Abstract
The gradual accumulation of chronic lymphocytic leukemia (B-CLL) cells is presumed to derive from proliferation centers in lymph nodes and bone marrow. To what extent these cells possess the purported antiapoptotic phenotype of peripheral B-CLL cells is unknown. Recently, we have described that, in B-CLL samples from peripheral blood, aberrant apoptosis gene expression was not limited to protective changes but also included increased levels of proapoptotic BH3-only member Noxa. Here, we compare apoptosis gene profiles from peripheral blood B-CLL (n = 15) with lymph node B-CLL (> 90% CD5+/CD19+/CD23+ lymphocytes with Ki67+ centers; n = 9). Apart from expected differences in Survivin and Bcl-xL, a prominent distinction with peripheral B-CLL cells was the decreased averaged level of Noxa in lymph nodes. Mcl-1 protein expression showed a reverse trend. Noxa expression could be reduced also in vitro by CD40 stimulation of peripheral blood B-CLL. Direct manipulation of Noxa protein levels was achieved by proteasome inhibition in B-CLL and via RNAi in model cell lines. In each instance, cell viability was directly linked with Noxa levels. These data indicate that suppression of Noxa in the lymph node environment contributes to the persistence of B-CLL at these sites and suggest that therapeutic targeting of Noxa might be beneficial.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by a progressive accumulation of monoclonal CD5+ CD23+ mature B cells in the secondary lymphoid tissues, bone marrow, and blood.1 Previously, it was assumed that B-CLL is associated with a defective regulation of programmed cell death (apoptosis), rather than uncontrolled cell proliferation.2 Indeed, high expression of the antiapoptotic proteins Bcl-2 and Mcl-1 has been associated with rapid disease progression and a poor response to chemotherapy.3,4 Paradoxically, investigation of virtually all direct apoptosis regulators known at present revealed that, in addition to these antiapoptotic alterations, the proapoptotic proteins Noxa and Bmf are also abundantly expressed in B-CLL.5,6 How the elevated expression of these proapoptotic proteins is associated with the reputed increased life span of the B-CLL cells is currently unknown.
The vast majority of the circulating B-CLL cells are arrested in G0/G1 phase of the cell cycle,7 which has contributed to the view that B-CLL is an indolent disease. However, isotopic labeling of leukemic cells in vivo revealed that a substantial fraction of the B-CLL cells does proliferate.8 It seems logical to assume that the generation of new cells takes place in so-called proliferation centers frequently found in lymph nodes and bone marrow of patients with B-CLL. This is supported by the numerous Ki67+ and Survivin+ cells present in these structures.1,9 The microenvironment not only plays an essential role in the induction of proliferation but presumably also in the suppression of apoptosis. In vitro experiments revealed that various cell types can support the survival of B-CLL cells. Apart from follicular dendritic cells (FDCs), bone marrow stromal cells, IL-6–producing endothelial cells, VCAM-1, and SDF-producing nurselike cells, CD4+ T cells can also aid in providing a microenvironment where B-CLL cells can survive and proliferate.10-14 The importance of the microenvironment for the survival of B-CLL cells is also shown by the finding that, despite the relentless accumulation of the B-CLL cells in vivo, culturing the leukemic cells in vitro results in spontaneous apoptosis.15,16 In vitro culture of B-CLL cells in the presence of CD40L rescues the cells from spontaneous and drug-induced apoptosis, suggesting that such costimulatory signals play a role in the survival of B-CLL cells in vivo and even in the response to treatment.9,17-19
To date, B-CLL is an incurable disease. Although multiagent treatment can result in a profound peripheral lymphocyte depletion, the B-CLL cells in the bone marrow, lymph nodes, or both are less effectively targeted.20 Persistence of B-CLL cells in the bone marrow is associated with an increased risk of relapse.21 Therefore, more molecular data about the B-CLL cells in the lymphoid tissues and bone marrow are necessary, preferably coupled with assessment of efficacy of therapeutics toward B-CLL residing in those niches. We here initiated such an effort by comparing a large panel of apoptosis regulators in circulating B-CLL cells and B-CLL cells residing in lymph nodes. Although the expression of most apoptosis regulators was remarkably comparable, a prominent difference was the expression of the BH3-only protein Noxa. Furthermore, we demonstrate that CD40 engagement of peripheral B-CLL cells can largely reproduce the altered apoptosis profile found in lymph node B-CLL cells. Finally, we show that in vitro manipulation of Noxa expression has a significant and direct effect on B-CLL cell survival. Together, these data provide a new link between the antiapoptotic microenvironment in the lymph nodes and suppression of Noxa, which suggests that drugs that increase Noxa levels, such as proteasome inhibitors,22,23 may be of therapeutic benefit in B-CLL.
Materials and methods
Patient material and cell lines
Patient material was obtained after routine diagnostic or follow-up procedures at the departments of Hematology and Pathology of the Academic Medical Center Amsterdam. All patients were diagnosed according to the WHO classification system.1 Lymph node (LN) material diffusely infiltrated by B-CLL cells was freshly frozen in liquid nitrogen directly after surgical removal. Immunohistochemical analysis (see “Immunohistochemistry”) of these lymph nodes revealed that greater than 90% of the tissue consisted of tumor cells. Peripheral blood (PB) mononuclear cells (PBMCs) of patients with B-CLL were obtained after Ficoll density centrifugation (Pharmacia Biotech, Roosendaal, The Netherlands). PBMCs from patients with B-CLL contained greater than 75% CD5+, CD19+ cells as assessed by flow cytometry and were stored in liquid nitrogen as cell suspensions in 10% DMSO (Merck, Darmstadt, Germany) in heat-inactivated FCS (Invitrogen, Breda, The Netherlands). Clone FSA of the Burkitt lymphoma cell line Ramos with enhanced response to CD95 has been described previously.24 Cell lines were cultured in Iscove modified Dulbecco medium (IMDM; Invitrogen), supplemented with 10% (vol/vol) heat-inactivated FCS (ICN Biomedicals GmbH, Meckenheim, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 mM L-glutamine (Invitrogen). This study was conducted in accordance with the ethical standards in our institute and approved by the AMC medical committee on human experimentation, and informed consent was obtained from patients in agreement with the Helsinki Declaration of 1975, revised in 1983.
RNA isolation and reverse transcription–multiplex ligation-dependent probe amplification assay
Total RNA was isolated using the Nucleospin RNA isolation kit (Macherey-nagel, Düren, Germany). Reverse transcription–multiplex ligation-dependent probe amplification assay (RT-MLPA) procedure was performed as described previously.5,25 Briefly, 100 ng total RNA was reverse transcribed using a gene-specific probe mix. The resulting cDNA was annealed overnight at 60°C to the MLPA probes. Annealed oligonucleotides were covalently linked by Ligase-65 (MRC, Amsterdam, The Netherlands) at 54°C. Ligation products were amplified by polymerase chain reaction (PCR; 33 cycles, 30 seconds at 95°C, 30 seconds at 60°C, and 1 minute at 72°C) using one unlabeled and one 6-carboxy-fluorescein–labeled primer (10 pM). PCR products were run on an ABI 3100 capillary sequencer in the presence of 1 pM ROX 500 size standard (Applied Biosystems, Warrington, United Kingdom). Results were analyzed using the programs Genescan analysis and Genotyper (Applied Biosystems). Category tables containing the area for each assigned peak (scored in arbitrary units) were compiled in Genotyper and exported for further analysis with Excel spreadsheet software (Microsoft, Redman, WA). Data were normalized by setting the sum of all signals at 100% and expressing individual peaks relative to the 100% value.
Immunohistochemistry
Monoclonal antibodies specific for CD5 (clone 4C7), CD3 (clone SP7), CD23 (clone 1B12), Bcl-6 (clone PG-B67) (all from Lab Vision, Neomarkers, Fremont, CA), were used on formalin-fixed paraffin-embedded lymph node specimens. When necessary, antigen retrieval was achieved using a Tris-EDTA buffer, pH 9.2. Antibody detection was performed with the Powervision+ system (ImmunoVision Technologies, Daly City, CA) which was succeeded, for the single antibody staining, by peroxidase visualization with 3,3′-diaminobenzidine (DAB; Sigma, St Louis, MO), 0.03% H2O2 in Tris-HCl, pH 7.6. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted in pertex. For the CD3/Ki67 double stainings the Ki67 MIB-1 clone (Dako, Glostrup, Denmark) was used, for the CD20/Ki67 double staining the Ki67 SP6 clone (Neomarkers), and the CD20 L26 clone from Dako. After antibody detection with the Powervision+ system (ImmunoVision) the Liquid permanent red kit (Dako) was used, followed by peroxidase visualization with DAB (Sigma). Finally, the slides were counterstained with hematoxylin and mounted in Vectamount. Samples were visualized through an Olympus BX51 microscope (Olympus, Zoeterwoude, The Netherlands; 40×/0.85 NA objective) and photographed with an Olympus DP70 camera using Olympus DP manager image acquisition software. Pictures were processed with Photoshop CS software (Adobe Systems Benelux BV, Amsterdam, The Netherlands).
Flow cytometry
Purified B-CLL cells were incubated with FITC- or PE-conjugated mAbs directed against CD5 (Sanquin, Amsterdam, The Netherlands), CD19 (Sanquin), and CD3 (Becton Dickinson, San Jose, CA) and analyzed by flow cytometry with the CellQuest program on a fluorescence-activated cell sorting (FACS) Calibur (Becton Dickinson).
In vitro CD40 stimulation
B-CLL samples were enriched to greater than 95% purity from PBMCs via negative depletion as described previously.5 In brief, T cells, monocytes, and granulocytes were depleted using anti-CD3, anti-CD14, and anti-CD16 immunomagnetic beads on a magnetic particle concentrator (Dynal AS, Oslo, Norway). The B-CLL cells were stimulated for 3 days in culture-treated 24-wells plates (Costar, Corning, NY). Each well contained 5 × 106 B-CLL cells and 1.5 × 105 irradiated (30 Gy) CD40L-transfected or untransfected fibroblasts (NIH3T3).
Retroviral constructs and transduction
To knock down Noxa, pRetro-super was used, which contains the polymerase III H1-RNA promoter (pol3) for transcription of the siRNA probe and the phosphoglycerin kinase (pgk)1 promoter driving GFP expression.26 The siRNA sequences were N7, 5′-GAAGGTGCATTCATGGTG-3′, and N8, 5′-GTAATTATTGACACATTTC-3′.27 The retroviral plasmids were transfected into the helper virus amphotropic producer cell line Phoenix with Fugen-6 (Roche Diagnostics, Almere, The Netherlands). For transduction, Ramos-FSA cells were exposed overnight to viral supernatant (containing vector GFP-only or one of the 2 Noxa RNAi-targeting sequences) on retronectin-coated (Takara Shuzo, Otso, Japan) 24-well plates. GFP-positive cells were sorted using a FACS-Aria (BD Biosciences, Alphen aan de Rijn, the Netherlands) cell sorter to greater than 90% purity for further experiments.
Analysis of apoptosis
PB B-CLL cells were stimulated at a concentration of 5 × 106 cells/mL with 20 nM bortezomib (Janssen-Cilag, Tilburg, The Netherlands) for 4 hours. The cells were washed twice with IMDM (when indicated) and incubated at given time points with FITC-labeled annexin V (IQ Products, Groningen, The Netherlands) for 20 minutes. Prior to analyses, propidium iodide (PI) was added (final concentration 5 μg/mL). Viable cells were defined by annexin V−/PI− staining. Ramos.FSA clones expressing either control-GFP or Noxa-RNAi (N7 or N8) were stimulated at a concentration of 5 × 105 cells/mL with 30 nM bortezomib for 24 hours, harvested, and incubated with 200 nM MitoTracker Orange (Molecular Probes, Leiden, The Netherlands) for 30 minutes at 37°C, washed, and double-stained with APC-labeled annexin V (IQ Products). Fludarabine, staurosporine, and PI were purchased from Sigma. Anti–human Fas10 (agonistic antibody to the CD95 receptor) was a kind gift from Prof Dr L. Aarden (Sanquin).
Western blotting
Western blotting was performed as described previously.5 Protein samples were separated by 13% sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Western blotting. Blots were probed with the following antisera: polyclonal Mcl-1 (catalog no. 554103; Pharmingen, BD Biosciences), monoclonal anti-Noxa (clone 114C307.1; Imgenex, San Diego, CA), monoclonal anti-Bim (clone 14A8; Chemicon, Temecula, CA), and antiserum to β-actin (clone I-19; Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were quantified using high-resolution (1200 dpi) scanned images of exposed films and AIDA image analyzer software v3.5 (Raytest Gmbh, Straubenhardt, Germany). Exposed films were only considered when software indicated that bands were not overexposed. In each sample, Mcl-1 and Noxa bands were normalized by correcting for actin levels.
Statistical analyses
The Mann Whitney U test was used to analyze whether differences in gene expression between the peripheral blood and lymph node samples were statistically significant. P values below .01 were considered statistically significant. Densitometric scans of Western blots and MLPA analyses of CD40-triggered CLL cells were analyzed with Student t test. P values less than .05 were considered statistically significant.
Results
Patient characteristics and immunohistochemistry
Lymph nodes from 9 patients with B-CLL and peripheral blood (PB) samples from 15 patients with B-CLL were included in the study. From 2 patients (B-CLL25 and B-CLL31) both lymph node (LN) tissue and PB samples were available (Table 1). All B-CLL expressed CD5, CD23, and CD19/CD20. The B-CLL cells of the patients with LN involvement expressed unmutated immunoglobulin heavy chain (IgVH) genes. Of the peripheral blood B-CLL, 10 expressed mutated IgVH genes and 8 unmutated IgVH genes (Table 1). In the peripheral blood samples, at least 75% of the leucocytes were lymphocytes. Because of low levels of CD5 expression, the standard FACS gating yielded low percentages of CD5+ CD19+ cells in some patients; patient 30 had in addition low numbers of circulating cells and was first diagnosed as small lymphocytic leukemia (SLL). Immunohistochemistry demonstrated that greater than 90% of the LNs consisted of leukemic lymphocytes. Ki67+ cells were present in all LNs, either diffusely or in proliferation centers. These cells were of B-cell origin, as demonstrated by double staining which showed that all Ki67+ cells were also CD20+. In contrast, the scattered CD3+ T cells were generally negative for Ki67. Absence of clusters of Bcl-6+ or CD21+ (data not shown) cells excluded the presence of germinal center remnants in these LNs (Figure 1).
Patient and B-CLL sample characteristics
| Patient . | Location . | Age, y . | Rai stage . | Lymph,* % . | CD5+CD19+, % . | CD19+, % . | CD3+, % . | IgVH mutations† . |
|---|---|---|---|---|---|---|---|---|
| B-CLL24 | LN | 52 | > 90 | ND | ND | ND | − | |
| B-CLL27 | LN | 64 | 1 | > 90 | 76 | 76 | 10 | ND |
| B-CLL28 | LN | 80 | 4 | > 90 | 64 | 93 | 8 | − |
| B-CLL30 | LN | 46 | 3 | > 90 | 37§ | 72 | 26 | − |
| B-CLL33 | LN | 76 | 2 | > 90 | ND | ND | ND | − |
| B-CLL35 | LN | 67 | 2 | > 90 | ND | ND | ND | − |
| B-CLL36 | LN | 59 | 4 | > 90 | 93 | 93 | 5 | − |
| B-CLL37 | PB | 62 | 1 | ND | ND | ND | ND | + |
| B-CLL38 | PB | 62 | 0 | 98 | 97 | 97 | 2 | + |
| B-CLL39 | PB | 72 | 3 | ND | 94 | 98 | 3 | + |
| B-CLL40 | PB | 70 | 3 | 93 | 48‡ | 98 | 1 | − |
| B-CLL41 | PB | ND | 4 | 94 | 99 | 99 | 1 | − |
| B-CLL42 | PB | 48 | 0 | 69 | 76 | 76 | 17 | − |
| B-CLL43 | PB | 54 | 2 | 91 | 96 | 96 | 4 | − |
| B-CLL44 | PB | 55 | 2 | 86 | 91 | 91 | 0 | + |
| B-CLL45 | PB | 63 | 4 | 82 | 98 | 98 | 2 | + |
| B-CLL46 | PB | ND | ND | 82 | ND | ND | ND | ND |
| B-CLL47 | PB | 54 | 2 | 83 | ND | ND | 7 | − |
| B-CLL48 | PB | 59 | 2 | 75 | 89 | 89 | 4 | + |
| B-CLL50 | PB | 69 | 0 | ND | 99 | 99 | 3 | + |
| B-CLL167‡ | PB | 70 | 0 | 62 | 84 | 86 | 11 | + |
| B-CLL183‡ | PB | 78 | 0 | 47 | 94 | 95 | 4 | + |
| B-CLL226‡ | PB | 64 | 1 | 70 | 98 | 98 | 1 | − |
| B-CLL261‡ | PB | 77 | 4 | 32 | 92 | 92 | 4 | + |
| B-CLL25 | PB | 68 | 2 | ND | ND | 78 | 13 | − |
| B-CLL25 | LN | 68 | 2 | > 90 | 82 | 83 | 20 | − |
| B-CLL31 | PB | 72 | 2 | ND | 81 | 81 | 8 | − |
| B-CLL31 | LN | 72 | 2 | > 90 | 72 | 81 | 12 | − |
| Patient . | Location . | Age, y . | Rai stage . | Lymph,* % . | CD5+CD19+, % . | CD19+, % . | CD3+, % . | IgVH mutations† . |
|---|---|---|---|---|---|---|---|---|
| B-CLL24 | LN | 52 | > 90 | ND | ND | ND | − | |
| B-CLL27 | LN | 64 | 1 | > 90 | 76 | 76 | 10 | ND |
| B-CLL28 | LN | 80 | 4 | > 90 | 64 | 93 | 8 | − |
| B-CLL30 | LN | 46 | 3 | > 90 | 37§ | 72 | 26 | − |
| B-CLL33 | LN | 76 | 2 | > 90 | ND | ND | ND | − |
| B-CLL35 | LN | 67 | 2 | > 90 | ND | ND | ND | − |
| B-CLL36 | LN | 59 | 4 | > 90 | 93 | 93 | 5 | − |
| B-CLL37 | PB | 62 | 1 | ND | ND | ND | ND | + |
| B-CLL38 | PB | 62 | 0 | 98 | 97 | 97 | 2 | + |
| B-CLL39 | PB | 72 | 3 | ND | 94 | 98 | 3 | + |
| B-CLL40 | PB | 70 | 3 | 93 | 48‡ | 98 | 1 | − |
| B-CLL41 | PB | ND | 4 | 94 | 99 | 99 | 1 | − |
| B-CLL42 | PB | 48 | 0 | 69 | 76 | 76 | 17 | − |
| B-CLL43 | PB | 54 | 2 | 91 | 96 | 96 | 4 | − |
| B-CLL44 | PB | 55 | 2 | 86 | 91 | 91 | 0 | + |
| B-CLL45 | PB | 63 | 4 | 82 | 98 | 98 | 2 | + |
| B-CLL46 | PB | ND | ND | 82 | ND | ND | ND | ND |
| B-CLL47 | PB | 54 | 2 | 83 | ND | ND | 7 | − |
| B-CLL48 | PB | 59 | 2 | 75 | 89 | 89 | 4 | + |
| B-CLL50 | PB | 69 | 0 | ND | 99 | 99 | 3 | + |
| B-CLL167‡ | PB | 70 | 0 | 62 | 84 | 86 | 11 | + |
| B-CLL183‡ | PB | 78 | 0 | 47 | 94 | 95 | 4 | + |
| B-CLL226‡ | PB | 64 | 1 | 70 | 98 | 98 | 1 | − |
| B-CLL261‡ | PB | 77 | 4 | 32 | 92 | 92 | 4 | + |
| B-CLL25 | PB | 68 | 2 | ND | ND | 78 | 13 | − |
| B-CLL25 | LN | 68 | 2 | > 90 | 82 | 83 | 20 | − |
| B-CLL31 | PB | 72 | 2 | ND | 81 | 81 | 8 | − |
| B-CLL31 | LN | 72 | 2 | > 90 | 72 | 81 | 12 | − |
LN indicates lymph node; PB, peripheral blood; ND, not done.
Lymphocytes were investigated in the lymph node samples by immunohistochemistry and in the peripheral blood samples by FACS analysis.
IgVH mutations were positive if ≥ 2% of the IgVH gene was mutated.
These samples were used only for Western blot analyses.
These samples displayed low CD5 staining; therefore, the combined CD5/CD19 gate yielded low values.
Histology of lymph node infiltrated by B-CLL cells. Ubiquitously present B-CLL cells were positive for CD23 and CD5. Scattered CD3+ T cells were present throughout the LN. The absence of clusters of BCL-6+ cells excluded the presence of germinal center remnants in the LNs. Ki67/CD20 and Ki67/CD3 double staining indicate that the majority of cycling Ki67+ cells (pink) were also CD20+ (brown) (see inset), whereas the CD3+ T cells were predominantly Ki67 negative. Magnification ×40.
Histology of lymph node infiltrated by B-CLL cells. Ubiquitously present B-CLL cells were positive for CD23 and CD5. Scattered CD3+ T cells were present throughout the LN. The absence of clusters of BCL-6+ cells excluded the presence of germinal center remnants in the LNs. Ki67/CD20 and Ki67/CD3 double staining indicate that the majority of cycling Ki67+ cells (pink) were also CD20+ (brown) (see inset), whereas the CD3+ T cells were predominantly Ki67 negative. Magnification ×40.
Profiling of apoptosis genes in peripheral blood and LN samples of B-CLL
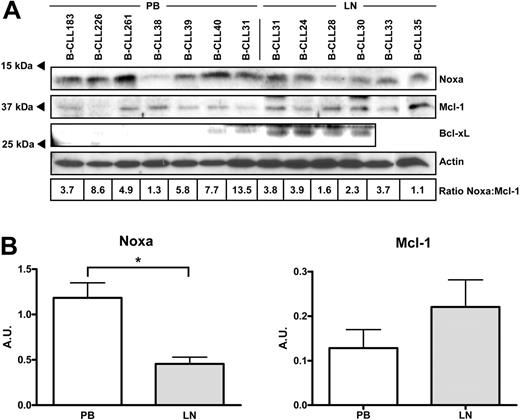
The relative expression of 34 known apoptosis regulators was investigated by RT-MLPA5,25,28 in PB samples of 13 patients with B-CLL, LN samples of 7 patients with B-CLL (Figure 2A) and paired PB and LN samples of 2 patients with B-CLL (Figure 2B; data not shown). The relative expression of the majority of the investigated genes was remarkably comparable between the PB and LN samples. We have described previously that, compared with normal tonsillar B-cell fractions, in PB B-CLL several antiapoptosis and proapoptosis genes (eg, Flip, Bcl-2, Noxa, and Bmf) are aberrantly expressed,5 and this was also found in LN samples of B-CLL. Interestingly, 3 genes were differentially expressed in PB B-CLL cells as compared with LN samples (Figure 2C). In agreement with previous reports, the IAP family member Survivin was not expressed in any of the PB B-CLL samples, whereas it was clearly expressed in LN B-CLL5,9 (P < .001). Also, the antiapoptotic Bcl-2 family member Bcl-xL was more abundantly expressed in the LN samples (P < .001). The most striking difference in expression was observed for the BH3-only member Noxa. As found previously, this apoptogenic gene is abundantly expressed in PB B-CLL cells,5 but its expression was clearly lower in LN B-CLL cells (averaged relative expression of 15.6 ± 9.8 in PB B-CLL versus 3.0 ± 1.1 in LN B-CLL; P < .001; Figure 2C). Of note, a difference in Noxa expression was also observed between the paired PB and LN samples of an individual patient (relative expression 9.6 in the PB sample versus 3.4 in the LN sample) (Figure 2B). Western blot analyses confirmed that the differences in Bcl-xL and Noxa mRNA expression were also present at the protein level. The B-CLL LN samples generally expressed lower levels of Noxa than did the PB B-CLL samples, and the reverse was observed for Bcl-xL (Figure 3). Comparison of the RT-MLPA data with the Western blot data revealed a clear correlation between the levels of Noxa mRNA and Noxa protein. As reported previously, no differences were observed in expression of these apoptosis genes among IgVH-mutated versus unmutated cases.5
Apoptosis gene expression profile of B-CLL cells in peripheral blood and lymph nodes. (A) Relative expression of 34 apoptosis regulators was investigated in 15 PB B-CLL (▪) and 9 LN B-CLL (⊡) cells. Results of individual apoptosis regulatory genes are shown as expression relative to the total signal in the sample, with standard deviation (error bars). Nonapoptosis genes included as housekeeping genes are β2-microglobulin (B2M), ferritin light chain (FLT), β-glucoronidase (GUS), and poly(A)-specific ribonuclease (PARN). (B) RT-MLPA data from PB and LN samples of B-CLL25. (C) The expression of Noxa, Survivin, Bcl-xL, and Mcl-1 in individual patients are depicted as dots. *indicates statistical significance (P < .001) of differences in gene expression between PB and LN B-CLL. Lines indicate averages.
Apoptosis gene expression profile of B-CLL cells in peripheral blood and lymph nodes. (A) Relative expression of 34 apoptosis regulators was investigated in 15 PB B-CLL (▪) and 9 LN B-CLL (⊡) cells. Results of individual apoptosis regulatory genes are shown as expression relative to the total signal in the sample, with standard deviation (error bars). Nonapoptosis genes included as housekeeping genes are β2-microglobulin (B2M), ferritin light chain (FLT), β-glucoronidase (GUS), and poly(A)-specific ribonuclease (PARN). (B) RT-MLPA data from PB and LN samples of B-CLL25. (C) The expression of Noxa, Survivin, Bcl-xL, and Mcl-1 in individual patients are depicted as dots. *indicates statistical significance (P < .001) of differences in gene expression between PB and LN B-CLL. Lines indicate averages.
Comparison of Noxa, Mcl-1, and Bcl-xL protein in PB versus LN B-CLL. Protein lysates of 7 PB samples and 6 LN samples were subjected to Western blot analyses. (A) Blots were stained with antibodies directed against Noxa, Mcl-1, or Bcl-xL and reprobed with an antibody against β-actin as a loading control. In case of Bcl-xL, a specific staining at the upper cutting edge of the blot is visible and precluded analysis of the rightmost 2 samples. Densitometric scanning was performed, and Noxa/Mcl-1 ratios, corrected for actin levels, are indicated below the samples. The averaged Noxa/Mcl-1 ratio was significantly different between PB and LN (P = .011). (B) Averaged Noxa/actin and Mcl-1/actin ratios are separately plotted for PB and LN samples. Unpaired t test showed that Noxa ratios were statistically significant (P = .002), and Mcl-1 ratios showed a nonsignificant trend. Error bars indicate standard deviation.
Comparison of Noxa, Mcl-1, and Bcl-xL protein in PB versus LN B-CLL. Protein lysates of 7 PB samples and 6 LN samples were subjected to Western blot analyses. (A) Blots were stained with antibodies directed against Noxa, Mcl-1, or Bcl-xL and reprobed with an antibody against β-actin as a loading control. In case of Bcl-xL, a specific staining at the upper cutting edge of the blot is visible and precluded analysis of the rightmost 2 samples. Densitometric scanning was performed, and Noxa/Mcl-1 ratios, corrected for actin levels, are indicated below the samples. The averaged Noxa/Mcl-1 ratio was significantly different between PB and LN (P = .011). (B) Averaged Noxa/actin and Mcl-1/actin ratios are separately plotted for PB and LN samples. Unpaired t test showed that Noxa ratios were statistically significant (P = .002), and Mcl-1 ratios showed a nonsignificant trend. Error bars indicate standard deviation.
Because Noxa can selectively interact with the antiapoptotic protein Mcl-1 and this may influence the degradation of Mcl-1,29 we investigated the expression of this Bcl-2 family member in PB B-CLL and LN B-CLL. Although RT-MLPA showed no difference in mRNA expression (Figure 2C), Western blot analyses revealed that in most LN B-CLL, where Noxa levels were low, Mcl-1 was slightly elevated. Furthermore, the PB samples that expressed higher levels of Noxa showed a decreased expression of Mcl-1 (Figure 3). This is further illustrated by a paired PB/LN protein sample, where in fact Noxa expression was almost equal, but in this case Mcl-1 protein levels were clearly higher in the LN compartment. Densitometric scanning of Noxa and Mcl-1 protein levels (ratio indicated below individual lanes in Figure 3A) also showed divergence between LN and PB, of which the averaged differences in Noxa levels were statistically significant (Figure 3B). In summary, the majority of the apoptotic regulators are expressed equally in PB and LN B-CLL, but a novel and prominent distinction in the Noxa level was found.
Noxa expression is modulated by CD40 engagement in B-CLL cells
In the LNs the CD40+ B-CLL cells are in close contact with T cells that may express CD40L2 (Figure 1). To investigate the effect of this interaction on the expression of the apoptotic regulators, PB B-CLL samples (n = 11) were cocultured for 1 to 5 days with CD40L-transfected or untransfected 3T3 fibroblasts (Figure 4). As reported previously, CD40 stimulation resulted in increased expression of Bcl-xL, A1/Bfl-1, Bid, and Survivin.9,17,18 Interestingly, in accordance with our findings in the LN B-CLL cells, CD40L-stimulated PB B-CLL cells also showed a diminished expression of Noxa (Figure 4A). The effects were observed after 1 day of CD40 stimulation, and RT-MLPA performed at day 3 and day 5 showed that the expression of the apoptosis regulators did not alter significantly after that (Figure 4B). It should be noted that the levels of Noxa mRNA as compared with t = 0 also decreased after culture on the control 3T3 cells (P = .019). The reason for this is not known; however, a stronger decline in Noxa levels was consistently observed after CD40 ligation (P = .004), and the difference between control and CD40-treated cells was statistically significant (P = .016). These differences were further investigated at the protein level for 3 patients (see Figure 4C). Concordant with RT-MLPA analyses, Noxa levels decreased after 3 days of culture in the presence of CD40L-expressing cells, and Bcl-xL levels increased. Mcl-1 protein levels also clearly increased on CD40-triggering, although this was not observed via RT-MLPA. So, similar to findings in LN samples (Figure 3), Mcl-1 levels were apparently under posttranscriptional control.
CD40 stimulation of peripheral blood B-CLL results in an apoptosis gene expression profile similar to lymph node B-CLL. (A) Apoptosis gene expression profile was investigated by RT-MLPA in PB samples of 11 freshly isolated B-CLL patient samples without culturing (▪) and after 3 days of culturing on either 3T3 cells (▽) or CD40L-transfected 3T3 cells (□). Data plus standard deviation are presented as in Figure 2. (B) The expression of Bcl-xL, Bfl-1/A1, Bid, and Noxa are shown at day 1, 3, and 5 of culturing on 3T3 cells (▿) or CD40L-transfected 3T3 cells (•). Statistical analysis of day 0 versus day 1 samples showed that in all cases the CD40L-treated values were significantly different (P < .01). In case of Noxa, there was also a small but significant decrease for the 3T3 control cells (P = .019), and a more pronounced effect for CD40L-treated cells (P = .004; **P = .015, difference between 3T3 and CD40L-treated cells). Error bars indicate standard deviation. (C) Western blot of t = 0 samples in comparison with CD40L-treated cells at day 3 for Noxa, Mcl-1, and Bcl-xL showed that Noxa protein levels decrease, whereas Mcl-1 and Bcl-xL increase. For B-CLL sample 226 the Mcl-1 levels at t = 0 were in fact undetectable (see also Figure 3).
CD40 stimulation of peripheral blood B-CLL results in an apoptosis gene expression profile similar to lymph node B-CLL. (A) Apoptosis gene expression profile was investigated by RT-MLPA in PB samples of 11 freshly isolated B-CLL patient samples without culturing (▪) and after 3 days of culturing on either 3T3 cells (▽) or CD40L-transfected 3T3 cells (□). Data plus standard deviation are presented as in Figure 2. (B) The expression of Bcl-xL, Bfl-1/A1, Bid, and Noxa are shown at day 1, 3, and 5 of culturing on 3T3 cells (▿) or CD40L-transfected 3T3 cells (•). Statistical analysis of day 0 versus day 1 samples showed that in all cases the CD40L-treated values were significantly different (P < .01). In case of Noxa, there was also a small but significant decrease for the 3T3 control cells (P = .019), and a more pronounced effect for CD40L-treated cells (P = .004; **P = .015, difference between 3T3 and CD40L-treated cells). Error bars indicate standard deviation. (C) Western blot of t = 0 samples in comparison with CD40L-treated cells at day 3 for Noxa, Mcl-1, and Bcl-xL showed that Noxa protein levels decrease, whereas Mcl-1 and Bcl-xL increase. For B-CLL sample 226 the Mcl-1 levels at t = 0 were in fact undetectable (see also Figure 3).
A prominent distinction between CD40-stimulated B-CLL and LN B-CLL was found for expression of the apoptogenic BH3-only protein Bid. In contrast to LN B-CLL, CD40L-stimulated B-CLL showed a strong and continuous induction of Bid (Figure 4B). Thus, the altered gene expression in LNs can be mimicked largely but not entirely by in vitro CD40 engagement of B-CLL cells.
Bortezomib-induced Noxa up-regulation causes apoptosis of PB B-CLL cells
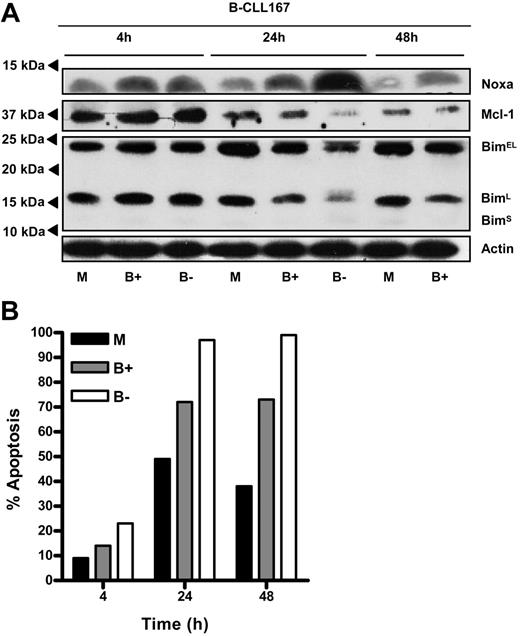
To establish a functional relationship between Noxa expression levels and apoptosis sensitivity of B-CLL cells, we made use of recent findings that proteasome inhibitors rapidly and specifically up-regulate Noxa.22,23,30 To reduce a widespread effect of proteasome inhibition on protein levels and transcription-dependent processes,31 PB B-CLL cells were transiently exposed to bortezomib for 4 hours. The reversible proteasome inhibitor was then either washed away or incubation was continued. As expected, a pulse of bortezomib treatment already caused a rise in Noxa protein, and this was sufficient to impair survival of B-CLL cells (Figure 5). Continuous exposure to bortezomib resulted in a massive increase in Noxa levels that was accompanied by almost 100% cell death at 48 hours. Over the course of this experiment, Mcl-1 protein levels first increased (4-hour time point), most likely because of proteasome inhibition, and then declined when cells went into apoptosis. This decline could be prevented by blocking caspase activity with z-VAD (data not shown) and is thus in accord with reports that Mcl-1 is a caspase substrate.32,33 Next, we investigated whether the level of Bim, another proapoptotic binding partner of Mcl-1,34 was also subject to change on bortezomib treatment and might thereby trigger apoptosis. However, Bim levels were unaffected, both as detected by RT-MLPA (data not shown) and by Western blotting (Figure 5A). Thus, pharmacologic manipulation of the levels of Noxa protein in B-CLL cells appeared to be directly related to viability in an in vitro setting.
Noxa up-regulation via transient treatment with bortezomib affects CLL survival. Freshly isolated peripheral blood B-CLL cells were treated for 4 hours with 20 nM proteasome inhibitor bortezomib. Cells were then washed and cultured in fresh medium, or incubation was continued. (A) At the indicated time points, cell lysates were prepared and probed for expression of Noxa, Mcl-1, Bim, and Actin protein by Western blot. Indicated below the lanes: untreated (M), bortezomib washed away after 4 hours (B+), and bortezomib without washing (B-). The decrease in Mcl-1 levels in bortezomib-treated cells at 24 and 48 hours could be inhibited by the pan-caspase inhibitor z-VAD (data not shown). Because of massive cell death after 48 hours in the presence of bortezomib, these lysates did not yield sufficient protein for analysis. (B) Apoptosis of cells was determined by annexin V staining. Spontaneous apoptosis in medium was approximately 50%, which was increased by bortezomib treatment. Results are representative for 3 separate experiments.
Noxa up-regulation via transient treatment with bortezomib affects CLL survival. Freshly isolated peripheral blood B-CLL cells were treated for 4 hours with 20 nM proteasome inhibitor bortezomib. Cells were then washed and cultured in fresh medium, or incubation was continued. (A) At the indicated time points, cell lysates were prepared and probed for expression of Noxa, Mcl-1, Bim, and Actin protein by Western blot. Indicated below the lanes: untreated (M), bortezomib washed away after 4 hours (B+), and bortezomib without washing (B-). The decrease in Mcl-1 levels in bortezomib-treated cells at 24 and 48 hours could be inhibited by the pan-caspase inhibitor z-VAD (data not shown). Because of massive cell death after 48 hours in the presence of bortezomib, these lysates did not yield sufficient protein for analysis. (B) Apoptosis of cells was determined by annexin V staining. Spontaneous apoptosis in medium was approximately 50%, which was increased by bortezomib treatment. Results are representative for 3 separate experiments.
Noxa-deficient cells exhibit resistance to bortezomib-induced cell death
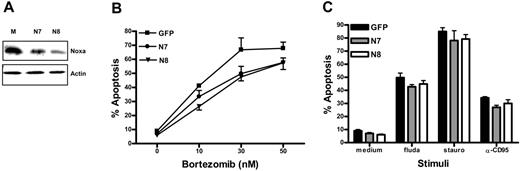
Apoptosis regulatory genes as detected by RT-MLPA were not affected during the short-term bortezomib treatment in the previous experiments (data not shown). Yet, it cannot be excluded that other genes and proteins besides Noxa that might effect survival were affected by bortezomib. Therefore, to investigate a direct role for Noxa in bortezomib-induced apoptosis, we used a model system. Ramos B cells (clone FSA)24 were transduced with distinct retroviral constructs encoding Noxa siRNAs (N7 or N827 ) or control GFP. GFP-positive cells were sorted, and Western blot analysis revealed a suppression of Noxa-levels to approximately 50% to 75% compared with the control-GFP (Figure 6A). Both Ramos FSA cell lines expressing Noxa RNAi exhibited a significant resistance to bortezomib-induced apoptosis compared with the mock-transduced cells (Figure 6B). The partial resistance to proteasome inhibitor-mediated apoptosis matched the partial knock-down of Noxa protein. Of note, also in Noxa RNAi cells, bortezomib treatment caused a rapid increase in Noxa protein (data not shown), thus explaining that apoptosis still occurred at a higher concentration of the drug. These data are in good agreement with effects of Noxa knock-down in other cell types (melanoma, mantle cell lymphoma, and T-cell leukemia).22,23,30 In addition, we obtained similar findings with another protease inhibitor (MG132; data not shown). In contrast, no effect of Noxa protein reduction was observed on apoptosis triggered by other pathways, such as fludarabine or staurosporin treatment, or triggering of the CD95 receptor (Figure 6C). In conclusion, these data demonstrate that decreased expression of Noxa has a direct and specific effect on the susceptibility to apoptosis induced by proteasome inhibitors. Conversely, the death-inducing effect of proteasome inhibition observed in B-CLL cells may therefore rely predominantly on shifts in Noxa expression.
Noxa reduction by RNAi specifically prevents apoptosis induction by proteasome inhibitors. Ramos Burkitt lymphoma cells were retrovirally transduced with 2 RNAi constructs targeting Noxa (N7 or N8) or GFP control. (A) Western blot demonstrating reduced Noxa expression in Ramos-N7 and-N8. Equal protein loading is shown by reprobing for β-actin. (B) Mock-, N7-, and N8-transduced Ramos FSA cells were cultured 24 hours in the presence of indicated concentration of bortezomib. Viability was assessed by annexin V/MitoTracker staining and FACS analysis. Data represent mean ± SD from 3 independent experiments. (C) Cells were incubated for 24 hours in medium containing 100 μM fludarabine (fluda), 0.25 μM staurosporine (stauro), or 5 μg/mL α-CD95, and analyzed as in panel B.
Noxa reduction by RNAi specifically prevents apoptosis induction by proteasome inhibitors. Ramos Burkitt lymphoma cells were retrovirally transduced with 2 RNAi constructs targeting Noxa (N7 or N8) or GFP control. (A) Western blot demonstrating reduced Noxa expression in Ramos-N7 and-N8. Equal protein loading is shown by reprobing for β-actin. (B) Mock-, N7-, and N8-transduced Ramos FSA cells were cultured 24 hours in the presence of indicated concentration of bortezomib. Viability was assessed by annexin V/MitoTracker staining and FACS analysis. Data represent mean ± SD from 3 independent experiments. (C) Cells were incubated for 24 hours in medium containing 100 μM fludarabine (fluda), 0.25 μM staurosporine (stauro), or 5 μg/mL α-CD95, and analyzed as in panel B.
Discussion
There is increasing awareness that the B-CLL population in lymphoid proliferation centers differs fundamentally from the well-studied fraction in PB and that this distinction may have clinical relevance.8,35 Here, we present a first direct comparison of these 2 populations, focusing on the expression of 34 apoptosis regulatory genes. Apart from expected differences in proliferation-related genes (Survivin and Ki67) and antiapoptotic Bcl-xL, we observed a prominent divergence in the expression of proapoptotic Noxa. Previously, we described that, compared with nonmalignant tonsil or peripheral B-cell fractions, B-CLL cells in the periphery display significantly increased levels of this BH3-only member of the Bcl-2 family, in a p53-independent manner.5 The high levels of Noxa and another BH3-only member Bmf6 contrasted with the purported antiapoptotic phenotype of B-CLL cells2 but remained functionally unexplained. Our new findings show that the Noxa level is considerably lower in LN CLL and that this is linked with survival capacity. Therefore, targeting Noxa expression or function could be of clinical benefit, also in p53-deficient cases.
In vitro CD40 stimulation of PB B-CLL cells resulted in a clear reduction of Noxa expression. Within the LN microenvironment, CD40 stimulation is most likely delivered by CD40L+ T cells. Several groups have investigated the effects of in vitro CD40 engagement in B-CLL cells,9,14,17,18,36-39 but an effect on Noxa expression was not yet reported. It is well known that CD40-stimulated B-CLL cells are more resistant to spontaneous or drug-induced apoptosis. This is most probably due to the induction of the transcription factor nuclear factor κB (NFκB) and, as a consequence, the expression of various antiapoptotic genes, such as Bcl-xL, cIAP2, A20, and Flip.36 Previously, Noxa was proposed to be a p53-response gene,40 but in B-CLL cells, Noxa is apparently not under the control of p53, as illustrated by the clearly divergent expression of Puma and Noxa on p53 stimuli.5,41 Later, various transcription factors were proposed to regulate Noxa such as E2F1, p73, and hypoxia-inducible factor HIF-1α.42-45 Therefore, at present it is difficult to definitely assign a specific signaling route that mediates Noxa expression. Very recently though, it was reported that HIF-1α is overexpressed in peripheral B-CLL cells,45 which may constitute a potential link to the increased Noxa levels in B-CLL.
Although CD40 stimulation of PB B-CLL cells resulted in a similar apoptosis gene expression profile to LN B-CLL, several genes deviated from this profile, most prominently Bid, as reported previously,17 but also A1/Bfl-1. This indicates that in the LN, B-CLL cells also receive other stimuli than CD40. Indeed, apart from CD4+ T cells expressing CD40L, other cell types can support survival of B-CLL cells. In vitro culture with an FDC cell line or dendritic cells protects B-CLL cells from spontaneous apoptosis.14,46 FDC-mediated survival was reported to depend on the expression of the Bcl-2 family member Mcl-1,14 and in vitro experiments revealed that Mcl-1 levels decline in B-CLL cells undergoing apoptosis.3,47 Interestingly, recent data indicate that Mcl-1 is a preferred binding partner of Noxa,34 and we have indeed observed association of Mcl-1 with Noxa in primary B-CLL samples (D.Y.H.H., manuscript in preparation). Furthermore, in 293T cells, Noxa has been described to mediate the degradation of Mcl-1.29 If this mechanism also holds true for B-CLL cells, it may explain the increase in Mcl-1 protein we observed in LN B-CLL, which was not accompanied by an increase in Mcl-1 mRNA. Accordingly, augmented Mcl-1 protein levels are a consequence of the down-regulation of Noxa in the LNs, rather than a environmental effect on Mcl-1 RNA expression. In addition, in vitro triggering of CD40 on B-CLL cells also influenced Mcl-1 levels in a posttranscriptional fashion (Figure 4). Thus, the differences in protein levels observed by us for Noxa, Mcl-1, and Bcl-xL levels in the B-CLL LN environment, correspond with current models based on the differential interaction potential of these Bcl-2 family members,29,34 and support the antiapoptosis phenotype of B-CLL cells at this location compared with PB. In addition, spontaneous apoptosis of B-CLL cells in vitro may be connected with the high levels of Noxa which eventually saturate the short-lived Mcl-1 protein.29,48
Two separate experimental approaches supported a direct role for Noxa in survival capacity of B-CLL cells. First, we used the recently discovered rapid and direct effect of bortezomib on Noxa levels22,23,30 to demonstrate that short-term bortezomib exposure also quickly induced Noxa protein in B-CLL cells, with a corresponding decrease in viability (Figure 5). Although the levels of Bim did not change on bortezomib treatment, a role for Bim during the actual triggering phase of apoptosis cannot be excluded. In model systems, Bim is capable of actively triggering Bax activation, whereas Noxa functions as “sensitiser.”49,50 Second, a complementary experiment was performed in a model system in which only Noxa levels were modified by RNAi. Here, a clear inhibitory effect of Noxa reduction toward apoptosis mediated by proteasome inhibition was observed, whereas other apoptosis pathways were unaffected (Figure 6).
Taken together, our data support a model in which the viability of the malignant B-CLL clone within the LNs, and possibly also the bone marrow, corresponds with low levels of Noxa and an up-regulation of Bcl-xL and Mcl-1. In addition to these antiapoptotic gene expression alterations, the B-CLL cells also receive proliferative stimuli as indicated by the Ki-67+ and Survivin+ cells. When the B-CLL cells enter the circulation, these stimuli are lost; Noxa is up-regulated; and Bcl-xL, Mcl-1, and Survivin are down-regulated. As a result, the B-CLL cells may become prone to apoptosis, which can however still be prevented by the continuous high expression of Bcl-2. To what extent circulating B-CLL cells are actually undergoing apoptosis is difficult to detect directly. Freshly isolated CLL cells are mostly nonapoptotic but undergo rapid “spontaneous” apoptosis in vitro, and recent calculations point to appreciable in vivo death rates.8 Together, this suggests that apoptotic B-CLL cells are rapidly cleared from circulation in vivo. It is generally assumed that in the LNs and bone marrow the B-CLL cells are relatively protected against therapeutic drugs.20 The circulating B-CLL cells that are already prone to apoptosis are more easily targeted, but the residual B-CLL cells in the LN/bone marrow will eventually lead to a relapse. Currently, there is much interest in application of novel, p53-independent drugs to treat B-CLL.51-53 Our data provide new insight into the regulation of the apoptotic behavior of B-CLL cells and also afford new clues for therapeutic intervention by targeting Noxa expression.
Authorship
Contribution: L.A.S. and D.Y.H.H. performed the experiments, analyzed the data, and wrote the paper; R.S., B.dG., A.J. and A.P.K. performed the experiments; M.H.vO. and C.vNo. designed and supervised the research; E.E. designed and supervised the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
L.A.S. and D.Y.H.H. contributed equally to the manuscript.
Correspondence: Eric Eldering, Departments of Hematology and Experimental Immunology, Academic Medical Center, Rm K0-144, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: e.eldering@amc.uva.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients for donating samples and the clinicians involved for their collaboration. This study was initiated after suggestions from Professor Steven Pals (Department of Pathology of the AMC) that investigation into survival and apoptosis of B-CLL cells should include lymph nodes. We thank Dr C.M. van der Loos, J.B.G. Mulder, and A.R. Musler for immunohistochemical stainings.
This work was supported by the Dutch Cancer Foundation (DCF).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal