Abstract
We have previously demonstrated that STAT3 hyperactivation via the interleukin 6 (IL-6) cytokine family receptor gp130 in gp130Y757F/Y757F mice leads to numerous hematopoietic and lymphoid pathologies, including neutrophilia, thrombocytosis, splenomegaly, and lymphadenopathy. Because IL-6 and IL-11 both signal via a gp130 homodimer, we report here a genetic approach to dissect their individual roles in these pathologies. Neutrophilia and thrombocytosis were absent in gp130Y757F/Y757F mice lacking either IL-6 (gp130Y757F/Y757F: IL-6−/−) or the IL-11 receptor α subunit (gp130Y757F/Y757F: IL-11Rα1−/−), and this was associated with a normalized bone marrow compartment. The elevated myelopoiesis and megakaryopoiesis in bone marrow of gp130Y757F/Y757F mice was attributable to an increase by either IL-6 or IL-11 in the STAT3-driven impairment of transforming growth factor β (TGF-β) signaling, which is a suppressor of these lineages. In contrast, the absence of IL-6, but not IL-11 signaling, prevented the splenomegaly, abnormal lymphopoiesis, and STAT3 hyperactivation in lymphoid organs of gp130Y757F/Y757F mice. Furthermore, hyperactivation of STAT3 in lymphoid organs was associated with increased expression of IL-6Rα, and IL-6Rα expression was reduced in gp130Y757F/Y757F: Stat3+/− mice displaying normal levels of STAT3 activity. Collectively, these data genetically define distinct roles of IL-6 and IL-11 in driving pathologic hematopoietic and lymphoid responses mediated by STAT3 hyperactivation.
Introduction
Interleukin-6 (IL-6) and IL-11, members of the IL-6 cytokine family, display both unique and overlapping biologic activities on multiple hematopoietic lineages.1,2 For instance, IL-6 and IL-11 can both synergize with other cytokines such as stem-cell factor (SCF) and IL-3 to enhance the production of immature hematopoietic progenitor cells,3,4 as well as act directly on megakaryocytes to stimulate their functional maturation.5,6 However, IL-6 plays a broader role during hematopoiesis than IL-11, as demonstrated by genetic deletion of IL-6 in mice, which leads to aberrant production of both immature and committed progenitors from multiple lineages in spleen and bone marrow.7 In contrast, despite the widespread expression of the ligand-binding IL-11 receptor α subunit (IL-11Rα) in the lymphohematopoietic compartment,8 lymphopoiesis and hematopoiesis in mice lacking the IL-11Rα1 gene are normal.9 On the other hand, IL-6 overproduction in transgenic mice results in plasmacytoma formation and extramedullary hematopoiesis.10,11 This phenotype is exacerbated in mice double transgenic for both IL-6 and the soluble (s) IL-6Rα subunit, with these mice also displaying splenomegaly and an expansion of circulating white blood cells and platelets.12
The biologic activities of IL-6 and IL-11 are elicited via their common use of the gp130 signal-transducing receptor β subunit. Specifically, binding of either IL-6 or IL-11 to its specific receptor α subunit induces gp130 homodimerization13,14 and subsequent activation of Janus kinases (JAKs).15 Intracellular signaling is initiated by JAKs on phosphorylation of specific tyrosine residues (pY) in the gp130 cytoplasmic domain, which ultimately facilitate activation of signal transducers and activators of transcription 1 (STAT1) and STAT3,16 and SHP2-mediated mitogen activated protein kinase (MAPK) and PI3K pathways.17 Notably, the SHP2 tyrosine phosphatase competes with the suppressor of cytokine signaling 3 (SOCS3) protein to bind to the membrane-proximal pY757 in murine gp130, thus implicating this residue in the negative regulation of gp130 signaling.18
The importance of regulatory mechanisms to maintain STAT3 activity at physiologic levels for hematopoietic homeostasis is evidenced by the critical role STAT3 plays in mediating cellular responses involved in the production of immature and committed hematopoietic progenitors.19,21–23 This is further emphasized by the numerous human lymphoproliferative and myeloproliferative diseases, including multiple myeloma (MM), non-Hodgkin lymphoma (NHL), and acute myeloid leukemia (AML), that display deregulated STAT3 activation.24–27 Although the precise mechanisms responsible for regulating STAT3 signaling during these pathophysiologic responses remain unresolved, in MM and a subset of AML cells from patients, constitutive activation of STAT3 is driven by autocrine production of IL-6.24,27 In these disease settings, increased STAT3 activation contributes to disease pathogenesis by preventing apoptosis.24–26
We have previously demonstrated the physiologic importance of tightly regulated gp130-dependent activation of STAT3 for hematopoiesis. Gp130Y757F/Y757F mice homozygous for a knock-in Y757F mutation that abolishes binding of SHP2 and SOCS3 to gp130 develop numerous hematopoietic and lymphoid abnormalities, including thrombocytosis, splenomegaly, and lymphadenopathy, as a consequence of gp130-dependent STAT3 hyperactivation.19,20 Here, we have generated gp130Y757F/Y757F mice lacking either the IL-6 or IL-11Rα1 gene to identify the specific roles IL-6 and IL-11 play in driving the pathologic consequences of gp130-dependent STAT3 hyperactivation during hematopoiesis. We found that normal hematopoiesis and lymphopoiesis were restored in gp130Y757F/Y757F:IL-6−/− mice, and this correlated with normalized gp130-dependent STAT3 activation. In contrast, preventing hematopoietic perturbations in gp130Y757F/Y757F:IL-11Rα1−/− mice was primarily restricted to bone marrow and involved a mechanism common to both IL-6 and IL-11 based on cross-talk between gp130-STAT3 and transforming growth factor β (TGF-β) signaling. The specific requirement of IL-6–dependent STAT3 hyperactivation for aberrant lymphopoiesis in gp130Y757F/Y757F mice correlated with increased expression of IL-6Rα, but not IL-11Rα, in lymphoid organs. Collectively, these data genetically implicate IL-6 as the primary cytokine crucial for controlling gp130-mediated STAT3 activation during lymphohematopoietic homeostasis in vivo.
Materials and methods
Mice and treatments
Gp130Y757F/Y757F mice homozygous for the gp130Y757F knock-in mutant receptor, and gp130Y757F/Y757F mice on an IL-6 null background (gp130Y757F/Y757F:IL-6−/−), were generated as previously described.28,29 Mice null for the IL-11Rα1 gene9 were crossed with gp130Y757F/Y757F mice. All experiments were fully approved by the Ludwig Institute for Cancer Research/Department of Surgery and Monash Medical Centre “A” Animal Ethics Committees, and included wild-type (gp130+/+) littermate controls that were genetically matched. Animals were housed under specific pathogen-free conditions.
For biochemical analyses, mice were subjected to a single intraperitoneal injection with human (h) IL-11 (5 μg).
Cytokines and antibodies
Recombinant hIL-6 and hIL-11 were kindly provided by C. G. Begley (Amgen, Thousand Oaks, CA) and L. Robb (Walter and Eliza Hall Institute, Melbourne, Australia), respectively. Recombinant murine (m) SCF, mGM-CSF, TGF-β1 and mIL-3 were purchased from PeproTech (Rocky Hill, NJ). Commercially available antibodies were purchased as follows: ERK1/2, STAT3, and phospho(Tyr705)STAT3 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), biotin-IL-6Rα antibody from R&D Systems (Minneapolis, MN), and IgG Fc receptor, FITC-B220, FITC-CD4, PE-Thy1.2, and biotin-CD8a from PharMingen (San Diego, CA).
Hematologic and hematopoietic progenitor-cell analyses
Blood from the retro-orbital plexus of mice was collected into EDTA-coated tubes and analyzed on an Advia 120 System quantitative hematology analyser (Bayer Diagnostics, Tarrytown, NY). Manual differential cell counts were performed on May-Grünwald-Giemsa–stained blood smears and cytocentrifuge preparations of spleen and bone marrow single-cell suspensions.
Clonal cultures of hematopoietic progenitors from unfractionated bone marrow cells (2.5 × 104 to 5 × 104) or spleen cells (2.5 × 105) were assayed in semisolid agar as previously described.19 The following cytokines were used at the indicated concentrations: SCF (50 ng/mL), IL-3 (10 ng/mL), GM-CSF (10 ng/mL), TGF-β1 (10 ng/mL), IL-6 (100 ng/mL), and IL-11 (100 ng/mL).
Analysis of megakaryocyte liquid cultures
Megakaryocytes were grown ex vivo from 107 bone marrow cells over 4 days as described previously23 in the absence or presence of IL-6 (100 ng/mL), IL-11 (100 ng/mL), and TGF-β1 (10 ng/mL). Cells (5 × 104) from each liquid culture were transferred onto glass slides, fixed with acetone, and then stained for acetylcholinesterase.
Protein extraction and immunoblot analysis
Lysates were prepared from snap-frozen cell suspensions and tissues in ice-cold lysis buffer, following which they were subjected to immunoblot analyses with the indicated primary antibodies as previously described.30 Proteins were visualized using either the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, NJ) or Odyssey Infrared Imaging System (LI-COR, Lincoln, NE) with the appropriate secondary antibodies as per the manufacturer's instructions.
Flow cytometry
Flow cytometric analyses were performed on single-cell suspensions from thymus and lymph node with various biotin-labeled, FITC-labeled, or PE-labeled antibodies using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) as described previously.19
RNA isolation and quantitative expression analysis
Total RNA was extracted from snap-frozen cell pellets and tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. cDNA was prepared from 5 μg total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen) as per the manufacturer's instructions. Quantitative reverse transcription-polymerase chain reaction (Q-PCR) target gene expression analyses were performed on triplicate samples in a Rotor-Gene 3000 Real Time Thermal Cycler (Corbett Research, Mortlake, NSW, Australia) using SYBR Green (Invitrogen) over 40 cycles (94°C/20 seconds, 62°C/30 seconds, 72°C/30 seconds), following an initial denaturation step at 95°C/10 minutes. Primers to specifically amplify 18S were used to normalize cDNA concentrations of target genes. Data acquisition and analyses were performed with the Rotor-Gene 3000 software. Sequences for the mouse primer sets used are in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Statistical analyses
Comparisons between mean values were performed using ANOVA and Student t tests as appropriate. P < .05 was considered statistically significant.
Results
Absence of IL-6 or IL-11 signaling prevents thrombocytosis and neutrophilia in gp130Y757F/Y757F mice
We have previously reported that gp130Y757F/Y757F mice display numerous hematopoietic abnormalities, including thrombocytosis, neutrophilia, splenomegaly, and lymphadenopathy, as a consequence of gp130-dependent STAT3 hyperactivation.19 Although this highlights the importance of regulatory mechanisms to maintain physiologic gp130-dependent STAT3 activity for hematopoietic homeostasis, the identity of IL-6 family cytokines that elicit pathologic STAT3 activity during hematopoiesis in gp130Y757F/Y757F mice remained unknown. To address this, we used a genetic approach to examine the contribution of either IL-6– or IL-11–induced STAT3 hyperactivity to the hematopoietic phenotype of gp130Y757F/Y757F mice by crossing these mice onto a background deficient in either the IL-6 or IL-11Rα1 gene.
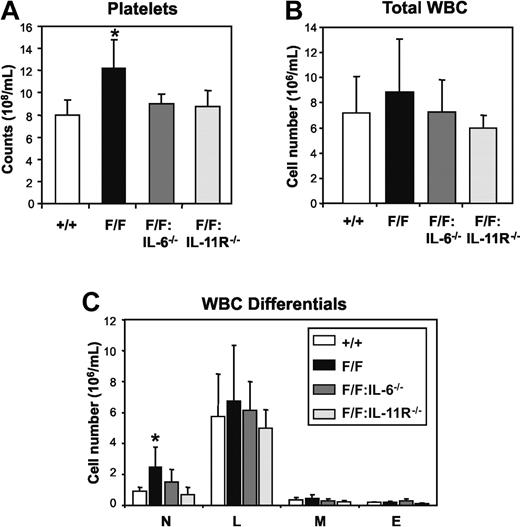
Hematologic analyses of mice at 12 to 16 weeks of age revealed that the numbers of circulating platelets in gp130Y757F/Y757F:IL-6−/− mice (868 ± 60 × 108/mL, mean ± SD) and gp130Y757F/Y757F:IL-11Rα1−/− mice (850 ± 132 × 108/mL) were approximately 30% lower than in gp130Y757F/Y757F mice (1274 ± 264 × 108/mL, P < .05) and indistinguishable from gp130+/+ controls (831 ± 121 × 108/mL; Figure 1A). Similarly, in contrast to the 3-fold increase in the number of neutrophils in peripheral blood of gp130Y757F/Y757F mice (2.81 ± 1.50 × 106/mL, mean ± SD) compared to gp130+/+mice (0.92 ± 0.24 × 106/mL, P < .05), there was no evidence of neutrophilia in either gp130Y757F/Y757F:IL-6−/− (1.32 ± 0.50 × 106/mL) or gp130Y757F/Y757F:IL-11Rα1−/− mice (0.68 ± 0.46 × 106/mL; Figure 1C). All other peripheral blood parameters from gp130Y757F/Y757F, gp130Y757F/Y757F:IL-6−/−, and gp130Y757F/Y757F:IL-11Rα1−/− mice were normal (data not shown), thus revealing that either IL-6 or IL-11 signaling in gp130Y757F/Y757F mice triggers neutrophilia and thrombocytosis without affecting the production of other circulating blood-cell lineages.
Peripheral blood profile of gp130Y757F/Y757F (F/F) mice lacking IL-6 or IL-11Rα. (A) Platelet counts, (B) total white blood cell (WBC) counts, and (C) WBC differentials. Data from 5 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from gp130+/+ mice; n = neutrophils, L = lymphocytes, M = monocytes and E = eosinophils.
Peripheral blood profile of gp130Y757F/Y757F (F/F) mice lacking IL-6 or IL-11Rα. (A) Platelet counts, (B) total white blood cell (WBC) counts, and (C) WBC differentials. Data from 5 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from gp130+/+ mice; n = neutrophils, L = lymphocytes, M = monocytes and E = eosinophils.
Hematopoietic perturbations in gp130Y757F/Y757F bone marrow are prevented by the loss of either IL-6 or IL-11 signaling
We have previously reported that thrombocytosis in gp130Y757F/Y757F mice correlated with increased production of mature megakaryocytes.19 Consistent with the absence of thrombocytosis in gp130Y757F/Y757F:IL-6−/− and gp130Y757F/Y757F:IL-11Rα1−/− mice, the numbers of mature megakaryocytes in bone marrow from gp130Y757F/Y757F:IL-6−/− and gp130Y757F/Y757F:IL-11Rα1−/− mice were similar to those in bone marrow from gp130+/+ controls and represented a 44% to 55% reduction in the elevated number of mature megakaryocytes present in gp130Y757F/Y757F bone marrow (Table 1)The cellular composition of gp130Y757F/Y757F bone marrow is also characterized by excessive myelopoiesis at the expense of erythropoiesis and lymphopoiesis (Table 1).19 However, morphologic analysis of bone marrow cells from gp130Y757F/Y757F:IL-6−/− and gp130Y757F/Y757F:IL-11Rα1−/− mice revealed that the frequency and absolute numbers of neutrophils and lymphocytes in both genotypes had been restored to wild-type levels (Table 1; Figure S1). In contrast, the absence of IL-6, but not IL-11Rα, in compound gp130Y757F/Y757F bone marrow prevented an increase in the numbers of nucleated red blood cells (RBCs) that was observed in the single gp130Y757F/Y757F mutant bone marrow.
Characterization of bone marrow and splenic hematopoiesis in gp130Y757F/Y757F mice lacking either IL-6 or IL-11Rα
| Parameter . | +/+ . | F/F . | F/F:IL-6−/− . | F/F:IL-11R−/− . |
|---|---|---|---|---|
| Bone marrow per femur* | ||||
| Cellularity, × 107 | 4.15 ± 0.89 | 4.06 ± 0.53 | 3.98 ± 0.69 | 3.71 ± 1.46 |
| Blasts, % | 6 ± 3 | 8 ± 1 | 5 ± 3 | 8 ± 6 |
| Promyelocytes/myelocytes, % | 6 ± 2 | 13 ± 5‡ | 12 ± 3‡ | 7 ± 1 |
| Metamyelocytes/neutrophils, % | 30 ± 8 | 43 ± 5‡ | 32 ± 8 | 35 ± 9 |
| Lymphocytes, % | 35 ± 9 | 19 ± 5‡ | 31 ± 9 | 34 ± 11 |
| Monocytes, % | 4 ± 2 | 3 ± 2 | 3 ± 1 | 3 ± 2 |
| Eosinophils, % | 3 ± 1 | 3 ± 2 | 2 ± 1 | 2 ± 2 |
| Nucleated RBCs, % | 16 ± 4 | 10 ± 5 | 15 ± 2 | 11 ± 4 |
| Megakaryocytes, × 104 | 1.54 ± 0.35 | 2.83 ± 0.64§ | 1.59 ± 0.37 | 1.28 ± 0.35 |
| Total CFC† | 27 ± 8 | 40 ± 9‡ | 26 ± 9 | 30 ± 12 |
| Spleen* | ||||
| Mass, mg | 87 ± 8 | 222 ± 36§ | 132 ± 22‡ | 201 ± 26§ |
| Cellularity, × 108 | 1.35 ± 0.54 | 3.82 ± 1.57§ | 1.52 ± 0.60 | 2.90 ± 0.98‡ |
| Blasts, % | 1 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 0 |
| Promyelocytes/myelocytes, % | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Metamyelocytes/neutrophils, % | 3 ± 2 | 8 ± 5§ | 6 ± 2 | 10 ± 4§ |
| Lymphocytes, % | 89 ± 3 | 76 ± 8‡ | 89 ± 3 | 80 ± 8 |
| Monocytes, % | 1 ± 1 | 3 ± 2 | 1 ± 1 | 4 ± 2 |
| Eosinophils, % | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 1 |
| Nucleated RBCs, % | 5 ± 3 | 14 ± 5‡ | 3 ± 2 | 7 ± 3§ |
| Megakaryocytes, × 103 | 1.66 ± 0.31 | 15.94 ± 3.69§ | 4.19 ± 1.10§ | 18.06 ± 7.82§ |
| Total CFC† | 3 ± 2 | 13 ± 8‡ | 3 ± 1 | 7 ± 2‡ |
| Parameter . | +/+ . | F/F . | F/F:IL-6−/− . | F/F:IL-11R−/− . |
|---|---|---|---|---|
| Bone marrow per femur* | ||||
| Cellularity, × 107 | 4.15 ± 0.89 | 4.06 ± 0.53 | 3.98 ± 0.69 | 3.71 ± 1.46 |
| Blasts, % | 6 ± 3 | 8 ± 1 | 5 ± 3 | 8 ± 6 |
| Promyelocytes/myelocytes, % | 6 ± 2 | 13 ± 5‡ | 12 ± 3‡ | 7 ± 1 |
| Metamyelocytes/neutrophils, % | 30 ± 8 | 43 ± 5‡ | 32 ± 8 | 35 ± 9 |
| Lymphocytes, % | 35 ± 9 | 19 ± 5‡ | 31 ± 9 | 34 ± 11 |
| Monocytes, % | 4 ± 2 | 3 ± 2 | 3 ± 1 | 3 ± 2 |
| Eosinophils, % | 3 ± 1 | 3 ± 2 | 2 ± 1 | 2 ± 2 |
| Nucleated RBCs, % | 16 ± 4 | 10 ± 5 | 15 ± 2 | 11 ± 4 |
| Megakaryocytes, × 104 | 1.54 ± 0.35 | 2.83 ± 0.64§ | 1.59 ± 0.37 | 1.28 ± 0.35 |
| Total CFC† | 27 ± 8 | 40 ± 9‡ | 26 ± 9 | 30 ± 12 |
| Spleen* | ||||
| Mass, mg | 87 ± 8 | 222 ± 36§ | 132 ± 22‡ | 201 ± 26§ |
| Cellularity, × 108 | 1.35 ± 0.54 | 3.82 ± 1.57§ | 1.52 ± 0.60 | 2.90 ± 0.98‡ |
| Blasts, % | 1 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 0 |
| Promyelocytes/myelocytes, % | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Metamyelocytes/neutrophils, % | 3 ± 2 | 8 ± 5§ | 6 ± 2 | 10 ± 4§ |
| Lymphocytes, % | 89 ± 3 | 76 ± 8‡ | 89 ± 3 | 80 ± 8 |
| Monocytes, % | 1 ± 1 | 3 ± 2 | 1 ± 1 | 4 ± 2 |
| Eosinophils, % | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 1 |
| Nucleated RBCs, % | 5 ± 3 | 14 ± 5‡ | 3 ± 2 | 7 ± 3§ |
| Megakaryocytes, × 103 | 1.66 ± 0.31 | 15.94 ± 3.69§ | 4.19 ± 1.10§ | 18.06 ± 7.82§ |
| Total CFC† | 3 ± 2 | 13 ± 8‡ | 3 ± 1 | 7 ± 2‡ |
*Values represent the mean ± SD from at least 5 mice for each genotype.
†Number of colony-forming cells (CFCs) in semisolid agar cultures containing 2.5 × 104 bone marrow cells or 2.5 × 105 spleen cells. Cells were stimulated with IL-3 and SCF.
‡P < .05 and
§P < .001 versus data from gp130+/+ mice. No other statistically significant differences were observed between genotypes.
Increased myelopoiesis and neutrophilia in gp130Y757F/Y757F mice most likely arises from a marked increase in the production of hematopoietic progenitors representing the various myeloid lineages in gp130Y757F/Y757F bone marrow.19 Because there was no evidence of neutrophilia in either gp130Y757F/Y757F:IL-6−/− or gp130Y757F/Y757F:IL-11Rα1−/− mice, we compared the frequency of myeloid colony-forming cells (CFCs) from gp130Y757F/Y757F, gp130Y757F/Y757F:IL-6−/−, and gp130Y757F/Y757F:IL-11Rα1−/− bone marrow in agar cultures containing SCF and IL-3 (Table 1). In contrast to the increase in absolute number of myeloid CFCs in gp130Y757F/Y757F bone marrow (gp130Y757F/Y757F, 6.43 ± 0.66 × 104 CFC/femur, mean ± SD versus gp130+/+, 4.18 ± 0.97 × 104 CFC/femur, P < .05), the absolute number of myeloid progenitors in bone marrow from gp130Y757F/Y757F:IL-6−/− mice (4.45 ± 1.12 × 104 CFC/femur) and gp130Y757F/Y757F:IL-11Rα1−/− mice (4.79 ± 1.00 × 104 CFC/femur) mice was comparable to that observed in gp130+/+ controls (Table 1). Collectively, the data earlier in this section identify significant overlap in the pathologic activities of IL-6 and IL-11 in driving deregulated bone marrow megakaryopoiesis and myelopoiesis.
Impaired TGF-β1 activity during bone marrow hematopoiesis in gp130Y757F/Y757F mice is driven by either IL-6 or IL-11
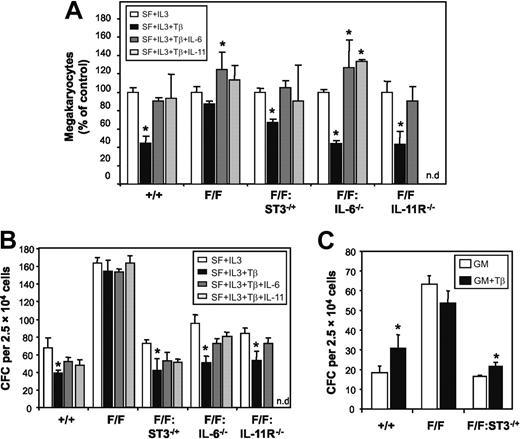
We have previously demonstrated that TGF-β1–driven biologic responses are impaired in gp130Y757F/Y757F mice as a consequence of STAT3 hyperactivation, which desensitizes gp130Y757F/Y757F cells to the biologic actions of TGF-β1 through increased expression of inhibitory Smad7.20 Considering that TGF-β1 suppresses megakaryopoiesis and therefore platelet production,31 we reasoned the thrombocytosis in gp130Y757F/Y757F mice may be due, at least in part, to the impaired ability of TGF-β1 to suppress megakaryocyte production. We therefore examined the effect of TGF-β1 on the production of gp130Y757F/Y757F bone marrow-derived megakaryocytes in serum-free liquid cultures containing IL-3 over 4 days. In cultures derived from gp130+/+ bone marrow, TGF-β1 reduced the number of megakaryocytes by 55%, whereas no such TGF-β1–mediated inhibition was observed in the presence of either IL-6 or IL-11 (Figure 2A). In contrast, TGF-β1 had minimal inhibitory effect on megakaryocyte production in gp130Y757F/Y757F bone marrow-derived cultures, and the presence of either IL-6 or IL-11 led to an increase in megakaryocyte numbers. Consistent with our previous observations,20 the failure of TGF-β1 to inhibit megakaryopoiesis correlated with STAT3 hyperactivation because TGF-β1 suppressed megakaryocyte production by 34% in cultures derived from gp130Y757F/Y757F:Stat3+/− bone marrow (Figure 2A), in which the level of STAT3 activation is reduced to that in gp130+/+ bone marrow.19 Again, TGF-β1 failed to reduce megakaryocyte numbers in gp130Y757F/Y757F:Stat3+/− liquid cultures containing either IL-6 or IL-11, suggesting that either of these cytokines can impair the inhibitory actions of TGF-β1 on megakaryocyte production. In support, TGF-β1 suppressed megakaryocyte production in cultures from gp130Y757F/Y757F:IL-6−/− and gp130Y757F/Y757F:IL-11Rα1−/− bone marrow by 56% and 57%, respectively, and addition of either IL-6 or IL-11 to these cultures negated the suppressive actions of TGF-β1 on megakaryocyte production (Figure 2A).
STAT3 hyperactivation suppresses TGF-β activity on hematopoietic progenitor cells in gp130Y757F/Y757F bone marrow. (A) Liquid cultures of bone marrow-derived megakaryocytes were grown in serum-free media containing either IL-6 or IL-11 (100 ng/mL) in the presence of TGF-β1 (10 ng/mL) for 4 days. Cell cultures were cytocentrifuged and megakaryocytes scored by staining for acetylcholinesterase activity. (B-C) In vitro colony formation of bone marrow cells in semisolid agar cultures containing (B) SCF (50 ng/mL), IL-3 (10 ng/mL), TGF-β1 (10 ng/mL), IL-6 (100 ng/mL), and IL-11 (100 ng/mL), and (C) GM-CSF (10 ng/mL) and TGF-β1 (10 ng/mL). Data from 4 to 6 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from cultures within each genotype without TGF-β1.
STAT3 hyperactivation suppresses TGF-β activity on hematopoietic progenitor cells in gp130Y757F/Y757F bone marrow. (A) Liquid cultures of bone marrow-derived megakaryocytes were grown in serum-free media containing either IL-6 or IL-11 (100 ng/mL) in the presence of TGF-β1 (10 ng/mL) for 4 days. Cell cultures were cytocentrifuged and megakaryocytes scored by staining for acetylcholinesterase activity. (B-C) In vitro colony formation of bone marrow cells in semisolid agar cultures containing (B) SCF (50 ng/mL), IL-3 (10 ng/mL), TGF-β1 (10 ng/mL), IL-6 (100 ng/mL), and IL-11 (100 ng/mL), and (C) GM-CSF (10 ng/mL) and TGF-β1 (10 ng/mL). Data from 4 to 6 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from cultures within each genotype without TGF-β1.
In addition to the inhibitory actions of TGF-β1 on megakaryopoiesis,31 TGF-β1 also inhibits colony formation in soft agar by early hematopoietic progenitors stimulated with SCF and IL-3.32 In contrast, TGF-β1 can synergize with GM-CSF to promote colony formation by CFU-granulocyte, and to a lesser extent CFU-macrophage, progenitors.33 To examine if these activities of TGF-β1 on other hematopoietic-cell lineages were suppressed by the gp130Y757F mutant receptor, we compared the frequency of CFCs in bone marrow from gp130+/+ and gp130Y757F/Y757F mice using soft agar cultures containing either GM-CSF alone or SCF plus IL-3, in the absence or presence of TGF-β1 with IL-6 or IL-11. In gp130+/+ marrow cultures containing SCF and IL-3, TGF-β1 reduced the total number of colonies formed by 43% (Figure 2B). The inhibitory effect of TGF-β1 on colony formation in gp130+/+ cultures was slightly impaired by the addition of either IL-6 or IL-11. In contrast, TGF-β1 failed to inhibit colony formation in gp130Y757F/Y757F bone marrow cultures even in the absence of either IL-6 or IL-11 (Figure 2B). However, the capacity of TGF-β1 to inhibit colony formation in cultures from gp130Y757F/Y757F:IL-6−/−, gp130Y757F/Y757F:IL-11Rα1−/−, and gp130Y757F/Y757F:Stat3+/− bone marrow by 47%, 36%, and 43%, respectively, was comparable to TGF-β1–mediated colony suppression in gp130+/+ cultures (Figure 2B). Similar to gp130+/+ cultures, addition of either IL-6 or IL-11 to these cultures also suppressed the inhibitory actions of TGF-β1 on colony formation. Furthermore, the presence of TGF-β1 increased the number of GM-CSF–induced colonies in gp130+/+ and gp130Y757F/Y757F:Stat3+/↑ marrow cultures by 70% and 30%, respectively, whereas TGF-β1 had no effect on GM-CSF–induced colony formation in gp130Y757F/Y757F marrow cultures (Figure 2C). Thus, from these data we propose that activation of STAT3 via IL-6– or IL-11–induced gp130 homodimerization desensitizes bone marrow hematopoietic progenitor cells to the growth inhibiting or promoting actions of TGF-β1 in a lineage-specific manner.
Loss of IL-6, but not IL-11, signaling in gp130Y757F/Y757F mice prevents splenomegaly
By 12 weeks of age, gp130Y757F/Y757F mice develop splenomegaly characterized by increased numbers of mature megakaryocytes, nucleated RBCs, and neutrophils.19 In contrast, gp130Y757F/Y757F:IL-6−/− mice at 12 weeks of age and beyond show no sign of splenomegaly, and the distribution of mature cells in gp130Y757F/Y757F:IL-6−/− spleens resembled that of gp130+/+ controls (Table 1; Figure S1). However, the cellularity of spleens from gp130Y757F/Y757F:IL-11Rα1−/− mice was increased by 2.1-fold compared to age-matched gp130+/+ mice and resembled the 2.8-fold increase in gp130Y757F/Y757F splenic cellularity. Similar to gp130Y757F/Y757F mice, splenomegaly in gp130Y757F/Y757F:IL-11Rα1−/− mice was characterized by increases in the frequency of nucleated RBCs (1.4-fold) and neutrophils (3.3-fold) at the expense of lymphocytes (Table 1). Compared to gp130+/+ mice, there were even greater increases in absolute numbers of nucleated RBCs (2.8-fold) and neutrophils (7.3-fold), as well as increases in megakaryocytes (10.8-fold) and lymphocytes (1.9-fold), all of which were comparable to the increases observed in gp130Y757F/Y757F spleens (7.3-fold for nucleated RBCs, 7.5-fold for neutrophils, 9.6-fold for megakaryocytes, and 2.3-fold for lymphocytes; Table 1; Figure S1).
A similar trend among genotypes was also observed when we assessed the production of myeloid progenitors in spleens from age-matched gp130Y757F/Y757F, gp130Y757F/Y757F:IL-6−/−, and gp130Y757F/Y757F:IL-11Rα1−/− mice. Clonal cultures of spleen cells revealed that the absolute number of myeloid CFCs in gp130Y757F/Y757F:IL-6−/− spleens (1.88 ± 0.60 × 103 CFCs/spleen, mean ± SD) was similar to that observed in gp130+/+ controls (1.37 ± 0.34 × 103 CFCs/spleen) and represented an 88% reduction compared to gp130Y757F/Y757F spleens (15.15 ± 6.80 × 103 CFCs/spleen, P < .05). In contrast, the absolute number of splenic CFCs in gp130Y757F/Y757F:IL-11Rα1−/− mice (6.63 ± 2.24 × 103 CFCs/spleen) was only reduced by 56% compared to gp130Y757F/Y757F mice and remained 4.8-fold and 3.5-fold higher than in gp130+/+ and gp130Y757F/Y757F:IL-6−/− mice, respectively. Thus, these data reveal that hyperactivation of STAT3 by IL-6 is primarily responsible for deregulated splenic hematopoiesis in gp130Y757F/Y757F mice.
Loss of IL-6 and to a lesser extent IL-11 signaling in gp130Y757F/Y757F mice alleviates lymphadenopathy and abnormal lymphopoiesis
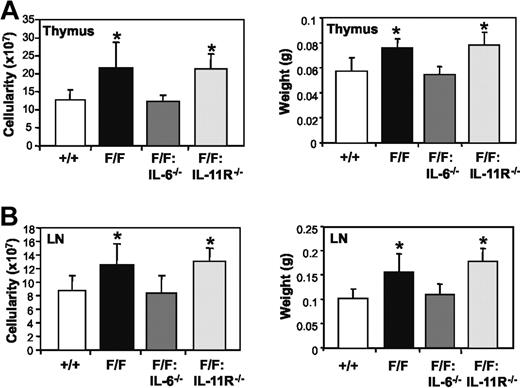
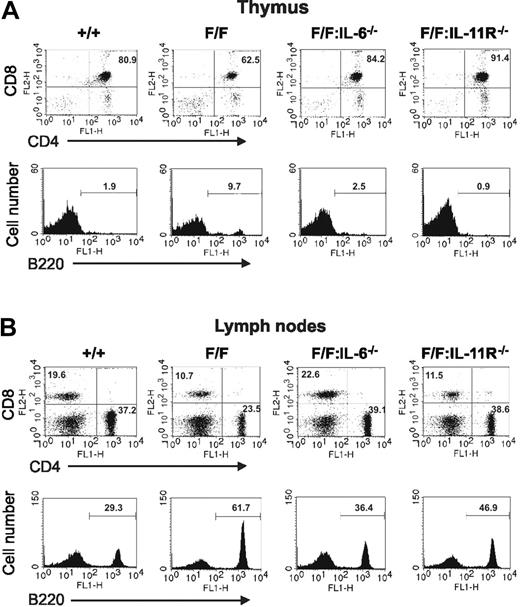
We next examined whether IL-6 or IL-11-induced activation of the gp130Y757F receptor was responsible for the lymphadenopathy and altered cellular distribution in thymus and lymph nodes in gp130Y757F/Y757F mice. The mass and cellularity of thymus and lymph nodes from gp130Y757F/Y757F:IL-6−/− mice were indistinguishable from those of gp130+/+ mice (Figure 3). Flow cytometric analyses also demonstrated that the increased frequency of B220+ B cells in gp130Y757F/Y757F thymus (Figure 4A) and lymph nodes (Figure 4B) was not apparent in these lymphoid organs from gp130Y757F/Y757F:IL-6−/− mice. Similarly, the frequency of double-positive CD4+CD8+ T cells in thymus and single-positive CD4+ and CD8+ T cells in lymph nodes from gp130Y757F/Y757F:IL-6−/− mice was normal, whereas the frequency of these T-cell subsets in gp130Y757F/Y757F lymphoid organs was lower (Figure 4). In contrast, the mass and cellularity of thymus and lymph nodes from gp130Y757F/Y757F:IL-11Rα1−/− mice remained increased (Figure 3). Although the distribution of thymic B220+ B cells and CD4+CD8+ T cells in gp130Y757F/Y757F:IL-11Rα1−/− mice was normal (Figure 4A), the increased frequency of B cells in gp130Y757F/Y757F lymph nodes was only partially rescued by the absence of IL-11Rα (Figure 4B). Interestingly, the frequency of CD4+ T cells, but not CD8+ T cells, in lymph nodes of gp130Y757F/Y757F:IL-11Rα1−/− mice was increased when compared to that observed in gp130+/+ lymph nodes. Together, these data imply that IL-6 is the primary cytokine responsible for the lymphoid abnormalities in gp130Y757F/Y757F mice, whereas deregulated IL-11 signaling only affects the differentiation/maturation of lymphoid precursors into B- and T-cell subsets.
Absence of IL-6 but not IL-11Rα in gp130Y757F/Y757F mice prevents lymphoid organ enlargement. Cellularity and mass of (A) thymus and (B) lymph nodes from gp130+/+, gp130Y757F/Y757F, gp130Y757F/Y757F:IL-6−/−, and gp130Y757F/Y757F:IL-11Rα1−/− mice. Data from 6 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from gp130+/+ mice.
Absence of IL-6 but not IL-11Rα in gp130Y757F/Y757F mice prevents lymphoid organ enlargement. Cellularity and mass of (A) thymus and (B) lymph nodes from gp130+/+, gp130Y757F/Y757F, gp130Y757F/Y757F:IL-6−/−, and gp130Y757F/Y757F:IL-11Rα1−/− mice. Data from 6 mice of each genotype are expressed as the mean ± SD. *P < .05 versus data from gp130+/+ mice.
Flow cytometric analysis of the cellular distribution in thymus and lymph nodes of gp130Y757F/Y757F mice lacking either IL-6 or IL-11Rα. (A) Thymocytes and (B) lymph node cells were subjected to dual-color flow cytometric analysis with the indicated antibodies. For dot plots, x-axis represents FITC intensity, and y-axis represents PE intensity, and numbers indicate the percent of positive cells in each quadrant. For histograms, x-axis represents either FITC or PE intensity, and y-axis represents relative cell number. The numbers indicate the percent of positive cells in the marked regions. These flow cytometric profiles are representative of at least 3 mice of each genotype.
Flow cytometric analysis of the cellular distribution in thymus and lymph nodes of gp130Y757F/Y757F mice lacking either IL-6 or IL-11Rα. (A) Thymocytes and (B) lymph node cells were subjected to dual-color flow cytometric analysis with the indicated antibodies. For dot plots, x-axis represents FITC intensity, and y-axis represents PE intensity, and numbers indicate the percent of positive cells in each quadrant. For histograms, x-axis represents either FITC or PE intensity, and y-axis represents relative cell number. The numbers indicate the percent of positive cells in the marked regions. These flow cytometric profiles are representative of at least 3 mice of each genotype.
Increased expression of IL-6 and IL-6Rα in gp130Y757F/Y757F mice correlates with STAT3 hyperactivation and disease severity
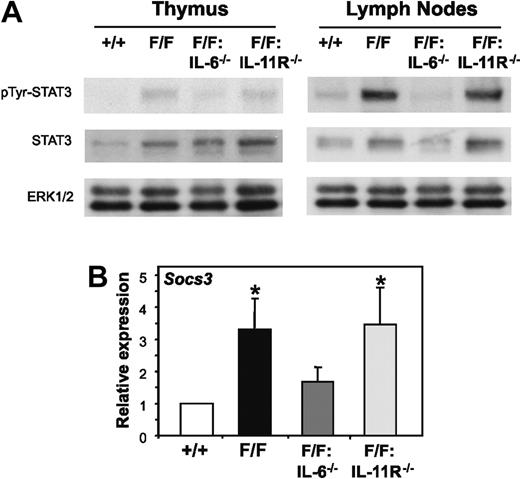
We have previously demonstrated that STAT3 hyperactivation was responsible for the perturbed lymphopoiesis in gp130Y757F/Y757F mice because genetically reducing the elevated level of gp130-dependent STAT3 activation in gp130Y757F/Y757F:Stat3+/− mice prevented the lymphoid abnormalities.19 Consistent with this finding, immunoblot analyses of STAT3 tyrosine phosphorylation in thymocytes and splenocytes, as well as whole thymus and lymph node tissue, revealed a correlation between organ cellularity and the level of STAT3 tyrosine phosphorylation, the latter of which was elevated in lymphoid organs from gp130Y757F/Y757F and gp130Y757F/Y757F:IL-11Rα1−/− mice, but not from gp130Y757F/Y757F:IL-6−/− mice (Figure 5A and data not shown). Furthermore, Q-PCR analyses confirmed enhanced expression of a representative gp130-induced STAT3-response gene, Socs3, in lymphocytes from gp130Y757F/Y757F and gp130Y757F/Y757F:IL-11Rα1−/− mice compared with gp130Y757F/Y757F:IL-6−/− mice and gp130+/+ control mice (Figure 5B).
Selective reduction of gp130-dependent STAT3 hyperactivation in gp130Y757F/Y757F lymphoid organs lacking IL-6 but not IL-11Rα. (A) Lysates prepared from thymus or lymph node cells were run out on 10% polyacrylamide electrophoresis (PAGE) gels, transferred to nitrocellulose membranes, and then immunoblotted with the indicated antibodies. (B) Q-PCR analyses of Socs3 gene expression was performed on cDNA derived from total RNA prepared from thymocytes. Expression data from 3 samples per genotype are shown following normalization for 18S expression, and are presented from replicate analysis as the mean fold induction ± SD relative to expression in gp130+/+ samples. *P < .05 versus expression in gp130+/+ samples.
Selective reduction of gp130-dependent STAT3 hyperactivation in gp130Y757F/Y757F lymphoid organs lacking IL-6 but not IL-11Rα. (A) Lysates prepared from thymus or lymph node cells were run out on 10% polyacrylamide electrophoresis (PAGE) gels, transferred to nitrocellulose membranes, and then immunoblotted with the indicated antibodies. (B) Q-PCR analyses of Socs3 gene expression was performed on cDNA derived from total RNA prepared from thymocytes. Expression data from 3 samples per genotype are shown following normalization for 18S expression, and are presented from replicate analysis as the mean fold induction ± SD relative to expression in gp130+/+ samples. *P < .05 versus expression in gp130+/+ samples.
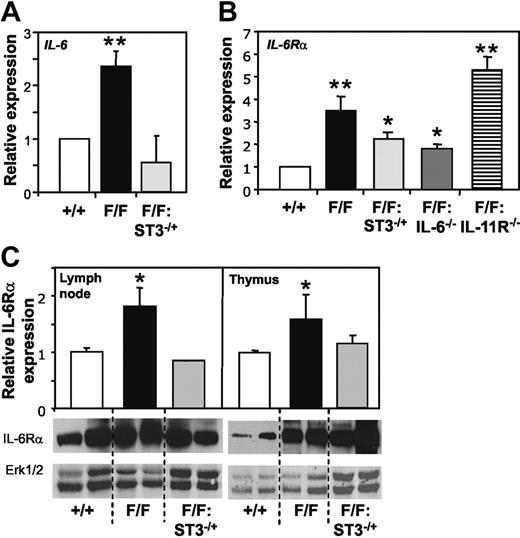
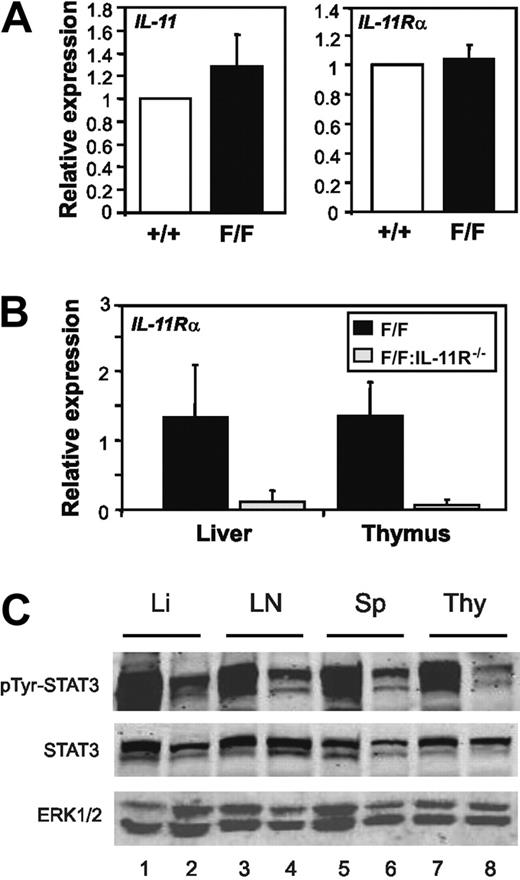
Several abnormalities observed in gp130Y757F/Y757F but not gp130Y757F/Y757F:IL-6−/− mice, such as splenomegaly, and enlargement of thymus and lymph nodes characterized by B-cell expansion, phenocopy transgenic overexpression of IL-6.10,11 We therefore investigated whether expression of IL-6 as well as IL-6Rα was elevated in the lymphoid compartment of gp130Y757F/Y757F mice. Indeed, Q-PCR analyses revealed a 2.3-fold and 2.8- to 3.4-fold increase in IL-6 and IL-6Rα gene expression, respectively, in thymocytes from gp130Y757F/Y757F mice compared to gp130+/+ mice (Figure 6A-B). Furthermore, IL-6Rα gene expression was significantly reduced in gp130Y757F/Y757F:Stat3+/− and gp130Y757F/Y757F:IL-6−/− thymus, but remained elevated in the gp130Y757F/Y757F:IL-11Rα1−/− lymphoid compartment (Figure 6B). Importantly, Western blot analyses with an IL-6Rα antibody that detects both membrane-bound and soluble versions of IL-6Rα confirmed the elevated expression of membrane-bound IL-6Rα protein in gp130Y757F/Y757F but not gp130Y757F/Y757F:Stat3+/− lymphoid tissues (Figure 6C), whereas no detectable increase in sIL-6Rα was observed (data not shown). As shown in Figure 7A, IL-11 and IL-11Rα gene expression was unchanged in gp130Y757F/Y757F thymocytes. Although it has previously been reported that a second IL-11Rα locus (IL-11Rα2) with restricted expression in the testes, thymus, and lymph nodes exists in some strains of mice,8 Q-PCR analyses failed to detect expression of either IL-11Rα locus in thymus and, as expected liver, of gp130Y757F/Y757F:IL-11Rα−/− mice (Figure 7B). Furthermore, unlike gp130Y757F/Y757F mice, IL-11 treatment of gp130Y757F/Y757F:IL-11Rα1−/− mice failed to induce STAT3 tyrosine phosphorylation in lymphoid and nonlymphoid organs (Figure 7C), thus confirming that IL-11 does not elicit a signal in gp130Y757F/Y757F:IL-11Rα1−/− mice. From these data, we conclude that expression levels of IL-6 and its ligand-binding α subunit correlate with the magnitude of STAT3 activation and disease severity in mice, and therefore provide a likely molecular explanation for the specific requirement of IL-6 in driving the lymphoid and indeed other abnormalities in gp130Y757F/Y757F mice.
Increased expression of IL-6 and IL-6Rα in gp130Y757F/Y757F mice correlates with STAT3 hyperactivation. Q-PCR analyses of (A) IL-6 and (B) IL-6Rα gene expression was performed on cDNA derived from total RNA prepared from thymocytes. Expression data from 3 samples per genotype are shown following normalization for 18S expression and are presented from replicate analysis as the mean fold induction ± SD relative to expression in gp130+/+ samples. (C) Immunoblot analysis of IL-6Rα levels in lysates prepared from lymph node and thymus tissue. Densitometric quantitation of IL-6Rα in each of 2 representative samples per genotype was performed and normalized against ERK1/2, and data are presented as the mean fold induction ± SD relative to expression in gp130+/+ samples. **P < .001 and *P < .05 versus expression in gp130+/+ samples.
Increased expression of IL-6 and IL-6Rα in gp130Y757F/Y757F mice correlates with STAT3 hyperactivation. Q-PCR analyses of (A) IL-6 and (B) IL-6Rα gene expression was performed on cDNA derived from total RNA prepared from thymocytes. Expression data from 3 samples per genotype are shown following normalization for 18S expression and are presented from replicate analysis as the mean fold induction ± SD relative to expression in gp130+/+ samples. (C) Immunoblot analysis of IL-6Rα levels in lysates prepared from lymph node and thymus tissue. Densitometric quantitation of IL-6Rα in each of 2 representative samples per genotype was performed and normalized against ERK1/2, and data are presented as the mean fold induction ± SD relative to expression in gp130+/+ samples. **P < .001 and *P < .05 versus expression in gp130+/+ samples.
Comparative analysis of IL-11 and IL-11Rα expression and IL-11–induced STAT3 activation in gp130Y757F/Y757F and gp130Y757F/Y757F:IL-11Rα1−/− mice. Q-PCR analysis of (A) IL-11 and IL-11Rα gene expression in thymocytes and (B) IL-11Rα gene expression in whole thymus and liver tissue was performed on cDNA derived from total RNA. Expression data from 3 samples per genotype are shown following normalization for 18S expression and are presented from replicate analysis as the mean fold induction ± SD relative to expression in (A) gp130+/+ and (B) gp130Y757F/Y757F samples. (C) Immunoblot analysis of STAT3 tyrosine phosphorylation in liver (Li), lymph node (LN), spleen (Sp), and thymus (Thy) lysates prepared from gp130Y757F/Y757F (lanes 1, 3, 5, 7) and gp130Y757F/Y757F:IL-11Rα1−/− (lanes 2, 4, 6, 8) mice.
Comparative analysis of IL-11 and IL-11Rα expression and IL-11–induced STAT3 activation in gp130Y757F/Y757F and gp130Y757F/Y757F:IL-11Rα1−/− mice. Q-PCR analysis of (A) IL-11 and IL-11Rα gene expression in thymocytes and (B) IL-11Rα gene expression in whole thymus and liver tissue was performed on cDNA derived from total RNA. Expression data from 3 samples per genotype are shown following normalization for 18S expression and are presented from replicate analysis as the mean fold induction ± SD relative to expression in (A) gp130+/+ and (B) gp130Y757F/Y757F samples. (C) Immunoblot analysis of STAT3 tyrosine phosphorylation in liver (Li), lymph node (LN), spleen (Sp), and thymus (Thy) lysates prepared from gp130Y757F/Y757F (lanes 1, 3, 5, 7) and gp130Y757F/Y757F:IL-11Rα1−/− (lanes 2, 4, 6, 8) mice.
Discussion
In this paper, we report that STAT3 hyperactivation and the resultant complex lymphohematopoietic phenotype of gp130Y757F/Y757F mice are prevented by genetic deletion of IL-6, thus implicating IL-6 as the predominant gp130-dependent cytokine that activates STAT3 during lymphopoiesis and hematopoiesis. In bone marrow of gp130Y757F/Y757F mice, the increased myelopoiesis and megakaryopoiesis responsible for the observed neutrophilia and thrombocytosis, respectively, were also corrected by genetic ablation of IL-11 signaling. These observations are consistent with the elevated myelopoiesis and megakaryopoiesis reported in mice injected with either IL-634 or IL-11,35 and together with our earlier findings that gp130-dependent STAT3 hyperactivation has broad pathologic consequences on marrow hematopoiesis,19 reveal redundancy between IL-6 and IL-11 in maintaining the STAT3 signal output at levels for steady-state homeostasis of these lineages. Because IL-6, IL-11, and other IL-6 family cytokines such as LIF and OSM have overlapping functions in promoting myelopoiesis and megakaryopoiesis both in vitro3–6,36 and in vivo,34,35,37,38 generating gp130Y757F/Y757F mice on backgrounds lacking LIF and OSM will ultimately provide a definitive genetic tool to determine their specific contribution to the deregulated marrow hematopoiesis in gp130Y757F/Y757F mice.
Importantly, our current studies define a physiologically relevant mechanism by which gp130-dependent STAT3 signaling impairs the actions of TGF-β during marrow hematopoiesis. For instance, TGF-β suppresses both the production of megakaryocyte progenitors and their subsequent differentiation into mature platelet-shedding megakaryocytes,31,39 which contrasts the activities of IL-6 family cytokines to promote both these early and late stages of megakaryopoiesis. We have previously shown that TGF-β–mediated biologic responses, specifically the suppression of fibroblast and gastric epithelial-cell proliferation, are impaired in gp130Y757F/Y757F mice because of gp130-dependent STAT3 hyperactivation, which in turn promotes increased expression of the TGF-β signaling inhibitory molecule Smad7.20 The ability of TGF-β to suppress megakaryocyte production from gp130Y757F/Y757F:IL-6−/−, gp130Y757F/Y757F:IL-11Rα1−/−, and gp130Y757F/Y757F:Stat3+/− bone marrow, but not gp130Y757F/Y757F bone marrow, correlates with the normal production of bone marrow megakaryocytes and platelets in gp130Y757F/Y757F:IL-6−/−, gp130Y757F/Y757F:IL-11Rα1−/−, and gp130Y757F/Y757F:Stat3+/− mice, and suggests a novel mechanism by which IL-6 family cytokines impair the suppressive effects of TGF-β on megakaryocyte and platelet production in a STAT3-dependent manner. We also show in gp130Y757F/Y757F bone marrow that TGF-β failed to synergize with GM-CSF to promote the growth of committed CFU-GM progenitors,33 supporting the likelihood of a more generalized mechanism by which the actions of TGF-β on other hematopoietic lineages are also influenced by the level of gp130-dependent STAT3 activation. The relevance of our current findings to human hematologic diseases, including MM, NHL, and AML, is also underscored by the fact that disease pathogenesis correlates with a desensitization to the homeostatic actions of TGF-β.40 In light of the link between these diseases and persistent STAT3 activation,24–27 it is highly likely that their desensitization to TGF-β may also be mediated by, at least in part, increased STAT3 activation.
Another important finding from our current study is that genetic deletion of IL-6 in gp130Y757F/Y757F mice prevents STAT3 hyperactivation, increased cellularity, and altered cellular composition of thymus and lymph nodes, thus emphasizing the importance for strict control of IL-6–dependent STAT3 signaling to ensure physiologic development and homeostasis of thymocytes and lymph node cells. In this regard, the enlargement and altered composition of gp130Y757F/Y757F lymphoid organs are phenocopied in a related gp130F759/F759 mouse strain that expresses a mouse/human chimeric gp130 receptor containing a phenylalanine substitution at position 759 in the SHP2/SOCS3 docking site of human gp130.41 By analogy with the lymphoid abnormalities in gp130F759/F759 mice, which also display elevated STAT3 activity, the abnormal distribution of thymocytes and peripheral T cells in our gp130Y757F/Y757F mice is likely to result from both impairment of negative selection of immature thymic CD4+CD8+ T cells and clonal deletion of peripheral T cells in lymph nodes.42 However, although we observed a positive correlation between STAT3 hyperactivation and increased cellularity in thymus from gp130Y757F/Y757F and gp130Y757F/Y757F:IL-11Rα1−/− mice, but not gp130Y757F/Y757F:IL-6−/− mice, the thymic T-cell subset distributions in gp130Y757F/Y757F:IL-6−/− and gp130Y757F/Y757F:IL-11Rα1−/− mice remained normal irrespective of the level of STAT3 activation (normal in gp130Y757F/Y757F:IL-6−/− and elevated in gp130Y757F/Y757F:IL-11Rα1−/− thymocytes). These data suggest that the proliferation rather than maturation of thymocytes is more dependent on the STAT3 signal output. Indeed, we (Christine Brender, Gillian Tannahill, B.J.J., Joel Fletcher, Ruth Columbus, Christiaan Saris, M.E., Nicos Nicola, Douglas Hilton, Warren Alexander, Robyn Starr, “SOCS 3 regulates CD8 T cell proliferation by inhibition of IL-6 and IL-27,” manuscript submitted, August 2006) and others42 have observed that T cells expressing the gp130Y757F (or gp130Y759F in humans) mutant receptor are hyperproliferative, which may explain the increased cellularity of lymphoid organs in gp130Y757F/Y757F mice. Consistent with this notion, STAT3 promotes expression of antiapoptotic and cell-cycle progression genes,43 and such genes are up-regulated in gp130Y757F/Y757F tissues in a STAT3-dependent manner.19,20
Because the lower frequency of T cells in thymus and lymph nodes of gp130Y757F/Y757F mice was accompanied by an increase in the frequency of B cells, it is also possible that increased IL-6 signaling in gp130Y757F/Y757F B cells may influence a bias toward mature B-cell expansion. In support, B-cell hyperplasia (plasmacytosis) in thymus and lymph nodes is induced by continuous activation of gp130 in transgenic mice overexpressing IL-6.10,11 In addition to IL-6 acting as a late maturation factor on activated B cells expressing IL-6Rα,44 it was proposed that plasmacytosis was the direct result of IL-6 stimulating maturation and proliferation of these B cells.11 The normal frequency of B cells in, for example, thymus of gp130Y757F/Y757F:IL-11Rα1−/− mice, would also suggest the involvement of IL-11 signaling in maturation, which is supported by the limited effect of IL-11 on the B-cell lineage to promote maturation.45
We demonstrate that the positive correlation between increased cellularity and STAT3 hyperactivation in gp130Y757F/Y757F lymphoid organs is paralleled by increased expression of IL-6Rα. It has recently been shown that IL-6–induced activation of gp130 in hematopoietic progenitor cells transiently promotes IL-6Rα gene expression, whereas IL-11 has no effect on IL-6Rα mRNA levels,46 analogous to our observations that IL-6Rα expression remained elevated in lymphocytes from gp130Y757F/Y757F:IL-11Rα1−/− but not gp130Y757F/Y757F:IL-6−/− mice. Interestingly, it was hypothesized that the failure of prolonged gp130 activation to increase IL-6Rα expression may be the result of inhibitory feedback mechanisms on gp130 signaling, such as SOCS3.46 In this respect, because SOCS3 is unable to inhibit activation of the gp130Y757F receptor, IL-6Rα expression levels would therefore be predicted to remain high on prolonged exposure to IL-6, as is the case in gp130Y757F/Y757F mice. We also note that STAT3 DNA-binding motifs were identified in the human and mouse promoters for IL-6Rα, but not IL-11Rα, using MATCH software47 to search for putative transcription factor-binding motifs in the TRANSFAC sequence database.48 Finally, another possible explanation for increased IL-6Rα expression in gp130Y757F/Y757F lymphoid organs may relate to the STAT3-driven functional impairment of TGF-β1, because blockade of TGF-β1 in hematopoietic progenitors has been shown to increase IL-6Rα expression.49
In summary, the data presented here, together with those from a previous study demonstrating that reducing STAT3 activity to normal levels in gp130Y757F/Y757F:Stat3+/− mice prevented the lymphohematopoietic phenotype,19 identify IL-6 as a crucial controller of STAT3 activation for maintaining homeostasis in bone marrow, spleen, thymus, and lymph nodes. We also observed similarities between the pathologic actions of deregulated IL-6 and IL-11 STAT3 signaling, primarily in bone marrow, which involved a novel mechanism by which STAT3 suppressed the actions of TGF-β1 on myeloid and megakaryocyte lineages. In light of the broad role of TGF-β1 during hematopoiesis, as well as on the development and function of lymphoid lineage cells,50 the gp130Y757F/Y757F mice provide a novel experimental model to determine whether impaired TGF-β–mediated responses, as a consequence of STAT3 hyperactivation, disrupt normal lymphopoiesis. Importantly, the STAT3-driven lymphomyeloproliferative phenotype in gp130Y757F/Y757F mice also mimics the increased activation of STAT3 observed in human lymphomyeloproliferative diseases such as MM, NHL, and AML,24–27 thus suggesting a potential causative role for deregulation of gp130-STAT3 signaling in these human hematologic diseases. Collectively, these observations validate our gp130Y757F/Y757F mice to further investigate the role of STAT3 hyperactivation in the pathogenesis of these lymphomyeloproliferative diseases, including assessing the efficacy of specific STAT3 inhibitors as potential therapeutics, such as peptidomimetics51 or decoy oligonucleotides,52 which neutralize STAT3 activity by inhibiting dimerization or DNA binding, respectively.
Authorship
Contribution: B.J.J. designed and performed research, analyzed data, and wrote the paper; A.W.R. designed research and analyzed data; D.G., M.N., C.G., and T.L.M. performed research; L.R. contributed vital new reagents; and M.E. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brendan J. Jenkins, Centre for Functional Genomics and Human Disease, Monash Institute of Medical Research, Monash University, 27-31 Wright St, Clayton, Victoria 3168, Australia; e-mail: brendan.jenkins@med.monash.edu.au.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by a research grant from the National Health and Medical Research Council (NHMRC) of Australia (B.J.J and M.E.). B.J.J is supported by both an R.D. Wright Biomedical Fellowship awarded by the NHMRC, and a Monash University Fellowship. A.W.R is supported by an NHMRC Practitioner Fellowship and also received support from the Cancer Council of Victoria Sir Edward Dunlop Clinical Research Fellowship. L.R and M.E are supported by Principle and Senior Research Fellowships, respectively, from the NHMRC.
We are grateful to P. Hertzog and R. Starr for critical reading of the manuscript. We also thank S. Samarajiwa, E. Richardson, B. Teal, T. Thorne, J. Cohen, and M. Arnold for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal