improve outcome in patients with aggressive NHL.
At the time we initiated this study, clinical risk factors had been characterized by HOVON that could identify patients with aggressive NHL with a low-, intermediate-, or high-risk profile with corresponding cure rates of approximately 70%, 40%, and 20%, respectively, based on data from our previous study.7 We concluded from these data that patients with a high-risk profile might need other experimental approaches than CHOP-21 because of the dismal prognosis, and that patients with a low-risk profile were doing relatively well with this regimen. Therefore, we initiated this multicenter, randomized study to investigate whether I-CHOP has an additional benefit over CHOP-21 in patients with intermediate-risk aggressive NHL, which is also the largest cohort of patients with aggressive NHL.
Patients, materials, and methods
Patients
Between November 1994 and February 2004, a total of 513 patients with previously untreated aggressive NHL according to the intermediate-or high-grade Working Formulation (groups D, E, F, G, and H)8 and an intermediate-risk profile according to the HOVON criteria (see the third paragraph of this section) were included in the study (HOVON-26 study). Patients with lymphoblastic and Burkitt (-like) NHL (groups I and J) were excluded. Eligible patients were between 16 and 65 years old and were fully staged, including computed tomography scanning of thorax and abdomen, bone marrow biopsy, and other investigational procedures according to clinical symptoms. Patients were excluded if they had central nervous system (CNS) lymphoma, positive serology to HIV, history of low-grade NHL, major organ dysfunctions not directly related to NHL, prior malignancy except in situ cervix carcinoma or skin epithelioma, prior chemotherapy or radiotherapy, or a World Health Organization (WHO) performance score of 3 or 4 (not related to the NHL).
Central pathology review was performed by D.J., and lymphomas were reclassified according to the WHO classification.9
Intermediate-risk profile was defined according to HOVON criteria as either stage II disease with serum lactate dehydrogenase (LDH) levels greater than 1.5 times the upper limit of normal (ULN) of the participating center, or as stage III or IV disease with LDH levels less than 1.5 times ULN.7 At the time the HOVON-26 study was designed, the International Prognostic Index (IPI) score was not yet in use.10 The intermediate-risk profile according to HOVON criteria corresponds almost perfectly to the combined low- and high-intermediate-risk groups of the age-adjusted (aa) IPI. For the purpose of this report, patients were also classified retrospectively into low-intermediate- and high-intermediate-risk groups according to the aa-IPI,10 because the IPI is the generally used risk score at present.
The study was conducted in accordance with the Helsinki declaration. The protocol was approved by the ethics review committee of each participating center, and all patients gave informed consent for study participation.
Treatment protocol
Standard treatment, the CHOP-21 regimen, consisted of cyclophosphamide (750 mg/m2 intravenously), doxorubicin (50 mg/m2 intravenously), and vincristine (2 mg intravenously) on day 1, and prednisone (100 mg orally) given on days 1 to 5. Patients were treated every 3 weeks for 8 cycles. The experimental treatment, the I-CHOP regimen, consisted of cyclophosphamide (1000 mg/m2 intravenously), doxorubicin (70 mg/m2 intravenously), and vincristine (2 mg intravenously) on day 1, and prednisone (100 mg orally) given on days 1 to 5. Granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA), at a dose of 5 μg/kg, was given subcutaneously from days 2 to 11 in the I-CHOP arm only. Patients were treated every 2 weeks for 6 cycles. The treatment design was intended to offer the same total amount of cyclophosphamide and doxorubicin in 12 weeks of I-CHOP compared with 24 weeks of CHOP-21, thus doubling dose intensity without increasing cumulative dose. The first CHOP-21 or I-CHOP cycle was administrated at 100% dose, regardless of blood cell counts. The next chemotherapy cycles, both in CHOP-21 and in I-CHOP, were administered at 100% when white blood cell (WBC) counts were 3 × 109/L or higher and platelet (PLT) counts were 100 × 109/L or higher. If blood cell counts were below these values, the next cycle was delayed from 2 to 7 days. If blood cell counts were still below these values, the dose of cyclophosphamide and doxorubicin was attenuated based on the actual counts as follows: if the WBC count was 2 to 3 × 109/L and the PLT count was greater than 100 × 109/L, dosage was attenuated to 75%; if the WBC count was 1 to 2 × 109/L or the PLT count was 50 to 100 × 109/L, dosage was attenuated to 50%; and if the WBC count was less than 1 × 109/L or the PLT count was less than 50 × 9/L, the dosage was attenuated to 0%.
Dose modifications for vincristine were only made if neurotoxicity occurred, and this was at the discretion of the treating physician.
Dose intensity
Dose intensity (milligrams per square meter per week) and total dose (milligrams per square meter) of cyclophosphamide and doxorubicin, as intended to be delivered to the patients in both CHOP-21 and I-CHOP, are shown in Table 1.
Dose intensity and total dose of cyclophosphamide and doxorubicin as planned and actual doses administered in CHOP-21 and I-CHOP
| Regimen . | Dose intensity, median mg/m2/wk (range) . | Total dose, mg/m2 (range) . | ||
|---|---|---|---|---|
| Cyclophosphamide . | Doxorubicin . | Cyclophosphamide . | Doxorubicin . | |
| Planned | ||||
| CHOP-21 | 250 | 17 | 6000 | 400 |
| I-CHOP | 500 | 35 | 6000 | 420 |
| Administered | ||||
| CHOP-21 | 243 (139-267) | 16.2 (9.3-17.8) | 5966 (417-6461) | 398 (28-428) |
| I-CHOP | 463 (155-532) | 32.3 (10.4-37.4) | 5984 (1000-7544) | 415 (71-561) |
| Regimen . | Dose intensity, median mg/m2/wk (range) . | Total dose, mg/m2 (range) . | ||
|---|---|---|---|---|
| Cyclophosphamide . | Doxorubicin . | Cyclophosphamide . | Doxorubicin . | |
| Planned | ||||
| CHOP-21 | 250 | 17 | 6000 | 400 |
| I-CHOP | 500 | 35 | 6000 | 420 |
| Administered | ||||
| CHOP-21 | 243 (139-267) | 16.2 (9.3-17.8) | 5966 (417-6461) | 398 (28-428) |
| I-CHOP | 463 (155-532) | 32.3 (10.4-37.4) | 5984 (1000-7544) | 415 (71-561) |
Actual treatment duration was calculated as the number of weeks between day 1 of the first CHOP cycle until day 22 after the last CHOP cycle in the CHOP-21 arm and between day 1 of the first I-CHOP cycle until day 15 after the last I-CHOP cycle in the I-CHOP arm. Actual dose intensity of the delivered cytotoxic drugs was measured as the median of the actual delivered dose of the drug per square meter of body surface per week over actual treatment duration. The calculation of the actual dose intensity was based on the article of Hryniuk et al.11
Assessment of response and toxicity
All patients underwent restaging after 3 and 8 cycles of CHOP-21 and after 3 and 6 cycles of I-CHOP. This included tumor response evaluation of all involved sites by appropriate methods. Tumor responses were classified as complete remission (CR; including unconfirmed [CRu]), partial remission (PR), stable disease, or progressive disease according to the International Workshop Criteria.12
Patients went off protocol treatment if response after 3 cycles of CHOP-21 or I-CHOP was less than PR, if there was progression/relapse of disease during treatment, when the patient declined to continue, or at the discretion of the treating physician in case of intercurrent diseases or excessive toxicity.
All nonhematologic toxicities, except hair loss, were graded according to the WHO Common Toxicity Criteria.
Statistical analysis
The calculation of the required number of patients to be included in the study was based on the CR rate and overall survival, both primary endpoints. Disease-free survival (DFS) and event-free survival (EFS) were secondary endpoints. In 5 to 7 years, 500 patients were expected to enter the study. Given this number of patients, a 2-sided significance level of α = .05 and a power of 1-β = .80, an increase in CR rate of 12% (from 65% to 77%), could be detected. Around 130 events were needed to detect a similar difference in overall survival (OS).
Random assignments were done, stratified by hospital, with a minimization procedure whereby the probability of allocation to the arm with lowest number of patients increased gradually from 0.5 to 1.0 with the size of the calculated imbalance.
The data were analyzed according to the intention-to-treat principle (all eligible patients were analyzed according to the treatment arm to which they were assigned).
Patient characteristics were compared between the 2 treatment arms using the Pearson χ2 in case of discrete variables, or the Kruskal-Wallis test in case of ordinal or continuous variables. OS was measured from the date of randomization until death. Patients still alive at the date of last contact were censored. EFS was measured from random assignment until there was progression, relapse, or death from any cause, whichever came first. DFS was calculated from date of CR until relapse or death from any cause. Death during therapy or within 1 month after the end of therapy from causes other than NHL was classified as treatment-related death.
OS, EFS, and DFS were estimated by the Kaplan-Meier method. Kaplan-Meier curves were generated to illustrate differences between the 2 treatment arms, and the log-rank test was used to compare the survival curves. Logistic regression was used to analyze differences in CR rate between subgroups with respect to patient characteristics at registration. Furthermore, survival analysis was performed with Cox regression to determine differences in survival between subgroups.
At the final analysis, subgroup analyses of low-intermediate- and high-intermediate-risk patients according to the aa-IPI score (upper age limit, 65 years) were done; we did this also for the subgroup of patients with diffuse large B-cell lymphoma (DLBCL).
The following variables were included in the analysis of prognostic factors: treatment arm, sex, age at registration (continuous), WHO performance status (0 vs 1 vs 2-4), stage (Ann Arbor II versus III/IV), extranodal sites involved (none vs 1 vs more than 1), B-symptoms (yes vs no), bulky disease (tumor size > 10 cm), bone marrow involvement (yes vs no), and a logarithmic transformation of LDH/ULN (log transformation because of nonnormal distribution of LDH). All analyses of prognostic factors were performed while adjusting for arm and aa-IPI score.
A small percentage of values of some prognostic factors were missing (ranging from 0.2%-2.3%). They were imputed by means of an expectation-maximization algorithm.
An interim analysis was planned and performed after 100 patients were evaluable in both arms to exclude large differences in outcome and to ensure that toxicity was acceptable.
All reported P values are 2-sided, and a significance level of α = .05 was used.
Results
Patients
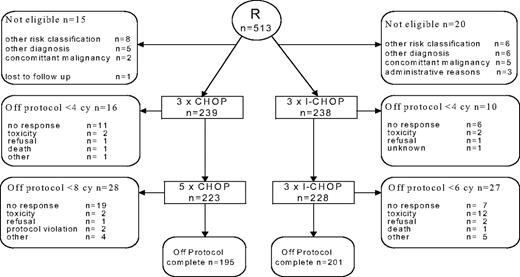
This study was conducted at 70 centers. A total of 513 patients were enrolled in the study; of these, 35 patients were found to be ineligible because they did not fulfill the entry criteria, mainly because they did not belong to the intermediate-risk group, because they suffered from concomitant malignancies, or because a different diagnosis was given. One patient was excluded from the analyses because he was lost to follow-up (Figure 1). At July 1, 2005, median follow-up of those patients still alive was 50 months (range, 16 to 128 months). Of 477 eligible patients, 239 were randomized to CHOP-21 and 238 were randomized to I-CHOP. Central pathology review (according to the WHO classification) was achieved in 85% of the patients; most patients (64%) had DLBCL, while 7% of the patients proved to have low-grade (indolent) lymphomas, the same in both arms. However, results were analyzed on the basis of the original inclusion criteria, so none of the eligible patients were excluded by data acquired later on. As can be seen in Table 2, the patients were well balanced in both treatment arms in clinical and pathologic characteristics, except for WHO performance status (there were more patients with a score of 2 in the I-CHOP arm; P = .05).
Flow diagram of 513 patients with NHL by treatment arm. Per treatment arm, the number of patients who received specific induction treatment and the number of patients who went off protocol and the reasons for going off protocol are shown. R indicates randomization.
Flow diagram of 513 patients with NHL by treatment arm. Per treatment arm, the number of patients who received specific induction treatment and the number of patients who went off protocol and the reasons for going off protocol are shown. R indicates randomization.
Patient characteristics
| Characteristic . | CHOP-21 . | I-CHOP . |
|---|---|---|
| No. of patients | 239 | 238 |
| No. of male patients (%) | 135 (56) | 133 (56) |
| Median age, y (range) | 50 (16-65) | 50 (16-65) |
| 60 y or younger, no. (%) | 210 (88) | 205 (86) |
| Older than 60 y, no. (%) | 29 (12) | 33 (14) |
| Histologic findings according to WHO, no. (%) | ||
| Not reviewed | 35 (15) | 38 (16) |
| Reviewed | 204 (85) | 200 (84) |
| Diffuse large B-cell lymphoma | 126 (62) | 133 (67) |
| Mantle cell lymphoma | 21 (10) | 14 (7) |
| Mediastinal B-cell lymphoma | 1 (0) | 1 (0) |
| Follicular lymphoma, grade 3 | 18 (9) | 15 (8) |
| Follicular lymphoma, grades 1-2 | 14 (7) | 12 (6) |
| Small lymphocytic lymphoma | 1 (0) | 2 (1) |
| Aggressive B-cell lymphoma, unspecified | 5 (2) | 6 (3) |
| Peripheral T-cell lymphoma | 1 (0) | 5 (3) |
| Angioimmunoblastic T-cell lymphoma | 4 (2) | 3 (2) |
| Unclassifiable T-cell | 2 (1) | |
| Anaplastic large cell lymphoma | 11 (5) | 9 (5) |
| WHO performance score, no. (%) | ||
| 0 | 139 (58) | 118 (50) |
| 1 | 83 (35) | 84 (35) |
| 2 | 8 (3) | 22 (9) |
| 3 | 3 (1) | 3 (1) |
| 4 | 1 (0) | 0 (0) |
| Unknown | 5 (2) | 11 (5) |
| LDH level, no. (%) | ||
| Less than 1.5 times ULN | 194 (81) | 206 (87) |
| 1.5 times ULN or more | 45 (19) | 32 (13) |
| LDH level greater than ULN, no. (%) | 111 (46) | 110 (46) |
| Stage, no. (%) | ||
| II | 44 (18) | 32 (13) |
| III | 90 (38) | 78 (33) |
| IV | 105 (44) | 128 (54) |
| B symptoms, no. (%) | 88 (37) | 103 (44) |
| More than 1 extranodal site, no. (%) | 46 (19) | 42 (18) |
| Bulky disease at least 10 cm present, no. (%) | 52 (22) | 51 (21) |
| Age-adjusted IPI score,* no. (%) | ||
| Low-intermediate | 165 (69) | 145 (61) |
| High-intermediate | 69 (29) | 83 (35) |
| High | 5 (2) | 10 (4) |
| Characteristic . | CHOP-21 . | I-CHOP . |
|---|---|---|
| No. of patients | 239 | 238 |
| No. of male patients (%) | 135 (56) | 133 (56) |
| Median age, y (range) | 50 (16-65) | 50 (16-65) |
| 60 y or younger, no. (%) | 210 (88) | 205 (86) |
| Older than 60 y, no. (%) | 29 (12) | 33 (14) |
| Histologic findings according to WHO, no. (%) | ||
| Not reviewed | 35 (15) | 38 (16) |
| Reviewed | 204 (85) | 200 (84) |
| Diffuse large B-cell lymphoma | 126 (62) | 133 (67) |
| Mantle cell lymphoma | 21 (10) | 14 (7) |
| Mediastinal B-cell lymphoma | 1 (0) | 1 (0) |
| Follicular lymphoma, grade 3 | 18 (9) | 15 (8) |
| Follicular lymphoma, grades 1-2 | 14 (7) | 12 (6) |
| Small lymphocytic lymphoma | 1 (0) | 2 (1) |
| Aggressive B-cell lymphoma, unspecified | 5 (2) | 6 (3) |
| Peripheral T-cell lymphoma | 1 (0) | 5 (3) |
| Angioimmunoblastic T-cell lymphoma | 4 (2) | 3 (2) |
| Unclassifiable T-cell | 2 (1) | |
| Anaplastic large cell lymphoma | 11 (5) | 9 (5) |
| WHO performance score, no. (%) | ||
| 0 | 139 (58) | 118 (50) |
| 1 | 83 (35) | 84 (35) |
| 2 | 8 (3) | 22 (9) |
| 3 | 3 (1) | 3 (1) |
| 4 | 1 (0) | 0 (0) |
| Unknown | 5 (2) | 11 (5) |
| LDH level, no. (%) | ||
| Less than 1.5 times ULN | 194 (81) | 206 (87) |
| 1.5 times ULN or more | 45 (19) | 32 (13) |
| LDH level greater than ULN, no. (%) | 111 (46) | 110 (46) |
| Stage, no. (%) | ||
| II | 44 (18) | 32 (13) |
| III | 90 (38) | 78 (33) |
| IV | 105 (44) | 128 (54) |
| B symptoms, no. (%) | 88 (37) | 103 (44) |
| More than 1 extranodal site, no. (%) | 46 (19) | 42 (18) |
| Bulky disease at least 10 cm present, no. (%) | 52 (22) | 51 (21) |
| Age-adjusted IPI score,* no. (%) | ||
| Low-intermediate | 165 (69) | 145 (61) |
| High-intermediate | 69 (29) | 83 (35) |
| High | 5 (2) | 10 (4) |
No more than 65 y.
There was no perfect balance between treatment arms with respect to subgroups defined by the aa-IPI score. In the low-intermediate-risk aa-IPI group (n = 310), 165 (53%) patients were treated with CHOP-21 and 145 (47%) patients with I-CHOP; in the high-intermediate aa-IPI group (n = 152), 69 (45%) patients were treated with CHOP-21 and 83 (55%) patients were treated with I-CHOP. However, these differences were not significant.
Response to treatment
In the CHOP-21 arm, 195 (82%) of the 239 patients completed the total planned number of 8 cycles of CHOP according to protocol. In the I-CHOP arm, 201 (84%) of 238 patients completed the total planned number of 6 I-CHOP cycles (Figure 1). Reasons for not having completed the protocol were no response after 3 cycles or progression/relapse during treatment (30 patients in the CHOP-21 arm and 13 patients in the I-CHOP arm), toxicity of the treatment (4 patients in the CHOP-21 arm and 14 patients in the I-CHOP arm), death during treatment (1 patient in each arm), refusal of the patient (2 patients in the CHOP-21 arm and 3 patients in the I-CHOP arm) and other reasons (7 patients in the CHOP-21 arm and 6 patients in the I-CHOP arm). So, primary treatment failure occurred more often in the CHOP-21 arm (13% vs 5%; P < .01), and going off treatment due to toxicity occurred more often in the I-CHOP arm (6% vs 2%; P < .05). In the I-CHOP arm, going off treatment due to toxicity occurred mainly after 4 or more cycles.
In the CHOP-21 arm, 218 (91%) of 239 patients achieved at least PR, including 117 (49%) patients with CRu, while in the I-CHOP arm, 224 (94%) of 238 patients achieved at least PR, including 126 (53%) patients with CRu (P = .14 and .41, respectively).
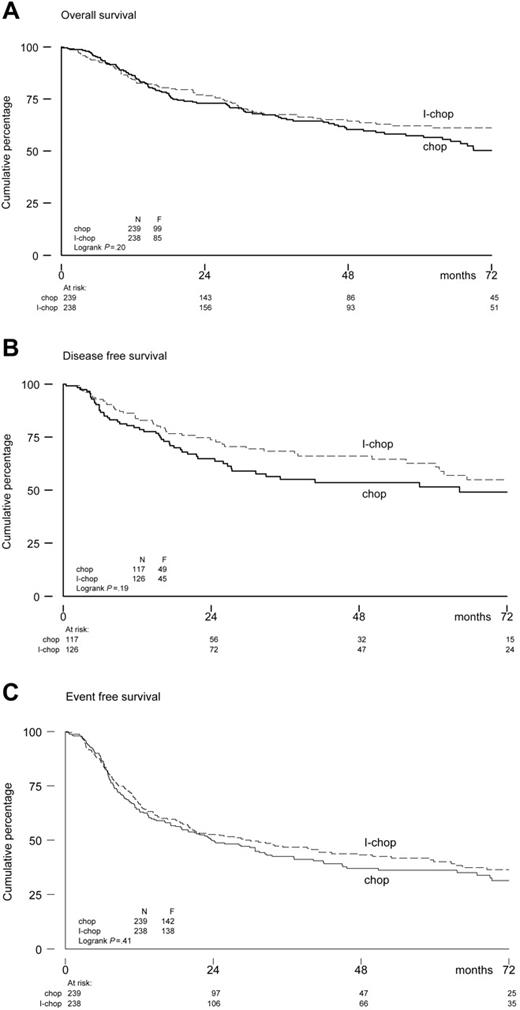
The Kaplan-Meier estimates of the endpoints of OS, DFS, and EFS for both study arms are shown in Figure 2. The observed differences between the arms were not statistically significant. The 6-year estimated OS rates were 50% in the CHOP-21 arm and 61% in the I-CHOP arm (hazard ratio, 0.83; 95% confidence interval [CI], 0.62-1.11). The 6-year estimated DFS and EFS were 49% and 31%, respectively, after CHOP-21, and 55% and 36%, respectively, after I-CHOP (DFS hazard ratio, 0.76; 95% CI, 0.51-1.15; EFS hazard ratio, 0.90; 95% CI, 0.72-1.14).
Baseline prognostic factors were analyzed by multivariate Cox regression analysis for effects on OS, DFS, and EFS, and by logistic regression for effects on the CR rate. All analyses were adjusted for arm and aa-IPI score. Factors associated with a lower CR rate were a WHO performance status of 2 to 4 (P < .05) and bulky disease (P < 0.01). Factors associated with a shorter OS were male sex (P < .01), increased age (P < .01), and higher levels of LDH/ULN (P < .01). Factors associated with a shorter EFS were Ann Arbor stage III/IV (P < .05), higher levels of LDH/ULN (P < .01), and bone marrow involvement (P < .05). Bone marrow involvement was also significantly associated with shorter DFS (P < .01).
Subgroup analyses
Subgroup analyses of low-intermediate- and high-intermediate-risk groups according to the aa-IPI score were also performed. The results should be interpreted as explorative only because the analyses were not planned. The interaction term between treatment arm and aa-IPI score was not statistically significant in a Cox regression model (analyzing OS, DFS, and EFS) or in logistic regression (analyzing CR rate), with all P values greater than .05.
The response rates with CHOP-21 and I-CHOP in the low-intermediate- and high-intermediate-risk groups according to the aa-IPI score revealed comparable data: 50% and 55% CR rates with CHOP-21 and I-CHOP, respectively, in the aa-IPI low-intermediate-risk group and 49% and 51% CR rates with CHOP-21 and I-CHOP, respectively, in the aa-IPI high-intermediate-risk group. Overall response rates (CR and PR) were higher than 90% in both aa-IPI risk groups after either CHOP-21 or I-CHOP.
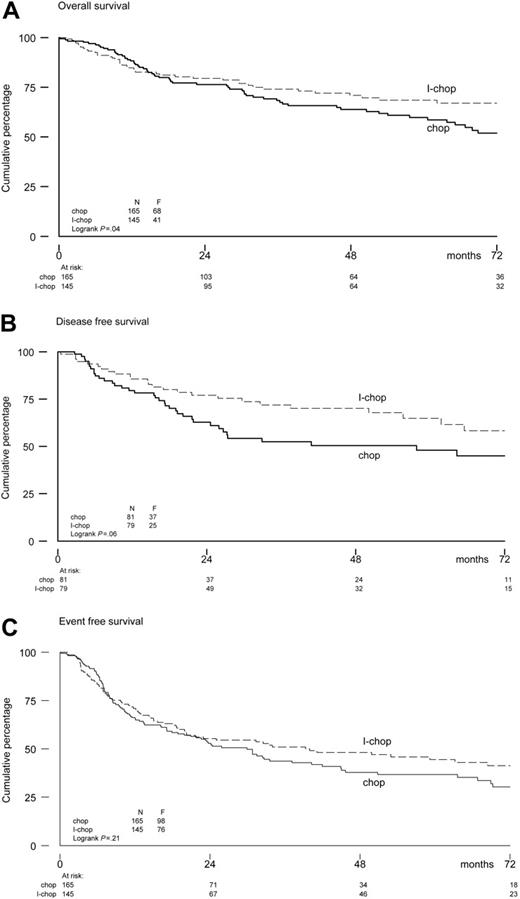
In the aa-IPI low-intermediate risk group, a benefit of I-CHOP was observed with a 6-year estimated OS rate of 52% after CHOP-21 versus 67% after I-CHOP. The 6-year estimated DFS and EFS rates in the low-intermediate-risk group were 45% and 30%, respectively, after CHOP-21 and 58% and 41%, respectively, after I-CHOP. Hazard ratios were 0.67 (95% CI, 0.46-0.99; P = .05) for OS, 0.61 (95% CI, 0.37-1.02; P = .06) for DFS, and 0.82 (95% CI, 0.61-1.11; P = .21) for EFS. The corresponding survival curves are shown in Figure 3. In the aa-IPI high-intermediate-risk group, no differences were found between the 2 treatment arms. Estimated OS, DFS, and EFS at 6 years were 47%, 60%, and 35% in the CHOP-21 arm and 53%, 49%, and 29% in the I-CHOP arm, respectively. Hazard ratios were 0.99 (95% CI, 0.61-1.60) for OS, 1.11 (95% CI, 0.54-2.28) for DFS, and 0.98 (95% CI, 0.66-1.46) for EFS.
OS (A), DFS (B), and EFS (C) for low-intermediate risk patients according to aa-IPI-criteria.
OS (A), DFS (B), and EFS (C) for low-intermediate risk patients according to aa-IPI-criteria.
The effects of CHOP-21 and I-CHOP in patients with DLBCL were also analyzed: 126 patients received CHOP-21 and 133 patients received I-CHOP (Table 2). Within the subgroup of patients with DLBCL, no significant differences between the 2 treatment arms were found. Although there was a tendency in favor of I-CHOP in low-intermediate-risk patients (DFS of 64% vs 50%), significant differences were not found between the treatment arms within the subgroups of aa-IPI low-intermediate-risk and high-intermediate-risk patients with DLBCL (data not shown).
Delivered dose intensity
Of the patients who completed the chemotherapy cycles according to protocol (82% in the CHOP-21 arm, and 84% in the I-CHOP arm), no reduction in dose or delay between cycles occurred in 72% of the cycles in the CHOP-21 arm and in 69% of the cycles in the I-CHOP arm. The main reasons for deviation were hematopoietic toxicity (64% in CHOP-21 and 71% in I-CHOP) and mistakes in dose administration (15% in CHOP-21 and 7% in I-CHOP). Nearly all (90% or more) of the planned doses of cyclophosphamide, doxorubicin, vincristine, and prednisone were given to patients in 97%, 96%, 89%, and 96% of the cycles of the CHOP-21 arm, respectively, and in 88%, 87%, 91%, and 96% of the cycles in the I-CHOP arm, respectively.
Of note, 20 patients in the CHOP-21 arm were treated with G-CSF (not according to protocol) for a median of 4 cycles (range, 1-6 cycles) because of leukopenia. Furthermore, 7 patients received treatment of the other arm by mistake (4 patients received I-CHOP, and 3 patients received CHOP). These 7 patients, and 1 patient for whom no treatment data were available, were left out the analysis of delivered dose intensity.
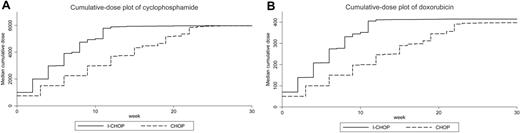
The planned dose intensity and the median and range of the delivered dose intensity of cyclophosphamide and doxorubicin given to the patients in each treatment arm are shown in Table 1. In summary, patients in the I-CHOP arm received an actual increase in dose intensity of cyclophosphamide and doxorubicin of 190% and 199%, respectively, compared with patients in the CHOP-21 arm. The total delivered dose was similar between the arms. Cumulative dose plots for cyclophosphamide and doxorubicin are shown in Figure 4.
Cumulative dose plots for cyclophosphamide and doxorubicin. Median delivery in milligrams per square meter calculated weekly from the start of chemotherapy. The slopes of the plots represent dose intensity, and the plateaus represent median total dose delivered. The delivered dose intensity of the I-CHOP arm is higher than the delivered dose intensity of the CHOP arm, but the median total delivered dose is quite similar. The cycles (steps) are less distinguishable after 4 or 5 cycles, which means that from that point onwards most dose delays or dose reductions occur.
Cumulative dose plots for cyclophosphamide and doxorubicin. Median delivery in milligrams per square meter calculated weekly from the start of chemotherapy. The slopes of the plots represent dose intensity, and the plateaus represent median total dose delivered. The delivered dose intensity of the I-CHOP arm is higher than the delivered dose intensity of the CHOP arm, but the median total delivered dose is quite similar. The cycles (steps) are less distinguishable after 4 or 5 cycles, which means that from that point onwards most dose delays or dose reductions occur.
Nonhematologic toxicity
Table 3 summarizes all reported grades 3 and 4 nonhematologic toxicities. The percentages of patients developing a toxic event in at least 1 cycle were comparable between the 2 arms, but the percentage of patients developing an infection was almost 4 times higher in the I-CHOP arm. Treatment-related mortality (TRM) was similar in both arms: 4 patients in the CHOP-21 arm and 6 patients in the I-CHOP arm died from TRM. In detail, 2 patients (1 in each arm) died from hemorrhage, 1 patient (in the I-CHOP arm) died from liver toxicity, and 7 patients died from infection (3 in the CHOP-21 arm and 4 in the I-CHOP arm).
Nonhematologic toxicity grades 3 and 4 observed in patients treated with CHOP-21 or I-CHOP
| Event . | CHOP-21, % . | I-CHOP, % . |
|---|---|---|
| Mucositis | 4 | 8 |
| Nausea or vomiting | 6 | 5 |
| Liver toxicity | 1 | 2 |
| Cardiac toxicity | 3 | 4 |
| Neurotoxicity | 1 | 3 |
| Fever | 1 | 2 |
| Infections | 7 | 26 |
| Other | 7 | 14 |
| Event . | CHOP-21, % . | I-CHOP, % . |
|---|---|---|
| Mucositis | 4 | 8 |
| Nausea or vomiting | 6 | 5 |
| Liver toxicity | 1 | 2 |
| Cardiac toxicity | 3 | 4 |
| Neurotoxicity | 1 | 3 |
| Fever | 1 | 2 |
| Infections | 7 | 26 |
| Other | 7 | 14 |
Results are given as the percentage of patients with an event in at least 1 cycle.
Furthermore, among causes of death occurring during follow-up, 7 patients died from cardiac toxicity (5 in the CHOP-21 arm and 2 in the I-CHOP arm), and 5 patients died from secondary malignancies (2 in the CHOP-21 arm and 3 in the I-CHOP arm), as is shown in Table 4 Apart from the 5 secondary malignancies that caused death, there were 7 patients with other secondary malignancies observed during follow-up (Table 4).
Patients with cardiotoxic death or with secondary malignancies during follow-up
| Event . | CHOP-21, no. of patients . | I-CHOP, no. of patients . |
|---|---|---|
| Cardiotoxic death | 5 | 2 |
| Death from secondary malignancies | 2 (1 to lung cancer, 1 to gastric cancer) | 3 (1 to acute myeloid leukemia, 1 to ureter cancer, 1 to prostate cancer) |
| Alive with secondary malignancies | 4 (1 with breast cancer, 2 with skin cancer, 1 with cervical cancer) | 3 (1 with bladder cancer, 2 with skin cancer) |
| Event . | CHOP-21, no. of patients . | I-CHOP, no. of patients . |
|---|---|---|
| Cardiotoxic death | 5 | 2 |
| Death from secondary malignancies | 2 (1 to lung cancer, 1 to gastric cancer) | 3 (1 to acute myeloid leukemia, 1 to ureter cancer, 1 to prostate cancer) |
| Alive with secondary malignancies | 4 (1 with breast cancer, 2 with skin cancer, 1 with cervical cancer) | 3 (1 with bladder cancer, 2 with skin cancer) |
Discussion
In this randomized trial we assessed whether a substantial dose intensification of both cyclophosphamide and doxorubicin, considered the most important chemotherapeutic agents for patients with NHL, might improve the cure rate in younger patients aged 18 to 65 years with intermediate-risk aggressive NHL. The treatment arms were either “standard” CHOP-21 or I-CHOP, in which the doses of cyclophosphamide and doxorubicin were escalated and the time intervals between the CHOP cycles were reduced without changing total cumulative dose. We found no statistically significant differences between CHOP-21 and I-CHOP in CR rates or OS, DFS, and EFS estimates. Although the number of eligible patients (477) was smaller than originally planned (500), the study did not suffer from a lack of power. With respect to OS, more events occurred (184) than anticipated (130), increasing the study's power to 0.91. Furthermore, given 477 patients, the study's power still was 0.80 to detect the anticipated difference in CR rate.
Although treatment with I-CHOP was accompanied by more infections than treatment with CHOP-21, there were no differences in TRM or in incidence of secondary malignancies such as myelodysplastic syndrome/acute myeloid leukemia. The higher toxicity of I-CHOP, however, might be caused in part by the fact that the number of patients with a poor performance status in the I-CHOP arm was slightly higher. Notably, no increase in cardiotoxicity was observed after I-CHOP treatment compared with that of CHOP-21.
When this study was initiated, eligible patients had to belong to the intermediate-risk group of aggressive NHL, as defined by criteria of the HOVON Lymphoma Study Group. Practically all (97%) patients belonging to the intermediate-risk group according to the HOVON criteria can be subdivided into low-intermediate-risk and high-intermediate-risk patients according to the aa-IPI score, the risk score uniformly used at present. Therefore, we also analyzed the study endpoints according to these aa-IPI groups, which were, however, unplanned analyses. An improved outcome, with an approximate 15% increase in overall survival, was observed for patients in the aa-IPI low-intermediate-risk group if they were treated with I-CHOP compared with CHOP-21. This improvement was largely attributable to the quality of CR by the more intensive treatment, demonstrated by the increase of DFS after I-CHOP. However, as the data are coming from secondary analyses and the P values are of borderline significance, these results need independent confirmation.
No impact of this type of dose intensification for patients in the aa-IPI high-intermediate-risk group was observed. The paradoxical findings of the study (ie, possible benefit of dose intensification of conventional chemotherapy in low-intermediate-risk patients, whereas this seemed to be absent in high-intermediate-risk patients) are hard to explain. It should be noted first that the treatment comparison in the high-intermediate subgroup was underpowered; thus, the absence of a possible benefit for I-CHOP in this subgroup might also be the result of the fact that the number of patients in this subgroup was too limited. Nevertheless, there are more paradoxical findings with dose-intensified therapies: aa-IPI low- and low-intermediate-risk patients with aggressive NHL do not benefit from much higher dose escalations of cytotoxic drugs to levels that require stem cell support (autologous stem cell transplantation), whereas aa-IPI high-intermediate- and high-risk patients seem to profit from this approach.7,13-17 Furthermore, in other hematologic malignancies (eg, in patients with acute myeloid leukemia) comparable paradoxical findings are observed: dose intensifications with cytarabine in induction treatment only benefits low-risk patients and not high-risk patients.18
The dose intensification of both cyclophosphamide and doxorubicin in this multicenter phase 3 trial can be considered substantial, as the actual dose intensity was almost doubled with I-CHOP compared with that of CHOP-21. There are a few previously published phase 2 studies of dose intensifications of both agents in CHOP (-like) regimens, varying from lower to higher dose intensifications than those used in this phase 3 study, in patients of all risk categories.19-21 These studies suggested a possible benefit for dose intensification although toxicity of these regimens, especially the occurrence of secondary leukemias, raised concern about their final results. In accordance with our study is the German NHL-B1 trial,22 in which dose densification was investigated in younger patients with both IPI low- and low-intermediate-risk NHL, by comparing shortening of treatment interval (3 vs 2 weeks; CHOP-21 vs CHOP-14) between the CHOP cycles. A survival benefit for patients receiving the every-2-weeks regimen was shown.
Finally, it should be noted that this study did not include the addition of rituximab (anti-CD20 monoclonal antibody) to CHOP. Rituximab added to CHOP-21 has proven superiority over CHOP-21 alone in elderly patients with aggressive B-cell lymphomas.3 In younger low-risk patients with aggressive B-cell lymphomas a phase 3 trial comparing CHOP-21 plus rituximab with CHOP-21 alone was stopped early because of a superior outcome for those who received the combined treatment.23 Furthermore, 2 trials comparing (dose-dense) CHOP-14 alone with CHOP-14 plus rituximab in elderly patients with aggressive B-cell lymphomas were also stopped early because of the significantly better outcome for the combined treatment.24,25 Therefore, optimal treatment of aggressive B-cell NHL should include the addition of anti–B-cell monoclonal antibody to the best chemotherapy regimen.
We conclude that I-CHOP is an effective chemotherapy regimen for younger patients with aa-IPI low- and low-intermediate-risk aggressive NHL. This regimen should be combined with rituximab in patients with B-cell lymphoma. Another advantage of I-CHOP is the fact that patients can complete their chemotherapy in 12 weeks, twice as fast compared with treatment with CHOP-21. For younger patients with high-intermediate- and high-risk aggressive NHL, the optimal dose and timing of chemotherapy still needs to be clarified.
Authorship
Contribution: L.F.V. and G.W.v.I. designed the research protocol; L.F.V., D.D.d.J., M.A.M., G.E.G.V., M.H.H.K., G.J.O., J.K.D., P.S., and G.W.v.I. were involved in performing the clinical research; L.F.V., A.N., D.D.d.J., and G.W.v.I. collected and analyzed the data; L.F.V., A.N., and G.W.v.I. wrote the paper; and all other authors critically contributed to the final preparation of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of HOVON appears as a data supplement to the online version of this article (Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article).
Correspondence: Leo F. Verdonck, University Hospital Medical Center Utrecht, Heidelberglaan 100/PO Box 85500, 3584 CX/3508 GA Utrecht, the Netherlands; e-mail: l.f.verdonck@umcutrecht.nl.
Presented orally and in abstract form at the 47th annual meeting of the American Society of Hematology, Atlanta, Georgia, December 11, 2005.26
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal