Abstract
Neurofibromatosis type 1 (NF1) syndrome is caused by germline mutations in the NF1 tumor suppressor, which encodes neurofibromin, a GTPase activating protein for Ras. Children with NF1 are predisposed to juvenile myelomonocytic leukemia (JMML) and lethally irradiated mice given transplants with homozygous Nf1 mutant (Nf1−/−) hematopoietic stem cells develop a fatal myeloproliferative disorder (MPD) that models JMML. We investigated the requirement for signaling through the GM-CSF receptor to initiate and sustain this MPD by generating Nf1 mutant hematopoietic cells lacking the common β chain (Beta c) of the GM-CSF receptor. Mice reconstituted with Nf1−/−, beta c−/− stem cells did not develop evidence of MPD despite the presence of increased number of immature hematopoietic progenitors in the bone marrow. Interestingly, when the Mx1-Cre transgene was used to inactivate a conditional Nf1 mutant allele in hematopoietic cells, concomitant loss of beta c−/−reduced the severity of the MPD, but did not abrogate it. Whereas inhibiting GM-CSF signaling may be of therapeutic benefit in JMML, our data also demonstrate aberrant proliferation of Nf1−/−myeloid progenitors that is independent of signaling through the GM-CSF receptor.
Introduction
Neurofibromatosis type 1 (NF1) is a common familial cancer syndrome that is associated with increased risk of specific neoplasms.1 Children with NF1 are predisposed to juvenile myelomonocytic leukemia (JMML), a myeloproliferative disorder (MPD) characterized by leukocytosis, organomegaly with myeloid infiltration, hypersensitivity of myeloid progenitors to granulocyte-macrophage colony-stimulating factor (GM-CSF), and a poor prognosis.2,3 NF1 encodes neurofibromin, a GTPase-activating protein (GAP) that accelerates the hydrolysis of active GTP-bound Ras to inactive Ras-GDP.4,5 NF1-associated tumors frequently demonstrate somatic loss of the normal NF1 allele, and extensive data support the idea that NF1 functions as a tumor suppressor by negatively regulating Ras signaling.6
Mice with a heterozygous germline Nf1 mutation develop many of the tumors seen in persons with NF1, which show loss of the normal allele.7 Homozygous Nf1 inactivation is lethal at about E137,8 ; however, fetal hematopoietic cells from these embryos are hypersensitive to GM-CSF in vitro and induce a JMML-like MPD when transplanted into irradiated recipient mice.9,10 Recently, a conditional Nf1 mutant allele and the interferon-inducible Mx1-Cre strain were harnessed to induce somatic Nf1 inactivation in hematopoietic cells. Like recipients given transplants with Nf1−/− fetal liver cells, these Mx1-Cre, Nf1flox/flox mice consistently develop MPD.11
The GM-CSF receptor shares a common signaling β subunit (beta c) with the receptors for interleukins 3 and 5.12 Unlike humans, the mouse has 2 homologous β subunits: beta c and βIL-3. The GM-CSF and IL-5α subunits only pair with beta c, whereas IL-3α forms heterodimers with both beta c and βIL-3.13 Bone marrow cells from homozygous beta c mutant (beta c−/−) mice respond to IL-3 but not GM-CSF. These mice maintain normal leukocyte counts.14 We intercrossed beta c and Nf1 mutant mice to determine the effects on progenitor populations and the MPD phenotype. We find that loss of beta c attenuates the severity of MPD in Nf1 mutant mice. However, we also demonstrate that Nf1 inactivation expands immature progenitors even in the absence of beta c.
Materials and methods
Mouse strains
Studies of Nf1 mutant fetal liver cells
The experimental procedures for intercrossing mice, genotyping fetal tissues using the polymerase chain reaction (PCR), and adoptive transfer have been described.9 The primer sequences and PCR conditions are available on request. Briefly, fetal livers from E12.5 Nf1−/−embryos and Nf1−/−, beta c−/−; Nf1+/−, beta c−/−; and Nf1+/+, beta c−/− embryos were pooled and injected into congenic recipients (B6.SJL-PtprcaPep3b/BoyJ) that had been lethally irradiated with 900 rad. At 4 to 8 weeks after transplantation, bone marrow was harvested from primary recipients and intravenously injected into lethally irradiated secondary recipients (B6.SJL-PtprcaPep3b/BoyJ). Mice were housed in the University of Minnesota Research Animal Resources facilities and all procedures were approved by Institutional Animal Care and Use Committee.
Studies in Mx1-Cre, Nf1flox/flox, beta c mutant mice
Mx1-Cre, Nf1flox/flox mice of all 3 beta c genotypes were generated by intercrossing. Pups that received a single intraperitoneal injection of polyinosinic-polycytidylic acid (pI-pC) at 3 to 5 days of age to activate Cre recombinase expression from the Mx1 promoter were genotyped and monitored as described previously.11 Mx1-Cre, Nf1flox/flox mice were maintained in a sterile animal care facility under a protocol that was approved by the University of California San Francisco Committee on Animal Research.
Complete blood counts, flow cytometry, and pathological analysis
Blood counts were measured in Coulter ZBI (Beckman Coulter, Fullerton CA) or Hemavet 850 (CDC Technologies, Oxford, CT) instruments. Bone marrow and blood leukocytes were analyzed by flow cytometry on a FACSCalibur with data analysis via CELLQuest Pro software (BD Biosciences, Franklin Lakes, NJ). Tissue sections from control and mutant mice were stained with hematoxylin and eosin, visualized using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) equipped with either a 4×/0.10 numerical aperture (NA) or a 50×/0.90 NA objective. Images were photographed using a Nikon Coolpix 5000 digital camera.
Colony-forming unit assays
Colony-forming unit granulocyte-erythrocyte-megakaryocyte-macrophage (CFU-GEMM) assays were performed by seeding bone marrow (3 × 104) or spleen cells (1 × 105) in Methocult (M3434; StemCell Technologies, Vancouver, BC, Canada). Colonies were enumerated at day 7. Spleen colony-forming unit (CFU-S) assays were performed by injecting bone marrow cells (6 × 104) or splenocytes (2 × 105) intravenously into C57BL/6 recipients that were irradiated with 750 rad. Spleens were harvested from day 8 and fixed in Tellyesniczky solution (64% ethanol, 5% acetic acid, 2% formaldehyde) to enumerate surface colonies. Splenic CFU-GMs were enumerated from Mx1-Cre, Nf1flox/flox and control mice in M3231 medium containing a saturating concentration of IL-3.
Statistical analysis
All graphical and statistical analyses were performed using StatView (SAS Institute, Cary, NC) software with statistical significance determined by ANOVA with Fisher post-hoc tests.
Results
beta c inactivation suppresses MPD in recipients of Nf1 mutant fetal liver cells
Nf1+/−, beta c−/− compound mutant mice on an inbred C57BL/6 background were mated to generate E12.5 embryos (Figure 1A). Fetal livers were removed and made into single-cell suspensions while a portion of embryonic tissue was used to genotype the Nf1 locus. Nf1−/−, beta c−/− and Nf1+/−, beta c−/−cells were pooled separately and 2 × 106 cells were injected intravenously into lethally irradiated congenic mice. The C57BL6/J recipients were syngenic to the donor mice, but expressed a variant allele of the CD45 cell surface antigen (CD45.1) to distinguish donor-derived from recipient hematopoietic cells by flow cytometry. To increase the number of recipient mice for subsequent analysis, the primary recipients were killed 4 to 8 weeks after adoptive transfer and 5 million bone marrow cells were injected into irradiated congenic CD45.1+ hosts (Figure 1B). These secondary recipients were monitored for at least 13 months or until they developed overt MPD. Flow cytometric analysis of primary and secondary recipients demonstrated high levels of CD45.2 chimerism in recipient bone marrows, thereby confirming that they were derived from the transplanted fetal livers (Figure 2A). None of the recipients repopulated with Nf1−/−, beta c−/− or Nf1+/−, beta c−/− cells showed leukocytosis, splenomegaly, or any other evidence of MPD (Figure 2B). Qualitative measurements of disease burden in mice with MPD correlate with increased numbers of mature myeloid lineage cells that express the surface markers Gr-1 and Mac-1. Animals from both cohorts exhibited 52% to 68% Gr-1/Mac-1 double-positive cells in the bone marrow and 12% to 20% in the peripheral blood. In addition, flow cytometric analysis of splenic cells revealed only 2% to 7% Gr-1/Mac-1 cells (data not shown). By contrast, recipients given transplants with C57BL/6 Nf1−/− fetal liver cells develop leukocytosis and splenomegaly with myeloid infiltration 4 to 6 months after adoptive transfer in primary and secondary recipients.17-19
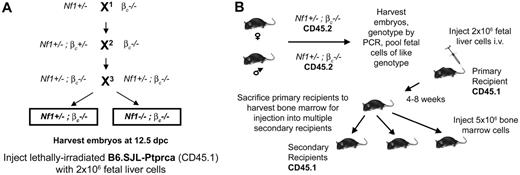
Experimental design of transplants. (A) Three classes of embryos result from the genetic crosses. X1 is an outcross of Nf1+/− -to βc−/− mice. This is to establish Nf1 and βc null alleles within the same animal. X2 is another outcross to βc−/− mice to homozygose the βc allele in Nf1+/−, βc−/− mice. By intercrossing, Nf1+/−, βc−/− mice X3, the 2 classes of embryos are generated. (B) To test the effect loss of βc has on Nf1−/−-induced MPD, Nf1+/−, βc−/− mice are intercrossed and fetal livers from embryos of the correct genotype are harvested. Lethally irradiated recipients are injected with 2 million fetal liver cells and allowed a 4- to 8-week recovery period. These primary recipients are then killed to provide bone marrow for secondary recipient transplantation.
Experimental design of transplants. (A) Three classes of embryos result from the genetic crosses. X1 is an outcross of Nf1+/− -to βc−/− mice. This is to establish Nf1 and βc null alleles within the same animal. X2 is another outcross to βc−/− mice to homozygose the βc allele in Nf1+/−, βc−/− mice. By intercrossing, Nf1+/−, βc−/− mice X3, the 2 classes of embryos are generated. (B) To test the effect loss of βc has on Nf1−/−-induced MPD, Nf1+/−, βc−/− mice are intercrossed and fetal livers from embryos of the correct genotype are harvested. Lethally irradiated recipients are injected with 2 million fetal liver cells and allowed a 4- to 8-week recovery period. These primary recipients are then killed to provide bone marrow for secondary recipient transplantation.
Engraftment but lack of MPD in Nf1−/−, beta c−/− recipients. (A) Bone marrow from 3 representative irradiated mice given transplants with Nf1−/−, beta c−/− (top panel) and Nf1+/−, beta c−/− (bottom panel) cells was mostly comprised of cells expressing donor CD45.2, with only a few cells staining positive for CD45.1. Recipients that do not receive donor cells do not stain for the donor-cell marker (data not shown). (B) Flow cytometric analysis of 2 representative mice. Bone marrow and peripheral blood stained for the cell-surface markers Gr-1 and Mac-1, markers commonly associated with mature granulocytes. Similar staining profiles were seen in all recipients. Ranges of double-positive Gr-1/Mac-1 cells in both cohorts, bone marrow 52% to 68% and peripheral blood 12% to 20%. (C) Peripheral-blood analysis monitored over 46 weeks Nf1+/−, beta c−/−and Nf1−/−, beta c−/− secondary recipients. Animals that display symptoms of MPD would show drastic increases in total WBC counts outside of the range indicated by the bar. Left graph illustrates peripheral-blood analysis monitored over 56 weeks Nf1+/−, beta c−/− and Nf1−/−, beta c−/− secondary recipients. Upper lines are lymphocyte percentages and lower lines are neutrophil percentages. Animals that display symptoms of MPD would show drastic increases in neutrophil percentages and reductions in lymphocytes, with values outside of the normal ranges indicated by the dotted (lymphocyte) and solid (neutrophil) bars. Four data points included for Nf1+/+, beta c+/+ primary recipients to illustrate normal values.
Engraftment but lack of MPD in Nf1−/−, beta c−/− recipients. (A) Bone marrow from 3 representative irradiated mice given transplants with Nf1−/−, beta c−/− (top panel) and Nf1+/−, beta c−/− (bottom panel) cells was mostly comprised of cells expressing donor CD45.2, with only a few cells staining positive for CD45.1. Recipients that do not receive donor cells do not stain for the donor-cell marker (data not shown). (B) Flow cytometric analysis of 2 representative mice. Bone marrow and peripheral blood stained for the cell-surface markers Gr-1 and Mac-1, markers commonly associated with mature granulocytes. Similar staining profiles were seen in all recipients. Ranges of double-positive Gr-1/Mac-1 cells in both cohorts, bone marrow 52% to 68% and peripheral blood 12% to 20%. (C) Peripheral-blood analysis monitored over 46 weeks Nf1+/−, beta c−/−and Nf1−/−, beta c−/− secondary recipients. Animals that display symptoms of MPD would show drastic increases in total WBC counts outside of the range indicated by the bar. Left graph illustrates peripheral-blood analysis monitored over 56 weeks Nf1+/−, beta c−/− and Nf1−/−, beta c−/− secondary recipients. Upper lines are lymphocyte percentages and lower lines are neutrophil percentages. Animals that display symptoms of MPD would show drastic increases in neutrophil percentages and reductions in lymphocytes, with values outside of the normal ranges indicated by the dotted (lymphocyte) and solid (neutrophil) bars. Four data points included for Nf1+/+, beta c+/+ primary recipients to illustrate normal values.
Bone marrow CFU-GEMMs and CFU-Ss are increased in recipients of Nf1−/−, beta c−/− fetal liver cells
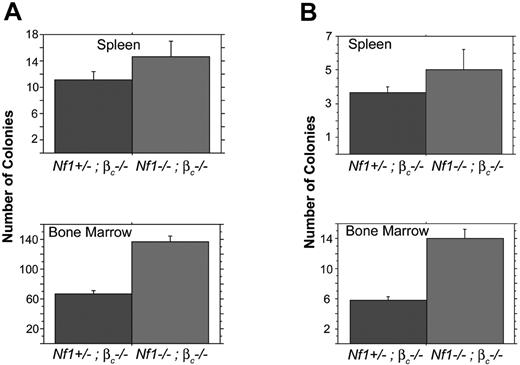
Nf1−/− fetal liver cells form abnormal numbers of CFU-GM colonies in methylcellulose cultures containing low concentrations of GM-CSF.9-11 The absolute number of immature progenitors is also elevated in the bone marrows of mice repopulated with Nf1 mutant fetal liver cells.20 Based on these data, we investigated progenitor colony growth in recipients of transplants with Nf1−/−, beta c−/− and Nf1+/−, beta c−/− fetal liver cells. Whereas splenocytes from Nf1−/−, beta c−/− and Nf1+/−, beta c−/− recipients yielded similar numbers of progenitor colonies, bone marrow cells from Nf1−/−, bta c−/− recipients contained significantly more CFU-GEMMs (Figure 3). We also observed increased numbers of day 8 CFU-S colonies in the bone marrows of recipients of transplants with Nf1−/−, beta c−/− versus Nf1+/−, beta c−/− cells (Figure 3).
Recipients of Nf1−/−βc−/− bone marrow have increased CFCs and CFU-Ss. (A) Spleen and bone marrow cells from 5 secondary mice given transplants with Nf1−/−, βc−/− or 4 secondary mice given transplants with Nf1+/−, βc−/− fetal stem cells were assayed for CFU-GMs, -Gs, -Ms, and -GEMMs and erythroid burst-forming units. Spleen cells from animals given transplants do not show a significant difference from sample to sample, P = .164. Bone marrow plated from 5 mice given transplants with Nf1−/−, βc−/−or Nf1+/−, βc−/− fetal stem cells have a significantly higher number of cells capable of colony formation than the Nf1+/−, βc−/− transplants, P < .001. (B) Spleen and bone marrow cells from 6 mice given transplants with Nf1−/−, βc−/−or 9 mice given transplants with Nf1+/−, βc−/− fetal stem cells were injected intravenously into mice irradiated with 750 rads to assay for CFU-Ss. Injected spleen cells do not show a significant difference from sample to sample, P = .228. Bone marrow cells in animals given Nf1−/−, βc−/− transplants have a significantly higher number of CFU-S progenitor cells than the Nf1+/−, βc−/− transplants, P < .001. Error bars indicate SE.
Recipients of Nf1−/−βc−/− bone marrow have increased CFCs and CFU-Ss. (A) Spleen and bone marrow cells from 5 secondary mice given transplants with Nf1−/−, βc−/− or 4 secondary mice given transplants with Nf1+/−, βc−/− fetal stem cells were assayed for CFU-GMs, -Gs, -Ms, and -GEMMs and erythroid burst-forming units. Spleen cells from animals given transplants do not show a significant difference from sample to sample, P = .164. Bone marrow plated from 5 mice given transplants with Nf1−/−, βc−/−or Nf1+/−, βc−/− fetal stem cells have a significantly higher number of cells capable of colony formation than the Nf1+/−, βc−/− transplants, P < .001. (B) Spleen and bone marrow cells from 6 mice given transplants with Nf1−/−, βc−/−or 9 mice given transplants with Nf1+/−, βc−/− fetal stem cells were injected intravenously into mice irradiated with 750 rads to assay for CFU-Ss. Injected spleen cells do not show a significant difference from sample to sample, P = .228. Bone marrow cells in animals given Nf1−/−, βc−/− transplants have a significantly higher number of CFU-S progenitor cells than the Nf1+/−, βc−/− transplants, P < .001. Error bars indicate SE.
Loss of βc attenuates MPD in Mx1-Cre, Nf1flox/flox mice
While our adoptive transfer experiments were in progress, Zhu and colleagues reported a conditional mutant Nf1 allele, which they generated by flanking exons 31 and 32 with loxP sites.16 Somatic inactivation of this Nf1flox allele, which is functionally wild-type in the basal state, can be achieved by expressing Cre recombinase in specific tissues. Mx1-Cre, Nf1flox/flox mice in a mixed C57BL/6 × 129/Sv genetic background consistently develop a JMML-like MPD that closely resembles the disorder seen in mice given transplants with Nf1−/− fetal liver cells.11 We generated Mx1-Cre, Nf1flox/flox mice of all 3 possible βc genotypes to examine the effects of ablating GM-CSF signaling on the MPD phenotype in this model. Cre recombinase was induced in offspring of Mx1-Cre, Nf1flox/flox, beta c+/− intercrosses at 3 to 5 days of age by injection of pI-pC.11 PCR analysis of DNA extracted from blood leukocytes at 6 weeks of age demonstrated efficient Mx1-Cre transgene-dependent inactivation of Nf1 (data not shown).
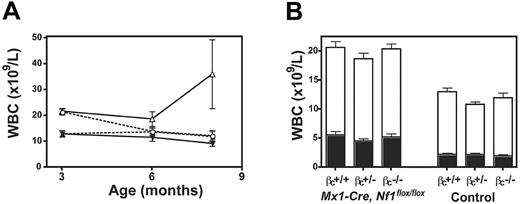
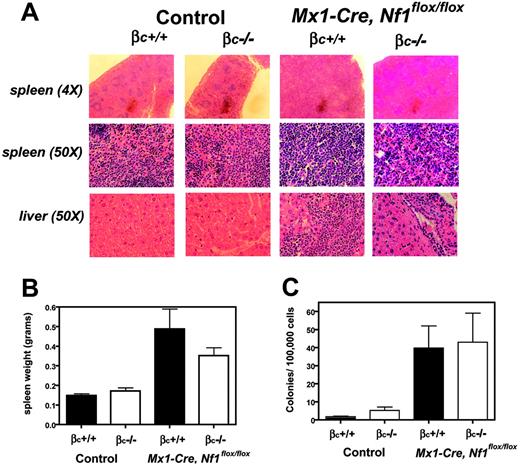
Blood counts were compared over time in Mx1-Cre+ and Mx1-Cre−Nf1flox/flox mice of different beta c genotypes. Because mice with heterozygous inactivation of beta c were similar to beta c+/+ animals (data not shown), we focused on 4 cohorts: (1) Mx1-Cre, Nf1flox/flox, beta c+/+; (2) Mx1-Cre, Nf1flox/flox, beta c−/−; (3) Nf1flox/flox, beta c+/+; and (4) Nf1flox/flox, beta c−/−. Leukocyte counts were significantly elevated in the Mx1-Cre, Nf1flox/flox animals by 3 months of age irrespective of beta c genotype, which was due to increased numbers of lymphoid and myeloid cells (Figure 4A-B). The leukocyte counts of Mx1-Cre, Nf1flox/flox, beta c+/+ mice were persistently elevated at 6 months of age, whereas the counts of Mx1-Cre, Nf1flox/flox, beta c−/− mice were similar to those of beta c+/+ and beta c−/− mice that did not inherit the Mx1-Cre transgene and therefore retained wild-type Nf1 function (Figure 4A). Mx1-Cre, Nf1flox/flox, beta c+/+ mice developed MPD between 5 and 7 months of age, characterized by hunching, abnormal gait, a disheveled appearance, and rising white blood cell (WBC) counts (Figure 4A). These sick mice were humanely killed and analyzed with littermates of the other 3 groups. Importantly, none of the Mx1-Cre, Nf1flox/flox, beta c−/− animals were killed due to signs of systemic illness. The bone marrows of Mx1-Cre, Nf1flox/flox, beta c+/+ mice were highly cellular with myeloid cells at various stages of differentiation and a paucity of erythroid elements, whereas Mx1-Cre, Nf1flox/flox, beta c−/− marrows showed less myeloid proliferation and residual erythropoiesis (data not shown). Pathologic analysis of sick Mx1-Cre, Nf1flox/flox, beta c+/+ mice also uncovered extensive splenic infiltration by myeloid and erythroid cells with effacement of germinal centers (Figure 5A). Splenic infiltration was also present in some age-matched Mx1-Cre, Nf1flox/flox, beta c−/− mice, but was typically less prominent than in Mx1-Cre, Nf1flox/flox, beta c+/+ littermates (Figure 5A). Fluorescence-activated cell sorting (FACS) analysis demonstrated substantial numbers of erythroid (Ter119+ or CD71+ or both) and myeloid (Gr-1+ or Mac-1+ or both) cells in the spleens of Mx1-Cre, Nf1flox/flox, beta c+/+ and Mx1-Cre, Nf1flox/flox, beta c−/− mice that had morphologic evidence of infiltration (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). We also observed extensive periportal invasion within the livers of Mx1-Cre, Nf1flox/flox, beta c+/+ animals that was markedly reduced in Mx1-Cre, Nf1flox/flox, beta c−/− mutant mice (Figure 5A). The spleens of Mx1-Cre, Nf1flox/flox, beta c+/+ and Mx1-Cre, Nf1flox/flox, beta c−/− mice were enlarged (Figure 5B) and contained granulocyte-macrophage progenitors (Figure 5C).
Effects of beta c genotype on leukocyte counts in Mx1-Cre, Nf1flox/flox mice. (A) Data from 6 Mx1-Cre, Nf1flox/flox, beta c−/−(○) and 6 Mx1-Cre, Nf1flox/flox, beta βc+/+ mice (▵) show a modest elevation in total leukocyte counts relative to 5 beta c−/−(•) or 7 wild-type (▴) animals. Leukocyte counts are significantly higher in the Mx1-Cre, Nf1flox/flox, beta c+/+ group by 6 months of age. (B) Lymphoid (□) and myeloid (▪) cell counts are shown at 3 months of age for mice of each genotype: 12 Mx1-Cre, Nf1flox/flox, beta c+/+; 12 Mx1-Cre, Nf1flox/flox, beta c+/−; 8 Mx1-Cre, Nf1flox/flox, beta c−/−; 8 beta c+/+; 12 beta c+/−; 9 beta c−/−. Error bars indicate SD from the mean.
Effects of beta c genotype on leukocyte counts in Mx1-Cre, Nf1flox/flox mice. (A) Data from 6 Mx1-Cre, Nf1flox/flox, beta c−/−(○) and 6 Mx1-Cre, Nf1flox/flox, beta βc+/+ mice (▵) show a modest elevation in total leukocyte counts relative to 5 beta c−/−(•) or 7 wild-type (▴) animals. Leukocyte counts are significantly higher in the Mx1-Cre, Nf1flox/flox, beta c+/+ group by 6 months of age. (B) Lymphoid (□) and myeloid (▪) cell counts are shown at 3 months of age for mice of each genotype: 12 Mx1-Cre, Nf1flox/flox, beta c+/+; 12 Mx1-Cre, Nf1flox/flox, beta c+/−; 8 Mx1-Cre, Nf1flox/flox, beta c−/−; 8 beta c+/+; 12 beta c+/−; 9 beta c−/−. Error bars indicate SD from the mean.
Splenic infiltration in Mx1-Cre, Nf1flox/flox, beta c−/− and Mx1-Cre, Nf1flox/flox, beta c+/+ mice. (A) Inactivation of Nf1 leads to increased numbers of myeloid lineage cells in spleen and liver, which is most pronounced in 6 Mx1-Cre, Nf1flox/flox, beta c+/+ animals and absent in 7 wild-type and 5 beta c−/− spleens. (B) Spleen weights in 7 control and 6 Mx1-Cre, Nf1flox/flox mice. Loss of Nf1 results in splenomegaly and is attenuated 6 Mx1-Cre, Nf1flox/flox, beta c−/− mice. (C) CFU-GM colony numbers from 4 individual experiments of mice of all 4 genotypes demonstrate infiltration in the Nf1 mutant animals.
Splenic infiltration in Mx1-Cre, Nf1flox/flox, beta c−/− and Mx1-Cre, Nf1flox/flox, beta c+/+ mice. (A) Inactivation of Nf1 leads to increased numbers of myeloid lineage cells in spleen and liver, which is most pronounced in 6 Mx1-Cre, Nf1flox/flox, beta c+/+ animals and absent in 7 wild-type and 5 beta c−/− spleens. (B) Spleen weights in 7 control and 6 Mx1-Cre, Nf1flox/flox mice. Loss of Nf1 results in splenomegaly and is attenuated 6 Mx1-Cre, Nf1flox/flox, beta c−/− mice. (C) CFU-GM colony numbers from 4 individual experiments of mice of all 4 genotypes demonstrate infiltration in the Nf1 mutant animals.
Discussion
Birnbaum et al intercrossed Gmcsf and Nf1 mutant mice to investigate whether GM-CSF was essential for MPD.18 Whereas GM-CSF production by either donor hematopoietic cells or by the irradiated recipients was sufficient to induce MPD, inactivating Gmcsf in both donor cells and recipients attenuated the disease phenotype. However, only 6 primary Gmcsf−/− recipients of Nf1, Gmcsf doubly mutant fetal liver cells survived due to an unexpected requirement for GM-CSF signaling for efficient engraftment.21 Moreover, 4 of 6 Gmcsf mutant mice that were repopulated with doubly mutant fetal liver cells developed MPD with prolonged latency.18 The α and β subunits of the GM-CSF receptor form ligand-independent complexes, and it has been suggested that these preformed receptors normally provide a weak constitutive survival signal in hematopoietic cells.22 This raised the possibility that cytokine-independent signaling accounts for the observation that most of the recipients of Nf1, Gmcsf doubly mutant fetal liver cells ultimately develop MPD.18 Resolving this question has therapeutic implications because inhibitors of GM-CSF signaling have been proposed as a potential strategy for treating JMML.23,24
We first used a fetal liver cell adoptive transfer model to demonstrate that loss of Nf1 results in expansion of early hematopoietic progenitors (CFU-GEMMs and day 8 CFU-Ss) that is independent of beta c function. Consistent with these data, competitive repopulation experiments showed Nf1-deficient donor cells have a proliferative advantage in all hematopoietic lineages, which is greatest in the myeloid compartment.20 Our data extend these studies by demonstrating that Nf1 perturbs myelopoiesis independent of signaling through beta c.
Despite increased numbers of immature hematopoietic progenitors in recipients of Nf1−/−, beta c−/− fetal liver cells, loss of beta c suppressed any evidence of MPD for over 13 months. Although the lack of a positive control is a potential concern, we did not transplant Nf1−/−, beta c+/+ cells in parallel because previous studies found that Nf1−/− fetal livers consistently cause MPD in primary and secondary recipients.17-19 In contrast to recipients of doubly mutant fetal liver cells, somatic inactivation of Nf1 in Mx1-Cre, Nf1flox/flox, beta c−/− mice attenuated, but did not eliminate, the MPD phenotype. These data indicate that beta c function is not essential for myeloid disease. Deregulated signaling from other cytokine receptors might contribute to MPD that arises in some Nf1−/−, beta c−/− fetal liver cells. Interestingly, Nf1−/− progenitors are hypersensitive to stem-cell factor (SCF), which signals through the c-kit receptor.20 Although Nf1−/− myeloid progenitors demonstrate a normal pattern of colony growth in response to IL-3 alone, these cells are hypersensitive to the combination of IL-3 plus SCF.20 Importantly, despite phenotypic differences between recipients of Nf1−/−, beta c−/− fetal liver cells and Mx1-Cre, Nf1flox/flox, beta c−/− mice, both models infer an important role of GM-CSF signaling in modulating myeloproliferation in vivo.
The absence of MPD in the adoptive transfer model may have been due to decreased replicative potential of the donor cells after 2 rounds of transplantation, particularly given the interaction between GM-CSF signaling and fetal liver cell engraftment.21 It is also possible that transplant recipients would have ultimately developed MPD had we observed them beyond 13 months. On the other hand, because the Mx1-Cre promoter is active in multiple cell types, it is possible that nonhematopoietic cells contribute to MPD pathogenesis in Mx1-Cre, Nf1flox/flox, beta c−/− mice by secreting abnormal amounts of cytokines. This idea is consistent with studies showing that heterozygous inactivation of the Nf1 gene in mice can induce a “field effect” that promotes neurofibroma formation due to interactions between Schwann cells that have inactivated both Nf1 alleles and heterozygous mutant mast cells and fibroblasts.25
Based on our data and the existing literature, we speculate that immature and lineage-specific hematopoietic progenitors require neurofibromin to negatively regulate multiple extracellular stimuli in the bone marrow microenvironment that orchestrate the differentiation of stem cells into progenitors and then mature myeloid cells. Loss of Nf1 results in increased numbers of cells at all stages of myeloid differentiation. Although we have shown that immature hematopoietic progenitors accumulate in the absence of beta c, our data also demonstrate that GM-CSF signaling is required for a fully penetrant MPD phenotype. Previous in vitro and in vivo data support the idea that interfering with GM-CSF signaling may inhibit the growth of JMML cells.18,23,24 However, the GM-CSF–independent proliferation of immature Nf1 mutant progenitors also infers that curing this aggressive MPD will require inhibiting beta c in combination with other strategies.
Authorship
Contribution: K.M., D.A.L., and K.S. designed research; A.K., K.M., D.E.H., S.M.W., and D.T.L. performed research; A.K., K.M., J.O.L., and S.C.K. analyzed data; D.E.H., J.L.G., and M.D.D. maintained the mouse colony; S.C.K. and L.F.P. contributed reagents; and A.K., K.M., J.O.L., K.S., and D.A.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.K. and K.M. authors contributed equally to the study.
Correspondence: David A. Largaespada, 6-160 Jackson Hall, 321 Church St SE, Minneapolis, MN 55455; e-mail: larga002@tc.umn.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the National Cancer Institute (R29 CA78269-01; D.A.L.), American Cancer Society (RPG LIB-106632; D.A.L.), Leukemia and Lymphoma Society of America Specialized Center of Research (LLS 7019-04; D.A.L. and K.S.), National Cancer Institute (R01 CA72614; K.S.), and the US Army Neurofibromatosis Research Program (Project DAMD17-02-1-0638; K.S.). K.M. was supported by a predoctoral training grant (Young Investigator Award) from the National Neurofibromatosis Foundation, now called the Children's Tumor Foundation, and J.O.L. was supported by a training grant from the National Institutes of Health.
We would like to thank the members of the Largaespada and Shannon laboratories for technical advice and input on experimental design.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal