Abstract
The incorporation of blood-borne forms of tissue factor (TF) into a growing blood clot is necessary for normal fibrin generation and stabilization of the blood clot. Tissue factor pathway inhibitor (TFPI) is the primary physiologic inhibitor of tissue factor and is present within platelets. Expression of TFPI on the platelet surface may be the optimal location for it to abrogate blood-borne TF activity that incorporates within the blood clot, balancing the need for adequate hemostasis while preventing development of occlusive thrombosis. TFPI is produced by megakaryocytes but is not expressed on the platelet surface. Activation of platelets with thrombin receptor activation peptide does not cause release or surface expression of TFPI, demonstrating that TFPI is not stored within platelet α granules. TFPI is expressed on the platelet surface following dual-agonist activation with convulxin plus thrombin to produce coated platelets. In association with its expression on the surface of coated platelets TFPI is also released in microvesicles or as a soluble protein.
Introduction
Vascular injury results in the exposure of collagen and tissue factor, present within the vessel wall, to flowing blood. Platelets rapidly adhere to the collagen within the subendothelial tissue of the injured vessel wall where they are activated; subsequently, platelet aggregation provides an initial barrier against blood loss. Tissue factor within the subendothelial tissue binds to factor VIIa, leading to the production of thrombin, which further activates platelets and generates fibrin. Platelets exposed to dual-agonist stimulation with collagen plus thrombin form a distinct subpopulation of platelets, called coated platelets, that express high levels of procoagulant proteins, including factor V, fibrinogen, fibronectin, and VWF, providing a procoagulant surface that supports further fibrin generation.1–3 In this manner, collagen and tissue factor work synergistically at the site of vascular injury to produce a platelet-fibrin plug that closes the wound and prevents severe hemorrhage.
In addition to its presence within the subendothelial tissues of the vessel wall, tissue factor is also present on circulating microvesicles released from activated leukocytes or endothelial cells. Incorporation of these tissue factor–bearing microvesicles into the blood clot is thought to be necessary for effective stabilization within the vasculature.4–6 The recruitment of tissue factor–bearing microvesicles into growing thrombi is mediated by interactions between P-selectin glycoprotein ligand-1 present on the microvesicles and P-selectin expressed on the surface of activated platelets within the blood clot.7 It has been demonstrated that the tissue factor–bearing microvesicles can fuse with activated platelets.8 In addition, splicing of tissue factor pre-mRNA within activated platelets is another potential mechanism for expression of tissue factor on the platelet surface.9 However, overexpression of tissue factor or inadequate regulation of its procoagulant activity can lead to propagation of a hemostatic plug to an occlusive thrombus.
The primary regulator of tissue factor activity is tissue factor pathway inhibitor (TFPI). TFPI is a trivalent Kunitz-type serine protease inhibitor that is produced primarily by endothelial cells,10 but it also is present within platelets11,12 and monocytes13 and circulating in plasma.14 It rapidly inhibits both factor Xa and the factor VIIa/tissue factor catalytic complex with its second and first Kunitz domains, respectively.15 TFPI present in platelets may play a critical role in down-regulating tissue factor activity within a growing blood clot and preventing formation of an occlusive thrombus. Here, we have characterized the production, surface expression, and activity of platelet TFPI, demonstrating that TFPI is produced by megakaryocytes but retained intracellularly in quiescent platelets. TFPI is then expressed on the platelet surface following dual activation with convulxin plus thrombin to produce coated platelets.
Materials and methods
Reagents
Ethyl methanesulfonate, 3,4-dichloroisocoumarin (DCI), PGE1, and 1-(l-trans-epoxysuccinylleucylamino)-4-guanidinobutane (E-64) were obtained from Sigma Chemical (St Louis, MO). The chromogenic factor Xa substrate (methoxycarbonyl-d-cyclohexylglycyl-glycyl-arginine p-nitroanilide acetate) and anti–mouse TFPI polyclonal antibody were from American Diagnostica (Greenwich, CT). The monoclonal antibody against human CD59 was from the Blood Center of Wisconsin core laboratory, and anti–GP-IIb/IIIa was from Dr Thomas Kunicki. The anti-TFPI 2H8 monoclonal antibody and the TFPI polyclonal antibodies directed against the entire protein and the C-terminus were from Dr George Broze, Jr. The sheep polyclonal anti–rat VWF was from Cederlane Laboratories (Ontario, Canada). Rat anti–mouse Lamp-1, ID4B, was from BD Pharmingen (San Diego, CA). The mouse IgG1κ (MOPC-21), anti–rabbit IgG (whole molecule) peroxidase conjugate, and anti–mouse phycoerythrin conjugate were from Sigma. The recombinant human tissue factor was from Ortho Diagnostic Systems (Toronto, Ontario, Canada). Human factor VIIa was from Haematologic Technologies (Essex Junction, VT); human factor X was from Enzyme Research Laboratories (South Bend, IN). Deglycosylation enzymes were from Prozyme (San Leandro, CA). Human recombinant TPO and IL-11 and mouse recombinant IL-3 were from PeproTech (Rocky Hill, NJ); streptavidin magnetic beads were from Miltenyi Biotech (Auburn, CA).
Buffers
ACD was 38.1 mM citric acid, 74.8 mM sodium citrate, 136 mM glucose; BSGC was 129 mM NaCl, 13.6 mM sodium citrate, 11.1 mM glucose, 1.6 mM KH2PO4, 8.6 mMNaH2PO4, pH 6.5. PBS was 150 mM NaCl, 10 mM NaH2PO4, pH7.5. CHAPS lysis buffer was 30 mM CHAPS, 10 mM EDTA, PBS. HBSA was 50 mM HEPES + 100 mM NaCl + 0.1% BSA, pH 7.4, + 10 mM CaCl2.
Platelets
The use of human donors to provide whole blood for platelet isolation was approved by the Blood Center of Wisconsin Institutional Review Board, and all donors gave informed consent in accordance with the Declaration of Helsinki. Human platelets were obtained from whole blood drawn from the anticubital vein. Mouse platelets were obtained from whole blood drawn from the inferior vena cava. Human and mouse blood were collected in ACD and diluted 1:2 into BSGC. Platelet-rich plasma (PRP) was prepared immediately by centrifugation at 170g for 8 minutes at 23°C. Platelets were washed in BSCG or gel filtered by layering 2 mL PRP onto a 25 × 60 m (30 mL) column of Sepharose CL-2B.
Activation of platelets
In flow cytometry and Western blot experiments, platelets were activated with 8 μM thrombin receptor activation peptide, SFLLRN (TRAP), for 10 minutes in PBS. In activity assays, platelets were activated with 8 or 20 μM TRAP containing 10 mM EDTA, 25 μM DCI, and 50 μM E-64 to prevent adventitious proteolysis of TFPI from the platelet surface. Control experiments demonstrated that the presence of 10 mM EDTA has no effect on surface expression of P-selectin following activation of platelets with either TRAP or thrombin, demonstrating that α granule secretion by TRAP does not require calcium. For all experiments, coated platelets were prepared in 10 mM HEPES, pH 7.5, 1 mg/mL BSA, 2 mM CaCl2, 1 mM MgCl2, 140 mM NaCl. Platelets were stimulated with 0.5 U/mL thrombin (final) and either 500 ng/mL convulxin or 2 μM A23187. After 2 minutes, 10 U/mL hirudin was added, and the incubation continued for 8 minutes at 37°C.
Flow cytometry
Flow cytometry was performed using CELLQUEST software on a Becton Dickinson FACSCalibur. For TRAP-activated platelets, gel-filtered platelets were suspended in primary antibodies (anti–TFPI-FITC and anti–P-selectin-PE) in PBS with 1% BSA for 30 minutes at 4°C. The platelets were washed and analyzed. For coated platelets, gel-filtered platelets were activated with various agonists in the presence of biotin-fibrinogen as previously described.16 After fixation with 1% formalin for 25 minutes, platelets were diluted in PBS with 0.1% BSA and centrifuged at 1500g for 15 minutes. Primary antibody was applied to the cells, followed by washing and the addition of FITC anti–mouse IgG and PE-streptavidin secondary antibody for 30 minutes at room temperature. The platelets were washed and analyzed.
TFPI precipitation, SDS–polyacrylamide gel electrophoresis, and Western blot analysis
Platelets were lysed in CHAPS lysis buffer. The platelet lysate was centrifuged at 16 000g for 10 minutes to remove cellular debris. TFPI was precipitated from the ultracentrifuged supernatant and CHAPS pellet lysate using bovine factor Xa-agarose for 1 hour at room temperature. The agarose beads were washed 3 times with PBS/0.5% Tween, washed once with PBS, and boiled for 3 minutes in SDS sample buffer. The sample was then subjected to 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose for Western blot analysis. Nitrocellulose was immunostained for TFPI with the indicated primary antibodies and with antirabbit conjugated to horseradish peroxidase. Proteins were visualized using chemiluminescent substrate Western blotting reagents. In some experiments TFPI was precipitated from human plasma or the factor Xa–precipitated platelet TFPI was subjected to deglycosylation as described previously.17 To quantify the relative amount of platelet and plasma 45 kDa TFPI, defined amounts of platelet lysate and plasma from the same person were precipitated with multiple rounds of bovine factor Xa agarose to ensure precipitation of all TFPI, subjected to SDS-PAGE, Western blot for TFPI, and analyzed by quantitative densitometry.
Differential centrifugation of platelets
Quiescent or dual-agonist–activated platelets were subjected to 1500g for 15 minutes; the supernatant was removed and subjected to 10 000g for 15 minutes; the supernatant was removed and subjected to 200 000g for 1 hour. The pellets from each step, as well as the supernatant from the 200 000g supernatant, were subjected to Western blot analysis for TFPI.
Real-time PCR
These experiments were performed using leukocyte-reduced platelet concentrates obtained from the Blood Center of Wisconsin. Following gel filtration to remove plasma, the platelets (3 × 106/mL) were incubated with biotinylated anti-IIb/IIIa for 30 minutes at room temperature. Platelets were further purified by positive selection using streptavidin magnetic beads. Total RNA was isolated from these highly purified, leukocyte-reduced platelets using RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was produced with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time polymerase chain reaction (PCR) primers were selected using PrimerQuest (Integrated DNA Technologies, Coralville, IA). Primers specific for full-length TFPI were 5′-ATTTCACGGTCCCTCATGGTGTCT-3′ and 5′-GGCGGCATTTCCCAATGACTGAAT-3′. Primers specific for TFPIβ were 5′-GAAGGAACAAATGATGGTTGGAAGAATGCG-3′ and 5′-ATGGATGCATGAATGCAGAAGGCG-3′. Assays were performed in triplicate and normalized to ribosomal protein L-19 on a 7500 Real-Time PCR System using SYBR Green (Applied Biosystems, Foster City, CA).
Factor VIIa/tissue factor activity assays
Functional platelet TFPI activity was determined via kinetic assay. The reaction mix contained 0.2 nM human factor VIIa, 20 nM human factor X, and a 1:10 000 dilution of recombinant tissue factor, all diluted in HBSA, plus the platelet sample. After a 30-minute incubation period in which the samples generate Factor Xa, the reaction was quenched with 20 mM EDTA. Factor Xa generated was determined by monitoring the cleavage of 500 μM factor Xa substrate. The amount of TFPI in the samples was determined by comparison to a standard curve generated using known amounts of TFPI.
PIPLC treatment
Coated platelets were incubated with 1 U/mL phosphatidyl inositol–specific phospholipase C (PIPLC; Molecular Probes, Eugene, OR) for 1 hour at 37°C. Following treatment the cells or platelets were analyzed for surface-associated TFPI by flow cytometry.
Isolation and growth of mouse megakaryocytes
Mouse bone marrow cells were harvested from the femur and humerus. Cells were cultured at 5 × 106/mL in DMEM media with 10% human sera supplemented with rIL-3, rIL-11, and rTPO.18 Cells were fed every other day for 7 days. Cytospin preparations of the cultured megakaryocytes were fixed with 3.7% buffered formalin in preparation for staining and confocal microscopy.
Confocal microscopy
Cytospin preparations of mouse megakaryocytes/platelets and human platelets were washed with PBS and fixed with 3.7% buffered formalin. Cells were washed with PBS, blocked with 2% goat serum in PBS, and incubated for 1 hour in primary antibodies. Nonimmunized mouse, rabbit, sheep, or rat IgGs were used as negative controls. Some cells were permeablized in 1% Triton–X-100 in 20 mM HEPES, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, pH 7.0. Secondary antibodies used for immunofluorescence detection included goat anti–rabbit, anti–mouse, anti–rat, or anti–sheep IgG conjugated with Alexa Fluor 488 or Alexa Fluor 568 (Invitrogen, Eugene, OR). Images were acquired using a Leica TCS SP2 confocal Laser Imaging System (Leica, Heidelberg, Germany) equipped with a Leica 100×/1.4 numerical aperature oil objective as previously described.19 The imaging solution was ProLong Gold (Molecular Probes, Eugene, OR). Images were acquired electronically and were processed using Leica confocal software (LCS), version 2.61, Build 1537. The antibodies and stains are as described in “Reagents” and in the figure legends.
Results
Full-length TFPI is located within human platelets
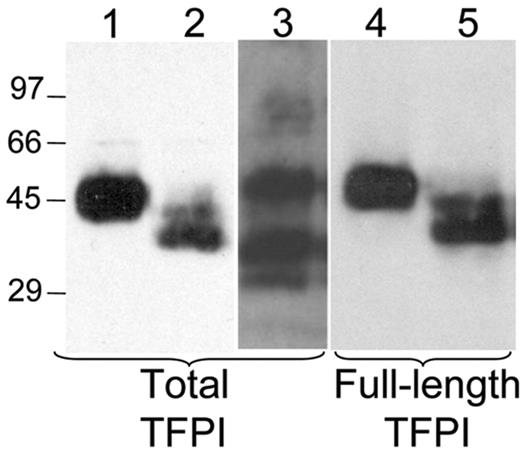
It has previously been reported that TFPI is present within platelets.11 This observation was confirmed by Western blot analysis of platelets following lysis in 30 mM CHAPS. Platelet TFPI migrates as a single 45-kDa band in SDS-PAGE, a finding that contrasts with the multiple plasma forms of TFPI (Figure 1). In addition, platelet TFPI reacts with an antibody directed against the C-terminal 12 amino acids of TFPI, indicating that it is not truncated within the C-terminal region (Figure 1). Quantitative densitometry of the 45-kDa band in standardized amounts of plasma and platelets from a single person indicates that about 10% of the TFPI in whole blood is within platelets and 90% in plasma (data not shown), consistent with the findings of Novotny et al11 who reported that 3 × 108 platelets have about 7% the amount of TFPI found in 1 mL serum.
Western blot analysis demonstrates the presence of full-length TFPI in platelets. TFPI from plasma or from platelets lysed in 30 mM CHAPS buffer were precipitated with bovine factor Xa agarose and subjected to SDS-PAGE and Western blot analysis with either an antitotal TFPI antibody or an anti-TFPI antibody recognizing only the C-terminal 12 amino acids. Lanes 1 to 3 are stained for total TFPI; lanes 4 to 5 are stained for the C-terminal 12 amino acids. Lane 1 indicates platelet TFPI; lane 2, deglycosylated platelet TFPI; lane 3, plasma TFPI; lane 4, platelet TFPI; lane 5, deglycosylated platelet TFPI.
Western blot analysis demonstrates the presence of full-length TFPI in platelets. TFPI from plasma or from platelets lysed in 30 mM CHAPS buffer were precipitated with bovine factor Xa agarose and subjected to SDS-PAGE and Western blot analysis with either an antitotal TFPI antibody or an anti-TFPI antibody recognizing only the C-terminal 12 amino acids. Lanes 1 to 3 are stained for total TFPI; lanes 4 to 5 are stained for the C-terminal 12 amino acids. Lane 1 indicates platelet TFPI; lane 2, deglycosylated platelet TFPI; lane 3, plasma TFPI; lane 4, platelet TFPI; lane 5, deglycosylated platelet TFPI.
TFPIβ is not detected within platelets
TFPIβ is an alternatively spliced form of TFPI that is truncated prior to the third Kunitz domain and contains a direct GPI-anchor attachment site within its C-terminal region.20 TFPIβ is highly glycosylated and migrates at the same molecular weight as full-length TFPI on SDS-PAGE.21 Western blot analysis of platelet TFPI performed following O- and N-linked deglycosylation demonstrates 2 bands. One band has undergone only partial deglycosylation and migrates at the approximate molecular weight of nondeglycosylated TFPI, whereas the second band has undergone extensive deglycosylation and migrates at approximately 35 kDa (Figure 1). Both of these bands react with an antibody directed against the C-terminal region of TFPI, indicating that they represent full-length TFPI. A band migrating at 21 kDa, the expected molecular weight of deglycosylated TFPIβ, is not observed, indicating that full-length TFPI is the predominant form of TFPI present within platelets.
TFPI is synthesized by mouse megakaryocytes and TFPI mRNA is present within human platelets
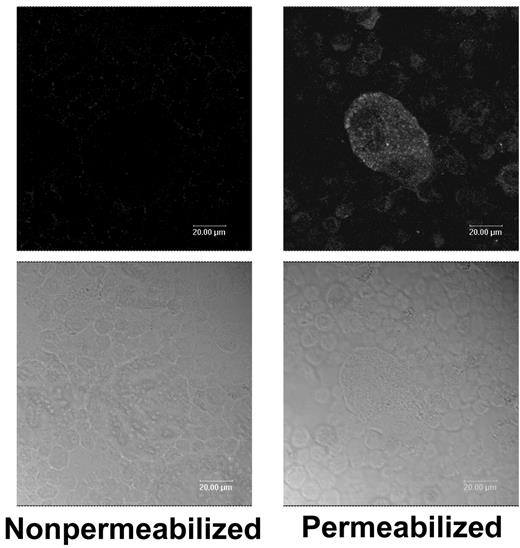
The presence of full-length TFPI in platelets, but not the C-terminally truncated forms of TFPI found in plasma, suggests that TFPI is either produced by megakaryocytes or that only the full-length form of TFPI is efficiently adsorbed from plasma by the platelet. Mouse TFPI was detected by immunofluoresence within permeablized, cultured mouse megakaryocytes grown in fetal bovine serum, demonstrating that mouse TFPI is synthesized by the megakaryocyte (Figure 2). Nonpermeablized megakaryocytes do not stain for TFPI, indicating that it is not expressed on the cell surface (Figure 2). TFPI production by human megakaryocytes was examined through isolation of mRNA from highly purified human platelets followed by real-time PCR analysis to quantify TFPI and TFPIβ expression. Signal for TFPI message was readily detected and present at approximately 46% the level of housekeeper gene, RPL-19. In contrast, message for TFPIβ was present at 0.082% that of RPL-19, essentially the same as the no-template negative control. This is quite different than expression in cultured endothelial cell lines in which TFPIβ mRNA is present at levels 5- to 10-fold lower than TFPI mRNA.17,21 Thus, it appears that human, as well as mouse, megakaryocytes produce TFPI, whereas TFPIβ mRNA is not produced, consistent with our inability to detect TFPIβ protein within platelets by Western blot analysis (Figure 1).
Fluorescence microscopy demonstrates TFPI within mouse megakaryocytes but not on their surface. Nonpermeablized and Triton–X-100–permeablized mouse megakaryocytes were immunostained for TFPI. Top panels are stained cells; bottom panels are bright field images of the cells.
Fluorescence microscopy demonstrates TFPI within mouse megakaryocytes but not on their surface. Nonpermeablized and Triton–X-100–permeablized mouse megakaryocytes were immunostained for TFPI. Top panels are stained cells; bottom panels are bright field images of the cells.
TFPI is not present on the surface of quiescent platelets and is not expressed on the platelet surface or secreted following TRAP stimulation
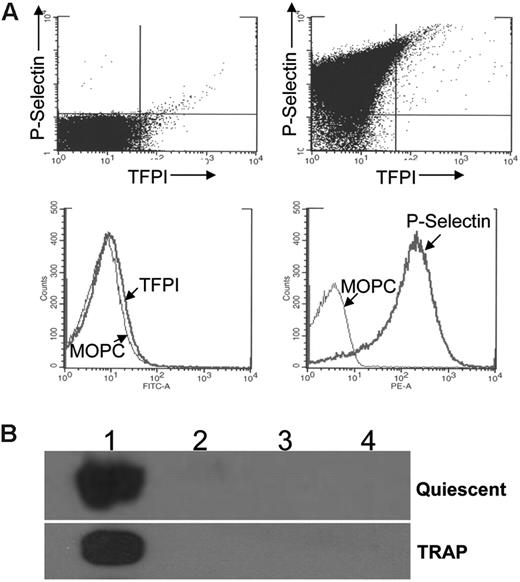
Similar to mouse megakaryocytes (Figure 2), TFPI is not expressed on the surface of quiescent human platelets when analyzed using flow cytometry (Figure 3A). When platelets are activated with TRAP to induce release of α granule proteins, P-selectin, a membrane-associated α granule protein is consistently present on the surface of the vast majority of platelets. In contrast, less than 1% of the activated platelets express surface TFPI (Figure 3A). Western blot analysis of TRAP-stimulated platelets subjected to differential centrifugation demonstrates that this agonist does not induce release of TFPI (Figure 3B).
Stimulation of platelets with TRAP does not cause surface expression or secretion of TFPI. (A) Flow cytometry analysis of platelets stained for TFPI and P-selectin. Quiescent platelets are in the upper left panel. Platelets stimulated with 8 μM TRAP are in the other 3 panels. Dot blot analysis of double-stained cells following activation indicates that 89% of the platelets have surface P-selectin, whereas less than 1% has surface TFPI. (B) Western blot analysis of quiescent and TRAP (8 μM) activated platelets subjected to differential centrifugation. Lane 1, 1500g pellet; lane 2, 10 000g pellet; lane 3, 200 000g pellet; lane 4, 200 000g supernatant.
Stimulation of platelets with TRAP does not cause surface expression or secretion of TFPI. (A) Flow cytometry analysis of platelets stained for TFPI and P-selectin. Quiescent platelets are in the upper left panel. Platelets stimulated with 8 μM TRAP are in the other 3 panels. Dot blot analysis of double-stained cells following activation indicates that 89% of the platelets have surface P-selectin, whereas less than 1% has surface TFPI. (B) Western blot analysis of quiescent and TRAP (8 μM) activated platelets subjected to differential centrifugation. Lane 1, 1500g pellet; lane 2, 10 000g pellet; lane 3, 200 000g pellet; lane 4, 200 000g supernatant.
TFPI is not localized within α granules or lysosomes
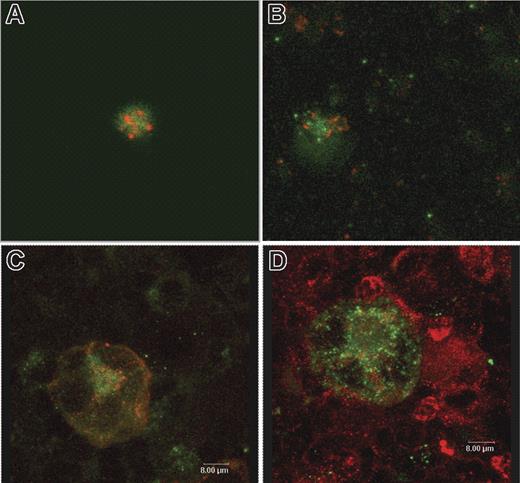
The lack of surface expression or secretion of TFPI from TRAP-activated platelets strongly suggests that platelet TFPI is not localized within the α granules. Confocal microscopy of human and mouse platelets and mouse megakaryocytes was performed to identify the intracellular location of TFPI. TFPI was present in all platelets inspected. In human platelets stained for TFPI and fibrinogen, used as a α granule marker, the 2 proteins do not colocalize, demonstrating that TFPI is not localized within platelet α granules (Figure 4A). Similar data were obtained, demonstrating that TFPI does not colocalize with VWF in α granules within mouse platelets (Figure 4B) or megakaryocytes (Figure 4C). TFPI also does not colocalize with LAMP-1 within lysosomes of mouse megakaryocytes (Figure 4D).
Confocal microscopy of mouse and human platelets and mouse megakaryocytes demonstrate that TFPI does not colocalize with either α granule or lysosomal protein. (A) Human platelets stained for TFPI (green) and fibrinogen as an α granule marker (red). (B) Mouse platelets stained for TFPI (green) and VWF as an α granule marker (red). (C) Mouse megakaryocytes stained for TFPI (green) and VWF as a α granule marker (red). (D) Mouse megakaryocytes stained for TFPI (green) and LAMP1 as a lysosome marker.
Confocal microscopy of mouse and human platelets and mouse megakaryocytes demonstrate that TFPI does not colocalize with either α granule or lysosomal protein. (A) Human platelets stained for TFPI (green) and fibrinogen as an α granule marker (red). (B) Mouse platelets stained for TFPI (green) and VWF as an α granule marker (red). (C) Mouse megakaryocytes stained for TFPI (green) and VWF as a α granule marker (red). (D) Mouse megakaryocytes stained for TFPI (green) and LAMP1 as a lysosome marker.
TFPI is expressed on the surface of coated platelets
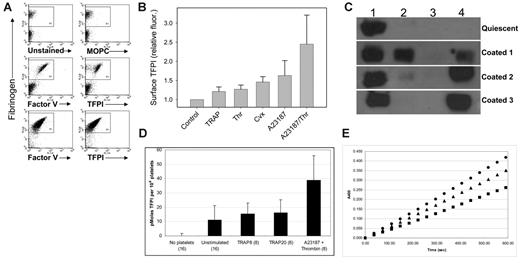
Dual-agonist stimulation of platelets with thrombin and convulxin, an activator of the platelet collagen receptor GPVI, produces a highly activated population of platelets known as coated platelets, in reference to the high levels of procoagulant proteins that coat the surface of these platelets.22 Coated platelets were produced as described in “Materials and methods” and analyzed for surface TFPI expression using flow cytometry. In contrast to TRAP-stimulated platelets, coated platelets consistently express TFPI on their surface (Figure 5A). Following activation with thrombin and convulxin only 35% to 50% of the platelets become coated.1 Surface expression of TFPI occurs exclusively on the coated-platelet population identified by the presence of fibrinogen on their surface (Figure 5A). When platelets are stimulated with thrombin and calcium ionophore greater than 95% of the platelets become coated1 and express surface TFPI (Figure 5A). Stimulation of platelets with a variety of other single agonists, including thrombin, calcium ionophore, and convulxin, did not produce significantly elevated cell-surface expression of TFPI (Figure 5B).
Active TFPI is expressed on the surface of coated platelets and secreted. (A) Platelets were simultaneously activated with convulxin plus thrombin (top 4 panels) or calcium ionophore and thrombin (bottom 2 panels) to make coated platelets and analyzed for surface TFPI expression using flow cytometry. Fibrinogen staining (y-axis) identifies the coated platelets. Staining on the x-axis is unstained cells (top left panel), MOPC (top right panel; negative control), factor V (middle and lower left panels; positive control), and TFPI (middle and lower right panels). (B) The relative fluorescence intensity of platelets determined by flow cytometry of platelets stained for TFPI following stimulation with different single agonists as indicated. Error bars represent the standard deviation of 3 or more experiments. (C) Western blot analysis of TFPI in quiescent and coated platelets produced by stimulation of platelets with thrombin and calcium ionophore following differential centrifugation. Lane 1, 1500g pellet; lane 2, 10 000g pellet; lane 3, 200 000g pellet; lane 4, 200 000g supernatant. Western blots from 3 coated-platelet preparations are shown to indicate the variable amounts of TFPI found in the 10 000g pellet and 200 000g supernatant. (D) Comparison of TFPI activity (surface and secreted) in quiescent platelets, platelets stimulated with 8 μM TRAP, 20 μM TRAP or coated platelets produced by stimulation with thrombin and calcium ionophore. TFPI activity was measured by inhibition of factor Xa generation by factor VIIa/TF. The number of persons tested in each category is indicated. Error bars represent the standard deviation of 3 or more experiments. (E) TFPI activity is reversed by a monoclonal antibody directed against the first Kunitz domain of TFPI. (•) No platelets; (▪) calcium ionophore plus thrombin activated platelets; (▴) calcium ionophore plus thrombin activated platelets with 100 μM anti-TFPI antibody.
Active TFPI is expressed on the surface of coated platelets and secreted. (A) Platelets were simultaneously activated with convulxin plus thrombin (top 4 panels) or calcium ionophore and thrombin (bottom 2 panels) to make coated platelets and analyzed for surface TFPI expression using flow cytometry. Fibrinogen staining (y-axis) identifies the coated platelets. Staining on the x-axis is unstained cells (top left panel), MOPC (top right panel; negative control), factor V (middle and lower left panels; positive control), and TFPI (middle and lower right panels). (B) The relative fluorescence intensity of platelets determined by flow cytometry of platelets stained for TFPI following stimulation with different single agonists as indicated. Error bars represent the standard deviation of 3 or more experiments. (C) Western blot analysis of TFPI in quiescent and coated platelets produced by stimulation of platelets with thrombin and calcium ionophore following differential centrifugation. Lane 1, 1500g pellet; lane 2, 10 000g pellet; lane 3, 200 000g pellet; lane 4, 200 000g supernatant. Western blots from 3 coated-platelet preparations are shown to indicate the variable amounts of TFPI found in the 10 000g pellet and 200 000g supernatant. (D) Comparison of TFPI activity (surface and secreted) in quiescent platelets, platelets stimulated with 8 μM TRAP, 20 μM TRAP or coated platelets produced by stimulation with thrombin and calcium ionophore. TFPI activity was measured by inhibition of factor Xa generation by factor VIIa/TF. The number of persons tested in each category is indicated. Error bars represent the standard deviation of 3 or more experiments. (E) TFPI activity is reversed by a monoclonal antibody directed against the first Kunitz domain of TFPI. (•) No platelets; (▪) calcium ionophore plus thrombin activated platelets; (▴) calcium ionophore plus thrombin activated platelets with 100 μM anti-TFPI antibody.
TFPI is released from coated platelets on microvesicles and as a soluble protein
Quiescent and dual-agonist–activated platelets were compared via differential centrifugation at 1500g, 10 000g, and 200 000g23 (Figure 5C). In quiescent platelets essentially all of the TFPI is found in the 1500g pellet, consistent with its presence within intact platelets. Experiments with dual-agonist–activated platelets produced results that varied depending on the persons donating the platelets and the day of the experiment. TFPI is always present within the 1500g pellet, consistent with its presence on the surface of coated platelets. In some experiments TFPI was found in the 10 000g pellet, whereas in others most of the TFPI was within the 200 000g supernatant. TFPI in the 10 000g pellet represents that being released in microvesicles, whereas TFPI in the 200 000g supernatant represents that released either in exosomes or as a soluble protein.23
TFPI expressed by dual-agonist–activated platelets inhibits tissue factor
Fresh platelets were stimulated with thrombin and calcium ionophore to produce coated platelets and were compared using TFPI activity assays to either quiescent platelets or platelets stimulated with 8 μM or 20 μM TRAP. Total platelet TFPI activity (surface and secreted) was measured in platelets from multiple persons in assays measuring the rate of factor X activation by factor VIIa/tissue factor. Comparison of quiescent and dual-agonist–activated platelets demonstrates a significant increase in the amount of TFPI activity in the dual-agonist–activated platelets (1.2 ± 0.7 versus 3.9 ± 1.3 pmole/10 million platelets; P = .006), but no significant increase in platelets activated with 8 or 20 μM TRAP (Figure 5D). In addition, stimulation of platelets with 2 μM A23187 did not produce significantly increased TFPI activity when compared with quiescent platelets (data not shown). The factor VIIa/tissue factor inhibitory activity is reversed by addition of an anti-TFPI monoclonal antibody, demonstrating that TFPI is responsible for the inhibitory activity observed (Figure 5E).17,24
TFPI on the surface of coated platelets is relatively protected from removal by PIPLC
TFPI on the surface of endothelial cells21,25,26 or fresh placenta24 is readily removed (80%-95%) by treatment with PIPLC, indicating that TFPI associates with the endothelial surface through a GPI-anchor. In contrast, analysis of coated platelets by flow cytometry before and after treatment with PIPLC revealed that only 22% of the surface TFPI is removed by PIPLC under conditions that remove 56% of the CD59 from the platelet surface (data not shown). Thus, compared with TFPI on the endothelial cell surface, platelet TFPI is relatively protected from removal by PIPLC. Previously the impact of transglutaminase inhibitors on coated-platelet formation was documented.2 For this study, we observed that 2 mg/mL acetyl-casein2 totally inhibited TFPI retention on the surface of coated platelets (data not shown), suggesting that the transglutaminase activity required for coated-platelet formation is also essential for TFPI expression. Whether this indicates direct derivatization of TFPI or derivatization of a cofactor required for TFPI retention is unclear at this time. However, this observation supports the finding that PIPLC was ineffective in removing TFPI from coated platelets because a PI-linkage would not be expected to be influenced by acetyl-casein.
Discussion
The platelet surface may be the optimal location for TFPI-mediated inhibition of the procoagulant activity of blood-borne forms of TF, thereby maintaining the appropriate procoagulant and anticoagulant balance that allows for adequate hemostasis without development of occlusive thrombosis. Novotny et al11 first demonstrated the presence of TFPI in platelets. Those investigators reported that platelets release soluble TFPI following stimulation with either thrombin or calcium ionophore but did not identify TFPI on the platelet surface. Since this initial description of platelet TFPI there have been many advances in our understanding of platelets and the biology of TFPI. These include the identification of protease-activated receptors on platelets and the production of artificial ligands for these receptors, such as TRAP, that allow platelet activation without active proteolysis by thrombin27,28 ; the description of a distinct population of highly procoagulant coated platelets that is produced following dual activation of platelets with collagen and thrombin22 ; the finding that TFPI is a cell-surface–associated protein bound indirectly to the endothelial surface through tight association with a GPI-anchored coreceptor25,26 ; and the identification of TFPIβ, an alternatively spliced, truncated form of TFPI that is directly GPI-anchored.20,21
Full-length TFPI is present within platelets, whereas the truncated forms of TFPI present in plasma are not. Thus, it appears that either platelet TFPI is produced by megakaryocytes or full-length TFPI is selectively adsorbed from plasma. TFPIβ protein is not detectable within platelets. Real-time PCR performed using mRNA from highly purified platelets readily detected full-length TFPI mRNA, but not TFPIβ mRNA, indicating that full-length TFPI is produced by megakaryocytes and is the predominant form in platelets. The presence of TFPI in megakaryocytes was confirmed by immunofluoresence microscopy. Platelet TFPI is not expressed on the surface of megakaryocytes or quiescent platelets.
Stimulation of platelets with thrombin, TRAP, convulxin, or calcium ionophore does not cause expression of TFPI on the platelet surface. This finding is consistent with those of Steiner et al29 who also did not observe TFPI on the surface of platelets in whole blood stimulated with TRAP. Although platelets have previously been reported to secrete soluble TFPI following activation with either thrombin or calcium ionophore,11 we did not observe secretion of TFPI following TRAP stimulation. The absence of TFPI surface expression or release following stimulation of platelets with TRAP strongly suggests that TFPI is not stored within platelet α granules. This was confirmed by confocal microscopy studies demonstrating that TFPI does not colocalize with the α granule proteins VWF or fibrinogen. Thus, the intracellular localization of TFPI remains unclear. The lack of colocalization of TFPI with LAMP-1 suggests that TFPI is not routed to the platelet lysosome. However, lysosomes in platelets are heterogenous in nature and may contain the membrane proteins LAMP-1, LAMP-2, or LAMP-3, as well as acid hydrolases and cathepsins.30 TFPI could potentially be localized to a lysosome that does not contain the membrane protein LAMP-1 or in a distinct, uncharacterized secretory vesicle. The studies presented here also do not exclude the possibility that TFPI is localized in multivesicular bodies that may be the precursor to α granules and dense granules.31 Activated platelets have also been shown to release exosomes, and it is possible that TFPI could be localized in an exosome.23
Platelets activated simultaneously with collagen and thrombin and examined by flow cytometry reveal 2 distinct populations of cells; one population expresses high levels of several procoagulant proteins on the cell surface, including factor V, fibrinogen, fibronectin, and VWF, whereas the other does not.1,3 Those cells retaining high levels of procoagulant proteins and phosphatidylserine are referred to as coated platelets because they are “coated” with these procoagulant proteins. Formation of coated platelets also is associated with secretion of both dense and α granules, transglutaminase activation, and glycoprotein IIb/IIIa activation.22 In contrast to stimulation of platelets with single agonists, platelets stimulated with thrombin plus convulxin or thrombin plus calcium ionophore to produce coated platelets consistently expressed TFPI on their surface. In platelets activated with thrombin plus convulxin, in which 35% to 50% of the platelets become coated, surface expression of TFPI occurred exclusively on the population of coated platelets (Figure 5A). Once on the platelet surface, TFPI is relatively resistant to removal by PIPLC, with only about 20% removed following 1 hour of incubation with the phospholipase. This is a somewhat unexpected result because TFPI expressed on the surface of both cultured endothelial cells and fresh placenta is highly susceptible to PIPLC with 80% to 95% removed during a 1-hour incubation.24–26 This relative protection of platelet TFPI from PIPLC cleavage suggests either that it is not GPI-anchored, as the inhibition of surface expression by acetyl-casein suggests, or that it is GPI-anchored but in a different lipid microenvironment than endothelial TFPI. It is also possible that there are structural differences between the GPI-anchor of platelet and endothelial TFPI. For example, in some proteins the inositol ring of the GPI-anchor is modified by palmitoylation or acylation, rendering it resistant to PIPLC.32,33
Platelet TFPI is transferred to the platelet surface and released following dual-agonist activation with thrombin plus collagen where it effectively inhibits tissue factor activity in factor Xa generation assays. This finding is consistent with that of other investigators who have observed TFPI activity in platelets that is reversed by anti-TFPI antibodies.34,35 Because TFPI is released from dual-agonist–activated platelets and dual-agonist–activated platelets release high amounts of microvesicles,36 differential centrifugation experiments23 were performed to characterize TFPI released from dual-agonist–activated platelets. These experiments produced variable results that were dependent on the platelet donor and on the day the experiment was performed. However, they suggest that TFPI is released in microvesicles and exosomes or as a soluble protein. The expression of TFPI on the surface of dual-agonist–activated platelets suggests that platelet TFPI may have a physiologic role in the inhibition of tissue factor activity at the edges of a vascular lesion where platelets are exposed to both collagen and thrombin. Further, in vivo experimentation will be necessary to confirm this hypothesis and to explore the physiologic roles for platelet TFPI in inhibition of the circulating forms of tissue factor that incorporate into a growing thrombus.
Platelet TFPI is not located within α granules, and the mechanism for its transfer to the platelet surface on activation is distinctly different from that of platelet α granule proteins such as P-selectin. Identification of the biochemical properties involved in its transfer from an intracellular location to the platelet surface may represent a new mechanism for how proteins can be expressed on the platelet surface. It has been demonstrated that simultaneous activation of platelets with thrombin plus collagen produces a sustained increase in platelet calcium influx in subpopulations of platelets that also have exposed surface phosphatidylserine,37 suggesting that the expression of TFPI on the platelet surface may result from changes in platelet membranes and/or cytoskeleton that occur only following sustained elevation of intracellular calcium that result from activation of both collagen and thrombin-signaling pathways.
Authorship
Contribution: S.A.M. designed, performed, supervised, and interpreted the real-time PCR, Western blot, confocal microscopy, and flow cytometry studies and wrote the paper; S.L.H. performed the confocal microscopy studies; P.F. performed the confocal microscopy and flow cytometry studies; M.L.C. performed the flow cytometry and Western blot studies; J.P.F. performed the deglycosylation and Western blot studies; G.L.D. designed the experiment, interpreted the results, and performed the flow cytometry experiments; and A.E.M. supervised the overall project, designed the experiment, interpreted the results, performed the TFPI activity assays, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan E. Mast, Blood Research Institute, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: alan.mast@bcw.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (Bethesda, MD) (grant HL68835, A.E.M.) and the American Heart Association Scientist Development Grant (0435466N, S.L.H.). A.E.M. is an Established Investigator of the American Heart Association.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal