Abstract
Von Willebrand factor (VWF) mediates the tethering/adhesion of platelets at sites of vascular injury. This function depends on its multimeric size, which is controlled by ADAMTS13. We measured plasma ADAMTS13 and VWF antigen levels by enzyme-linked immunosorbent assay (ELISA) in a large population-based case-control study (Study of Myocardial Infarctions Leiden [SMILE]), consisting of 560 men with a first myocardial infarction (MI) and 646 control subjects. Although ABO blood groups influenced VWF levels, they had no influence on ADAMTS13. Furthermore, there was no relationship between plasma ADAMTS13 and VWF levels. Similar to VWF, the estimated risk of MI was increased for every quartile of ADAMTS13 when compared to the lowest quartile (odds ratio, 1.5-1.6). If confirmed, the association of ADAMTS13 with MI may suggest an unexpected mechanistic action of ADAMTS13.
Introduction
Von Willebrand factor (VWF) binds exposed collagen to form a bridge between the site of vascular injury and platelets during the initiation of hemostasis.1,2 Due to its pivotal role in hemostatic plug formation, VWF function may directly influence the likelihood of a thrombotic event, as suggested by the association of VWF levels with an increased risk of ischemic heart disease (odds ratio [OR], approximately 1.5).3–8
Plasma VWF is comprised of multimers held together by intermolecular disulfide bonds. The molecular weight (MW)/multimeric composition of VWF is a key determinant of its platelet-tethering function.9,10 Larger multimers are the most reactive at the site of vessel injury. VWF multimeric size is modulated by ADAMTS13,11–14 which cleaves in the VWF A2 domain, thus reducing both its MW and platelet-tethering function. ADAMTS13 deficiency leads to VWF-induced platelet aggregation, resulting in thrombotic thrombocytopenia purpura (TTP).15 The level of ADAMTS13 in the blood may thus influence cardiovascular disease. Herein, we report on the relationships between ADAMTS13, VWF, and MI in a large population-based case-control study (Study of Myocardial Infarctions Leiden [SMILE]).16
Patients, materials, and methods
Subjects
We used plasma samples from the previously published population-based case-control study SMILE. We included 560 men (18-70 years), consecutively diagnosed with a first episode of myocardial infarction [MI], between 1994 and 1997, and 646 men without MI who had not received anticoagulants for more than 6 months. The control group was frequency matched to cases on 10-year age groups. Venous blood, anticoagulated using trisodium citrate (1:9 vol/vol), was collected at least 6 months after MI (median, 2.6 years).16 Aliquots of plasma samples were prepared and stored at −80°C. Approval for these studies was obtained from the institutional review boards of both the university and general hospitals in Leiden, The Netherlands. Informed consent was provided in accordance with the Declaration of Helsinki.
ELISAs for ADAMTS13, VWF, and C-reactive protein
The ADAMTS13 enzyme-linked immunosorbent assay (ELISA) used polyclonal antibodies raised in rabbits immunized with recombinant ADAMTS13 (rADAMTS13).17,18 Anti-ADAMTS13 thrombospondin type 1 repeat domains (2-4) (TSP1(2-4)) antibodies were affinity purified and biotinylated for use as the detection antibody. Anti-ADAMTS13 antibodies that had been fully depleted of anti-TSP1(2-4) IgG (5 μg/mL) were used as the capture antibody in 96-well plates. Wells were washed and blocked before the addition of 100 μL/well plasma samples (diluted 1:20 in PBS), or plasma standards in duplicate, and incubated overnight at 4°C. Wells were washed, and ADAMTS13 was detected using biotinylated anti-TSR1(2-4) antibodies (0.1 μg/mL), followed by a streptavidin-horseradish peroxidase conjugate (Amersham Pharmacia, Uppsala, Sweden). Highly purified rADAMTS1317,18 and pooled normal plasma (Technoclone, Vienna, Austria) were used in standard curves. Normal pooled plasma was determined to contain 900 ng/mL ADAMTS13, similar to previous estimates.19–21 Specificity of our ELISA was confirmed by the lack of signal derived from plasma from a patient with severe congenital ADAMTS13 deficiency (provided by Dr R. Leissner, Hospital for Sick Children, London, United Kingdom). Furthermore, this TTP plasma had no effect on standard curves when mixed with normal pooled plasma dilutions. Recovery of rADAMTS13 standards from TTP plasma was 94% ± 9.1%. The lower detection limit of the ELISA was 5% that of normal plasma. Intra-assay and interassay coefficients of variation were less than 5%. All outlying samples (< 50% or > 200%) were confirmed by repetition. Multiple freeze-thawing of control plasmas was found not to influence the ADAMTS13 ELISA.
The VWF ELISA was performed according to manufacturer's instructions (Diagnostica Stago, Asnieres, France). C-reactive protein (CRP) was measured as previously reported.16 Blood group was determined by questionnaire. Due to sample depletion, ADAMTS13 and VWF levels were both available for 534 of 560 patients and 607 of 646 control subjects.
Statistical analysis
Quartiles of ADAMTS13 and VWF were defined by control subjects. The lowest quartile was used as a reference category for the OR. A 95% confidence interval (95% CI) was calculated according to the method of Woolf.22 Unconditional logistic regression was performed to adjust for age. Further adjustments for ADAMTS13 or VWF levels were performed by using these as continuous variables. All computations were carried out with the SPSS for Windows Version 12.0.1 (SPSS, Chicago, IL) statistical package.
Results and discussion
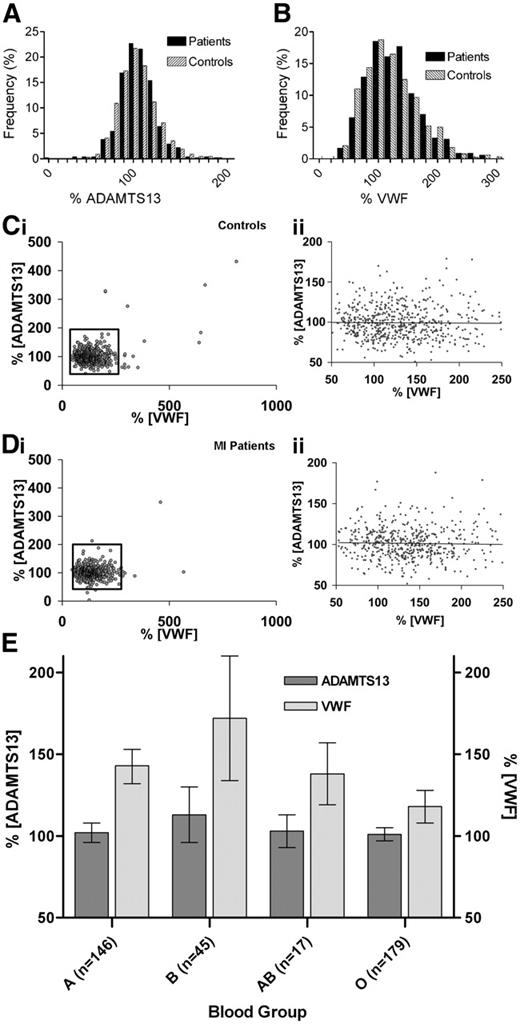
The mean ADAMTS13 level (± SD) in 551 male MI patient plasmas was 101% (22.9%; range, 4%-350%; 5th to 95th percentile, 70%-136%) and 100% (30.2%; range, 41%-432%; 5th to 95th percentile, 70%-139%) in 635 male control subjects (mean difference 0.0; 95% CI, −3.0% to 3.1%). The frequency plots in cases and controls (Figure 1A) showed normal distributions with tight groupings around the mean. Interestingly, one 45-year-old MI patient exhibited less than 5% ADAMTS13 (confirmed by activity assay; not shown), with no signs of TTP.
ADAMTS13 and VWF in male control and MI patients. Frequency distribution of ADAMTS13 (A) and VWF (B) levels in plasma of MI patients (▪) and control subjects (⊡). Data are presented as percentage of total plasma samples measured. Graphs do not show outlying samples more than 200% for (A) or 300% for (B). Association between plasma levels of ADAMTS13 and VWF in (Ci) control and (Di) MI patients. The association between these proteins in the normal range (boxed areas) are enlarged (Cii,Dii). The regression line is shown. (E) ADAMTS13 and VWF levels per blood group among control subjects. ADAMTS13 levels were not different between blood groups (ANOVA P = .21). VWF levels in blood groups A, B, and AB were all significantly different from blood group O (ANOVA P < .001).
ADAMTS13 and VWF in male control and MI patients. Frequency distribution of ADAMTS13 (A) and VWF (B) levels in plasma of MI patients (▪) and control subjects (⊡). Data are presented as percentage of total plasma samples measured. Graphs do not show outlying samples more than 200% for (A) or 300% for (B). Association between plasma levels of ADAMTS13 and VWF in (Ci) control and (Di) MI patients. The association between these proteins in the normal range (boxed areas) are enlarged (Cii,Dii). The regression line is shown. (E) ADAMTS13 and VWF levels per blood group among control subjects. ADAMTS13 levels were not different between blood groups (ANOVA P = .21). VWF levels in blood groups A, B, and AB were all significantly different from blood group O (ANOVA P < .001).
Mean VWF levels in plasma were similar, 138% (51.4%; range, 46%-567%; 5th to 95th percentile, 73%-229%) in MI patients and 135% (66.4%; range, 43%-813%; 5th to 95th percentile, 69%-199%) in control subjects (mean difference 3.50; 95% CI, −3.4% to 10.4%) with wide frequency distributions of results in both patients and control subjects (Figure 1B).
There was no clear association between ADAMTS13 and VWF in either control subjects or MI patients (Figure 1C-D). An inverse relationship between these proteins was previously reported in a small number of heterogeneous patients.23 However, because of homogeneity and the size of our study these data strongly suggest that ADAMTS13 plasma levels are not associated with VWF plasma levels in healthy men or male patients with MI. Blood group information was available for 387 control subjects. As previously reported,24 VWF levels were dependent on blood group (Figure 1E). No relationship was evident between blood group and ADAMTS13 levels.
Using the lowest quartile as the reference group, we found an increase in risk for MI in men for both ADAMTS13 and VWF for each quartile, after adjusting for age (Table 1), indicating a threshold rather than a dose relationship for ADAMTS13. Adjustment of ADAMTS13 results for VWF, and vice versa, did not materially change the risk estimate, suggesting that the proteins have separate effects on the risk of MI. Because inflammation can modulate risk of MI, adjustment was also made for CRP levels, but this did not alter the risk estimate for either ADAMTS13 or VWF levels.
ADAMTS13 and VWF levels in relation to MI
| . | No. of patients . | No. of controls . | OR 1 (95% CI)* . | OR 2 (95% CI)† . | OR 3 (95% CI)‡ . |
|---|---|---|---|---|---|
| ADAMTS13 level | |||||
| 3%-85% | 94 | 155 | 1§ | 1§ | 1§ |
| 86%-97% | 143 | 151 | 1.53 (1.08-2.16) | 1.54 (1.09-2.18) | 1.53 (1.08-2.16) |
| 98%-110% | 153 | 149 | 1.63 (1.16-2.30) | 1.64 (1.16-2.32) | 1.67 (1.18-2.36) |
| 111%-433% | 144 | 152 | 1.50 (1.06-2.12) | 1.48 (1.05-2.10) | 1.56 (1.10-2.22) |
| VWF level | |||||
| 40%-97% | 103 | 152 | 1§ | 1§ | 1§ |
| 98%-124% | 123 | 153 | 1.28 (0.90-1.81) | 1.28 (0.90-1.81) | 1.24 (0.87-1.76) |
| 125%-159% | 165 | 153 | 1.77 (1.26-2.49) | 1.77 (1.26-2.49) | 1.64 (1.16-2.32) |
| 160%-900% | 143 | 149 | 1.63 (1.15-2.33) | 1.64 (1.15-2.33) | 1.44 (1.00-2.07) |
| . | No. of patients . | No. of controls . | OR 1 (95% CI)* . | OR 2 (95% CI)† . | OR 3 (95% CI)‡ . |
|---|---|---|---|---|---|
| ADAMTS13 level | |||||
| 3%-85% | 94 | 155 | 1§ | 1§ | 1§ |
| 86%-97% | 143 | 151 | 1.53 (1.08-2.16) | 1.54 (1.09-2.18) | 1.53 (1.08-2.16) |
| 98%-110% | 153 | 149 | 1.63 (1.16-2.30) | 1.64 (1.16-2.32) | 1.67 (1.18-2.36) |
| 111%-433% | 144 | 152 | 1.50 (1.06-2.12) | 1.48 (1.05-2.10) | 1.56 (1.10-2.22) |
| VWF level | |||||
| 40%-97% | 103 | 152 | 1§ | 1§ | 1§ |
| 98%-124% | 123 | 153 | 1.28 (0.90-1.81) | 1.28 (0.90-1.81) | 1.24 (0.87-1.76) |
| 125%-159% | 165 | 153 | 1.77 (1.26-2.49) | 1.77 (1.26-2.49) | 1.64 (1.16-2.32) |
| 160%-900% | 143 | 149 | 1.63 (1.15-2.33) | 1.64 (1.15-2.33) | 1.44 (1.00-2.07) |
Levels of ADAMTS13 and VWF are depicted as quartiles, and OR was determined using the lowest quartiles as the reference groups. For patients, total n = 534; for total controls, n = 607.
Adjusted for age.
Adjusted for age, VWF, or ADAMTS13 as continuous variable.
Adjusted for age, log-transformed CRP.
Reference group.
Although a recent study has reported reduced ADAMTS13 in a small number of patients with acute MI,25 that study used data from samples taken on hospital admission. Consequently, whether reduced ADAMTS13 was a cause or effect of MI in those individuals could not be determined. Our data, using plasma samples obtained more than 6 months after the MI, reveal a positive rather than negative association of ADAMTS13 levels with risk of MI in men, and renders a detrimental effect of low levels of ADAMTS13 unlikely. This result is unexpected, and consequently merits additional investigation to ascertain whether this is specific to our study, or a general finding. If the latter, a mechanistic basis for the positive association must be identified, perhaps in the context of ADAMTS13 action in or around the atheromatous plaque.
Authorship
Contribution: C.K.N.K.C. designed and performed research, analyzed data, and wrote the paper; C.J.M.D. designed the original case-control study, analyzed data, and wrote the paper; J.T.B.C. designed and performed research, analyzed data, and wrote the paper; D.A.L. designed research, analyzed data, and wrote the paper; and F.R.R. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James T. B. Crawley, Department of Haematology, Imperial College London, 5th Floor Commonwealth Bldg, Hammersmith Hospital Campus, Du Cane Rd, London W12 ONN, United Kingdom; e-mail: j.crawley@imperial.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the British Heart Foundation and the Netherlands Heart Foundation (grant no. 92.345).
The authors thank the cardiologists at Leiden University Medical Center and Diaconessenhuis Leiden, F. J. M. van der Meer, T. Visser, J. J. Schreijer, and I. de Jonge for their assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal