Abstract
Angiogenesis is a complex, orchestrated process that plays a critical role in several conditions and has special relevance in the progression of cancer. Hypoxia is the major stimulus for angiogenesis, and hypoxia-inducible transcription factor–1 alpha (HIF-1α) is its key mediator. We set up a novel in vitro model of HIF-1α up-regulation by treating human umbilical vein endothelial cells (HUVECs) with the hypoxia-mimicking deferoxamine (DFO) and found that this condition was sufficient to promote angiogenesis, like the well-known HUVEC model cultured under low pO2. The proteasome inhibitor bortezomib, which induces strong apoptosis in cancer cells, abrogated proliferation and angiogenesis of HUVECs when used at a high concentration (100 nM), yet promoted both functions at a low dosage (10 nM). This double-edged effect appeared to be mediated by differential effects exerted by the different concentrations of bortezomib on 2 master regulators of tumor-associated angiogenesis, HIF-1α and nuclear factor kappa B (NF-kB). Significantly, when HUVECs were induced to express HIF-1α prior to bortezomib treatment, proliferative and angiogenic responses were abolished, and a greatly enhanced proapoptotic effect was promoted with both concentrations of the drug. These findings indicate that HIF-1α up-regulation may sensitize endothelial cells to the antiangiogenic and proapoptotic effects of bortezomib and might be exploited to target tumor-associated vessels in the course of antiangiogenic therapies.
Introduction
Angiogenesis is a highly complex, orchestrated process that plays a critical role in several processes, including normal development and wound repair.1 Aberrant angiogenesis helps the growth of tumors,2 making a deeper understanding of the underlying mechanisms mandatory. Among the mechanisms that control angiogenesis, hypoxia is the major pathophysiologic stimulus.2 Indeed, tumor-cell expansion requires new blood vessel formation to supply oxygen and nutrients. The expression of multiple angiogenic and antiangiogenic factors is dysregulated in tumors,3 an event known as “angiogenic switch” that leads normal angiogenesis to be replaced by the formation of immature vessels, which are structurally and functionally abnormal and prone to collapse. As a result, intratumoral hypoxia is common.4
In mammalian cells, exposure to a low-oxygen environment triggers an evolutionary conserved hypoxia-response pathway based on the regulated expression of hypoxia-inducible factor –1 α (HIF1α),5 which acts as a master transcription switch to regulate oxygen homeostasis.6 Under normal O2 conditions, HIF-1α is targeted for ubiquitination and rapid degradation by the proteasome.7,8 Under hypoxic conditions, HIF-1α subunits translocate to the nucleus and, together with their partner factor, the basic helix-loop-helix/PAS protein Arnt, bind to the promoters of the genes that mediate glycolysis and angiogenesis.6,9,10 In addition to vascular endothelial growth factor (VEGF), the HIF-1α transcription factor activates other genes that regulate the expression of angiopoietin-1 and -2, the VEGF receptor Flt-1, endothelin and the glucose transporter GLUT-1.11 Recently, HIF-1α hypoxic induction in endothelial cells has been shown to be essential to drive the angiogenesis of human umbilical vein endothelial cells (HUVECs) in vitro, via a basic fibroblast growth factor (bFGF)–dependent autocrine loop.12 Cumulatively, these results indicate that HIF-1α controls the expression of multiple angiogenic factors and suggest that targeting HIF-1α for therapy may result in a more comprehensive outcome than that achieved by any single antiangiogenic factor.13
The proteasome is a multicatalytic enzyme complex that is responsible for the degradation of the vast majority of intracellular proteins and is involved in diverse cellular processes such as proliferation, differentiation, and apoptosis.14,15 The latter function has been exploited for the development of specific proteasome inhibitors (PSIs), in particular the boronic acid dipeptide bortezomib (Velcade, formerly PS-341), which is used for the treatment of several malignancies, especially multiple myeloma (MM).16 Recent data have shown that in patients with MM, in addition to a direct effect on plasma cells, bortezomib exerts an antiangiogenic effect that appears to be mediated by a dose-dependent inhibition of VEGF and IL-6 secretion by endothelial cells.17 Little is known of the impact that bortezomib may have in a hypoxic microenvironment, which could ultimately be responsible for the outcome of the drug's effects.
Our study aimed at investigating the modifications induced by bortezomib in endothelial cells (HUVECs) exposed to stimuli that trigger HIF-1α–dependent angiogenesis.
Materials and methods
Proteasome inhibitors, reagents, and monoclonal antibodies
Bortezomib (Millennium Pharmaceuticals, Cambridge, MA) was dissolved in DMSO and stored at −20°C until use; it was diluted in culture medium immediately before use. DFO was from Novartis (Basel, Switzerland). Human VEGF (PeproTech, Rocky Hill, NJ) was used at 10 ng/mL. Anti–human HIF-1α mAb (clone OZ15) was from Novus Biologicals (Littleton, CO). Rabbit anti–GLUT-1 was from DAKO (Carpinteria, CA); DAPI (4,6 diamidino-2-phenyindole dihydrochloride) and the anti–human VEGFR2 mAb were from Sigma-Aldrich (Milan, Italy). Anti-P65 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell isolation and cultures
HUVECs were isolated from the human cord by collagenase treatment as described,18 and cultured in 1% gelatin-coated flasks using endotoxin-free Medium 199 (BioWhittaker, Cambrex Bio Science, Verviers, Belgium) containing 20% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), 1% bovine retinal-derived growth factor, 90 μg/mL heparin (Biochrom, Berlin, Germany), 100 U/mL penicillin, and 10 μg/mL streptomycin (Life Technologies, Grand Island, NY). All experiments were carried out with HUVECs during steps 1 to 4.
Hypoxia treatment
Hypoxia was mimicked by treatment with 380 μM of iron chelator DFO, established with titration. When indicated, cells were incubated in a temperature- and humidity-controlled environmental chamber IG750 (Jouan Italia, Cologno Monzese, Milan, Italy) in an atmosphere containing 5% O2, 5% CO2, and 90% N2.
Detection of apoptosis
Apoptosis in HUVECs was assessed by staining of surface phosphatidyl-serine with FITC-conjugated annexin V (Bender Medical Systems, Prodotti Gianni, Milan, Italy). The cell population undergoing apoptosis was identified by double-parameter flow cytometry using annexin V and the vital dye propidium iodide (PI). Caspase-3 activation was evaluated by the use of the cell-permeant substrate VAD-FMK-PE (BioVision Research Products, Mountain View, CA), conjugated according to the manufacturer's protocol. Mitochondrial membrane potential was measured by use of the lypophilic probe JC-1 (BIOMOL Research Lab, Plymouth Meeting, PA), which aggregates in healthy mitochondria and fluoresces red, but diffuses in the cytoplasm and fluoresces green upon mitochondrial damage. Cells were run on a FACScan instrument (Becton Dickinson, Mountain View, CA). Analysis of 104 cells per sample was done with CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Proliferation
Cell proliferation and viability were determined by the trypan blue dye exclusion test. Briefly, HUVECs (5 × 104) were seeded onto gelatin-coated 24-well flat plates (Costar, Cambridge, MA) and incubated with the indicated effectors. After trypsinization, cells that excluded trypan blue were counted in a Bürker chamber.
Confocal analysis
Detection of HIF-1α, GLUT-1, and p65 by confocal analysis was performed on HUVECs, fixed (2% paraformaldehyde), and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS). PBS (containing 1% FBS)–rinsed cells were incubated for 1 hour at room temperature with the relative mAbs followed by rabbit anti–mouse IgG AlexaFluor 594 for HIF-1α and for GLUT-1 and by goat anti–rabbit IgG AlexaFluor 488 for p65 (both from Molecular Probes, Eugene, OR) at a dilution of 1:500, and counterstained for nuclei with DAPI for 5 minutes. Microscopic analysis was performed using a Perkin-Elmer Ultraview ERS (Wellesley, MA) confocal laser microscope (Plan Apochromat 63× objective lens, 1.4 numeric aperture, oil). When indicated, average pixel intensity values were calculated within a defined area. Nuclear area was defined with DAPI staining. All analyses were performed with ImageJ software (National Institutes of Health, Bethesda, MD).
In vitro Matrigel capillaries formation
The experiments were performed using Matrigel (Collaborative Biomedical Products, BD Biosciences; 4 mg/mL in D-PBS) added to a 96-well plate and incubated at 37°C overnight to allow gel formation. HUVECs (2 × 104/well) were plated onto Matrigel in Medium 199 containing 1% FBS and incubated. After a 16-hour incubation, the HUVECs 2-dimensional organization and the network growth area were examined and photographed under an Eclipse TS100 microscope (Nikon, Melville, NY). The number of cords was measured using Nikon Lucia software.
Western blotting
HUVECs were grown to reach subconfluence on gelatin-coated flasks, treated with the indicated effectors, washed, and lysed with ice-cold lysis buffer (0.5% Nonidet P-40, 150 mM NaCl, 0.1% SDS, 50 mM NaF, 1 mM Na3VO4, 1 mM PMSF), supplemented with a complete protease inhibitors cocktail (Roche Diagnostics, Mannheim, Germany). Protein concentrations were determined by Bradford protein assay (Bio-Rad Laboratories, München, Germany) with bovine serum albumin as standard. Samples (30 μg protein) were electrophoresed on NuPAGE 4% to 12% acrylamide gel under reducing conditions using the Novex mini gel system (Invitrogen, Carlsbad, CA), transferred to nitrocellulose membranes (Amersham Biosciences, Amersham, United Kingdom) and reversibly stained with Ponceau S (Sigma Aldrich, St Louis, MO) to confirm complete transfer. Membranes were blocked with 10% nonfat dry milk in PBS-Tween and incubated with HIF-1α (clone H1alpha 67-7; Novus Biologicals) and NF-kB p65 antibodies. Membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary antibodies (Bio-Rad), developed by chemiluminescence (Amersham Biosciences) and visualized using the Chemi-doc Gel XRS System (Bio-Rad). Blots were stripped and reprobed with vinculin antibody (Sigma Aldrich) to normalize the results. Lysate of MDA-MB-231 cells (ATTC HTB 26) was used as positive control (30 μg protein) in HIF-1α Western blot analysis.
Statistical analysis
Data are presented as the mean plus or minus the standard error of the mean (SEM). Comparisons for each pair were calculated using a 2-tailed Student t test (JMP software; SAS, Cary, NC). P < .05 was considered statistically significant; P < .001 was considered highly significant.
Results
HIF-1α expression induced by deferoxamine drives angiogenesis
To evaluate the effects of HIF-1α activation on HUVEC angiogenic response, we first set up an experimental model by treating HUVECs with DFO (380 μM), an iron chelator recognized as a hypoxia-mimicking compound,19 and then compared it to the established HUVEC model cultured in low (5%) pO2.12 The levels of HIF-1α were measured by Western blot (Figure 1A) and compared with those expressed by the MDA-MB-231 human breast cancer cell line, in which both HIF-1α mRNA and protein are constitutively elevated.20
Induction of HIF-1α expression in HUVECs drives angiogenesis. HUVECs were treated with DFO (380 μM) and evaluated for HIF-1α expression by Western blot (A) and confocal (B) analyses. In panel A, the breast carcinoma MDAMB 231 cell line was used as a positive control. In panel B, HIF-1α (in red) colocalizes with nuclear DAPI (pseudo-colored in green; top panels); GLUT-1 staining demonstrated the transcriptional activity of HIF-1α (bottom panels). (C) HUVECs seeded on Matrigel layers in the presence or absence of DFO or hypoxia were analyzed for their capacity to form tubule-like structures. VEGF (10 ng/mL) was used as positive control. The contribution of the VEGF/VEGFR2 autocrine loop to hypoxia-induced tubule formation was assessed by using the blocking anti-VEGFR2 mAb. After 18 hours of incubation, images were captured under a Nikon TS 100 microscope (original magnification ×10), 3 independent experiments were performed, and structures were counted with Lucia software on 3 different fields (D).
Induction of HIF-1α expression in HUVECs drives angiogenesis. HUVECs were treated with DFO (380 μM) and evaluated for HIF-1α expression by Western blot (A) and confocal (B) analyses. In panel A, the breast carcinoma MDAMB 231 cell line was used as a positive control. In panel B, HIF-1α (in red) colocalizes with nuclear DAPI (pseudo-colored in green; top panels); GLUT-1 staining demonstrated the transcriptional activity of HIF-1α (bottom panels). (C) HUVECs seeded on Matrigel layers in the presence or absence of DFO or hypoxia were analyzed for their capacity to form tubule-like structures. VEGF (10 ng/mL) was used as positive control. The contribution of the VEGF/VEGFR2 autocrine loop to hypoxia-induced tubule formation was assessed by using the blocking anti-VEGFR2 mAb. After 18 hours of incubation, images were captured under a Nikon TS 100 microscope (original magnification ×10), 3 independent experiments were performed, and structures were counted with Lucia software on 3 different fields (D).
HIF-1α protein was undetectable in HUVECs under basal culture conditions, but was seen to be expressed early following DFO treatment (Figure 1A-B). Furthermore, under hypoxia HIF-1α translocates to the nucleus and binds to the gene promoters that mediate angiogenesis.8,10 Similarly, when HUVECs were cultured in the presence of DFO (Figure 1B), HIF-1α was translocated to the nucleus, as assessed by confocal microscopy, thus validating our in vitro model of HIF-1α activation.
The transcriptional activity of the protein was then investigated. Recent studies have demonstrated that the expression of the hypoxia-inducible GLUT-1 is critically dependent on the binding of HIF-1α to the hypoxia-responsive DNA element (HRE) in the promoter of the GLUT-1 gene.21 We observed an early expression of GLUT-1 (Figure 1B), which provides evidence for the initiation of the HIF-1α–dependent gene transcription.
The HIF-1α-driven angiogenic capability was then assessed. The spectacular organization of tubule-like structures reached with both DFO and low O2 (Figure 1C) clearly demonstrated that both conditions were themselves sufficient to initiate the angiogenic program, via a HIF-dependent autocrine pathway. The latter was shown to rely on VEGF; indeed, the inhibition of the VEGF cognate receptor (VEGFR2) abrogated the hypoxia-promoted angiogenic capability. It is important to note that the expression of VEGFRs is up-regulated by hypoxia and that the up-regulation of VEGFR2 is mediated by posttranscriptional regulation.22 The different organization on Matrigel substrata together with the increased number of tubule-like structures (Figure 1D) achieved following DFO treatment, as compared with that observed with VEGF alone (Figure 1C), suggested that other factors were also involved in the DFO model. Overall, these results demonstrate that the DFO-dependent translocation of HIF-1α from the cytoplasm to the nucleus is sufficient to generate a functional form of HIF-1α.
Bortezomib exerts a double-edged effect on HUVEC proliferation and angiogenesis, through HIF-1α and NF-kB. PSIs have entered clinical trials because of their capacity to induce cancer-cell apoptosis.23 As the efficacy of PIs depends on the type of cellular targets, we investigated the effect of bortezomib on HUVECs. HUVECs were exposed for 24 hours to 2 concentrations of bortezomib, 10 nM and 100 nM, both achievable in vivo (Millennium, Velcade prescribing information), and examined for proliferative rate and capillary organization on Matrigel. As shown in Figure 2A, bortezomib exerted a double-edged effect, in that proliferation was significantly impaired only in the presence of the high dose, while in the presence of the low dose it was enhanced at a level comparable to that achieved with VEGF, which we used as a positive control. Similar results were obtained with respect to capillary organization (Figure 2B), which was promoted at 10 nM and completely abrogated at 100 nM bortezomib. These results strongly suggest that the effects of bortezomib on HUVECs depend largely on the concentration used.
Bortezomib exerts double-edged effect on HUVECs, through HIF-1α and NF-kB. HUVECs were treated with bortezomib at 10 nM and 100 nM for 24 hours and then assessed for their proliferative activity (A) or for their ability to form capillaries on Matrigel (B) (original magnification ×10). Data are presented as the mean plus or minus standard error of the mean (SEM) from 3 independent experiments in duplicate in panel A; in panel B one experiment representative of 4 is depicted. (C) Quantification of total (dark column) and nuclear (gray column) HIF-1α expression as calculated by fluorescent pixel intensity at laser confocal microscopy. Data are presented as the mean plus or minus SEM from 20 cells derived from 3 different fields. (D) HIF-1α (green) and GLUT-1expression (red) were assessed in HUVECs treated with bortezomib at 10 nM and 100 nM by confocal analysis (original magnification ×60). Bortezomib up-regulated HIF-1α and NF-kB p65 expression as evaluated by Western blot analysis (E) and induced their nuclear accumulation as evaluated by confocal analysis through merge with DAPI (F) (original magnification ×60). **P < .001.
Bortezomib exerts double-edged effect on HUVECs, through HIF-1α and NF-kB. HUVECs were treated with bortezomib at 10 nM and 100 nM for 24 hours and then assessed for their proliferative activity (A) or for their ability to form capillaries on Matrigel (B) (original magnification ×10). Data are presented as the mean plus or minus standard error of the mean (SEM) from 3 independent experiments in duplicate in panel A; in panel B one experiment representative of 4 is depicted. (C) Quantification of total (dark column) and nuclear (gray column) HIF-1α expression as calculated by fluorescent pixel intensity at laser confocal microscopy. Data are presented as the mean plus or minus SEM from 20 cells derived from 3 different fields. (D) HIF-1α (green) and GLUT-1expression (red) were assessed in HUVECs treated with bortezomib at 10 nM and 100 nM by confocal analysis (original magnification ×60). Bortezomib up-regulated HIF-1α and NF-kB p65 expression as evaluated by Western blot analysis (E) and induced their nuclear accumulation as evaluated by confocal analysis through merge with DAPI (F) (original magnification ×60). **P < .001.
To evaluate the molecular mechanisms underlying the double-edged effect of bortezomib on HUVECs (ie, pro- vs antiproliferative/angiogenic), we assessed the effects of the 2 bortezomib concentrations on 2 master regulators of tumor angiogenesis, HIF-1α and NF-kB,24 which could both be affected by proteasome inhibition. We reasoned that proteasome blockade could inhibit HIF-1α degradation and hence cause its nuclear accumulation; on the other hand, blockade of the ubiquitin-dependent degradation of IkB, the inhibitor of NF-kB, could also determine its sequestration in the cytoplasm and prevent its translocation into the nucleus. Indeed, as shown in Figure 2C,D,F, we observed a significantly increased accumulation of HIF-1α upon exposure to the drug. Confocal analysis of HUVECs treated with 10 nM and 100 nM bortezomib demonstrated that within the increased expression of HIF-1α the nuclear fraction was prominent, and a significant nuclear translocation was observed in the presence of the lower dose (Figure 2C) as indicated by its colocalization with the nuclear dye DAPI (Figure 2F). HIF-1α accumulation was confirmed by Western blot analysis (Figure 2E). Notably, we could concomitantly detect the expression of GLUT-1, which is the signature of the transcriptional activity of HIF-1α, and which was again prominent at the low (10 nM) concentration of bortezomib (Figure 2D).
Bortezomib also affected the nuclear localization of the p65 member of the NF-kB family. Confocal microscopy (Figure 2F) demonstrated nuclear accumulation of p65 mainly with the low dose of bortezomib; indeed, treatment with 10 nM and 100 nM bortezomib resulted in nuclear accumulation of NF-kB p65 in 71.5% and 36% of HUVECs, respectively. These data were also confirmed by Western blot analysis (Figure 2E), in keeping with the “gain of functions” reached with low bortezomib concentration.
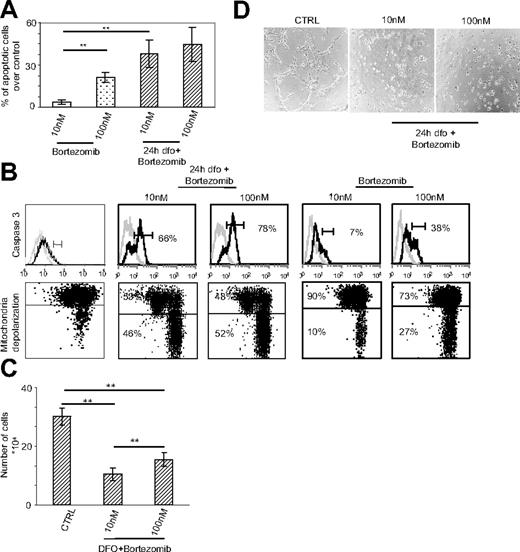
Induction of HIF-1α renders endothelial cells highly sensitive to the proapoptotic and antiangiogenic effects of bortezomib. As transient changes in oxygenation are a common finding in tumors,25 we aimed to determine whether HIF-1α up-regulation by endothelial cells might affect their sensitivity to bortezomib. For this purpose, HUVECs were preincubated with DFO for 24 hours to achieve the maximal expression of nuclear HIF-1α and were then treated for an additional 24 hours with bortezomib. The percentage of apoptotic cells, considered as propidium iodide/annexin V–double positive, was then assessed by means of cytofluorograph analysis. Under these conditions, bortezomib induced the death of HUVECs (Figure 3A) depending on the dose used. Interestingly, the effect exerted by bortezomib on DFO-treated HUVECs was significantly higher than the effect exerted on HUVECs grown in the absence of DFO. We then investigated the mechanism of bortezomib-induced death; specifically, we analyzed by FACS 2 key events associated with apoptosis, caspase activation and mitochondrial depolarization. As shown in Figure 3B, bortezomib treatment induced both caspase 3 activity and mitochondrial damage, as evaluated through the JC-1 mitochondrial dye, mostly when HUVECs were precultured in the presence of DFO. Moreover, following DFO treatment, proliferation (Figure 3C) and capillary formation (Figure 3D) were significantly impaired by bortezomib, irrespective of the dose used, showing that HIF-1α up-regulation sensitized HUVECs to the proapoptotic effect of bortezomib. In summary, these results show the fundamental involvement of HIF-1α in influencing the response of endothelial cells to bortezomib and reconcile with the data showing the antiangiogenic effect of bortezomib.17
HIF-1α up-regulation sensitizes HUVECs to the proapoptotic and antiangiogenic effects of bortezomib. (A) HUVECs, precultured in either the presence (left) or the absence (right) of DFO, were treated with 10 nM and 100 nM bortezomib and assessed for apoptosis induction by annexin V/PI staining and FACS analysis. Data, expressed as the percentage of annexin V/PI–positive cells, represent mean plus or minus SEM of 3 independent experiments. (B) Caspase 3 activity was evaluated by FACS analysis (top panels); mitochondrial membrane potential was evaluated by FACS analysis using JC-1 (bottom panels). The percentage of events associated with positive green and negative red (lower quadrants) was scored as mitochondrial depolarized cells. Data are representative of 3 independent experiments. (C) The 24-hour proliferation of HUVECs precultured with DFO was evaluated. Data are presented as the mean plus or minus SEM from 3 independent experiments in duplicate. Their ability to form capillaries on Matrigel layers was also assessed (D) (original magnification ×10). **P < .001.
HIF-1α up-regulation sensitizes HUVECs to the proapoptotic and antiangiogenic effects of bortezomib. (A) HUVECs, precultured in either the presence (left) or the absence (right) of DFO, were treated with 10 nM and 100 nM bortezomib and assessed for apoptosis induction by annexin V/PI staining and FACS analysis. Data, expressed as the percentage of annexin V/PI–positive cells, represent mean plus or minus SEM of 3 independent experiments. (B) Caspase 3 activity was evaluated by FACS analysis (top panels); mitochondrial membrane potential was evaluated by FACS analysis using JC-1 (bottom panels). The percentage of events associated with positive green and negative red (lower quadrants) was scored as mitochondrial depolarized cells. Data are representative of 3 independent experiments. (C) The 24-hour proliferation of HUVECs precultured with DFO was evaluated. Data are presented as the mean plus or minus SEM from 3 independent experiments in duplicate. Their ability to form capillaries on Matrigel layers was also assessed (D) (original magnification ×10). **P < .001.
Discussion
The concept of treating tumors by cutting off angiogenesis is sound. In practice, the results of clinical trials that have used angiogenesis inhibitors have not been satisfactory so far.24 Two major reasons might underlie these failures. First, the results of new therapies, including those that target angiogenesis, depend on their impact on the complexity of the tumor microenvironment.25 Second, angiogenesis results from a constellation of factors. Our data showing different morphologic features of capillary-like structures on Matrigel obtained upon culture of HUVECs in the presence of DFO or hypoxia (Figure 1C) may indicate that HIF-independent mechanisms operate in the latter condition, as previously suggested.26 Therefore, it is not surprising that targeting individual factors such as VEGF can be disappointing. A better understanding of the features associated with tumor microenvironment may lead to the development of novel and more effective therapeutic strategies. Hypoxia can be considered a unifying feature of the tumor microenvironment and the main stimulus for tumor-associated angiogenesis.27,28 The cellular response to hypoxia is mediated at the transcriptional level by HIF-1α, whose high levels in cancer specimens are associated with poor prognosis.29,30
The proteasome inhibitor bortezomib has shown antiproliferative and proapoptosis-promoting properties in different types of cancers in vitro, including melanoma, breast cancer, and hematologic malignancies,31,32 and is used clinically especially for the treatment of relapsing and refractory MM.33,34 Bortezomib is able to induce strong apoptosis of tumor cells, to affect their interaction with the microenvironment35,36 and to inhibit MM-associated angiogenesis.17 We here show that bortezomib exerts a double-edged effect on HUVECs: at low doses it allows cell proliferation and angiogenesis, whereas at high doses, still achievable in vivo, it induces growth arrest and angiogenesis blockade. The mechanism underlying these effects seems to be related to the bortezomib-induced blockade of protein degradation, which ultimately affects 2 master regulators of tumor-associated angiogenesis, HIF-1α and NF-kB.24 Indeed, bortezomib, at low doses, induces HIF-1α up-regulation and nuclear translocation with ensuing downstream effects, including Glut-1 expression. It is therefore conceivable that the proangiogenic pattern is sustained via an HIF-dependent pathway. NF-kB is a transcription factor that plays an important role in tumorigenesis via the transactivation of genes involved in cell proliferation, protection from apoptosis, and even in tumor-cell invasiveness and angiogenesis.24 Indeed, proteasome inhibition by bortezomib abrogates degradation of IkB, leading to cytoplasmic sequestration and inhibition of NF-kB, which is considered one of the main mechanisms in the induction of tumor apoptosis.37 Our data show instead that bortezomib induces a nuclear accumulation of NF-kB p65, which is most prominent in treatment with low doses. The mechanisms leading to this paradoxical induction following treatment with bortezomib are presently unclear and could be related to the emerging link between HIF and NF-kB,38 whose transcription was shown to be dependent on the presence of HIF-1α under hypoxic conditions. Irrespective of the mechanism(s), our data are in agreement with the recently postulated double-edged effect by proteasome inhibitors on tumor apoptosis/survival, which has been ascribed to the intrinsic status (eg activated vs quiescent) of the target cell as well as to the cellular context within which the tumor develops.24
It should be noted that we found an impressive increase in susceptibility to bortezomib-induced apoptosis when HUVECs were programmed to acquire a hypoxia-activated angiogenic profile, compared with the quiescent counterpart. Indeed, pretreatment with DFO (and hypoxia, as shown in Supplemental Figure S1) rendered HUVECs significantly more susceptible to apoptosis induction even by low-dose bortezomib. In this sense, HIF-1α could represent an element of discrimination between normal and tumor-associated vessels in terms of susceptibility to the antiangiogenic and proapoptotic effects of bortezomib. Hypoxia is indeed an important environmental factor directing the angiogenic switch through HIF-1α activation, and intratumoral hypoxia plays a crucial role in determining tumor progression and spreading. Moreover, a hypoxic condition is a common feature of both normal and MM bone marrow.39,40 In this context, the recently described antiangiogenic effect of bortezomib on multiple myeloma endothelial cells (MMECs)24 could be explained by the sensitizing effect to apoptosis induction exerted by HIF-1α.
In conclusion, our findings show the important role of hypoxia in influencing the response of endothelial cells to bortezomib and disclose the possibility that bortezomib can selectively target tumor-associated vessels, thus offering the possibility to more finely tune the antiangiogenic approach to cancer therapy.
Authorship
Contribution: L.V. performed salient experiments of the research, and collected and analyzed data; D.B. performed experiments relative to the death of HUVECs; C.F. supervised the confocal and morphologic analyses; G.C. performed Western blotting experiments; M.F. helped in discussing and writing the paper and in critical analysis of the data; F.C.-C. was the advisor, and participated in the interpretation and discussion of the results; E.F. designed and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabetta Ferrero, Laboratory of Tumor Immunology, Department of Oncology, DIBIT- San Raffaele H Scientific Institute, Via Olgettina, 60, Milan, Italy 20132; e-mail: ferrero.elisabetta@hsr.it.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Giacomo Ruotolo for his qualified help in performing statistical analyses. This work was supported by AIRC (Italian Association for Cancer Research) and by charity from a donor who wishes to remain anonymous.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal