Abstract
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous entity, with patients exhibiting a wide range of outcomes. The addition of rituximab to CHOP chemotherapy (R-CHOP)has led to a marked improvement in survival and has called into question the significance of previously recognized prognostic markers. Since randomized controlled trials of R-CHOP in DLBCL have included select subgroups of patients, the utility of the International Prognostic Index (IPI) has not been reassessed. We performed a retrospective analysis of patients with DLBCL treated with R-CHOP in the province of British Columbia to assess the value of the IPI in the era of immunochemotherapy. The IPI remains predictive, but it identifies only 2 risk groups. Redistribution of the IPI factors into a revised IPI (R-IPI) provides a more clinically useful prediction of outcome. The R-IPI identifies 3 distinct prognostic groups with a very good (4-year progression-free survival [PFS] 94%, overall survival [OS] 94%), good (4-year PFS 80%, OS 79%), and poor (4-year PFS 53%, OS 55%) outcome, respectively (P < .001). The IPI (or R-IPI) no longer identifies a risk group with less than a 50% chance of survival. In the era of R-CHOP treatment, the R-IPI is a clinically useful prognostic index that may help guide treatment planning and interpretation of clinical trials.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of all newly diagnosed cases and more than 80% of aggressive lymphomas.1 Recent insights into the pathogenesis of DLBCL suggest that it is a heterogeneous group of B-cell lymphomas rather than a single clinicopathologic entity.2 Multiple histologic subtypes and morphologic variants are recognized, a variety of molecular and genetic abnormalities are variably present, and patients exhibit a wide range of clinical presentations and outcomes. Gene-expression profiling studies have identified at least 3 distinct molecular subtypes of DLBCL, one with an expression profile similar to normal germinal center B cells (GCB subtype), one mimicking activated peripheral-blood B cells (ABC subtype), and a third, primary mediastinal large B-cell lymphoma (PMBCL), typically presenting with mediastinal lymphadenopathy and displaying some molecular genetic similarities to Hodgkin lymphoma.3–6 A small number of cases do not fit into any of these categories and have been designated as “unclassifiable.”7
The CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy regimen has been the mainstay of therapy for several decades. Attempts to improve outcomes with more intensive chemotherapy failed to show additional benefit.8 Recently, the development of monoclonal antibodies has transformed treatment practices for aggressive lymphoma and led to a marked improvement in outcome. The Groupe d'Etude de Lymphome d'Adultes (GELA) reported the first randomized controlled trial demonstrating the benefit of adding rituximab, a chimeric IgG1 monoclonal antibody targeting CD20, to CHOP chemotherapy (R-CHOP) for the treatment of elderly patients (age ≥ 60 years) with newly diagnosed DLBCL.9 A 5-year update of this trial demonstrates that the benefit seen with the addition of rituximab has been maintained over time, indicating an improvement in the cure rate for this patient population (5-year overall survival [OS] 58% vs 45%, P = .007).10
Three additional randomized controlled trials have confirmed this benefit in select groups of patients with DLBCL. The US Intergroup trial11 and the RICOVER-60 trial12 evaluated the use of rituximab and chemotherapy in elderly patients with DLBCL, while the MINT trial13 investigated its use in young patients (age ≤ 60 years) with a favorable prognostic profile. Results of a population-based study further demonstrated the value of the addition of rituximab to chemotherapy in an unselected population of patients with DLBCL in the province of British Columbia (BC).14 Although the adoption of R-CHOP as the new standard of care has led to improved outcomes for this curable lymphoma, patients whose lymphoma is not cured by first-line therapy continue to pose a difficult challenge. Early identification of poor-risk patients may allow for alternate treatment strategies to be considered.
The International Prognostic Index (IPI) has been the primary clinical tool used to predict outcome for patients with aggressive NHL.15 Based on the number of negative prognostic factors present at the time of diagnosis (age > 60 years, stage III/IV disease, elevated lactate dehydrogenase [LDH] level, Eastern Cooperative Oncology Group [ECOG] performance status ≥ 2, more than one extranodal site of disease), 4 discrete outcome groups were identified with a 5-year overall survival ranging from 26% to 73%. The introduction of a new therapy with superior effectiveness can alter the significance of previously recognized prognostic markers by virtue of its mechanism of action. Since the randomized controlled trials listed above were confined to select patient populations (either elderly patients or young patients with a good prognosis), the utility of the IPI in the era of immunochemotherapy could not be determined from the available randomized trials. We performed a retrospective analysis of patients with DLBCL treated with R-CHOP in the province of British Columbia to assess the current value of the IPI and to determine if a different grouping of the prognostic factor scores would permit more clinically relevant assignment of patients to prognostic groups.
Patients, materials, and methods
Study design
This study is a retrospective analysis of an unselected population of patients with DLBCL treated in the province of British Columbia. Cases were identified by searching the Lymphoid Cancer Database of the Center for Lymphoid Cancer of the BC Cancer Agency. This computerized research database contains clinical and pathologic information on more than 10 000 patients with lymphoma treated in BC since 1981. Patients were included for analysis in this study if they were at least 16 years of age with a biopsy-proven, newly diagnosed, CD20+ DLBCL prior to January 15, 2005, and were treated with R-CHOP with curative intent. Patients were excluded if they were HIV positive, had evidence of a secondary malignancy or an underlying indolent lymphoproliferative disorder, or if the presence of major coincident illness precluded an attempt to cure the lymphoma.
The BC Cancer Agency protocol for R-CHOP uses standard doses of chemotherapy and rituximab administered at a 21-day interval.16 Mid-cycle treatment with filgrastim is permitted if the neutrophil count on the day of treatment is less than 0.8 × 109/L, but is not otherwise required. Patients with advanced-stage disease defined as Ann Arbor stages III or IV, or stages I and II with B symptoms, bulky disease (≥ 10 cm), or disease that could not be encompassed within a single involved field radiation port were intended to receive 6 to 8 cycles of treatment (2 cycles beyond maximum response). All other patients were eligible to receive 3 cycles of R-CHOP and involved field radiation therapy unless they had a contraindication to radiation. All clinical and follow-up information was obtained from the Lymphoid Cancer Database, Cancer Agency clinical records, hospital records, or individual physicians' records. This study was approved by the University of British Columbia, BC Cancer Agency Research Ethics Board. Informed consent was not obtained because this was not a clinical trial but a retrospective review of standard care.
Statistics
This analysis is based on follow-up through June 15, 2006. This was an intention-to-treat analysis including all patients treated with curative intent who received at least one cycle of R-CHOP chemotherapy. Progression-free survival (PFS) was calculated from the date of diagnosis to documented disease progression; observations were censored on the date the patient was last known to be alive or, for patients dying as a result of causes unrelated to lymphoma or treatment, the date of death. Overall survival (OS) was calculated from the date of diagnosis until death as a result of any cause or date last known alive. PFS and OS were assessed using the Kaplan-Meier method and compared between risk groups using the log rank test.17,18 Data were analyzed using the Statistical Software Package for the Social Sciences (SPSS version 11.0 for Windows; SPSS, Chicago, IL).
Results
Patients
A total of 365 patients were identified. Central pathology review was performed by M.C., B.B., and R.D.G. on 95% of cases. Clinical characteristics at diagnosis, including the distribution of the individual IPI factors, are listed in Table 1 The median age at diagnosis was 61 years (range, 16-90 years).
Patient characteristics
| Characteristic . | Value . |
|---|---|
| Number | 365 |
| Median age, y (range) | 61 (16-90) |
| Male sex, % | 61 |
| IPI factors | |
| Age greater than 60 y, % | 51 |
| PS greater than 2, % | 41 |
| Elevated LDH, % | 55 |
| More than 1 extranodal site, % | 34 |
| Stage III/IV, % | 59 |
| Pathology, no. of patients | |
| DLBCL | 324 |
| PMBCL | 36 |
| IBL | 2 |
| IVDL | 2 |
| TCRBCL | 1 |
| Characteristic . | Value . |
|---|---|
| Number | 365 |
| Median age, y (range) | 61 (16-90) |
| Male sex, % | 61 |
| IPI factors | |
| Age greater than 60 y, % | 51 |
| PS greater than 2, % | 41 |
| Elevated LDH, % | 55 |
| More than 1 extranodal site, % | 34 |
| Stage III/IV, % | 59 |
| Pathology, no. of patients | |
| DLBCL | 324 |
| PMBCL | 36 |
| IBL | 2 |
| IVDL | 2 |
| TCRBCL | 1 |
PS indicates ECOG performance status; LDH, lactate dehydrogenase; PMBCL, primary mediastinal B-cell lymphoma; IBL, immunoblastic lymphoma; IVDL, intravascular diffuse large B-cell lymphoma; TCRBCL, T-cell/histiocyte-rich large B-cell lymphoma.
Treatment
Most patients (92%) were to receive 6 to 8 cycles of R-CHOP and 8% were to receive combined modality therapy with 3 cycles of R-CHOP followed by involved field radiation therapy for limited-stage disease. Of the patients receiving extended-course R-CHOP, 21% also received radiation therapy with their primary treatment, usually to sites of residual masses at the end of the chemotherapy. Patients whose lymphoma recurred despite first-line therapy were treated with a variety of secondary regimens at the discretion of the treating physician. Patients who were 65 years of age or younger with chemotherapy-responsive disease were eligible to undergo high-dose chemotherapy and stem-cell transplantation. Sixteen patients underwent stem-cell transplantation for relapsed disease.
Outcome according to standard IPI
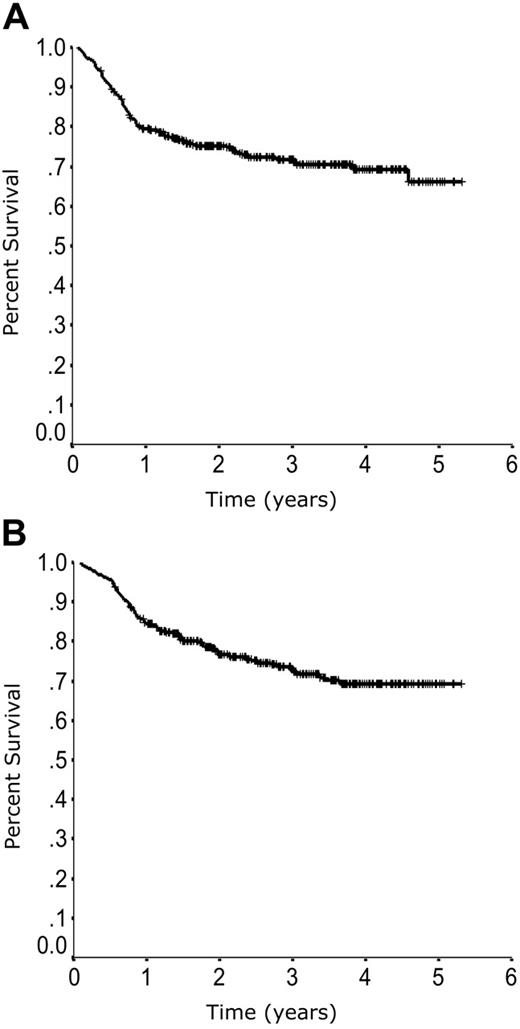
The medium follow-up time for living patients is 33 months (range, 7 to 64 months). The PFS and OS curves demonstrate the excellent outcome seen in patients treated with R-CHOP (Figure 1). The similarity between PFS and OS notably highlights the modest impact of secondary therapy in the general population of patients with DLBCL whose lymphoma is not cured with front-line therapy.
Overall outcome. Progression-free survival (A) and overall survival (B) in 365 patients with DLBCL treated with R-CHOP in British Columbia.
Overall outcome. Progression-free survival (A) and overall survival (B) in 365 patients with DLBCL treated with R-CHOP in British Columbia.
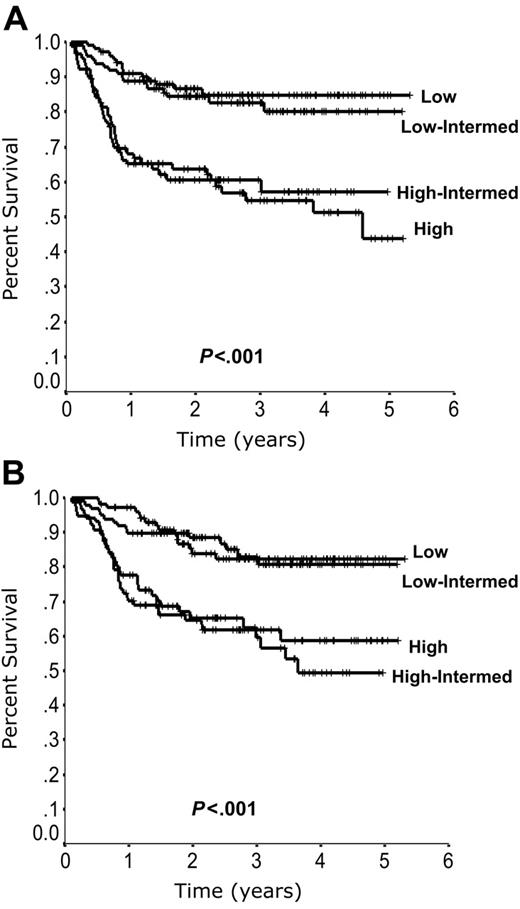
Outcome according to the standard IPI is listed in Table 2. Although the IPI remains predictive in patients treated with R-CHOP, it no longer distinguishes 4 risk groups. Instead, the 2 low-risk and 2 high-risk groups exhibit closely overlapping curves (Figure 2).
Outcome according to International Prognostic Index (IPI) factors in 365 patients treated with R-CHOP in British Columbia
| Risk group . | No. of IPI factors . | % Patients . | 4-year PFS, % . | 4-year OS, % . |
|---|---|---|---|---|
| Standard IPI | ||||
| Low | 0, 1 | 28 | 85 | 82 |
| Low-intermediate | 2 | 27 | 80 | 81 |
| High-intermediate | 3 | 21 | 57 | 49 |
| High | 4, 5 | 24 | 51 | 59 |
| Revised IPI | ||||
| Very good | 0 | 10 | 94 | 94 |
| Good | 1, 2 | 45 | 80 | 79 |
| Poor | 3, 4, 5 | 45 | 53 | 55 |
| Risk group . | No. of IPI factors . | % Patients . | 4-year PFS, % . | 4-year OS, % . |
|---|---|---|---|---|
| Standard IPI | ||||
| Low | 0, 1 | 28 | 85 | 82 |
| Low-intermediate | 2 | 27 | 80 | 81 |
| High-intermediate | 3 | 21 | 57 | 49 |
| High | 4, 5 | 24 | 51 | 59 |
| Revised IPI | ||||
| Very good | 0 | 10 | 94 | 94 |
| Good | 1, 2 | 45 | 80 | 79 |
| Poor | 3, 4, 5 | 45 | 53 | 55 |
Outcome according to the standard International Prognostic Index (IPI). Progression-free survival (A) and overall survival (B) according to the standard IPI.
Outcome according to the standard International Prognostic Index (IPI). Progression-free survival (A) and overall survival (B) according to the standard IPI.
Outcome according to revised IPI
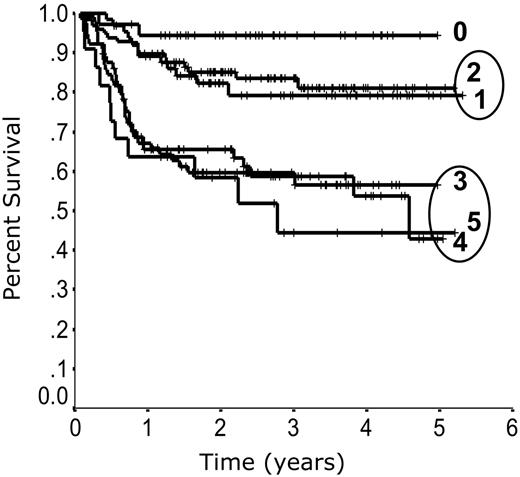
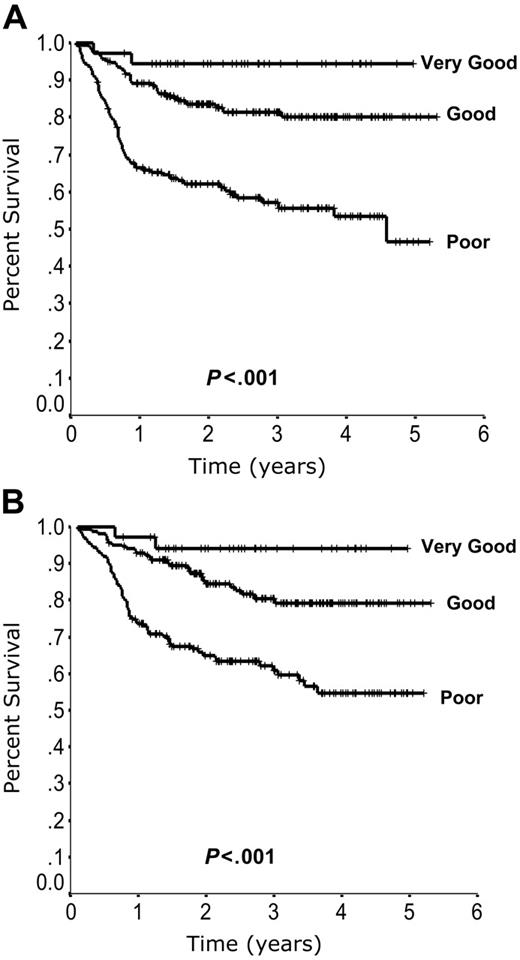
When outcome is plotted according to the number of individual IPI factors present at diagnosis, 3 risk groups emerge. Patients with zero risk factors have the best outcome, patients with 1 or 2 risk factors have a moderate outcome, and patients with 3, 4, or 5 risk factors have the poorest outcome (Figure 3). Redistribution of the IPI factors to account for this difference in outcome allows for a more simplified and accurate prediction model. Outcome according to the revised IPI (R-IPI) is listed in Table 2. The R-IPI distinguishes 3 separate prognostic groups with 4-year PFS ranging from 53% to 94% (P < .001) and 4-year OS ranging from 55% to 94% (P < .001; Figure 4).
Outcome according to the number of International Prognostic Index (IPI) factors. Progression-free survival according to the number of IPI factors present at diagnosis.
Outcome according to the number of International Prognostic Index (IPI) factors. Progression-free survival according to the number of IPI factors present at diagnosis.
Outcome according to the revised International Prognostic Index (R-IPI). Progression-free survival (A) and overall survival (B) according to the R-IPI.
Outcome according to the revised International Prognostic Index (R-IPI). Progression-free survival (A) and overall survival (B) according to the R-IPI.
Discussion
Given the marked heterogeneity of DLBCL, a reliable prediction tool is vital for optimizing patient care. An accurate estimate of survival serves to facilitate doctor-patient discussions and foster a realistic expectation of outcome, may help to guide the choice of initial treatment, and allows for appropriate stratification and interpretation of clinical trials. The IPI has been the primary prognostic model used in the management of patients with DLBCL since its publication in 1993. It has gained universal acceptance since it relies on information that is readily accessible and its predictive capacity has been validated in multiple studies. However, its value in the era of immunochemotherapy has not been re-examined.
It is important to recognize that risk assessment is a moving target. The introduction of a more effective new therapy can alter the significance of previously recognized prognostic factors. Depending on its mechanism of action, the benefit of a new drug may not be equally translated across all patient subgroups. The addition of rituximab to CHOP chemotherapy has resulted in a marked improvement in outcome for patients with DLBCL and has altered what was previously understood regarding risk assessment.
This study explores the utility of the IPI in an unselected population of patients treated with R-CHOP in the province of British Columbia. Although the IPI remains predictive it distinguishes only 2 risk groups, rather than the 4 groups originally described. Redistribution of the IPI factors into the R-IPI provides a more clinically relevant prediction of outcome. The R-IPI identifies 3 distinct prognostic groups with significantly different outcomes.
Patients with zero risk factors fall into a “very good” prognostic group with more than a 90% chance of long-term progression-free survival. With such an excellent outcome following R-CHOP, focusing on these patients in clinical trials would necessitate very large numbers of patients to demonstrate further therapeutic benefit. Patients with 1 or 2 risk factors fall into a “good” prognostic group with an approximately 80% chance of long-term progression-free survival. Although further progress is warranted in this group, care must be taken not to add excessive toxicity in view of the excellent outcome with R-CHOP. Finally, patients with 3, 4, or 5 risk factors fall into a “poor” risk group with a long-term chance of cure in the range of 50%. These patients should be considered for investigational approaches in the context of clinical trials that are designed to ensure that potentially curative therapy is not compromised.
Neither the IPI nor the R-IPI identifies a risk group with less than a 50% chance of survival. Therefore, other predictors must be elucidated to identify those patients most in need of alternate therapies. A number of molecular prognostic markers have been identified in patients with DLBCL; however, these will require validation in patients treated with R-CHOP.19 Several markers have been re-evaluated and appear to no longer retain significance, including Bcl-2 and Bcl-6.
Bcl-2 overexpression has been reported in approximately 40% to 60% of patients with DLBCL and has been associated with poorer survival when patients are treated with CHOP-type regimens.20,21 In vitro studies have shown that rituximab induces down-regulation of Bcl-2 protein expression and by this mechanism may abrogate some of the resistance to chemotherapy.22 The significance of Bcl-2 overexpression was re-evaluated in patients treated with R-CHOP in the GELA trial.23 In contrast to patients treated with CHOP alone, no correlation between Bcl-2 overexpression and survival was seen in patients treated with R-CHOP, implying that the addition of rituximab had overcome its negative influence. Other investigators have also reported that the addition of rituximab to chemotherapy has eliminated the prognostic significance of Bcl-2 overexpression in DLBCL.24,25
Bcl-6 protein expression, a marker of germinal center derivation, has been shown to predict a favorable outcome in DLBCL.26 A prospective correlative study performed in conjunction with the US Intergroup Trial examined the predictive value of Bcl-6 protein expression in patients treated with R-CHOP.27 In patients treated with CHOP alone, outcomes were superior for Bcl-6–positive patients relative to Bcl-6–negative patients; however, outcomes for patients treated with R-CHOP were not influenced by Bcl-6 status, implying that Bcl-6 protein expression is no longer a useful prognostic marker in DLBCL.
Currently, there are no molecular markers that have been revalidated and shown to remain prognostic in patients treated with R-CHOP. The predictive value of gene-expression profiling has not yet been adequately explored in this setting. Early restaging positron emission tomography (PET) scanning has shown promise as a prognostic tool but requires further investigation.28,29 Therefore, clinical models such as the IPI or R-IPI remain the only reliable tools for predicting outcome for patients with DLBCL.
The intent of this study was not to create a new prognostic model or identify new prognostic markers, but to assess the utility of a previously validated and widely used model in the setting of current treatment practices. Although this is not a randomized clinical trial, the patient cohort is representative of the general population of patients with DLBCL and was treated uniformly with a standard R-CHOP protocol. This should allow the results to be generalized to routine patient care. However, these findings should be validated prospectively in an independent population of patients. It is imperative that future studies in DLBCL incorporate correlative studies of clinical and biologic markers, which will allow the continuous reassessment of outcome predictors in the context of medical progress.
Authorship
Contribution: L.H.S. designed research, performed research, collected data, analyzed data, and wrote the manuscript; B.B. collected data; M.C., C.F., K.G., T.S., J.S., and R.D.G. collected data and reviewed the manuscript; P.H., R.K., and K.J.S. collected data; and J.M.C. designed research, collected data, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurie H. Sehn, BC Cancer Agency, Vancouver Clinic, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; e-mail: lsehn@bccancer.bc.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section 1734.
Acknowledgments
The Lymphoid Cancer Database is supported by the contributions of The Mary Toye Fund and The Turner Family Fund.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal