Abstract
The BH3-only protein Bim is required for maintaining the homeostasis of the immune system, since Bim regulates the down-modulation of T-cell responses, mainly through cytokine deprivation. Using T-cell blasts from healthy donors and also from patients with autoimmune lymphoproliferative syndromes (ALPSs) due to homozygous loss-of-function mutation of FasL (ALPS-Ic) or heterozygous mutation in the Fas/CD95 death domain (ALPS-Ia), it is shown that the induction of Bim expression during the process of human T-cell blast generation is strictly dependent on FasL/Fas-mediated signaling. The main pathway by which Fas signaling regulates the levels of Bim expression in human T-cell blasts is the death-domain– and caspase-independent generation of discrete levels of H2O2, which results in the net increase of Foxo3a levels. The present results connect the 2 main pathways described until the moment for the control of T-cell responses: death receptor–mediated activation-induced cell death and apoptosis by cytokine deprivation.
Introduction
The regulated termination of T-cell immune responses is one of the mechanisms responsible for peripheral tolerance. Cytokine deprivation as a consequence of antigen exhaustion1,2 and activation-induced cell death (AICD)3 are 2 major mechanisms implicated in this regulation. This last mechanism was reported to be dependent on the Fas/Fas ligand (FasL) system4-6 and also on APO2 ligand/TNF-related apoptosis-inducing ligand (APO2L/TRAIL) and its receptors DR4 and DR5.7,8
It is generally accepted that CD4+ T cells are deleted mainly through the Fas/FasL system,9 whereas CD8+ T-cell deletion seems to be more dependent on cytokine deprivation. In humans, an in vivo study analyzing the primary antiviral response in Epstein-Barr virus (EBV)–infected patients showed that the deletion of activated antiviral CD8+ T cells takes place through cytokine deprivation.10 Using Bim knockout mice, it has been shown that deletion of CD8+ T-cell responses is mainly dependent on cytokine deprivation after antigen clearance and that this process is strictly dependent on Bim expression.11,12 Using CD8+ human T-cell blasts we have confirmed that apoptosis by cytokine deprivation predominates over death receptor ligation and that the maintenance of high Bim levels is necessary for apoptosis to occur.13
Defects of the Fas pathway were identified in human patients and the disease was named autoimmune lymphoproliferative syndrome (ALPS).14,15 ALPS associates lymphoproliferative manifestations, such as lymphadenopathies and hepatosplenomegaly, with a specific immunologic disorder consisting of hypergammaglobulinemia G sometimes associated with hyper IgA and the presence of an expanded population of TcR+CD4−CD8− double-negative (DN) T lymphocytes. Autoimmune manifestations are observed in most cases. ALPS (Online Mendelian Inheritance in Man [OMIM] 601859) is classified according to the underlying genetic defect: homozygous Fas mutation in type 0; heterozygous Fas mutation in type Ia; and, more rarely, heterozygous FasL mutation in type Ib; caspase 10 mutation in type IIa or caspase 8 mutation in type IIb; or unknown mutations in type III.16
We previously characterized 3 different ALPS patients: (1) one with a new homozygous mutation in the FasL gene (patient 1), which resulted in loss of function, and that could be considered as the first reported case of ALPS-Ic17 ; (2) a child and his father (patients 2 and 3) with a new heterozygous mutation in the Fas death domain, who are included in the ALPS-Ia group of patients.54 As previously reported in similar cases, although the proapoptotic potential of Fas was completely abrogated in the ALPS-Ia patient, the autoimmune and lymphoproliferative manifestations of the disease were more acute in the ALPS-Ic patient, indicating that death domain–devoid Fas could still be able to partially participate in the regulation of T-cell activation. In the present study we show that this type of nonapoptotic Fas signaling during the process of T-cell blast generation is needed for the induction of Bim expression and the sensitization of these cells to death by cytokine deprivation.
Patients, materials, and methods
The protocols of this study were approved by the Institutional Review Board of Hospital 12 de Octubre (Madrid, Spain). Informed consent was provided according to the Declaration of Helsinki.
T-cell blast generation
Treatment of T-cell blasts with increasing doses of IL-2: effects of a blocking FasL mAb
In some experiments, day-6 T-cell blasts obtained from healthy donors were incubated during additional periods (between 4 and 48 hours) with increasing doses of IL-2 (0, 30, 100, or 300 IU/mL) in the presence of 1 μg/mL of the anti-FasL–blocking monoclonal antibody (mAb) NOK-1 (Pharmingen, Barcelona, Spain). At the end of the incubations, cell extracts were obtained and immunoblots performed as indicated below. In the same experiments, cell growth was estimated by counting trypan blue–negative cells and results were expressed as N(t)/N(0) (number of viable cells at a time given (t)/number of viable cells at time 0) and apoptosis by analyzing the loss of mitochondrial membrane potential (ΔΨm) by DiOC6(3) staining and flow cytometry, as indicated elsewhere.18
Flow cytometry analysis
Surface and whole-cell expression of FasL was analyzed by flow cytometry in CD4+ and CD8+ T-cell blasts by double labeling with PE-labeled anti-CD4 or anti-CD8 mAbs (Coulter, Barcelona, Spain) and by FITC-labeled anti-FasL mAb H11 (Bender Medsystems, Vienna, Austria), as indicated in Martínez-Lorenzo et al.19 Whole-cell FasL expression was analyzed in fixed cells permeabilized with 0.3% saponin.19 The intracellular production of H2O2 was analyzed by labeling with dichlorofluorescein diacetate (DCF-DA; Molecular Probes, Leiden, The Netherlands) and flow cytometry, as indicated in Devadas et al.20
T-cell blast death induction by IL-2 deprivation
Day-6 or day-8 T-cell blasts generated in the presence of 30 IU/mL of exogenous IL-2 were cultured in the absence of IL-2 during an additional period of at least 48 hours. Apoptosis was estimated each 24 hours by analyzing the loss of ΔΨm by DiOC6(3) (Molecular Probes) staining and flow cytometry.
Treatment with inhibitors
In some experiments, day-6 T-cell blasts were treated during additional periods (between 4 and 48 h) with the following inhibitors: PD098059 (10 μM; Calbiochem, La Jolla, CA), a specific inhibitor of Mek, which abrogates ERK activity21 ; l-buthionine-S, R-sulfoximine (BSO; 400 μM; Sigma, Barcelona, Spain), which is a specific inhibitor of Glu cysteine ligase and thus of GSH synthesis22 ; reduced glutathione (GSH; 5 mM; Sigma), which supports glutathione peroxidase activities to eliminate cellular H2O2; and the inhibitor of NADPH oxidase diphenylene iodonium chloride (DPI; 1 and 0.5 μM; Sigma).23 After the treatments, cell extracts were obtained and immunoblot analysis was performed as indicated below in “Immunoblot analysis.” The generation of cellular H2O2 by BSO or its reduction by GSH or NOK-1 was tested in parallel using the flow cytometry methods described.
Immunoblot analysis
Cells (5 × 106) were lysed at 4°C in 100 μL of a buffer containing 2% SDS; 20 mM Tris/HCl, pH 6.8; and EDTA 1 mM and boiled for 15 minutes. Lysed cells (1 × 106) were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels, and separated proteins were transferred to nitrocellulose membranes. Membranes were then blotted with specific anti-Bim (0.5 μg/mL; Calbiochem), anti-BclXS/L (0.4 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA; sc-1041), anti–Bcl-2 (0.4 μg/mL; Santa Cruz Biotechnology; sc-783), anti–Mcl-1 (0.4 μg/mL; Santa Cruz Biotechnology; sc-819), anti-Akt (0.2 μg/mL; Cell Signaling, Hitchin, United Kingdom), anti-Foxo3a (1 μg/mL; Upstate, Lake Placid, NY) rabbit polyclonal antibody (pAb) or with anti–β-actin (0.32 μg/mL; Sigma; clone AC-15) mouse mAb, in the conditions described in Bosque et al.18 The phosphorylation state of Akt, ERK1/2, JNK1/2, or Foxo3a, which is associated with enzymatic activation of the kinases and with inhibition of the transcription factor was also tested by immunoblot with 0.2 μg/mL of rabbit pAb against phospho-Akt, phospho-ERK1/2 (both from Cell Signaling), phospho-JNK1/2 (MBL, Woburn, MA), or against Foxo3a phosphorylated in threonine 32 (Upstate). The expression level of the proteins analyzed was quantified in a densitometer (Bio-Rad, Barcelona, Spain) and normalized to the same amount of β-actin.
Statistical analysis
Mean values were compared using Student 2-tailed t test for independent means. Differences were not regarded as significant if the P value was greater than .05.
Results
Defective Bim induction in T-cell blasts from an ALPS-Ic patient, but normal Bim expression in ALPS-Ia patients
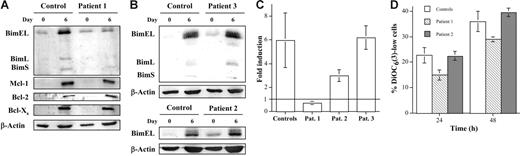
Patient 1 is an ALPS-Ic patient with a homozygous mutation in exon 4 of the FasL gene (A247E), corresponding to the FasL extracellular domain, which impairs FasL function. This has resulted in severe ALPS manifestations in the absence of systemic lupus erythematosus (SLE), characterized by lymphadenopathy, splenomegaly, hypergammaglobulinemia, and increased proportion of TcRαβ+ CD4−CD8− DN T lymphocytes. In vitro, T cells from patient 1 are characterized by impaired FasL-mediated cytotoxicity and resistance to PHA-induced AICD if compared with T cells from healthy donors, although they were equally sensitive to the effects of a cytotoxic anti-Fas mAb.17 As shown in Figure 1A and C, it was unexpectedly observed that although Mcl-1, Bcl-2, and Bcl-xS were induced during the process of T-cell blast generation at the same level as in healthy controls, Bim was not induced at all over the low levels present in freshly isolated peripheral-blood lymphocytes (PBLs).
The induction of Bim expression during human T-cell blast generation is abolished in an ALPS-Ic patient but not in ALPS-Ia patients. Sensitivity to death by IL-2 deprivation. Anti–Mcl-1, anti–Bcl-2, anti–Bcl-xS, anti-Bim, or anti–β-actin immunoblots were performed on extracts from fresh peripheral-blood mononuclear cells (PBMCs) (day 0) or from day-6 T-cell blasts (day 6), obtained from healthy donors (Control) or from the ALPS-Ic patient 1 (A) or ALPS-Ia patients 2 and 3 (B), as indicated. The positions of Mcl-1, Bcl-2, Bcl-xS, BimEL, BimL, BimS, and β-actin are indicated on the left of the corresponding blots. The extracts used correspond to 1 × 106 cells, and expression levels of the proteins analyzed were quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the BimEL/β-actin ratios in fresh PBMCs and in day-6 T-cell blasts from the same donors allowed the calculation of the fold induction of Bim in each case, and results are depicted in panel C. Results are the mean ± SD from 10 different healthy controls and from at least 2 different experiments performed with the individual ALPS patients 1, 2, or 3, as indicated. (D) Day-6 T-cell blasts were incubated at 2 × 106 cells/mL for 24 or 48 hours in the absence of IL-2. Apoptosis was estimated by analyzing ΔΨm loss by DiOC63 staining and flow cytometry, and results were expressed as the percentage of DiOC63-low cells. Results are the mean ± SD of duplicate determinations using T cells from 4 different healthy controls and of 2 different experiments using T cells from the patients. The samples shown in each immunoblot were run in the same gel.
The induction of Bim expression during human T-cell blast generation is abolished in an ALPS-Ic patient but not in ALPS-Ia patients. Sensitivity to death by IL-2 deprivation. Anti–Mcl-1, anti–Bcl-2, anti–Bcl-xS, anti-Bim, or anti–β-actin immunoblots were performed on extracts from fresh peripheral-blood mononuclear cells (PBMCs) (day 0) or from day-6 T-cell blasts (day 6), obtained from healthy donors (Control) or from the ALPS-Ic patient 1 (A) or ALPS-Ia patients 2 and 3 (B), as indicated. The positions of Mcl-1, Bcl-2, Bcl-xS, BimEL, BimL, BimS, and β-actin are indicated on the left of the corresponding blots. The extracts used correspond to 1 × 106 cells, and expression levels of the proteins analyzed were quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the BimEL/β-actin ratios in fresh PBMCs and in day-6 T-cell blasts from the same donors allowed the calculation of the fold induction of Bim in each case, and results are depicted in panel C. Results are the mean ± SD from 10 different healthy controls and from at least 2 different experiments performed with the individual ALPS patients 1, 2, or 3, as indicated. (D) Day-6 T-cell blasts were incubated at 2 × 106 cells/mL for 24 or 48 hours in the absence of IL-2. Apoptosis was estimated by analyzing ΔΨm loss by DiOC63 staining and flow cytometry, and results were expressed as the percentage of DiOC63-low cells. Results are the mean ± SD of duplicate determinations using T cells from 4 different healthy controls and of 2 different experiments using T cells from the patients. The samples shown in each immunoblot were run in the same gel.
Patients 2 and 3 are heterozygous ALPS-Ia patients with a mutation in the Fas/CD95 death domain. The clinical features in these patients are less severe than in the ALPS-Ic patient. In vitro, T cells from these patients are completely resistant to the effects on the anti-Fas cytotoxic mAb CH-11, whereas PHA-induced AICD is normal.54 The fact that apoptotic Fas signaling is completely abolished in T cells from these patients, although only 1 allele is mutated, indicates that the mutated form acts as a dominant-negative molecule, such as in the similar mice lprcg mutation.24 As shown in Figure 1B-C, Bim expression was induced during the process of T-cell blast generation at the same level in patients 2 and 3 and in healthy controls.
These results suggest that Bim induction during the process of human T-cell blast generation is dependent on Fas signaling that does not implicate the death domain.
Reduced Bim levels in T-cell blasts from the ALPS-Ic patient correlate with resistance to apoptosis by IL-2 deprivation
Results obtained in Bim knockout mice and also in human T-cell blasts have demonstrated that this proapoptotic protein is needed for the down-modulation of CD8+ T-cell responses, mainly dependent on cytokine deprivation.11-13,25 Since death by IL-2 deprivation in human T-cell blasts is typically dependent on the mitochondrial apoptotic pathway,2 we measured ΔΨm loss as a marker of apoptotic cell death. As shown in Figure 1D, T-cell blasts from patient 1 are more resistant than T-cell blasts from healthy donors to death by IL-2 deprivation or, at least, apoptosis is retarded. However, T-cell blasts from patient 2 are as sensitive to this type of deletion as T-cell blasts from healthy donors (Figure 1D).
In normal human T-cell blasts, Fas signaling is involved in the induction of Bim expression
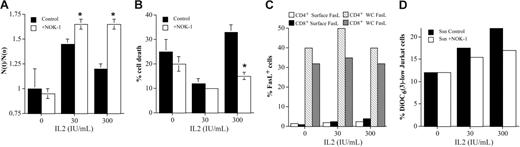
Next, experiments were performed using normal human T-cell blasts to confirm the physiologic validity of the observations made in cells from the patients. First, day-6 T-cell blasts from healthy donors were cultured for an additional 48-hour period in the presence of increasing doses of IL-2 (30, 100, or 300 IU/mL) and in the presence or absence of the blocking anti-FasL mAb NOK-1. In these experiments, the higher proliferation rate was obtained with 30 IU/mL of IL-2 (50% cell growth in 48 hours), whereas growth in the presence of 300 IU/mL was always lower (no more than 20% growth in 48 h). Culture in the presence of the anti-FasL–blocking antibody increased cell growth in the presence of 30 IU/mL to 65% and restored growth to the same level in the cultures made in the presence of 300 IU/mL (Figure 2A). In addition, while around 25% cell death was observed in the absence of IL-2, the basal level of cell death in T-cell blasts cultured in the presence of 30 IU/mL was clearly reduced, as expected. However, this percentage increased significantly to around 30% in cells cultured in the presence of 300 IU/mL of IL-2. This percentage of cell death was clearly reduced by the blocking anti-FasL mAb. Of note, cell death by IL-2 deprivation was not significantly prevented by the anti-FasL mAb (Figure 2B). Hence, culture of T-cell blasts with high concentrations of IL-2 favors growth control, and eventually cell death, by a FasL/Fas-dependent mechanism. As previously described,19,26,27 surface FasL expression was low in human T-cell blasts (below 5% FasL-positive cells in both CD4+ and CD8+ subsets), and the percentage of cells positive for whole-cell FasL staining was much higher (Figure 2C). This confirms that most of FasL is stored in intracytoplasmic compartments, characterized previously as multivesicular bodies.19,28 The low level of surface FasL expression was not increased by culture in the presence of increasing doses of IL-2 (Figure 2C). However, the cytotoxicity of supernatants from these cells on Jurkat cells increased with increasing doses of IL-2, and it was reduced in the presence of NOK-1 (Figure 2D). This demonstrates that the regulatory effect of the FasL/Fas system is mainly mediated by the secretion of bioactive FasL associated with exosomes, which is increased by high doses of IL-2.
Regulation by FasL of T-cell blast growth in the presence of increasing doses of IL-2. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, in the presence (□) or absence (▪) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1, and cell growth was estimated by counting trypan blue–negative cells. Results are expressed as the ratio between the number of viable cells at a given time (N(t)) and the number of viable cells at time 0 (N(o)). (B) In the same experiments, cell death was analyzed by counting trypan blue–positive cells, and results were expressed as the percentage of dead cells. Results are the mean ± SD of experiments performed using T-cell blasts from 3 different donors. *P < .05. (C) Using T-cell blasts from one of the donors used in panels A and B, the surface and whole-cell (WC) expression of FasL was evaluated by flow cytometry in CD4+ and CD8+ T-cell blasts. (D) Using T-cell blasts from the same donor, supernatants (Ssn) were collected after 48 hours of culture in the absence or presence of the indicated IL-2 concentrations and tested on Jurkat cells for 16 hours in the absence (Ssn control) or presence (Ssn+NOK-1) of 1 μg/mL of NOK-1. Results are expressed as the percentage of DiOC63-low Jurkat cells.
Regulation by FasL of T-cell blast growth in the presence of increasing doses of IL-2. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, in the presence (□) or absence (▪) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1, and cell growth was estimated by counting trypan blue–negative cells. Results are expressed as the ratio between the number of viable cells at a given time (N(t)) and the number of viable cells at time 0 (N(o)). (B) In the same experiments, cell death was analyzed by counting trypan blue–positive cells, and results were expressed as the percentage of dead cells. Results are the mean ± SD of experiments performed using T-cell blasts from 3 different donors. *P < .05. (C) Using T-cell blasts from one of the donors used in panels A and B, the surface and whole-cell (WC) expression of FasL was evaluated by flow cytometry in CD4+ and CD8+ T-cell blasts. (D) Using T-cell blasts from the same donor, supernatants (Ssn) were collected after 48 hours of culture in the absence or presence of the indicated IL-2 concentrations and tested on Jurkat cells for 16 hours in the absence (Ssn control) or presence (Ssn+NOK-1) of 1 μg/mL of NOK-1. Results are expressed as the percentage of DiOC63-low Jurkat cells.
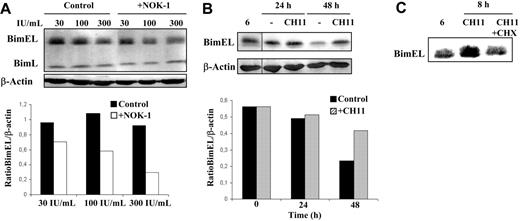
As shown in Figure 3A, in the presence of the different IL-2 doses, high Bim levels were maintained in the T-cell blast culture. However, the blocking of FasL interaction with Fas resulted in a net reduction in Bim levels, being the BimEL/β-actin ratio 3-fold lower at the higher IL-2 dose (see the quantification of Figure 3A). These data indicate that FasL/Fas signaling is needed in normal human T-cell blasts to maintain high levels of Bim expression. Another approach was to treat normal human T-cell blasts with 100 ng/mL of the anti-Fas mAb CH11 in the presence of IL-2 during different periods of time and follow the effect on Bim levels. In these conditions, CH11 does not induce a substantial amount of cell death on normal T-cell blasts but almost completely inhibits IL-2–dependent growth through a cell-cycle arrest in G2/M (data not shown for this experiment; Bosque et al18,29 ). As shown in the representative experiment of Figure 3B, Bim expression in human T-cell blasts was slowly decreasing with time in the presence of IL-2, being the BimEL/β-actin ratio after 48 hours 2.5-fold lower than at the beginning of the experiment. However, in the presence of CH11, that is, in the presence of forced additional Fas signaling, Bim levels were maintained at the same high level after at least 48 hours of culture. This Fas-mediated Bim induction was dependent on protein synthesis, since cycloheximide (CHX) inhibited the induction (Figure 3C).
FasL is implicated in maintaining high levels of Bim expression in normal human T-cell blasts. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, and in the presence (+NOK-1) or absence (Control) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. (B) Day-6 T-cell blasts were cultured for 24 or 48 hours, as indicated, in the absence (−) or presence of 100 ng/mL of the anti-Fas mAb CH-11 (CH11). (C) Day-6 T-cell blasts were cultured for 8 hours in the absence or presence of 100 ng/mL of the anti-Fas mAb CH-11, alone (CH11) or in combination with 1 μg/mL cycloheximide (CH11+CHX). After all the incubations described, cell extracts were obtained and anti-Bim or anti–β-actin immunoblots were performed. In panels B and C, extracts from day-6 T-cell blasts were also analyzed for comparison (noted as “6”). The extracts used correspond to 1 × 106 cells, and the expression level of BimEL was quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the BimEL/β-actin ratios are shown in panels A and B. Results are representative of experiments performed with T-cell blasts from at least 4 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the subpanels in panel B indicate that lanes were cut from the same immunoblot membrane.
FasL is implicated in maintaining high levels of Bim expression in normal human T-cell blasts. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, and in the presence (+NOK-1) or absence (Control) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. (B) Day-6 T-cell blasts were cultured for 24 or 48 hours, as indicated, in the absence (−) or presence of 100 ng/mL of the anti-Fas mAb CH-11 (CH11). (C) Day-6 T-cell blasts were cultured for 8 hours in the absence or presence of 100 ng/mL of the anti-Fas mAb CH-11, alone (CH11) or in combination with 1 μg/mL cycloheximide (CH11+CHX). After all the incubations described, cell extracts were obtained and anti-Bim or anti–β-actin immunoblots were performed. In panels B and C, extracts from day-6 T-cell blasts were also analyzed for comparison (noted as “6”). The extracts used correspond to 1 × 106 cells, and the expression level of BimEL was quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the BimEL/β-actin ratios are shown in panels A and B. Results are representative of experiments performed with T-cell blasts from at least 4 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the subpanels in panel B indicate that lanes were cut from the same immunoblot membrane.
Akt signaling could be implicated in Fas-mediated Bim induction in human T-cell blasts, but additional pathways are needed
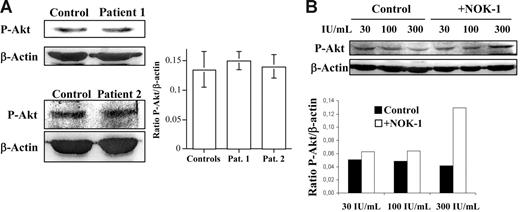
The most important pathway for transcriptional Bim induction is mediated by the activation of Forkhead (Foxo) transcription factors.30 Under survival signaling conditions, the PI3K-mediated activation/phosphorylation of Akt/PKB results in phosphorylation of Foxo transcription factors, leading to their sequestration in the cytoplasm. However, upon inactivation/dephosphorylation of Akt, Foxo gets dephosphorylated, migrates to the nucleus, and activates the transcription of specific genes.31 Therefore, we analyzed the phosphorylation status of Akt in T-cell blasts from healthy donors or from the ALPS patients studied by immunoblot. As shown in Figure 4A, at the same Akt expression level (data not shown) the phosphorylation status of Akt was not significantly greater in T-cell blasts from patients 1 or 2 than in T-cell blasts from healthy donors.
Normal phospho-Akt levels in T-cell blasts from ALPS patients. Role of FasL in controlling Akt activation. (A) Anti–phospho-Akt (P-Akt) or anti–β-actin immunoblots were performed on extracts from day-6 T-cell blasts, obtained from healthy donors (Control) or from ALPS-Ic patient 1 or ALPS-Ia patient 2, as indicated. (B) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, and in the presence (+NOK-1) or absence (Control) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. The extracts used correspond to 1 × 106 cells, and the levels of P-Akt were quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the P-Akt/β-actin ratios are shown in panels A and B. In panel A, results are the mean ± SD from 4 different healthy controls and from 2 different experiments performed with the individual ALPS patients, as indicated. In panel B, results are representative of experiments performed with T-cell blasts from 4 different healthy donors. The samples shown in each immunoblot panel were run in the same gel.
Normal phospho-Akt levels in T-cell blasts from ALPS patients. Role of FasL in controlling Akt activation. (A) Anti–phospho-Akt (P-Akt) or anti–β-actin immunoblots were performed on extracts from day-6 T-cell blasts, obtained from healthy donors (Control) or from ALPS-Ic patient 1 or ALPS-Ia patient 2, as indicated. (B) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of increasing doses of IL-2, as indicated, and in the presence (+NOK-1) or absence (Control) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. The extracts used correspond to 1 × 106 cells, and the levels of P-Akt were quantified in a densitometer and normalized to the same amount of β-actin. The results obtained for the P-Akt/β-actin ratios are shown in panels A and B. In panel A, results are the mean ± SD from 4 different healthy controls and from 2 different experiments performed with the individual ALPS patients, as indicated. In panel B, results are representative of experiments performed with T-cell blasts from 4 different healthy donors. The samples shown in each immunoblot panel were run in the same gel.
The phosphorylation level of Akt was also analyzed in experiments similar to those described in Figures 2 and 3A. As shown in Figure 4B, the level of phosphorylation of Akt decreased with increasing doses of IL-2, a decrease that was prevented by NOK-1, which, in the case of culture in the presence of 300 IU/mL, increased 3-fold the phospho-Akt/β-actin ratio.
Data in the literature point to down-modulation of Akt activity through caspase-dependent mechanisms.32,33 However, in similar experiments as those performed in Figures 3A and 4B, neither Z-DEVD-fmk nor ZIETD-fmk affected Bim levels at any IL-2 concentration (data not shown). These data, together with the normal status of Akt phosphorylation in T-cell blasts from the ALPS patients, indicate that the regulation of Akt activation through Fas ligation in human T-cell blasts, although occurring, is independent of the death domain and of caspase activation and is not the main pathway to regulate Bim levels.
Effect of FasL/Fas signaling on JNK and ERK activation in human T-cell blasts
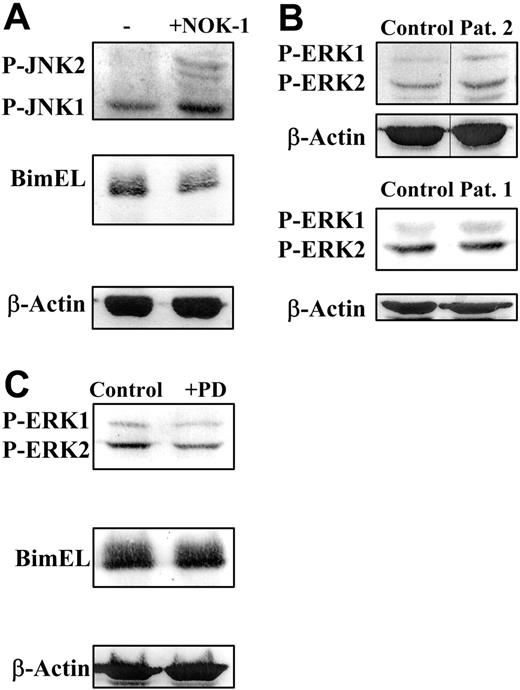
Another signaling pathway activated by Fas ligation, different from the activation of the caspase cascade, is the activation of JNK, at least in tumoral cells.34 However, no information exists on the regulation of JNK activation by Fas ligation in normal human T cells. In a first approach, we failed to detect a further JNK activation as a consequence of T-cell blast treatment with the cytotoxic anti-Fas mAb in the presence of IL-2 (data not shown). On the contrary, as shown in Figure 5A, while it was confirmed again that culture of human T-cell blasts for 48 hours in the presence of IL-2 and of the blocking anti-FasL mAb NOK-1 resulted in a net reduction in Bim levels, blocking of FasL/Fas signaling in the same conditions resulted in a net increase in the phosphorylation/activation of JNK1/2.
JNK and ERK are not the main pathways that control Bim levels in human T-cell blasts. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of 30 IU/mL of IL-2 and in the presence (+NOK-1) or absence (−) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. (B) Day-6 T-cell blasts, obtained from healthy donors (Control) or from ALPS-Ia patient 2 or ALPS-Ic patient 1, as indicated, were analyzed without further incubation. (C) Day-6 T-cell blasts from healthy donors were cultured for an additional period of 24 hours in the presence of 30 IU/mL of IL-2 and in the presence of either 0.2% DMSO (Control) or 10 μM PD098059, as indicated. After the incubations described, cell extracts were obtained and anti–phospho-JNK1/2 (P-JNK1/2; A), anti-Bim (A,C), anti–phospho-ERK1/2 (PERK1/2; B-C), or anti–β-actin (A-C) immunoblots were performed. The extracts used correspond to 1 × 106 cells, and the levels of P-ERK1 or P-ERK2 were quantified in a densitometer and normalized to the same amount of β-actin (not shown). Results are representative of experiments performed with T-cell blasts from 3 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the top subpanels in panel B indicate that lanes were cut from the same immunoblot membrane.
JNK and ERK are not the main pathways that control Bim levels in human T-cell blasts. (A) Day-6 T-cell blasts were cultured for an additional period of 48 hours in the presence of 30 IU/mL of IL-2 and in the presence (+NOK-1) or absence (−) of 1 μg/mL of the anti-FasL–blocking mAb NOK-1. (B) Day-6 T-cell blasts, obtained from healthy donors (Control) or from ALPS-Ia patient 2 or ALPS-Ic patient 1, as indicated, were analyzed without further incubation. (C) Day-6 T-cell blasts from healthy donors were cultured for an additional period of 24 hours in the presence of 30 IU/mL of IL-2 and in the presence of either 0.2% DMSO (Control) or 10 μM PD098059, as indicated. After the incubations described, cell extracts were obtained and anti–phospho-JNK1/2 (P-JNK1/2; A), anti-Bim (A,C), anti–phospho-ERK1/2 (PERK1/2; B-C), or anti–β-actin (A-C) immunoblots were performed. The extracts used correspond to 1 × 106 cells, and the levels of P-ERK1 or P-ERK2 were quantified in a densitometer and normalized to the same amount of β-actin (not shown). Results are representative of experiments performed with T-cell blasts from 3 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the top subpanels in panel B indicate that lanes were cut from the same immunoblot membrane.
Regarding ERK activation, only moderate increases or decreases in ERK phosphorylation were observed, respectively, in the presence of the anti-FasL–blocking mAb NOK-1 or of the agonistic anti-Fas mAb CH11 (data not shown), in agreement with similar experiments described in Jackson et al.27 However, the level of ERK activation in T-cell blasts from ALPS patients 1 or 2 was not significantly different from that observed in control T-cell blasts (Figure 5B). Finally, the Mek-specific inhibitor PD098059,21 which reduced ERK phosphorylation by more than 50%, did not at all affect Bim levels (Figure 5C). Thus, the status of ERK activation does not seem to play a main role in the regulation of Bim levels in human T-cell blasts.
Discrete H2O2 generation by Fas ligation is implicated in the induction of Bim expression in human T-cell blasts by increasing Foxo3a levels
Recently, several reports have described that Foxo transcription factors can be activated by reactive oxygen species (ROSs) production through Akt-independent pathways in different cellular settings.35-38 Since it has been demonstrated that Fas ligation during the process of human T-cell blast generation is associated with discrete ROSs production through activation of a phagocyte-like NADPH oxidase,20,27 we reasoned that this mechanism could also be acting on the transcriptional regulation of Bim expression. We used BSO, a specific inhibitor of GSH synthesis,22 and exogenous GSH, which supports glutathione peroxidase activities, to increase or decrease, respectively, the cellular content of H2O2. Neither BSO nor GSH was toxic at the concentrations used, indicating that the discrete levels of ROSs generated were well tolerated by cells and did not induce cell death. We measured the real amount of H2O2, by using the oxidation of DCF-DA, as described in Devadas et al.20 As shown in the representative experiment of Figure 6A, the total amount of H2O2 was clearly increased by BSO (77% increase) and somewhat reduced by GSH (around 20%). Interestingly, blocking FasL/Fas interaction reduced the total amount of H2O2 to the same extent as GSH (Figure 6A). To ascertain the effect of Fas ligation on the production of H2O2, we measured the intracellular amount of H2O2 after exposure of T-cell blasts to increasing concentrations of the anti-Fas mAb CH11. As shown in Figure 6B, the amount of H2O2 increased concomitantly with the mAb dose, arriving at a 21% increase at the highest dose used. As shown in Figure 6C for the same donor as in Figure 6A, BSO induced a remarkable increase in Bim levels compared with control T-cell blasts (75% increase). At the same time, it did not affect the Akt phosphorylation status, and the effect on the phosphorylation/activation status of ERK1/2 was very much limited. Conversely, GSH did reduce the levels of Bim (20% decrease) while not increasing the phosphorylation levels of Akt or ERK1/2 (Figure 6C). It is remarkable that the extent of the increase or reduction in Bim levels after treatment with BSO or GSH, respectively, normally correlated with the extent of increase or reduction exerted by these agents on the H2O2 levels. Finally, we also showed that the specific inhibitor of NADPH oxidase DPI23 effectively reduced Bim levels in T-cell blasts from healthy donors (Figure 6D) at the same time that it reduced H2O2 levels (23% reduction as a mean of 3 experiments; data not shown).
The levels of H2O2 control Bim expression in T-cell blasts are reduced in T-cell blasts from the ALPS-Ic patient. Day-6 T-cell blasts were cultured in the presence of 30 IU/mL of IL-2 for 24 hours in the absence of any additional treatment or in the presence of 400 μM BSO, 5 mM GSH, 1 μg/mL of NOK-1, or 0.5 μM DPI, as indicated. After the treatments, the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry (A), or extracts were obtained and anti–phospho-Akt, phospho-ERK1/2, anti-Bim, or anti–β-actin immunoblots performed (C-D). In panel B, day-6 T-cell blasts were treated with increasing doses of the agonistic anti-Fas mAb CH-11 for 16 hours, and intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. In panel A, results are expressed as the mean fluorescence intensity (MFI) values obtained in each case, and those values are also shown in panel B for each CH-11 concentration. In panel C, the levels of P-ERK1, P-ERK2, P-Akt, and BimEL were quantified in a densitometer and normalized to the same amount of β-actin, and the results obtained for the BimEL/β-actin, P-Akt/β-actin, and P-ERK1/2/β-actin ratios are shown. Results in panels A-D are representative of experiments performed with T-cell blasts from 3 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the subpanels in panel D indicate that lanes were cut from the same immunoblot membrane. (E) Day-6 T-cell blasts from a healthy control or from ALPS-Ic patient 1 were cultured for 24 hours in the presence of increasing doses of IL-2, as indicated, and the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. Results are expressed as the mean fluorescence intensity (MFI) values obtained in each case. (F) Day-6 T-cell blasts from a healthy control (black histogram) or from ALPS-Ic patient 1 (red histogram) were cultured for 48 hours in the presence of 300 IU/mL of IL-2, and the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. Numbers correspond to the MFI values in each case. Results are representative of 2 different experiments.
The levels of H2O2 control Bim expression in T-cell blasts are reduced in T-cell blasts from the ALPS-Ic patient. Day-6 T-cell blasts were cultured in the presence of 30 IU/mL of IL-2 for 24 hours in the absence of any additional treatment or in the presence of 400 μM BSO, 5 mM GSH, 1 μg/mL of NOK-1, or 0.5 μM DPI, as indicated. After the treatments, the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry (A), or extracts were obtained and anti–phospho-Akt, phospho-ERK1/2, anti-Bim, or anti–β-actin immunoblots performed (C-D). In panel B, day-6 T-cell blasts were treated with increasing doses of the agonistic anti-Fas mAb CH-11 for 16 hours, and intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. In panel A, results are expressed as the mean fluorescence intensity (MFI) values obtained in each case, and those values are also shown in panel B for each CH-11 concentration. In panel C, the levels of P-ERK1, P-ERK2, P-Akt, and BimEL were quantified in a densitometer and normalized to the same amount of β-actin, and the results obtained for the BimEL/β-actin, P-Akt/β-actin, and P-ERK1/2/β-actin ratios are shown. Results in panels A-D are representative of experiments performed with T-cell blasts from 3 different healthy donors. The samples shown in each immunoblot panel were run in the same gel. The vertical lines inside the subpanels in panel D indicate that lanes were cut from the same immunoblot membrane. (E) Day-6 T-cell blasts from a healthy control or from ALPS-Ic patient 1 were cultured for 24 hours in the presence of increasing doses of IL-2, as indicated, and the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. Results are expressed as the mean fluorescence intensity (MFI) values obtained in each case. (F) Day-6 T-cell blasts from a healthy control (black histogram) or from ALPS-Ic patient 1 (red histogram) were cultured for 48 hours in the presence of 300 IU/mL of IL-2, and the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry. Numbers correspond to the MFI values in each case. Results are representative of 2 different experiments.
Furthermore, the discrete basal or IL-2–generated levels of H2O2 are clearly lower in T-cell blasts from patient 1 than in comparable T-cell blasts from healthy donors at any IL-2 concentration tested (between 22% and 42% lower; Figure 6E). As shown in Figure 6F, after 48 hours of culture, the amount of H2O2 in T-cell blasts of patient 1 was greatly reduced (around 40%) with respect to the levels detected in control T-cell blasts.
Next, we analyzed whether the treatments described affected Foxo3a phosphorylation and could be correlated with Foxo3a's transcriptional activity on Bim, and, in parallel, if they affected the levels of the Foxo3a protein. As shown in the top panels of Figure 7A, while GSH, DPI, and NOK-1 clearly decreased the levels of Foxo3a, BSO increased them substantially. The reduction or increase of phospho-Foxo3a levels (Figure 7A bottom panels) correlated with the corresponding variation in the total amount of the protein in all cases except after the treatment with DPI, indicating that no additional regulation was exerted on Foxo3a activity apart from the regulation of its expression levels. In the case of DPI treatment, the phosphorylation level of Foxo3a was in addition almost completely abolished, in agreement with its inhibition of Akt phosphorylation (data not shown).
H2O2 control Bim expression through regulation of Foxo3a levels in T-cell blasts. (A) Day-6 T-cell blasts were cultured in the presence of 30 IU/mL of IL-2 for 24 hours in the absence of any additional treatment (Control) or in the presence of 5 mM GSH, 400 μM BSO, 1 μM DPI, or 1 μg/ml of NOK-1, as indicated. After the treatments, the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry, with similar percentages of increase or decrease as shown in Figure 6A (not shown), or extracts were obtained and anti-Foxo3a, anti–phospho-Foxo3a (P-Foxo3a), or anti–β-actin immunoblots performed. Results are representative of experiments performed with T-cell blasts from 4 different healthy donors. (B) Anti-Foxo3a or anti–β-actin immunoblots were performed on extracts from fresh PBMCs (day 0) or from day-6 T-cell blasts (day 6), obtained from healthy donors (Control) or from ALPS-Ia patient 2 (left panels), or from day-6 T cell blasts obtained from healthy donors (Control) or from ALPS-Ic patient 1 (right panels), as indicated. The extracts used correspond to 1 × 106 cells. Results are representative of experiments performed with T-cell blasts from 3 different healthy donors and of 2 different experiments performed with T cells from the ALPS patients. The vertical line in the left subpanel of panel B is due to smear of the sample, revealed in the immunoblot. The samples shown in each immunoblot panel were run in the same gel.
H2O2 control Bim expression through regulation of Foxo3a levels in T-cell blasts. (A) Day-6 T-cell blasts were cultured in the presence of 30 IU/mL of IL-2 for 24 hours in the absence of any additional treatment (Control) or in the presence of 5 mM GSH, 400 μM BSO, 1 μM DPI, or 1 μg/ml of NOK-1, as indicated. After the treatments, the intracellular levels of H2O2 were determined by DCF-DA staining and flow cytometry, with similar percentages of increase or decrease as shown in Figure 6A (not shown), or extracts were obtained and anti-Foxo3a, anti–phospho-Foxo3a (P-Foxo3a), or anti–β-actin immunoblots performed. Results are representative of experiments performed with T-cell blasts from 4 different healthy donors. (B) Anti-Foxo3a or anti–β-actin immunoblots were performed on extracts from fresh PBMCs (day 0) or from day-6 T-cell blasts (day 6), obtained from healthy donors (Control) or from ALPS-Ia patient 2 (left panels), or from day-6 T cell blasts obtained from healthy donors (Control) or from ALPS-Ic patient 1 (right panels), as indicated. The extracts used correspond to 1 × 106 cells. Results are representative of experiments performed with T-cell blasts from 3 different healthy donors and of 2 different experiments performed with T cells from the ALPS patients. The vertical line in the left subpanel of panel B is due to smear of the sample, revealed in the immunoblot. The samples shown in each immunoblot panel were run in the same gel.
As shown in the left panel of Figure 7B, Foxo3a levels are almost undetectable in freshly isolated PBLs from healthy donors, but Foxo3a expression is clearly induced in day-6 T-cell blasts, following a very similar pattern to that shown in Figure 1 or in previous studies18 for the induction of Bim expression. This pattern of induction was the same in T-cell blasts from patient 2, showing that the induction of Foxo3a, like that of Bim, is independent of signaling through the Fas death domain. However, as shown in the right panel of Figure 7B and again in agreement with the lack of Bim induction shown in Figure 1, no induction of Foxo3a was observed in T-cell blasts from patient 1.
These data indicate that the main pathway by which Fas signaling regulates the levels of Bim expression during the process of human T-cell blast generation is the production of discrete levels of H2O2, which regulates Foxo3a expression.
Discussion
The present study shows for the first time that the induction of Bim expression during the process of human T-cell blast generation is strictly dependent on FasL/Fas-mediated signaling. In addition, we show that the main pathway by which Fas signaling regulates the levels of Bim expression in these cells is the generation of discrete levels of H2O2, which results in the net increase of Foxo3a levels. It should be noted that this signaling pathway, activated by Fas ligation, is not related to apoptosis induction and should be differentiated from the death domain– and caspase-dependent apoptotic cascade.
The generation of discrete levels of ROSs by Fas ligation through the activation of a phagocyte-like NADPH oxidase was first described in several human leukemia cells.23 This rapid ROSs generation was differentiated from a second wave of ROSs generation that is caspase dependent, is associated with mitochondria depolarization, and is related to apoptosis induction. The initial wave of ROSs production, which is sensitive to DPI inhibition, was rather associated with hyper-polarization of mitochondria and has been interpreted as a protection mechanism.39-41 The generation of ROSs during the process of T-cell blast generation has recently been demonstrated to be dependent on Fas/FasL interaction through the activation of a phagocyte-like NADPH oxidase.27 Our present results indicate that the generation of discrete levels of H2O2 as a consequence of Fas ligation is the main pathway for the transcriptional control of Bim expression in human T-cell blasts. This induction of Bim expression seems to take place through the direct increase of Foxo3a levels by H2O2, something that has previously been reported in several cancer cell lines.38
How Fas ligation connects with the activation of NADPH oxidases has not been clarified yet. It does not seem to be dependent on caspase activation, since it is not inhibited by caspase inhibitors, and takes place in ALPS-Ia patients, in which proapoptotic signaling through the death domain is abrogated to the ALPS-Ia patients.54 In the case of TNF-α, it has been described that ROSs generation is associated with signaling through the kinase RIP.42 However, RIP recruitment to Fas is mediated by FADD43 or increased by c-FLIPL,44 which is also recruited to Fas through FADD.
Regarding other signaling pathways explored, our data indicate that although Fas signaling in T-cell blasts effectively down-modulates Akt activity and, hence, would increase Foxo3a activation and Bim transcription, this does not seem to be the main mechanism by which Fas regulates Bim levels in these cells. First of all, no significant differences in Akt activation levels were detected in T-cell blasts from the ALPS patients studied or from healthy donors. On the other hand, it has been reported in other cellular settings that caspases can directly or indirectly control Akt activation.32,33 However, in our experimental system, caspase inhibition did not at all affect Bim levels.
Recently, it has been shown that Foxo3a regulates the transcription of Bim and Puma during cytokine deprivation in mouse T cells.45 We have observed that both GSH and Z-DEVD-fmk reduce Puma expression in human T-cells blasts, indicating that caspase-mediated signal transduction could also be implicated (data not shown). However, we were not able to analyze Puma expression in T-cell blasts from the ALPS patients, so we cannot conclude regarding FasL/Fas implication in the regulation of Puma expression.
Fas ligation has also been reported to activate JNK, at least in tumoral cells.34 Although some controversy exists, JNK activation has been dissociated from Fas-induced apoptosis in several tumoral cell types.46 Our present data indicate that in normal human T-cell blasts, Fas ligation results in inhibition of JNK activation, in agreement with the idea that in these cells, JNK and AP-1 activation are proliferation signals activated as a consequence of stimulation through the TcR in the presence of cytokines.47 JNK could be inhibited by Fas ligation during long times of incubation, as is the case by the weak but sustained activation of NF-κB, which finally results in transcription, among other genes, of GADD45β, which binds to the JNK kinase MKK7/JNKK2, down-modulating JNK activation.48
On the other hand, it has been shown that ERK down-regulates Bim expression by direct phosphorylation, ubiquitination, and degradation in the proteasome49 and that Fas ligation down-modulates ERK activation in human T-cell blasts.27 However, our data indicate that this pathway does not seem to predominate in the control of Bim expression in these cells, although it could undoubtedly contribute to the fine-tuning of the system.
The present results have pathologic implications that can help us to understand the different phenotypes of ALPS or of other autoimmune diseases. The only 2 reported cases of ALPS due to loss-of-function mutations in FasL, in which Fas signaling is completely abrogated because it cannot be ligated, are especially serious: one of them is associated with development of SLE (ALPS-Ib50 ) and the other with elevated titles of antinuclear autoantibodies, autoimmune thrombopenia, and hemolytic anemia (ALPS-Ic17 ). On the other hand, in ALPS-Ia patients, in whom signaling through the death domain is abrogated but other nonapoptotic signaling like the one described in the present work (Fas-> ROSs > Bim) are maintained, disease is milder, with more cases reported (Rieux-Laucat et al16 ; Del Rey et al).
In addition, the present data connect the 2 main pathways that control T-cell responses: death receptor–mediated AICD and death by cytokine deprivation.51 Death by cytokine deprivation, dependent on Bim expression, has been demonstrated to be the main pathway for deletion of CD8+ T-cell blasts after a response against infection using Bim knockout mice11,12 or in humans infected with EBV.10 Our own studies using human CD8+ T-cell blasts have shown that apoptosis by cytokine deprivation predominates over death receptor ligation and that high levels of Bim expression are necessary for this process to occur.13 The expression of Bim and Bcl-xS clearly increases during the process of T-cell blast generation,13,18,52 arriving at maximum levels around day 5 or 6 of stimulation, just at the point where human T-cell blasts begin to be sensitive to apoptosis and AICD.8,53 Once the concentration of stimulatory cytokines decreases, prosurvival signals diminish and the levels of Bcl-2, Bcl-xL, and Mcl-1 rapidly decrease, whereas the levels of Bim expression are maintained high. In these conditions, T-cell blasts die through the activation of the mitochondrial apoptotic pathway by defect.13 The present data indicate that nonapoptotic Fas signaling during the process of human T-cell blast generation is responsible for the increase in Bim expression that prepares activated T cells to be deleted once infection is cleared and the amount of stimulatory cytokines decreases.
Authorship
Contribution: A.B., A.A., M.A.A., E.P.-A., J.N., and L.M.A. designed the research; A.B., J.I.A., and L.M.A. performed the research; A.B., J.I.A., M.A.A., and A.A. provided analytical tools; A.A., A.B., J.N., and L.M.A. analyzed the data; and A.A., A.B., L.M.A., and J.N. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Anel, Dept Bioquímica y Biología Molecular y Celular, Facultad de Ciencias, Universidad de Zaragoza, Zaragoza, E-50009, Spain; e-mail:anel@posta.unizar.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants SAF2004-03058 (A.A.) and BM2001-3865 (M.A.A.) from Ministerio de Educación y Ciencia/Fondo Social Europeo (Spain). A.B. was supported by a Formación del Personal Universitario (FPU) fellowship from Ministerio de Educación y Ciencia and J.I.A. by a Formación de Personal Investigador (FPI) fellowship ascribed to grant SAF2004-03058.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal