Abstract
We have previously reported that VEGF-A, in combination with MCP-5, contributes to leukemia progression within the splenic microenvironment of mice infected with F-MuLV. To study the influence of constitutively elevated VEGF-A levels on the progression of erythroleukemia, mice heterozygous for a VEGF-A “hypermorphic” allele (Vegfhi/+) were inoculated with F-MuLV. Unexpectedly, a significant delay in erythroleukemia was observed in Vegfhi/+ mice when compared with wild-type controls. These results suggested an altered physiologic response arising from elevated VEGF-A levels that decelerated erythroleukemic progression. Characterization of hematopoiesis in Vegfhi/+ spleens showed a higher natural killer cell activity, elevated B cells, and a decrease in T-cell number. Furthermore, higher erythroid progenitors (ie, CD34+, CD36+, and Ter119+ cells) were evident in the bone marrow, spleen, and peripheral blood of Vegfhi/+ mice. The CFU-E levels were significantly elevated in Vegfhi/+ bone marrow cultures, and this elevation was blocked by a neutralizing antibody to VEGF-A receptor (VEGFR-2). Moreover, erythroleukemic mice were treated with recombinant erythropoietin and, similar to diseased Vegfhi/+ mice, showed a delay in disease progression. We propose that a compensatory erythropoietic response combined with increased natural killer (NK) cell activity account for the extended survival of erythroleukemic, Vegfhi/+ mice.
Introduction
Sustained angiogenic activity is fundamental for the development of solid, malignant tumors (for review, see Folkman1 ). Studies showing increased microvessel density within the bone marrow of patients with multiple myeloma and pediatric acute lymphoblastic leukemia indicate that angiogenesis contributes to the progression of “liquid” hematologic malignancies as well.2,3 It does so by mechanisms that are different from solid tumors (eg, release of factors and cytokines from activated endothelial cells that promote growth and survival of the tumor cells). Several studies have shown that isoforms of the A family of vascular endothelial growth factor (VEGF-A) could promote leukemic expansion.4 It has also been demonstrated that concurrent inhibition of autocrine and paracrine signaling pathways mediated through binding of VEGF-A to its cognate receptor, vascular endothelial growth factor receptor-2 (VEGFR-2), lead to long-term remission of HL60 lymphoma in mice that received a xenotransplant.5 As such, a clear rationale for testing the efficacy of antiangiogenesis therapies in hematologic malignancies transpired.
The replication-competent, mammalian type C, Friend murine leukemia virus (F-MuLV)6,7 has been used as an experimental model for identifying genes involved in neoplastic transformation and progression, genetic susceptibility, and, more recently, erythroid differentiation.6 The induction and progression of this particular leukemia result from the insertional activation of oncogenes, such as Friend leukemia insertion-1 (Fli1) and inactivation of tumor suppressor genes, such as p53, both being relevant to human cancers as well.7,8 Injection of F-MuLV into newborn mice results in the transformation of its key target cells, erythroid burst-forming units (BFU-Es) to prompt the onset of erythroleukemia. Although it has been shown that within 48 hours of viral inoculation infected blasts depart the bone marrow and migrate to the spleen,9,10 according to our previous findings the actual insertional activation of the oncogene, Fli1, occurs between 4 and 6 weeks after viral infection, leading to induction of primary erythroleukemia.11
We have recently shown that VEGF-A and macrophage chemoattractant protein 5 (MCP-5) are up-regulated in the splenic microenvironment of erythroleukemic mice.12 Moreover, a combination of these 2 factors was mandatory and sufficient to drive leukemic cell proliferation and expansion in vitro. Finally, progression of the disease was delayed with the use of a neutralizing antibody to VEGF-A.12 Our findings therefore suggested that VEGF-A, in combination with MPC-5, is the key player in promoting the intrasplenic expansion of erythroleukemic cells in F-MuLV–induced disease.
A Vegf hypermorphic mouse model was initially generated by an insertion of an IRES-LacZ-pA vector into the 3′ UTR of Vegfa allele by homologous recombination, as previously described.13 Mice homozygous for this allele (Vegfhi/hi) are embryonic lethal, whereas those that are heterozygous (Vegfhi/+) survive to adulthood and exhibit modest elevations of VEGF-A isoforms 120 (VEGF120) and 164 (VEGF164) amino acids, compared with wild-type (wt) controls.13 We inoculated Vegfhi/+ and wt control mice with F-MuLV and followed leukemia progression. To our surprise, we found that the disease was significantly delayed in Vegfhi/+ mice. We therefore sought to analyze various erythropoietic and immune parameters in Vegfhi/+ mice that could potentially account for the deceleration of erythroleukemic growth.
Here, we report that Vegfhi/+ mice are characterized by enhanced erythropoietic and natural killer (NK) cell activity, both likely to slow the progression of F-MuLV–induced erythroleukemia. These 2 independent mechanisms appear to operate synergistically to delay erythroleukemia in Vegfhi/+ mice.
Materials and methods
Tumor induction and identification of Vegfhi/+ offspring
The Vegfhi/+ CD1 mice were mated with wt partners of identical background to obtain 50% of the offspring as heterozygous for Vegfa hypermorphic allele (Vegfhi/+). All neonates were infected with F-MuLV isolated from the supernatant of Clone B fibroblast cell line, as described.14 The neonates were injected intraperitoneally with 100 μL F-MuLV (500 focus-forming units) via a 1-cc U-100 insulin syringe (Becton Dickinson, Oakville, ON, Canada) 1 day after birth as previously described.12 At time of weaning all offspring were numerically tagged, and tissue sampling by ear punches was performed. Biopsies were then stained for the presence of β-galactosidase (LacZ) to confirm positive offspring as previously described.13 Both LacZ-positive and -negative mice were grouped and analyzed for survival. For tumor measurement, mice were killed on day 56 after viral infection, and spleen volume was measured using Vernier scale calipers with the formula (width2 × length × 0.5).
Hematocrit measurements and blood sampling
To measure the hematocrit values on a weekly basis tail blood from erythroleukemic Vegfhi/+ and wt mice was drawn into heparinized hematocrit tubes (Fisher Scientific, Nepean, ON, Canada) and centrifuged at 100g for 10 minutes. The packed cell volume was then measured using a hematocrit gauge. Moreover, for some experiments total exsanguination by cardiac puncture was performed to collect peripheral blood. All animal studies followed the institutional guidelines.
In vivo detection of plasma VEGF-A levels
Enzyme-linked immunosorbent assays (ELISAs) were performed on plasma collected from mice over a period of 4 weeks to establish an overall sustained elevation of VEGF-A in Vegfhi/+ mice compared with wt controls. The QuantikineM Kit (R&D Systems, Hornby, ON, Canada) was used in this analysis, and the assay was conducted according to the manufacturer's protocol. Absorbance was measured using a Benchmark Plus microplate spectrophotometer (BioRad, Mississauga, ON, Canada) at a wavelength of 450 nm.
Evaluation of cellular Vegfa RNA levels
To compare the expression level of VEGF-A in the spleens of either erythroleukemic or healthy uninfected Vegfhi/+ and wt mice, total cellular RNA from splenocytes was extracted using Trizol reagent isolation kit (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. cDNA synthesis was performed using Superscript II reverse transcriptase with random hexaprimers. For polymerase chain reactions (PCRs) 10 pM of each of the forward (5′-GGACCCTGGCTTTACTGC-3′) and reverse (5′-CGGGCTTGGCGATTTAG-3′) primers, 5 nM dNTP, and 0.4 U Taq DNA polymerase were used. The primers were synthesized by Sigma-Genosys (Oakville, ON, Canada). The PCR reaction consisted of 35 cycles of the following steps: denaturation at 94°C for 30 seconds, 1-minute annealing at 58°C, and 90-second extension at 72°C. PCR products were resolved in 2% agarose gel, stained with ethidium bromide, visualized under UV light, and photographed with Gel Doc 2000 digital camera (BioRad).
Evaluation of bone marrow, spleen, and peripheral blood mononuclear cells by flow cytometry
Femur, spleen, and peripheral blood were obtained from both Vegfhi/+ and wt mice (n ≥ 3 mice/group). Using a syringe and needle, PBS was flushed into femurs to obtain bone marrow cells. Spleens were put in PBS, chopped into approximately 5-mm2 pieces, and mashed by the plunger of a 3-mL syringe. Peripheral blood mononuclear cells (PBMCs) were obtained by a Ficoll (ICN, Costa Mesa, CA) gradient density centrifugation (400g, 30 minutes). Single-cell suspensions from femur, spleen, and PBMCs were washed in PBS and resuspended in fluorescence-activated cell sorting (FACS) staining buffer (PBS containing 1% bovine serum albumin) to the final concentration of 2 × 107 cells/mL. Fifty microliters (equal to 106 cells) of each sample was transferred to a corresponding Falcon round-bottom FACS tubes (Becton Dickinson, Franklin Lakes, NJ). To prevent nonspecific binding, before adding primary antibodies cellular Fc receptors were blocked with an anti-CD16/CD32 antibody (eBioscience, San Diego, CA) (0.5 μg/106 cells for 10 minutes). All primary antibodies were conjugated to phycoerythrin (PE), allophycocyanin (APC), or fluorescein isothiocyanate (FITC). APC-conjugated anti-Ter119 (eBiosciences), PE-conjugated anti-CD36 (Santa Cruz Biotechnology, Santa Cruz, CA), and PE-conjugated anti-CD34 (BD Pharmingen, San Diego, CA) were used at concentration of 0.5 μg/106 cells to stain bone marrow, spleen, and PBMC samples. Spleen cells were also stained with FITC-conjugated anti-B220, APC-conjugated anti-CD3 ϵ chain, PE-conjugated anti-NK1.1, and APC-conjugated anti-CD19 (all purchased from eBiosciences). After incubation for 30 minutes at 4°C cells were washed twice in FACS staining buffer (300g, 4°C, 30 minutes). Cells were resuspended in 400 μL FACS staining buffer, and 104 events were collected using a FACSCalibur flow cytometer (Becton Dickinson USA) and analyzed with Flow Jo (Treestar, San Carlos, CA) and CellQuest Pro (Becton Dickinson USA) software.
Methylcellulose assay for CFU-E and BFU-E measurement
The methylcellulose assay was carried out as previously described.15 Briefly, age-matched wt and Vegfhi/+ mice were killed, and bone marrow cells were flushed from femurs. Cells were then suspended in Iscove Modified Dulbecco Medium and Methocult M3334 (Stem Cell Technologies, Vancouver, BC, Canada) at a final concentration of 2 × 105 viable cells/mL, and a total of 1-mL suspension was plated into colony culture dishes (Stem Cell Technologies). Prior to plating, viability was assessed by trypan blue exclusion. Colonies were counted after 2 and 8 days for CFU-Es and BFU-Es, respectively. Incubation with 4 ng/mL recombinant murine VEGF, rmVEGF-A120, and rmVEGF-A164 (R&D Systems), or with 20 μg/mL of the neutralizing antibody to VEGFR-2, DC101 (given to R.S.K. by ImClone Systems, New York, NY), was performed for validation purposes where indicated. In a preliminary experiment, CFU-E– and BFU-E–derived cultures were supplemented with rat-IgG antibody (Jackson ImmunoResearch Laboratory, West Grove, PA) and used as a control group for DC101-supplemented culture. No differences in number or size of CFU-Es/BFU-Es were observed between rat IgG-supplemented and -unsupplemented control culture. For imaging, an inverted microscope (Leitz, Wetzlar, Germany) was used to view colonies (× 400) at room temperature, and photographs were obtained using a Nikon digital camera Coolpix 4500 (Nikon, Garden City, NY) with acquisition software OPTEX (Fredericton, NB, Canada).
Measurement of natural killer (NK) cell cytotoxicity
The killing activity of NK cells was measured as follows: Vegfhi/+ and age-matched CD-1 wt mice were inoculated intraperitoneally with 40 μg polyinosinic-polycytidylic acid (poly I:C; R&D Systems). Sixteen hours later all mice were killed, and single-cell suspensions of the spleen were prepared. Using red blood cell (RBC) lysis buffer (Sigma, Oakville, ON, Canada) RBCs were removed, and the cells were used as effectors in killing assay. To prepare target cells, Yac-1 cells (a mouse lymphoma cell line, obtained from ATCC, Manassas, VA) were labeled through incubating with chromium51 (Amersham Biosciences, Piscataway, NJ) to a final concentration of 25 μCi (0.925 MBq)/106 cells. The effector and target cells were then mixed at 200:1, 100:1, 50:1, and 25:1 ratios (effector to target) and incubated at 37°C for 4 hours in a Nunclon surface round-bottom 96-well plate (VWR, Mississauga, ON, Canada). Yac-1 cells alone or lysed with Triton X-100 were used as negative and positive controls, respectively. The plate was then centrifuged (500g, 4°C, 5 minutes), and the radioactivity of supernatants was determined using a gamma counter (Fisher Scientific). The percentage of specific lysis (PSL) was calculated according to the following formula and plotted against the effector-target ratios: PSL = [100 × (experimental release − spontaneous release)/(maximum release − spontaneous release)]. Spontaneous and maximum releases represent negative and positive controls, respectively.
In a similar experiment, three 6-week-old BALB/cJ mice (n = 3/group) were inoculated intraperitoneally with either 500 ng rmVEGF-A164 (R&D Systems) or vehicle control (PBS). Eight hours later, all mice were inoculated with 40 μg poly I:C. The procedure from this step onward followed the aforementioned methods.
Survival and statistical analyses
Survival of all mice was computed and plotted according to the nonparametric Kaplan-Meier analysis. All statistical analyses were performed using 2-tailed Student t test with significance considered at P less than .05. Results are shown as mean ± SD, where bars are presented.
Results
Quantification of VEGF in Vegfhi/+ mice
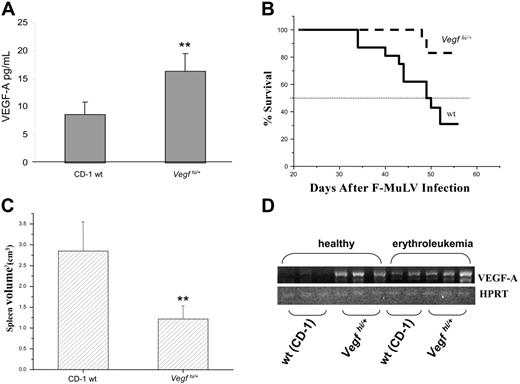
It has previously been reported that the VEGF-A levels in the embryonic lung, heart, brain, and the whole body of Vegfhi/+ embryos are moderately increased, averaging approximately 1.5- to 2-fold above those of wt littermates.13 However, the level of VEGF-A in adult Vegfhi/+ mice has not been determined. To do so, blood samples were collected from Vegfhi/+ mice on a weekly basis for a period of 4 weeks. Plasma was collected and processed for VEGF-A analysis by ELISA. As observed in Figure 1A, a significant increase of plasma VEGF in adult Vegfhi/+ mice was observed (averaged at approximately 2-fold higher) compared with wt controls. It is important to mention that Vegfhi/+ mice appeared to be as healthy as their age-matched, wt controls with no apparent physical anomalies throughout the experimental period.
Survival analysis for erythroleukemic, Vegfhi/+ mice. (A) Blood was sampled weekly (4-week period) by tail vein bleeding of 8-week-old Vegfhi/+ and wt mice. VEGF-A levels were detected by using the VEGF-A ELISA kit. (B) A Kaplan-Meier survival curve was plotted for Vegfhi/+ (n = 18) and wt (n = 16) mice inoculated by F-MuLV. Mice were monitored on a daily basis and killed according to institutional guidelines. The experiment was terminated on day 56 after viral inoculation. (C) Spleens were harvested and measured for volume as an indicator of tumor burden. (D) Splenocytes derived from 8-week-old healthy and F-MuL–induced erythroleukemic wt (CD-1) and Vegfhi/+ mice (n ≥ 3/group, only 2 samples of splenocytes derived from erythroleukemic wt mice are presented in the panel) were tested for VEGF-A RNA levels. **P < .05.
Survival analysis for erythroleukemic, Vegfhi/+ mice. (A) Blood was sampled weekly (4-week period) by tail vein bleeding of 8-week-old Vegfhi/+ and wt mice. VEGF-A levels were detected by using the VEGF-A ELISA kit. (B) A Kaplan-Meier survival curve was plotted for Vegfhi/+ (n = 18) and wt (n = 16) mice inoculated by F-MuLV. Mice were monitored on a daily basis and killed according to institutional guidelines. The experiment was terminated on day 56 after viral inoculation. (C) Spleens were harvested and measured for volume as an indicator of tumor burden. (D) Splenocytes derived from 8-week-old healthy and F-MuL–induced erythroleukemic wt (CD-1) and Vegfhi/+ mice (n ≥ 3/group, only 2 samples of splenocytes derived from erythroleukemic wt mice are presented in the panel) were tested for VEGF-A RNA levels. **P < .05.
The expression of VEGF-A transcript in splenocytes of both erythroleukemic and healthy mice was determined by reverse transcriptase (RT)–PCR. As shown in Figure 1A,D, a correlation exists between the protein and transcript expression profiles, respectively, of VEGF-A in healthy wt and Vegfhi/+ mice, hence corroborating with previous reports.13 However, it is important to note that wt mice infected with F-MuLV also exhibit elevated transcriptional expression over uninfected controls (Figure 1D). This is in agreement with the observations reported in a previous study in which the protein expression of VEGF is elevated in spleens of mice infected with F-MuLV (see “Discussion”).12
Vegfhi/+ erythroleukemic mice experience extended survival
In addition, it has been shown both by others and us that VEGF-A promotes leukemic cell proliferation in general4,5 and erythroleukemogenesis in particular.12 We therefore initially hypothesized that the progression of Friend erythroleukemia would be accelerated in Vegfhi/+ mice. Surprisingly, however, Vegfhi/+ mice experienced a significant deceleration of disease progression when compared with wt controls (Figure 1B, P < .05). To further ascertain this trend, spleen volumes of Vegfhi/+ (n = 18) and wt (n = 16) mice were measured, as an assessment of tumor burden at 8.5 weeks after F-MuLV infection. We have previously shown that splenomegaly correlates with leukemia progression.9,10 Indeed, spleens from F-MuLV–infected Vegfhi/+ mice were significantly smaller than those of wt controls, reflecting suppressed erythroleukemic expansion (Figure 1C), and correlating with increased survival rate in these mice.
Hematopoietic phenotype is altered in Vegfhi/+ mice
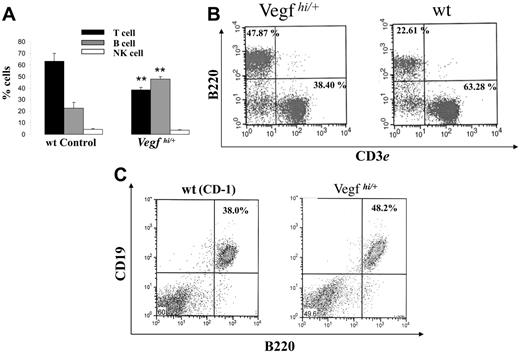
To investigate possible mechanisms involved in delaying tumor progression in Vegfhi/+ mice, we characterized their hematopoietic phenotype. We first searched for changes in RBC and white blood cell counts in peripheral blood of Vegfhi/+ mice. However, no differences were observed (data not shown). Because erythroleukemic cells expand in the spleen of F-MuLV–infected mice,12 we evaluated the proportion of hematopoietic subpopulations within the spleen of Vegfhi/+ mice. To this end, spleens from Vegfhi/+ and wt mice were harvested and weighed and single-cell suspensions were prepared. Total cell numbers were then determined, and the cells were stained for CD3, B220 (CD45R), and NK1.1 cell surface markers. In healthy mice wt and Vegfhi/+, no detectable differences existed in spleen volume, size, and total cell numbers (data not shown). However, as shown in Figure 2A-B, Vegfhi/+ spleen revealed a significant decrease in the percentage of CD3+ T lymphocytes and an increase in the percentage of B220+ cell population in comparison to wt controls. Double staining of spleen cells with B220 and CD19 antibodies, 2 markers of mature B cells,16 also showed the presence of a higher number of CD19+/B220+ cells in Vegfhi/+ mice than in the wt control mice (Figure 2C). This data indicated that VEGF-A alters the number of B220+/CD19+ cells. Analysis of Vegfhi/+ mice also revealed no differences in the percentage of NK cells (Figure 2A). These results suggested an altered hematopoietic phenotype in spleens of Vegfhi/+ mice, which likely affected the outcome of erythroleukemia progression, albeit by an unexplained mechanism.
Evaluation of hematopoietic phenotype of Vegfhi/+ and wt mice. Spleens from 8- to 10-week-old Vegfhi/+ and age-matched wt controls (n ≥ 3 mice/group) were harvested and prepared for enumeration of B, T, and NK cells by flow cytometry as described in “Materials and methods.” (A) Percentage of B, T, and NK cells from spleens of Vegfhi/+ and wt mice. **P < .05 when compared with wt control. (B) A representative dot plot of B220+ and CD3+ splenocytes from Vegfhi/+ and wt mice that were evaluated by flow cytometry. (C) A representative dot plot of B220+ and CD19+ splenocytes from Vegfhi/+ and wt control mice.
Evaluation of hematopoietic phenotype of Vegfhi/+ and wt mice. Spleens from 8- to 10-week-old Vegfhi/+ and age-matched wt controls (n ≥ 3 mice/group) were harvested and prepared for enumeration of B, T, and NK cells by flow cytometry as described in “Materials and methods.” (A) Percentage of B, T, and NK cells from spleens of Vegfhi/+ and wt mice. **P < .05 when compared with wt control. (B) A representative dot plot of B220+ and CD3+ splenocytes from Vegfhi/+ and wt mice that were evaluated by flow cytometry. (C) A representative dot plot of B220+ and CD19+ splenocytes from Vegfhi/+ and wt control mice.
Enhanced NK cell killing capacity in spleens of Vegfhi/+ mice
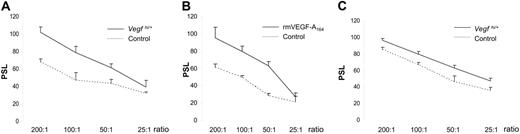
Because NK cells are effective in eliminating virally infected cells (for review, see Biron et al17 ), we determined NK activity of Vegfhi/+ spleen cells as a potential mechanism of tumor inhibition by F-MuLV. To this end, Vegfhi/+ and wt splenocytes were tested for NK activity against a sensitive target cell line (Yac-1). The results in Figure 3A showed a substantial increase in Vegfhi/+ NK activity compared with wt controls. In addition, we observed that CB3 cells, an F-MuLV–induced erythroleukemia cell line,18 were also more sensitive to Vegfhi/+ NK cells (Figure 3C).
NK cell activity analysis in Vegfhi/+ or BALB/cJ mice administered VEGF-A. NK cell activity analysis was performed for either Vegfhi/+ (A,C) or BALB/cJ wt (B) mice administered with 500 ng rmVEGF-A164. (C) Spleen cells were cocultured with either Yac-1 target cells (A,B) or CB3 erythroleukemia cell line. The effector-target ratios are shown on the x-axis. The PSL values are shown on the y-axis and calculated according to “Materials and methods.”
NK cell activity analysis in Vegfhi/+ or BALB/cJ mice administered VEGF-A. NK cell activity analysis was performed for either Vegfhi/+ (A,C) or BALB/cJ wt (B) mice administered with 500 ng rmVEGF-A164. (C) Spleen cells were cocultured with either Yac-1 target cells (A,B) or CB3 erythroleukemia cell line. The effector-target ratios are shown on the x-axis. The PSL values are shown on the y-axis and calculated according to “Materials and methods.”
To confirm whether the increased VEGF-A levels could account for the higher NK activity in the Vegfhi/+ mice, BALB/cJ mice were administered intraperitoneally with rmVEGF-A164 and 24 hours later tested for splenic NK activity. Similar results were obtained as demonstrated in Figure 3B. Thus, in both Vegfhi/+ and VEGF-A–inoculated BALB/cJ mice, a substantial increase in NK activity was observed. Overall, these findings demonstrated that VEGF augmented killing activity of poly I:C-activated NK cells and therefore supported the idea that a direct effect on the erythroleukemic population through increased NK cell activity reduced tumor burden in Vegfhi/+ mice.
Altered erythropoietic lineage in Vegfhi/+ mice
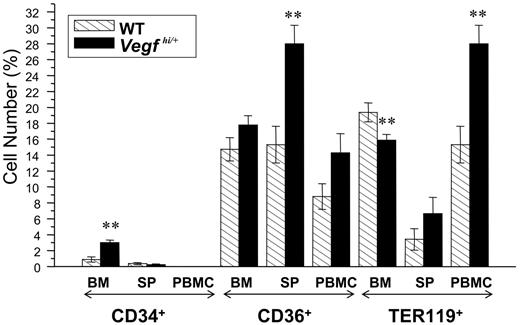
Another parameter characterized in Vegfhi/+ mice was the erythropoietic lineage. Bone marrow, spleen, and PBMCs of Vegfhi/+ and wt mice were stained with antibodies to the erythropoietic progenitor markers, CD34, CD36, and Ter119, and analyzed by flow cytometry as described in “Materials and methods.” As depicted in Figure 4, bone marrow and spleen of Vegfhi/+ mice demonstrated a different progenitor profile; bone marrow cells had a greater number of CD34+ and less Ter119+ cells, whereas CD36+ cells were found to be considerably higher in spleen cell populations. However, with the exception of lower Ter119+ and CD34+ cells in the bone marrow and spleen, respectively, Vegfhi/+ mice had elevated numbers of hematopoietic and erythroid progenitor cells. Overall, most notably, the number of erythroid precursors is induced in Vegfhi/+ mice.
Analysis of hematopoietic progenitor phenotype of Vegfhi/+ and wt mice. Femurs, spleens, and blood were harvested from 8- to 10-week-old Vegfhi/+ and age-matched wt mice. Cells from all tissues were prepared for flow cytometry evaluation for the hematopoietic progenitor markers CD34, CD36, and Ter119. The results are plotted as percentage of the total cell population in each tissue (BM indicates bone marrow; SP, spleen, and PBMCs, peripheral blood mononuclear cells). **P < .05.
Analysis of hematopoietic progenitor phenotype of Vegfhi/+ and wt mice. Femurs, spleens, and blood were harvested from 8- to 10-week-old Vegfhi/+ and age-matched wt mice. Cells from all tissues were prepared for flow cytometry evaluation for the hematopoietic progenitor markers CD34, CD36, and Ter119. The results are plotted as percentage of the total cell population in each tissue (BM indicates bone marrow; SP, spleen, and PBMCs, peripheral blood mononuclear cells). **P < .05.
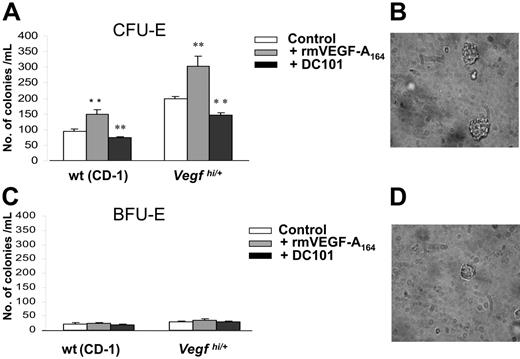
Increased size and number of erythroid colonies from Vegfhi/+-derived bone marrow cells in vitro
To further characterize the erythroid progenitor population found in Vegfhi/+ mice, we performed erythroid colony assays using bone marrow cells of Vegfhi/+ and wt mice. Our results showed a significant increase in the number of CFU-Es, but not BFU-Es, from Vegfhi/+ bone marrow–derived cells compared with control mice (Figure 5A and C, respectively). It was also observed that the sizes of the CFU-E colonies from Vegfhi/+ mice were considerably larger than those in the wt controls (Figure 5B and D, respectively).
Analysis of the number and size of CFU-Es in Vegfhi/+ mice. Eight- to 10-week-old Vegfhi/+ and wt mice (n > 3/group) were killed, and femurs were harvested and flushed. Bone marrow cells were cultured in methylcellulose for the evaluation of CFU-Es (A) (counted after 2 days) and BFU-Es (C) (counted after 8 days). Cells were cultured with no supplement (□), 4 ng/mL rmVEGF-A164 (⊡) or 20 μg/mL of DC101 (▪). CFU-Es derived from nonsupplemented culture (control) were imaged as described in “Methylcellulose assay for CFU-E and BFU-E measurement” for Vegfhi/+ (B) and wt mice (D). **P < .05.
Analysis of the number and size of CFU-Es in Vegfhi/+ mice. Eight- to 10-week-old Vegfhi/+ and wt mice (n > 3/group) were killed, and femurs were harvested and flushed. Bone marrow cells were cultured in methylcellulose for the evaluation of CFU-Es (A) (counted after 2 days) and BFU-Es (C) (counted after 8 days). Cells were cultured with no supplement (□), 4 ng/mL rmVEGF-A164 (⊡) or 20 μg/mL of DC101 (▪). CFU-Es derived from nonsupplemented culture (control) were imaged as described in “Methylcellulose assay for CFU-E and BFU-E measurement” for Vegfhi/+ (B) and wt mice (D). **P < .05.
To test whether the elevated CFU-E levels in Vegfhi/+ mice were related directly to the increased VEGF-A levels, we asked whether administration of rmVEGF-A164 in vitro could affect the proliferation of CFU-Es and thus potentially mimic the in vivo observations. Bone marrow cells from non–tumor-bearing wt mice were therefore cultured with either rmVEGF-A164 or rmVEGF-A120. Only rmVEGF-A164 (but not rmVEGF-A120) increased only CFU-E number and size of wt-derived bone marrow cells, hence mimicking Vegfhi/+ mouse phenotype (Figure 5A,C; data not shown for rmVEGF-A120).
VEGFR-2 antibody blocks the formation of leukemic CFU-E colonies in vitro
To verify the role of VEGF-A in CFU-E formation, bone marrow cells from Vegfhi/+ and wt mice were cultured in the presence of DC101, a rat antimouse antibody that blocks VEGFR-2.19 As shown in Figure 5A, addition of DC101 significantly decreased the number of CFU-Es in Vegfhi/+ mice. In addition, the size of the colonies was smaller in the presence of DC101 (data not shown). No effect of DC101 on the number of BFU-E colonies was observed (Figure 5C). These results indicated a direct effect of VEGF-A–driven erythropoietic response only at the CFU-E level. Furthermore, the data suggested that CFU-E expansion by VEGF-A was likely mediated by VEGFR-2.
Epo increases normal erythropoiesis and delays F-MuLV–induced erythroleukemia
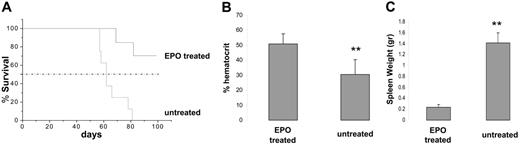
The data presented in the previous section suggested that increased VEGF-A levels in Vegfhi/+ mice delayed erythroleukemia progression, in part, by enhanced erythropoiesis. Because Epo is a strong inducer of CFU-E formation,20 we tested its effect on erythroleukemia progression in vivo. To this end, 6-week-old erythroleukemic BALB/cJ mice were treated with 50 IU recombinant Epo (3 times per week for a 3-week period). Because induction of erythroleukemias by F-MuLV has been shown to manifest by 4 to 6 weeks after viral infection,11 Epo treatment started after initial erythroid transformation to examine its effect on the expanding leukemic blasts. Mice were monitored for leukemia progression and survival. The results in Figure 6A showed that the administration of Epo increased median survival time of erythroleukemic mice. In addition, normal and sustained hematocrit values along with reduced splenomegaly were evident at 8 weeks after infection in Epo-treated erythroleukemic mice as opposed to untreated controls (Figure 6B and C, respectively). These results indicated that enhanced normal erythropoiesis offsets erythroleukemogenesis, leading to extended survival of diseased mice. Furthermore, prior to its exogenous administration, the serum levels of Epo were shown to be identical in Vegfhi/+ and wt mice (data not shown). This suggested an Epo-independent mechanism for VEGF-A–induced CFU-E expansion.
Survival analysis for Epo-administered, erythroleukemic mice. BALB/cJ neonates were inoculated with F-MuLV and grouped as experimental and control (n > 4 mice/group). On week 6 after viral infection, the mice in the experimental group were treated with 50 IU recombinant Epo, 3 times a week for a 3-week period. (A) A Kaplan-Meier survival curve was plotted for untreated and Epo-treated erythroleukemic mice. Mice were monitored on a daily basis and killed according to institutional guidelines. (B) On week 8 all mice were bled by tail veins, and hematocrits were measured using hematocrit tubes. (C) Spleens from all mice killed on day 100 (or at end point in the case of untreated controls) were harvested, and their weights were measured and plotted. **P < .05.
Survival analysis for Epo-administered, erythroleukemic mice. BALB/cJ neonates were inoculated with F-MuLV and grouped as experimental and control (n > 4 mice/group). On week 6 after viral infection, the mice in the experimental group were treated with 50 IU recombinant Epo, 3 times a week for a 3-week period. (A) A Kaplan-Meier survival curve was plotted for untreated and Epo-treated erythroleukemic mice. Mice were monitored on a daily basis and killed according to institutional guidelines. (B) On week 8 all mice were bled by tail veins, and hematocrits were measured using hematocrit tubes. (C) Spleens from all mice killed on day 100 (or at end point in the case of untreated controls) were harvested, and their weights were measured and plotted. **P < .05.
Discussion
The present study was undertaken to determine the role of VEGF-A in F-MuLV–induced erythroleukemia. It was motivated by our previous in vitro finding that VEGF-A and MCP-5, secreted by splenic stromal cells, increased the proliferation rate of F-MuLV–induced erythroleukemic cells in vitro.12 On the basis of this result, we hypothesized that either one or both of these proteins could exert the same effect in vivo and thus accelerate the progression of erythroleukemia in F-MuLV–infected mice. In the current study we used a VEGF-A–overexpressing mouse model to investigate the in vivo effect of VEGF-A on progression of F-MuLV–induced erythroleukemia. Contrary to our hypothesis, F-MuLV–infected Vegfhi/+ mice exhibited extended survival compared with wt mice. In addition, the spleens of these mice were considerably smaller than those of wt controls, indicating reduced tumor burden. Our result is in contradiction with many studies that have shown tumor-promoting capabilities of VEGF-A. For example, increased plasma VEGF-A levels have been correlated with a higher rate of tumor progression and poor prognosis in patients with multiple myeloma, chronic lymphoid leukemia, and chronic myelogenous leukemia (for review, see Podar and Anderson21 and Lim and Levine22 ). Despite these reports, however, there have also been studies that have not supported a positive connection between VEGF-A and tumor progression. For example, VEGF-A inhibited invasiveness of human breast carcinoma and trophoblast cells through decreasing the expression of urokinase-type plasminogen activator, which is required for invasion.23 In another study, no correlation was seen between the specific VEGF-A isoform expression and progression of ovarian cancer.24 To better understand the discrepancy, we proceeded to analyze the mechanisms underlying tumor retardation in Vegfhi/+ mice. Given our previous findings, and those of others, implicating the role of the spleen in F-MuLV–induced erythroleukemia, we examined the hematopoietic parameters within this organ. Our results showed a significant change in the hematopoietic phenotype in Vegfhi/+, compared with wt mice that could explain the delay in leukemogenesis seen in these mice.
Delayed erythroleukemia by enhanced NK cell activity
Natural killer cells are part of the innate immunity and are able to kill virally infected cells and tumor cells.25 Studies have demonstrated that VEGF-A165 considerably increases the binding affinity of NK cells to the tumor endothelium.26 Although the mechanism of NK activation by VEGF-A remains to be determined, a possible mechanism could involve an internal signaling pathway that stimulated its activity. In the present study we demonstrated that NK cell activity, but not proliferation, was increased in the wt, VEGF-A–treated splenic cells, akin to the activity observed for Vegfhi/+-derived splenocytes. Furthermore, it was previously demonstrated that NK cell activity played an even more critical role than T cells in conferring resistance to F-MuLV–induced erythroleukemia.27 Taken together, these results suggested that an increase in the NK cell activity detected in VEGF-A–treated and/or Vegfhi/+ mice delayed leukemia progression. This observation remains consistent with several reports indicating that administration of poly I:C and poly lysine, which are capable of boosting the NK cell activity, could delay tumor progression in several mouse tumor models. For example, this substance was effective in delaying the development of osteogenic sarcoma in C57BL/6 mice.28
Our findings also showed a significant decrease in the percentage of splenic CD3+ cells, a dramatic increase in B220+/CD19+ cell population that represent mature B cells. In agreement with our results, a previous study showed a dramatic increase in the B220+ population in the bone marrow of VEGF-A–infused mice.29 According to the same study, at pathophysiologic concentrations, VEGF-A interferes with the development of T cells from hematopoietic stem cells. Because of the importance of T cells in eliminating viral infections,30 their lower number in the spleens of Vegfhi/+ mice seems to contradict the higher survival rate seen in these mice following F-MuLV infection. However, it has previously been demonstrated that NK cells play a more important role than T cells in Friend erythroleukemia,27 which could explain the delay in erythroleukemia seen in Vegfhi/+ mice. Also, it has been shown that in Friend erythroleukemia, the requisite function of B cells is the production of virus-neutralizing antibodies rather than priming T cells. These antibodies play an essential role in promoting immunity against F-MuLV–induced erythroleukemic cells.31 Thus, the high number of B cells in Vegfhi/+ mice could also play a role in delaying leukemogenesis that requires further investigation.
Enhanced erythropoiesis by VEGF-A may also account for the delay in erythroleukemia progression
VEGF-A increased survival of hematopoietic stem cells and its ablation caused reduction in survival, colony formation, and their repopulating rates.32 Therefore, it was conceivable that VEGF-A, through increasing the erythropoiesis, could compete with the expansion of erythroleukemic cell precursors and thus delay the onset of erythroleukemia. Accordingly, we analyzed the levels of CFU-E and BFU-E progenitors in Vegfhi/+ mice and observed an increase in the number and size of CFU-Es, but not BFU-Es. This outcome was supported with earlier findings showing that in CD34+ human cord blood cells elevated VEGF-A was detected in BFU-Es.33 To further support this finding, we performed colony assays with wt bone marrow cells in VEGF-A–supplemented cultures and obtained the same results (ie, increased number and size of CFU-Es). Furthermore, the addition of DC101, an anti-VEGFR2 antibody, significantly suppressed erythropoiesis in vitro, as evidenced by the reduced colony number and size. These findings are supported by evidence indicating that, as a single agent, VEGF-A could promote erythroid lineage commitment of human embryonic stem cells in vitro.34 Overall, induction of erythropoiesis in erythroleukemic Vegfhi/+ mice may have contributed to the delay in disease progression.
Although the level of VEGF-A is much higher in plasma and spleen cells of healthy uninfected Vegfhi/+ than wt control mice, similar VEGF-A levels were found in erythroleukemic cells isolated from the spleens of erythroleukemic mice. Because erythroleukemic cells consist of the majority of cells in a spleen,11 it is possible that, in Vegfhi/+ mice, a selection occurs for expansion of a lower VEGF-A–expressing erythroleukemic cell clone. Lower VEGF-A secretion from erythroleukemic cells induced in the Vegfhi/+ mice may be necessary to override the compensatory hematopoiesis induced by higher VEGF-A which delays leukemic expansion.
Epo increases survival of erythroleukemic mice by inducing normal erythropoiesis
Erythroleukemia induced by F-MuLV leads to massive infiltration of leukemic cells into the spleen.9 As a result, diseased mice often die of splenic rupture. However, they may also succumb to a lethal drop in hematocrit values. Interestingly, a previous study demonstrated that transfusion of normal red blood cells into F-MuLV–infected mice delayed the progression of erythroleukemia.35 Our findings corroborated with this observation as we mimicked this response by inducing erythropoiesis in erythroleukemic mice through the administration of Epo. We similarly found a significant delay in erythroleukemogenesis both in Epo-treated and Vegfhi/+ mice. In this respect, VEGF-A was capable of inducing both erythropoiesis and leukemic proliferation in F-MuLV–infected mice. The fact that leukemic progression was delayed in Vegfhi/+ mice suggests that potentiating erythropoiesis also partook in delaying disease progression. Interestingly, it has previously been demonstrated that the activation of Fli1 in primary erythroleukemic cells could switch Epo-induced differentiation to Epo-induced proliferation.36 The fact that Epo administration to wt, leukemic mice delayed leukemogenesis, as observed in Vegfhi/+ mice, supported the notion that the balance between leukemic self-renewal versus erythroid differentiation governed the propensity of erythroleukemic burden.
As observed in our current study, alterations in erythropoiesis and the immune system by VEGF-A in erythroleukemic mice could suppress the expansion of leukemic cells, thereby leading to a delay in disease progression. Therefore, the administration of factors that protect or accelerate normal hematopoiesis could have clinical application in the treatment of hematologic malignancies. In agreement with these observations, a previous study demonstrated that administration of Epo to myeloma-bearing mice induced tumor regression as a result of an increase in immune responses.37
Conclusions and implications
In summary, although VEGF-A has generally been considered a tumor-promoting factor, the present study showed that it delayed progression of F-MuLV–induced erythroleukemia. Whether the observations reported here are inherent to viral-induced cancers only remains to be tested. The results presented here showed that increased NK cell activity and erythropoiesis were 2 factors that could delay leukemia progression in the Vegfhi/+ mouse. These observations may have potential clinical application relevant to patients with erythroleukemia and related hematologic malignancies, which are amenable to VEGF-A therapy.
Authorship
Contribution: D.C., Y.S., and M.H. designed and performed the research, analyzed the data, and wrote the manuscript; T.U. designed and performed experiments presented in Figure 5; C.R.L. performed experiments presented in Figure 3; J.J.H. performed experiments presented in Figure 1; A.N. contributed intellectually to the data obtained using Vegfhi/+ mice that were engineered in his laboratory; R.S.K. contributed intellectually to the overall data presented in the manuscript as well as financially supported this work; E.Y. designed experiments and participated in writing the manuscript; and Y.B.-D. is the principal investigator who was involved in the overall data presented in this study. He, too, financially supported the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
D.C., Y.S., and M.H. contributed equally to this study.
Correspondence: Yaacov Ben-David, Sunnybrook Health Sciences Centre, Division of Molecular and Cellular Biology, Research Bldg, Rm S-216, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada; e-mail: yaacov.bendavid@sri.utoronto.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Melissa Carroll for her excellent secretarial support.
This work was supported by grants from the Ontario Cancer Research Network (OCRN) and the Canadian Institutes of Health Research (CIHR) (Y.B.D.); by grants from the National Cancer Institute of Canada (NCIC), National Institutes of Health (NIH) (grant CA-41233), and the CIHR (R.S.K.); and by grants from NCIC (A.N.). D.C. is a recipient of a studentship from CIHR. Y.S. is a recipient of a postdoctoral fellowship award from the CIHR.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal