Abstract
Recent evidence from this laboratory indicated that reduced expression of caveolin-1 accompanied the diminished expression of tight junction (TJ)–associated proteins occludin and zonula occludens-1 (ZO-1) following stimulation of brain microvascular endothelial cells (BMECs) with the chemokine CCL2 (formerly called MCP-1). Because attenuated caveolin-1 levels have also been correlated with heightened permeability of other endothelia, the objective of this study was to test the hypothesis that reduced caveolin-1 expression is causally linked to the action of CCL2 on BMEC junctional protein expression and barrier integrity. This was achieved using adenovirus to nondestructively deliver caveolin-1 siRNA (Ad-siCav-1) to BMEC monolayers, which model the blood-brain barrier (BBB). Treatment with siRNA reduced the caveolin-1 protein level as well as occludin and ZO-1. Additionally, occludin exhibited dissociation from the cytoskeletal framework. These changes were attended by comparable alterations in adherens junction (AJ)–associated proteins, VE-cadherin and β-catenin, increased BMEC paracellular permeability, and facilitated the ability of CCL2 to stimulate monocytic transendothelial migration. Furthermore, treating BMECs with cavtratin, a synthetic cell-permeable peptide encoding the caveolin-1 scaffolding domain, antagonized effects of both Ad-siCav-1 and CCL2. These results collectively highlight caveolin-1 loss as a critical step in CCL2-induced modulation of BMEC junctional protein expression and integrity, and possibly serve a crucial role in regulating inflammation at the BBB.
Introduction
Elevated permeability of the normally highly restrictive blood-brain barrier (BBB) accompanies a variety of central nervous system (CNS) afflictions, including inflammation, infection, ischemia, seizures, and trauma.1-6 Nevertheless, mechanisms regulating BBB permeability in physiological and pathophysiological situations remain poorly defined. Among the many factors affecting BBB permeability, this laboratory recently reported that the beta-chemokine CCL2 (formerly known as monocyte chemoattractant protein-1 [MCP-1]), which is elevated in the CNS during a variety of neuroinflammatory conditions,7-10 plays an influential role.11 Specifically, it effects dissociation of tight junction (TJ)–associated proteins occludin and zonula occludens-1 (ZO-1) from the cytoskeletal framework of brain microvascular endothelial cells (BMECs) comprising the BBB as well as diminished expression of these proteins. Given the proposed role(s) of TJs in restricting solute and cellular passage across endothelial and epithelial barriers,12-14 such action could conceivably lie, in part, at the basis of the altered BBB permeability and accumulation of leukocytes in the CNS observed in certain neuroinflammatory episodes.
Aside from the loss of occludin and ZO-1 following BMEC exposure to CCL2, expression of caveolin-1 was also significantly down-regulated.11 This additional chemokine-associated loss may further contribute to BBB alteration, because caveolin-1 is the major structural protein of caveolae, membrane microdomains critically involved in various aspects of vesicular trafficking and cell signaling.15-17 Of particular significance in this regard, Nusrat et al18 reported that both occludin and ZO-1 might be organized within TJs by association with caveolin-1 in detergent-insoluble glycolipid rafts, membrane specializations closely related to caveolae. Association of caveolin-1 with CCL2 action on BMECs was additionally implied by our prior description that CCL2 internalization by these cells was dependent on caveolae integrity and the observation of CCL2–caveolin-1 colocalization.19 Further inferring a possible linkage between diminished caveolin-1 expression and heightened endothelial barrier permeability, caveolin-1 knockout mice suffer microvascular hyperpermeability.20 Also, the cell-permeable, caveolin-1–derived peptide cavtratin reduces microvascular hyperpermeability,21 as does endothelial-specific overexpression of caveolin-1.22 These reports collectively inspire the hypothesis that CCL2-induced down-regulated expression of caveolin-1 contributes to TJ alterations that accompany BMEC stimulation by this chemokine. Accordingly, caveolin-1 might be considered a critical determinant of BBB permeability.
To begin addressing the issue of whether down-regulation of caveolin-1 might be complicit in the alteration of occludin and ZO-1 provoked in BMECs by exposure to CCL2, we first sought to determine whether targeted loss of caveolin-1 in these cells is coupled to reduced expression of these TJ-associated proteins. Expression of caveolin-1 was directly and specifically knocked down in monolayer cultures by transduction with an adenoviral construct encoding caveolin-1 small interfering RNA (siRNA). By evoking down-regulation of caveolin-1 specifically in confluent BMECs, as opposed to disperse cultures, linkage of caveolin-1 expression to junctional integrity was established under conditions reflecting the BBB. Our findings reveal for the first time that targeted down-regulation of caveolin-1 leads to a loss and redistribution of junctional proteins in BMECs that is accompanied by heightened paracellular permeability and CCL2-stimulated monocytic transendothelial migration. Further underscoring the role of caveolin-1 loss in these responses, they were antagonized by application of a cell-permeable peptide that mimics several of caveolin-1's regulatory activities.
Materials and methods
Reagents and chemicals
Unless otherwise specified, regents were purchased from Sigma-Aldrich (St Louis, MO).
Animals
Mice (strain B6219PF2/J; Jackson Laboratory, Bar Harbor, ME) aged 3 to 5 weeks were used as the source of microvessels. Animals were killed by CO2 inhalation in accordance with measures stipulated by the Animal Care and Use Guidelines of the University of Connecticut Health Center.
Cell cultures
Mouse brain microvascular endothelial cells.
BMECs were prepared according to the method recently described by this laboratory.23 Cultures were at least 99% endothelial, as judged by staining with antibodies to CD31 and factor VIII–related antigen and uptake of diI-acetylated LDL. Freshly isolated cells were grown in DMEM/F12 containing 10% plasma-derived horse serum, 10% fetal bovine serum, 1% antibiotic-antimycotic (all from GIBCO BRL, Rockville, MD), 100 μg/mL heparin, and 100 μg/mL endothelial cell growth supplement (BD Biosciences, Bedford, MA) to confluence in 35-mm plates coated with collagen IV (BD Biosciences) and passaged only one time for experimentation. For all experiments, BMECs were plated onto Transwell filter inserts (Costar, Cambridge, MA). Unless stated otherwise, 12-well format, 1.0 μm–pore filters were used.
Cell lines.
The human embryonic kidney cell line HEK 293 and the mouse monocytic line WEHI-265.1 were obtained from American Type Culture Collection (ATCC; Manassas, VA) and maintained at 37°C in a 5% CO2 humidified atmosphere. HEK 293 cells were grown in DMEM containing 10% fetal bovine serum, 2 mM l-glutamine, and 50 U/mL penicillin/streptomycin. WEHI-265.1 was grown in DMEM containing 10% fetal bovine serum, 2 mM l-glutamine, 50 U/mL penicillin/streptomycin (all from GIBCO BRL), and 0.05 mM 2-mercaptoethanol.
Adenovirus-mediated delivery of caveolin-1 siRNA
Rationale.
To evaluate the role of caveolin-1 in regulating TJ alterations in a model of the BBB, it was necessary to transiently knock down caveolin-1 expression in a confluent monolayer of BMECs. This qualification precluded introducing siRNA by transfection, because nonproliferating epithelial/endothelial monolayers resist nucleic acid uptake and the transfection process itself significantly damages endothelial integrity.24,25 Hence, caveolin-1 knockdown was achieved by transduction of just subconfluent BMECs with recombinant adenovirus encoding a hairpin loop siRNA (Ad-siCav-1) that targeted the caveolin-1 transcript. This was feasible because mouse endothelial cells contain the requisite coxsackievirus and adenovirus receptor (CAR) that mediates viral attachment and infection.26 Exposing BMECs to virus at the just subconfluent state, when junctional complexes are not yet fully elaborated, served 2 purposes: (1) It enabled interaction of virus with the CAR, which has been reported to be associated with TJs of epithelial cells and therefore inaccessible to virus applied to the apical surface of tight, confluent monolayers27 ; and (2) it allowed BMECs to achieve confluence and establish a morphologic facsimile of the BBB within 96 hours after inoculation, at which time siRNA-induced reduction of caveolin-1 expression was maximal.
siRNA constructs.
The cDNA sequence of caveolin-1 was obtained from GenBank (accession no. NM_007616). A target sequence for siRNA was selected according to the siRNA oligonucleotide design criteria (BD Knockout RNAi Systems; BD Clontech, Palo Alto, CA) and further analyzed by basic local alignment sequence tool (BLAST) research to avoid a significant homology with other genes. This sequence for mouse caveolin-1 mRNA was 5′-GGAGATTGACCTGGTCAAC-3′, which started at position 221. The siRNA Shuttle constructs were established using the BD Knockout RNAi Systems kit (BD Clontech). In brief, sense and antisense oligonucleotides of self-complementary hairpin sequences (including the termination signal of 5 thymidines and an EcoRV site immediately downstream of the terminator sequence), which contain cohesive ends for BamHI and EcoRI at the 5′ and 3′ ends, were synthesized and annealed by consecutively heating at the following parameters: 95°C for 30 seconds, 72°C for 2 minutes, 37°C for 2 minutes, and 25°C for 2 minutes. The annealed oligonucleotides were then inserted into the BamHI and EcoRI sites of pShuttle, a plasmid containing the human U6 gene-based RNA polymerase III promoter. The resultant vector, pShuttlesiCav-1, was amplified by transforming into BD Fusion-Blue competent cells (BD Clontech). The constructs were selected by kanamycin resistance and confirmed by EcoRV restriction digest analysis. The plasmid pShuttlesiLuc, with similar structure to pShuttlesiCav-1 but encoding the luciferase gene, was used as a control. The luciferase siRNA oligonucleotides were provided with the kit.
Adenovirus constructs.
Each siRNA-expressing cassette (in pShuttle), flanked by I-CeuI and PI-SceI sites, was digested with these 2 enzymes and ligated to the E1- and E3-deleted Adeno-X Viral DNA (BD Adeno-X Expression System 1; BD Clontech). The resultant adenoviral DNA was transformed into BD Fusion-Blue competent cells and selected by ampicillin resistance. The adenoviral constructs were further confirmed by polymerase chain reaction (PCR) analysis. PCR was performed employing the Adeno-X PCR Primer Set 2 (BD Clontech) and using a Stratagene (La Jolla, CA) model Robocycler Gradient 40 PCR machine. The forward primer was provided in the kit, and the reverse primer was synthesized according to the insert (5′-GAGATTGACCTGGTCAAC-3′). The PCR cycles were 94°C for 2 minutes, 94°C for 30 seconds, followed by 69°C for 2 minutes for 30 cycles, and then 68°C for 5 minutes.
Adenovirus amplification and purification.
Recombinant adenoviral DNA was digested with PacI (New England Biolabs, Beverly, MA) to expose the inverted terminal repeats (ITRs) and then transfected into low-passage HEK 293 cells by a standard Lipofectamine-mediated method. Seven days following transfection, crude virus was prepared from the transfected cells by performing the following freeze-thaw cycle 3 consecutive times: (1) freeze cells in a dry ice/ethanol bath; (2) thaw cells by placing the tube in a 37°C water bath; and (3) vortex cells after thawing. To prepare high-titer stocks, recombinant adenovirus was amplified in HEK 293 cells by several rounds of infection. After 6 rounds of infection, cytopathic effect (CPE) was evident within 1 week. The virus stock prepared at this point was designated “primary amplification.” Further concentration and purification of virus were carried out by banding on a discontinuous CsCl density gradient.28 In brief, media from 10 infected 150-cm2 flasks were cleared by centrifugation at 10 000g for 10 minutes, and 6.5 mL of the resulting virus supernatant was layered atop a discontinuous CsCl gradient: 3 mL CsCl at 1.2 g/mL over 3 mL CsCl at 1.4 g/mL (both CsCl solutions were prepared in 10 mM Tris-HCl, pH 7.9). Centrifugation was carried out in an SW40 rotor at 155 000g at 4°C for 90 minutes. The lowest visible band containing infectious particles was harvested by needle side puncture, and the virus was dialyzed in a Pierce Slide-A-lyzer 10K cassette (Pierce, Rockford, IL) against several changes of 10% (vol/vol) glycerol, 10 mM Tris-HCl (pH 7.4), and 1 mM MgCl2, dispensed into 20-μL aliquots and frozen at −80°C. Virus stocks were titered by immunoperoxidase plaque assay (BD Adeno-X Rapid Titer Kit; BD Clontech) following the manufacturer's instructions.
Transduction of BMECs.
BMECs were grown to approximately 90% confluence (ie, 90% of maximal cell number) in normal growth medium on Transwell filter inserts. The growth medium was then removed, and 0.2 mL fresh medium containing adenovirus was added to the top chamber of each Transwell. Plates containing the Transwells were tipped several times to make the virus spread evenly. The plates were incubated at 37°C in a 5% CO2 humidified atmosphere for 4 hours to allow the virus to infect the cells before the addition of 0.3 mL fresh growth medium to each well. After incubation at these conditions for 96 hours, cells were assayed as indicated in the text.
Peptides
Cavtratin, the fusion peptide of caveolin-1 scaffolding domain (amino acids 82 to 101, DGIWKASFTTETVTKYWFYR) with Antennapedia internalization sequence (RQIKIWFQNRRMKWKK), was custom synthesized by the molecular biology resource facility at the University of Oklahoma Health Sciences Center with sequence identical to that published by Bucci et al.29 A 10-mM stock solution was prepared with 100% DMSO. The final concentration of the peptide used for cell treatment was 2 μM diluted in the cell-culture medium.
Immunocytochemistry
Immunocytochemical detection of caveolin-1 and junctional proteins in confluent BMEC monolayers was performed as detailed previously.11,23 Primary antibodies, their sources, and dilutions are listed in Table 1. Fluorescent detection was achieved with FITC-conjugated secondary antibody (at 1:200 dilution). Labeled antirabbit, antimouse, and antirat secondary antibodies were purchased from Vector Labs (Burlingame, CA). Images were viewed and photographed under a Zeiss LSM 410 confocal microscope (20×/0.5 NA objective; Oberkochen, Germany).
Primary antibodies used for Western blotting and immunocytochemistry
| Name . | Source . | Dilution . | |
|---|---|---|---|
| WB . | ICC . | ||
| ZO-1 rabbit polyclonal IgG | ZYMED Laboratories (Carlsbad, CA) | 1:500 | 1:50 |
| Occludin rabbit polyclonal IgG | ZYMED Laboratories | 1:1000 | 1:50 |
| β-catenin mouse monoclonal IgG1 | BD Transduction Labs (Lexington, KY) | 1:1000 | 1:50 |
| VE-cadherin rat monoclonal IgG2a | BD Pharmingen (San Diego, CA) | 1:500 | 1:50 |
| Caveolin-1 rabbit polyclonal IgG | BD Transduction Labs | 1:10 000 | 1:1000 |
| Clathrin rabbit polyclonal IgG | BD Transduction Labs | 1:1000 | — |
| β-tubulin mouse monoclonal IgG1 | ZYMED Laboratories | 1:500 | — |
| Name . | Source . | Dilution . | |
|---|---|---|---|
| WB . | ICC . | ||
| ZO-1 rabbit polyclonal IgG | ZYMED Laboratories (Carlsbad, CA) | 1:500 | 1:50 |
| Occludin rabbit polyclonal IgG | ZYMED Laboratories | 1:1000 | 1:50 |
| β-catenin mouse monoclonal IgG1 | BD Transduction Labs (Lexington, KY) | 1:1000 | 1:50 |
| VE-cadherin rat monoclonal IgG2a | BD Pharmingen (San Diego, CA) | 1:500 | 1:50 |
| Caveolin-1 rabbit polyclonal IgG | BD Transduction Labs | 1:10 000 | 1:1000 |
| Clathrin rabbit polyclonal IgG | BD Transduction Labs | 1:1000 | — |
| β-tubulin mouse monoclonal IgG1 | ZYMED Laboratories | 1:500 | — |
WB indicates Western blotting; ICC, immunocytochemistry; —, not done.
Western blotting
Cells were grown to confluence on Transwell filter inserts as for immunocytochemistry (ICC). For experiments in which total cellular protein was analyzed, cells were lysed in 200 μL of 1 × boiling sample buffer (6.3 mM Tris base [pH 6.8], 10% [wt/vol] glycerol, and 1% SDS) and equal amounts of protein subjected to Western blotting as described.11,23 Primary antibodies, their sources, and dilutions are listed in Table 1. Blots were developed with corresponding HRP-conjugated secondary antibodies (Cappel, Aurora, OH) and the chemiluminescent HRP substrate kit (SuperSignal West Femto Maximum Sensitivity; Pierce) according to the manufacturer's specifications and immediately exposed to Kodak X-OMAT AR film (Eastman Kodak, Rochester, NY). Relative quantification of proteins was determined by measuring the mean pixel intensity associated with individual bands (of constant pixel size) on scanned autoradiographs using Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA).
To gauge the relative association of TJ- and adherens junction (AJ)–associated proteins with the cytoskeleton, detergent-soluble (SOL) and cytoskeletal (CSK) fractions were generated as reported.11,23 Equal volumes of the respective fractions, constituting cellular equivalents, were then subjected to Western blotting. By analyzing cellular equivalents rather than equal protein amounts, the relative distributions of TJ and AJ proteins between the 2 fractions were determined by the SOL/CSK ratio for each protein.
BMEC permeability
BMECs were grown to confluence on Transwell filter inserts (24-well format, 1.0-μm pore), and monolayer paracellular permeability was determined as reported by Mark and Davis.30 Briefly, 100 μg/mL fluorescein dextran, Mwr 40 000 (FDX-40000; Molecular Probes, Eugene, OR), in assay buffer (0.1% BSA in DMEM) was added to the upper chamber. Samples (50 μL) were removed from the lower chamber at 0, 30, 60, and 120 minutes and replaced with equal volumes of assay buffer. The concentration of FDX-40000 applied to the upper chamber was assessed by retrieving a 50-μL sample at 0 minutes and similarly replacing this with 50 μL assay buffer. Retrieved samples were analyzed using a Victor Wallac 1420 multilabel plate reader (Perkin Elmer/Wallac, Gaithersberg, MD) with fluorescence-detecting capabilities (excitation λ 488 nm; emission λ 510 nm). A permeability coefficient (PC) for FITC-dextran was determined by the following equation: PC (cm/min) = V/(SA × Cd) × (Cr/T), where V is the volume in the receiver (lower) chamber (1.5 cm3), SA is surface area of the cell monolayer (0.3 cm2), Cd is the concentration of marker in the donor chamber at time 0, and Cr is the concentration of marker in the receiver at sampling time T. PC was determined for replicate samples and a mean value established. Change in permeability following Ad-siCav-1 treatment was reported as percent of mean control PC value.
Transendothelial migration
Transendothelial migration was carried out and analyzed as recently described by this laboratory.31 In short, BMECs were subcultured onto Transwell filter inserts (24-well format, 8.0-μm pore) that had been previously coated with a thin layer of hydrated collagen gel (1.93 mg/mL collagen type I and 0.07 mg/mL collagen type IV; BD Biosciences). Coating in this manner serves to block penetration of BMECs across the membrane while not affecting transchamber migration of monocytic cells.32 After washing the cells with migration medium (DMEM containing 0.2% BSA and 10 mM HEPES, pH 7.4), the assay commenced by adding WEHI 265.1 cells (1 × 105 in 500 μL migration medium) to the upper chamber and CCL2 (10 nM in migration medium) to the lower chamber. Following incubation at 37°C for designated times, the upper chamber was aspirated and the bottom filter surface rinsed with 0.5 mL media from the lower chamber to dislodge any adherent, transmigrated cells. The entire contents of the lower chamber were then transferred to a conical bottom tube and centrifuged at 250g for 5 minutes at room temperature. After centrifugation, all but approximately 100 μL media was removed, leaving behind the pelleted WEHI cells. The pellet was then resuspended in the remaining media by gentle pipetting and the dissociated cells transferred to a 96-well black-assay plate (Corning, Corning, NY). The relative amount of viable, migrated cells was then quantified using the Cell Titer-Glo Cell viability kit (Promega, Madison, WI), which is based on generation of a luminescent signal proportional to the amount of ATP present and, therefore, a reflection of the number of viable cells. Samples were read on a Victor Wallac 1420 multilabel plate reader (Perkin Elmer/Wallac) with luminescence-detecting capabilities.
Statistics
Statistical comparison of the data was performed using the Student t test. A P value of less than .05 was considered to be significant.
Results
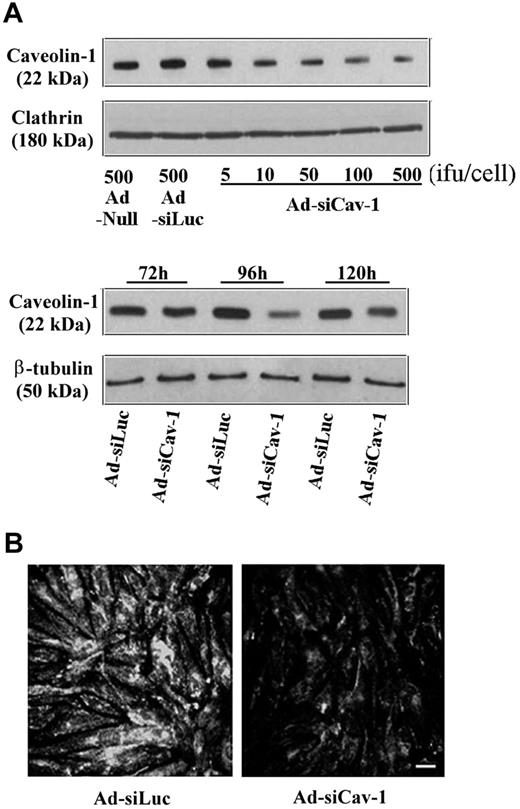
The ability of Ad-siCav-1 to specifically knock down caveolin-1 protein is shown in Figure 1. By Western blotting (Figure 1A), a reduction in caveolin-1 protein level was detected at 96 hours after inoculation with as little as 10 infectious units (ifu) per cell of Ad-siCav-1 when compared with cells inoculated with null virus (harboring no siRNA coding sequence). The 96-hour time point was intentionally selected for performing analysis because BMECs reached full confluence by this time and, in the noninfected state, would have exhibited maximal elaboration of TJ proteins.11,23 Moreover, the effect of Ad-siCav-1 in down-modulating caveolin-1 protein level was maximal at this time. Higher level of viral exposure yielded greater loss of caveolin-1 protein, but beyond 500 ifu per cell there was noticeable change in endothelial morphology (data not shown). Hence, 500 ifu per cell was the inoculum used in subsequent experiments. At this level of viral exposure, adenovirus-encoding siRNA for luciferase, an irrelevant enzyme for these cells, yielded no caveolin-1 loss when compared with null virus. This highlighted that diminished caveolin-1 did not merely result from viral exposure or the presence of any double-stranded RNA. That Ad-siCav-1 failed to affect the level of clathrin heavy chain, a prominent protein in the formation of clathrin-coated vesicles,33 which along with caveolae form the major recognized types of endocytic vesicles,34 further exemplifies the specificity of this siRNA approach. Confirmation of Ad-siCav-1–induced loss of caveolin-1 was provided by the attenuated immunofluorescent staining following viral exposure (Figure 1B).
Ad-siCav-1 knocks down caveolin-1 expression in BMEC monolayers. Just subconfluent BMECs were transduced with Ad-siCav-1 as described in “Materials and methods.” (A) Western blotting showing dosage (top; 96 hours) and time course (bottom; 500 ifu per cell) effects of Ad-siCav-1 exposure. Ad-siLuc refers to an adenoviral construct expressing luciferase siRNA, which served as a control for Ad-siCav-1. An inoculum of 500 ifu per cell produced the most dramatic effect, which was achieved after 96 hours of viral exposure. (B) Immunofluorescent staining with antibody to caveolin-1 of BMEC monolayers transduced with Ad-siCav-1 (500 ifu per cell, 96 hours). A significant reduction in staining intensity is readily apparent. Scale bar represents 50 μm.
Ad-siCav-1 knocks down caveolin-1 expression in BMEC monolayers. Just subconfluent BMECs were transduced with Ad-siCav-1 as described in “Materials and methods.” (A) Western blotting showing dosage (top; 96 hours) and time course (bottom; 500 ifu per cell) effects of Ad-siCav-1 exposure. Ad-siLuc refers to an adenoviral construct expressing luciferase siRNA, which served as a control for Ad-siCav-1. An inoculum of 500 ifu per cell produced the most dramatic effect, which was achieved after 96 hours of viral exposure. (B) Immunofluorescent staining with antibody to caveolin-1 of BMEC monolayers transduced with Ad-siCav-1 (500 ifu per cell, 96 hours). A significant reduction in staining intensity is readily apparent. Scale bar represents 50 μm.
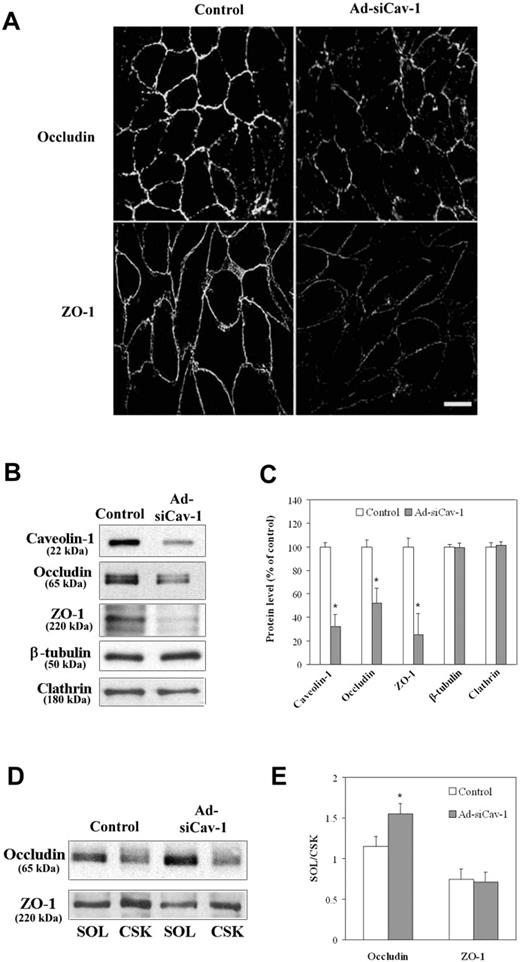
Upon substantiating knockdown of caveolin-1 protein by Ad-siCav-1, we proceeded to evaluate whether caveolin-1 loss could precipitate alterations in TJ-associated proteins occludin and ZO-1 similar to those observed following BMEC exposure to CCL2. Figure 2A shows that Ad-siCav-1 treatment did, in fact, promote reduced and fragmented immunocytochemical staining of both TJ-associated proteins at intercellular borders. Western blotting (Figure 2B-C) further confirmed quantitative loss of these proteins accompanying caveolin-1 knockdown by Ad-siCav-1 but no change in either β-tubulin or clathrin heavy chain levels.
Ad-siCav-1 causes loss and redistribution of TJ-associated proteins. BMECs were exposed to Ad-siCav-1 or Ad-siLuc (control) at 500 ifu per cell for 96 hours and then subjected to analysis. (A) Immunofluorescent staining for occludin and ZO-1 showing reduced and fragmented staining patterns following Ad-siCav-1 exposure. Scale bar represents 50 μm. Western blotting (B) and corresponding quantification (C) showing loss of occludin and ZO-1 in response to Ad-siCav-1. Western blotting (D) and corresponding quantification (E) revealing a significant shift in the association of occludin from the cytoskeletal framework (CSK) to the soluble (SOL) fraction of BMECs (increased SOL/CSK ratio) as a result of Ad-siCav-1 treatment. Because equal amounts of total protein were analyzed in panel B, while equal aliquots (cellular equivalents) were analyzed in panel D, protein intensities between these 2 figures are not comparable. Blots are representative of 3 experiments. Data are presented as mean ± SE of all 3 experiments. *P < .01.
Ad-siCav-1 causes loss and redistribution of TJ-associated proteins. BMECs were exposed to Ad-siCav-1 or Ad-siLuc (control) at 500 ifu per cell for 96 hours and then subjected to analysis. (A) Immunofluorescent staining for occludin and ZO-1 showing reduced and fragmented staining patterns following Ad-siCav-1 exposure. Scale bar represents 50 μm. Western blotting (B) and corresponding quantification (C) showing loss of occludin and ZO-1 in response to Ad-siCav-1. Western blotting (D) and corresponding quantification (E) revealing a significant shift in the association of occludin from the cytoskeletal framework (CSK) to the soluble (SOL) fraction of BMECs (increased SOL/CSK ratio) as a result of Ad-siCav-1 treatment. Because equal amounts of total protein were analyzed in panel B, while equal aliquots (cellular equivalents) were analyzed in panel D, protein intensities between these 2 figures are not comparable. Blots are representative of 3 experiments. Data are presented as mean ± SE of all 3 experiments. *P < .01.
Given that treatment of BMECs with CCL2 was additionally found to shift the subcellular distribution of occludin and ZO-1 from the detergent-resistant cytoskeletal framework (CSK) to the soluble fraction (SOL)11 as a possible prelude to these junctional proteins' degradation, we next sought to determine if caveolin-1 knockdown promoted similar effects. Figure 2D-E shows that the subcellular distribution of occludin also shifted in the direction of the SOL fraction in response to caveolin-1 knockdown, as indicated by a heightened SOL/CSK ratio. However, the SOL/CSK ratio for ZO-1 did not display variation under these same conditions despite significant loss of this protein. It may be that a shift in ZO-1, a peripheral TJ-associated protein with direct links to the CSK,35 occurred at an earlier time point.
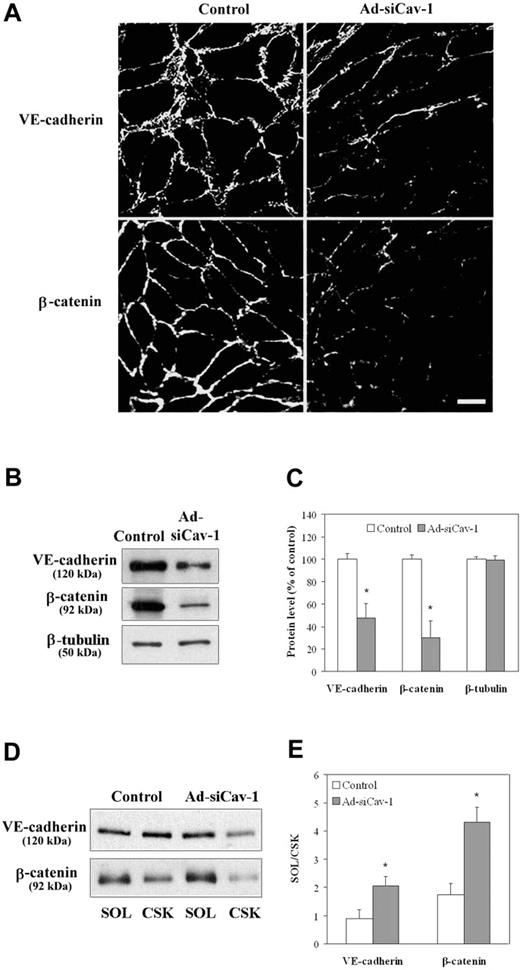
The effect of caveolin-1 knockdown on the expression of AJ-associated proteins VE-cadherin and β-catenin was also investigated, because both proteins are found intermingled with TJs along the entire intercellular cleft between endothelial cells at the BBB,36,37 and TJ function might depend on AJ structure. Figure 3A reveals that immunofluorescent staining of both VE-cadherin, an integral, membrane-spanning protein, and β-catenin, a peripheral membrane protein that, among other things, links VE-cadherin to the CSK,38 is also diminished at intercellular regions of BMECs subjected to caveolin-1 knockdown. Both proteins also showed a significantly reduced level, as assessed by Western blotting (Figure 3B-C). In addition, VE-cadherin and β-catenin exhibited a shift in subcellular distribution from the CSK fraction to the SOL fraction as a consequence of exposure to Ad-siCav-1 (Figure 3D-E).
Ad-siCav-1 causes loss and redistribution of AJ-associated proteins. BMECs were exposed to Ad-siCav-1 or Ad-siLuc (control) at 500 ifu per cell for 96 hours and then subjected to analysis. (A) Immunofluorescent staining for occludin and ZO-1 showing reduced and fragmented staining patterns following Ad-siCav-1 exposure. Scale bar represents 50 μm. Western blotting (B) and corresponding quantification (C) showing loss of VE-cadherin and β-catenin in response to Ad-siCav-1. Western blotting (D) and corresponding quantification (E) revealing a significant shift in the association of both VE-cadherin and β-catenin from the cytoskeletal framework (CSK) to the soluble (SOL) fraction of BMECs (increased SOL/CSK ratio) as a result of Ad-siCav-1 treatment. Because equal amounts of total protein were analyzed in panel B, while equal aliquots (cellular equivalents) were analyzed in panel D, protein intensities between these 2 figures are not comparable. Blots are representative of 3 experiments. Data are presented as mean ± SE of all 3 experiments. *P < .01.
Ad-siCav-1 causes loss and redistribution of AJ-associated proteins. BMECs were exposed to Ad-siCav-1 or Ad-siLuc (control) at 500 ifu per cell for 96 hours and then subjected to analysis. (A) Immunofluorescent staining for occludin and ZO-1 showing reduced and fragmented staining patterns following Ad-siCav-1 exposure. Scale bar represents 50 μm. Western blotting (B) and corresponding quantification (C) showing loss of VE-cadherin and β-catenin in response to Ad-siCav-1. Western blotting (D) and corresponding quantification (E) revealing a significant shift in the association of both VE-cadherin and β-catenin from the cytoskeletal framework (CSK) to the soluble (SOL) fraction of BMECs (increased SOL/CSK ratio) as a result of Ad-siCav-1 treatment. Because equal amounts of total protein were analyzed in panel B, while equal aliquots (cellular equivalents) were analyzed in panel D, protein intensities between these 2 figures are not comparable. Blots are representative of 3 experiments. Data are presented as mean ± SE of all 3 experiments. *P < .01.
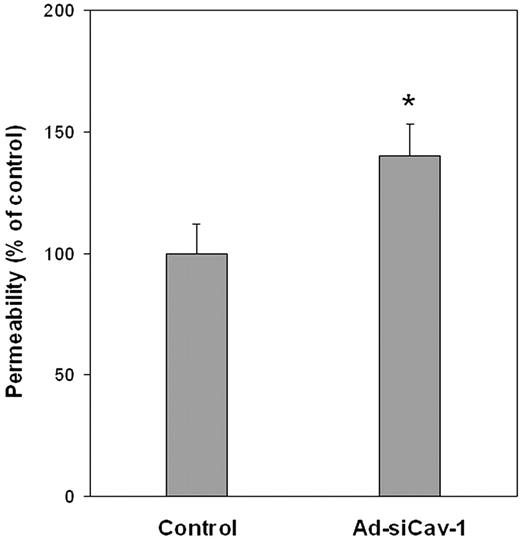
Given these Ad-siCav-1–induced changes in both TJ- and AJ-associated proteins, experiments were performed to assess whether caveolin-1 knockdown was associated with changes in paracellular flux, which serves as a functional index of junctional integrity. Figure 4 highlights that caveolin-1 knockdown in BMECs is accompanied by an approximately 42% increase in the flux of fluorescein dextran of Mwr 40 000. Thus, in addition to causing diminished expression and altered subcellular distribution of major junctional elements, loss of caveolin-1 is functionally coupled to diminished interendothelial adhesion, a condition that could potentially facilitate leukocyte extravasation.
Ad-siCav-1 increases paracellular permeability of BMECs. Paracellular flux of fluorescein dextran (Mwr 40 000) was performed and permeability coefficient (PC) determined for control (Ad-siLuc–treated) and Ad-siCav-1–treated cells, as described in “Materials and methods.” The change in permeability stemming from Ad-siCav-1 treatment was determined as percent change in PC compared with control (PCcontrol, 1.01 × 10−4 cm/min; PCAd-siCav-1, 1.42 × 10−4 cm/min). The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. *P < .05.
Ad-siCav-1 increases paracellular permeability of BMECs. Paracellular flux of fluorescein dextran (Mwr 40 000) was performed and permeability coefficient (PC) determined for control (Ad-siLuc–treated) and Ad-siCav-1–treated cells, as described in “Materials and methods.” The change in permeability stemming from Ad-siCav-1 treatment was determined as percent change in PC compared with control (PCcontrol, 1.01 × 10−4 cm/min; PCAd-siCav-1, 1.42 × 10−4 cm/min). The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. *P < .05.
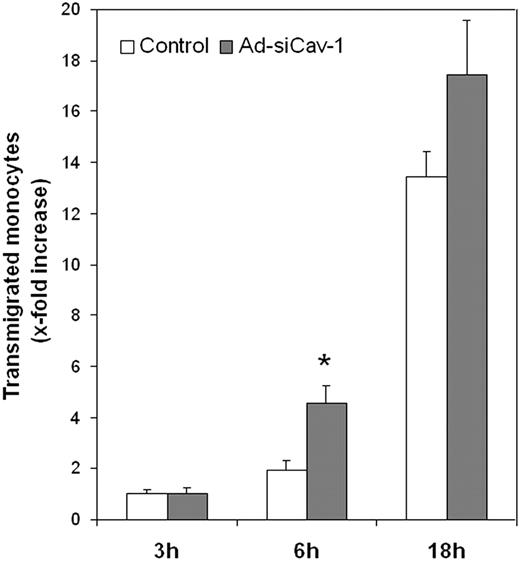
This possibility was tested directly by contrasting the ability of CCL2 to stimulate monocytic cell migration across control BMECs and BMECs subjected to caveolin-1 knockdown. As seen in Figure 5, by 6 hours CCL2 stimulated more than double the transendothelial migration of WEHI cells, a murine monocytic cell line,39 across BMECs transduced with Ad-siCav-1 compared with its respective control. By 18 hours of chemokine treatment, stimulated migration was still somewhat higher across caveolin-1 knockdown cells, but the difference with control cells was reduced. The smaller differential at 18 hours might reflect the fact that, by this time of CCL2 treatment, chemokine-induced alterations in junctional proteins are already maximal.11 We emphasize here that caveolin was targeted specifically in BMECs and that monocytic cells were unaffected. Thus, it may be argued that heightened WEHI transmigration across Ad-siCav-1–treated BMECs results from destabilized endothelial junctions, which, in addition to offering a less obtrusive pathway for diapedesis, might facilitate paracellular communication between WEHI cells and CCL2.
Ad-siCav-1 promotes CCL2-stimulated monocytic migration across BMECs. BMECs were transduced with Ad-siCav-1 or Ad-siLuc (control) and, after 96 hours of viral exposure, WEHI cells were added to the top Transwell chamber and CCL2 was (10 nM) applied to the lower chamber of all samples. At the indicated time points, transmigrated cells were collected from the lower chamber and quantified as detailed in “Materials and methods.” Relative transmigration was calculated as x-fold increase above 3 hours of control. The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. *P < .01 when compared with its respective control.
Ad-siCav-1 promotes CCL2-stimulated monocytic migration across BMECs. BMECs were transduced with Ad-siCav-1 or Ad-siLuc (control) and, after 96 hours of viral exposure, WEHI cells were added to the top Transwell chamber and CCL2 was (10 nM) applied to the lower chamber of all samples. At the indicated time points, transmigrated cells were collected from the lower chamber and quantified as detailed in “Materials and methods.” Relative transmigration was calculated as x-fold increase above 3 hours of control. The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. *P < .01 when compared with its respective control.
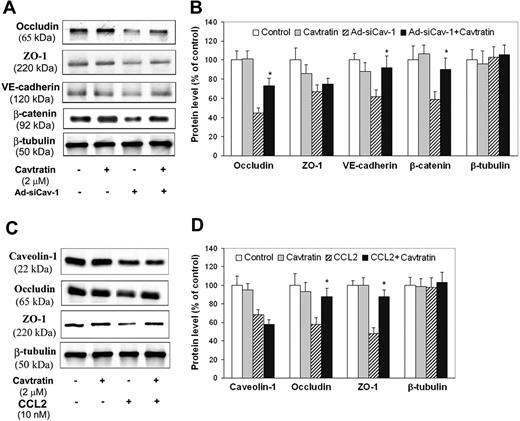
A final series of experiments was then performed to establish that effects of Ad-siCav-1 and CCL2 on BMECs resulted explicitly from caveolin-1 loss. To specifically validate the complicity of caveolin, we introduced into endothelial cultures the cell-permeable peptide cavtratin, which encodes the caveolin-1 scaffolding domain (amino acids 82 to 101) thought responsible for modulating several signaling systems.40 The objective here was to determine if cavtratin, acting as a functional surrogate for caveolin-1 activity, could at least partially antagonize the sequelae of Ad-siCav-1 and CCL2 treatment. Initial experiments focused on confirming caveolin's indispensable role in the loss of junctional proteins following BMEC challenge with Ad-siCav-1. Figure 6A-B indicates that delivery of cavtratin to si-RNA–treated BMECs trends in the direction of reversing the Ad-siCav-1–induced loss in occludin, ZO-1, VE-cadherin, and β-catenin, although the reversal effect on ZO-1 is the smallest and not statistically significant. That neither Ad-siCav-1 nor cavtratin affected the level of β-tubulin, a housekeeping gene, highlighted that these treatments were without general effect on protein expression. It is further significant that delivery of cavtratin alone yielded no significant change in expression of any of these junctional proteins. Apparently, cavtratin could partially substitute for the diminished caveolin-1 that resulted from Ad-siCav-1 treatment, though was without obvious effect when administered by itself to control BMECs.
Cavtratin reverses effects of CCL2 and Ad-siCav-1 on TJ- and AJ-associated proteins. Western blotting (A) and corresponding quantification (B) for Ad-siCav-1 effects. BMECs were transduced with Ad-siCav-1 or Ad-siLuc (control) as in Figure 5. After 48 hours of viral exposure, 2 μM cavtratin was added to both the top and bottom Transwell chambers and cells wells cultured for an additional 48 hours. *P < .01 when compared with Ad-siCav-1–treated cells. Western blotting (C) and corresponding quantification (D) for CCL2 effects. BMECs were cultured to confluence, pretreated with 2 μM cavtratin for 2 hours, and then exposed to 10 nM CCL2 for 18 hours (both cavtratin and CCL2 were added to top and bottom Transwell chambers). *P < .01 when compared with cells treated with CCL2 only.
Cavtratin reverses effects of CCL2 and Ad-siCav-1 on TJ- and AJ-associated proteins. Western blotting (A) and corresponding quantification (B) for Ad-siCav-1 effects. BMECs were transduced with Ad-siCav-1 or Ad-siLuc (control) as in Figure 5. After 48 hours of viral exposure, 2 μM cavtratin was added to both the top and bottom Transwell chambers and cells wells cultured for an additional 48 hours. *P < .01 when compared with Ad-siCav-1–treated cells. Western blotting (C) and corresponding quantification (D) for CCL2 effects. BMECs were cultured to confluence, pretreated with 2 μM cavtratin for 2 hours, and then exposed to 10 nM CCL2 for 18 hours (both cavtratin and CCL2 were added to top and bottom Transwell chambers). *P < .01 when compared with cells treated with CCL2 only.
A similar scenario was observed regarding CCL2 treatment. As it did with Ad-siCav-1, cavtratin specifically nullified, in part, the CCL2-induced loss of occludin and ZO-1 but, notably, did not reverse at all the caveolin-1 loss incurred by chemokine treatment (Figure 6C-D). Collectively, these results attest to the specificity of cavtratin in effectively substituting for caveolin-1 activity in regulating occludin and ZO-1 levels.
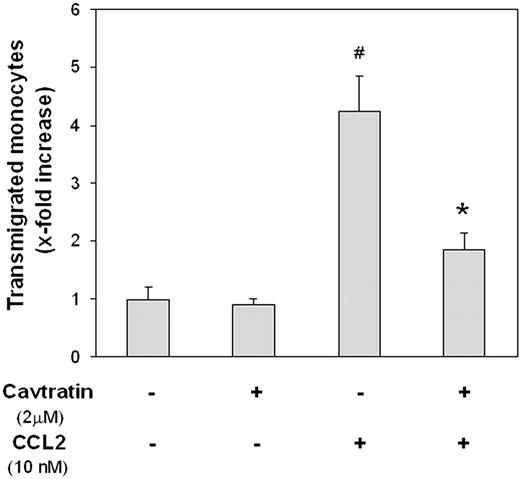
Lastly, the significance of reduced caveolin-1 level in the action of CCL2 to stimulate WEHI transendothelial migration was explored. Just as cavtratin thwarted CCL2 effects on occludin and ZO-1, so too did it obstruct the ability of this chemokine to stimulate the transmigration of WEHI cells across monolayer BMEC cultures (Figure 7). Cavtratin also failed to exert any effects on its own, underscoring that it did not negatively affect WEHI cell motility. Mirroring its action in antagonizing the consequences of Ad-siCav-1 and CCL2 treatment on junctional proteins, its apparent mode of action in this case was also to serve as proxy for caveolin-1 loss.
Cavtratin inhibits CCL2-stimulated migration of monocytic cells across BMECs. BMECs were cultured to confluence and then pretreated with 2 μm cavtratin added to both the top and bottom Transwell chambers. After 24 hours, monolayers were washed, fresh media containing WEHI cells were added to the top chamber, and CCL2 (10 nM) was applied to the lower chamber. At 18 hours after the application of chemokine, transmigrated WEHI cells were collected from the lower chamber and quantified as detailed in “Materials and methods.” Relative transmigration was calculated as x-fold increase over control (lacking both CCL2 and cavtratin). The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. #P < .01 when compared with control; *P < .01 when compared with 10 nM CCL2 treatment alone.
Cavtratin inhibits CCL2-stimulated migration of monocytic cells across BMECs. BMECs were cultured to confluence and then pretreated with 2 μm cavtratin added to both the top and bottom Transwell chambers. After 24 hours, monolayers were washed, fresh media containing WEHI cells were added to the top chamber, and CCL2 (10 nM) was applied to the lower chamber. At 18 hours after the application of chemokine, transmigrated WEHI cells were collected from the lower chamber and quantified as detailed in “Materials and methods.” Relative transmigration was calculated as x-fold increase over control (lacking both CCL2 and cavtratin). The data are presented as mean ± SE and represent 3 independent experiments performed in triplicate. #P < .01 when compared with control; *P < .01 when compared with 10 nM CCL2 treatment alone.
Discussion
The results described suggest a causal link between altered caveolin-1 expression and proinflammatory effects of CCL2 at the BBB. Specifically, targeted knockdown of caveolin-1 expression in BMECs by an adenoviral-mediated siRNA approach was accompanied by the following features: (1) reduced expression of TJ-associated proteins occludin and ZO-1 and AJ-associated proteins VE-cadherin and β-catenin; (2) shift in subcellular distribution of occludin, VE-cadherin, and β-catenin from the CSK fraction to the soluble fraction; (3) heightened paracellular permeability; and (4) elevated ability of CCL2 to stimulate transendothelial migration of mononuclear cells. Moreover, the effects of both siRNA and CCL2 on junctional protein expression, as well as CCL2-stimulated monocytic transendothelial migration, could be reversed by an exogenous peptide encoding the caveolin-1 scaffolding domain. These latter findings, in particular, serve to make clear that the lessened caveolin-1 pool is cause for bringing about the junctional alterations observed. Thus, our hypothesis is that the diminished caveolin-1 level is a critical, functional intermediary in the signal transduction pathway activated upon CCL2 binding to BMECs.
An important consideration in our approach was to acutely knock down caveolin-1 in a confluent BMEC monolayer, which would otherwise have exhibited restricted permeability and served as a facsimile of the BBB. This obliged us to avoid using BMECs from caveolin-1 knockout animals as well as refrain from transfection protocols to knock down caveolin-1 expression in BMECs from wild-type subjects. Use of knockout animals was specifically avoided, because a caveat of global gene disruption can be unanticipated compensatory mechanisms. Indeed, such adaptation may be at the basis for seemingly discordant reports of capillary transcytosis of albumin in 2 different caveolin-1 knockout lines.41,42 Transfection methods, on the other hand, are recognized as potentially toxic24 as well as inefficient when applied to nonproliferating epithelial/endothelial cells.25 And subjection of growing cells to transfection further posed the risk that confluence would not be achieved. The use of adenovirus to nondestructively deliver caveolin-1 siRNA to confluent BMECs by viral transduction, however, uniquely circumvented these complications. Because endothelial membranes at cell confluence undergo extensive remodeling crucial for regulating vascular permeability and caveolin-1 redistributes from a high-density to a low-density membrane compartment at this time,43 results obtained here are likely to reflect caveolin-1–mediated events that take place at the mature, CNS microvasculature in vivo.
Our results are in accord with those of other laboratories, which directly linked reduced caveolin-1 expression to elevated microvascular permeability and associated defects in endothelial junctional integrity. Schubert et al20 described heightened paracellular movement of ruthenium red across the lung microvasculature of caveolin-1 (−/−) knockout mice, a feature accompanied by the appearance of abnormal capillary TJs that were reduced in size and shape. Most recently, Miyawaki-Shimizu et al44 demonstrated that in vivo delivery of siRNA targeting caveolin-1 resulted in heightened lung microvascular permeability accompanied by dilated interendothelial junctions. A similar cavtratin approach to that used here was also used to correct other hyperpermeable states. For example, cavtratin has been shown to suppress carrageenan-induced acute inflammation and vascular leakage,29 attenuate the heightened permeability of rat mesenteric vessels following stimulation with platelet-activating factor,45 and inhibit tumor microvascular permeability.21 These latter findings additionally support an association of diminished caveolin-1 expression with compromised endothelial integrity. Likewise, when viewed in the context of the present observations, the observation that endothelial-specific expression of caveolin-1 impaired microvascular permeability and angiogenesis22 may also be interpreted as revealing that caveolin-1 acts to restrain processes that destabilize endothelial junctions. Absence or a reduced amount of caveolin-1 would therefore appear to release these processes from such constraint, liberating factors that negatively regulate the expression of junctional proteins and/or affect their disorganization at sites of intercellular adhesion. In what might further exemplify this scenario, Regina et al46 described down-regulation of caveolin-1 in glioma tumor microvasculature, the endothelium of which typically shows patent tight junctions and hyperpermeability.47
While the signal transduction pathways whereby loss of caveolin-1 leads to compromised junctional integrity and diminished barrier function await clarification, considerable and consistent evidence suggests that the Src-kinase family might be a key player. In this regard, Src tyrosine kinases are among the many signaling proteins caveolin-1 and its scaffolding domain have been shown to negatively regulate.48 Several inhibitor studies have also implicated Src tyrosine kinase as an effector of junctional destabilization, an activity that might require the yoke of caveolin-1 to be removed. For example, the Src-family kinase inhibitor PP1 has been shown to reduce the BBB permeability and brain edema found with subarachnoid hemorrhage.49 So, too, the related inhibitor PP2 reversibly abrogated the disruption of tight and adherens junction–associated brought about by constitutive expression of the activated form of the heterotrimeric G protein Galpha12.50 And, another Src family tyrosine kinase inhibitor, herbimycin A, was found to significantly improve barrier function of endothelial monolayers rendered hyperpermeable by treatment with the myosin phosphatase inhibitor calyculin A.51
Like the Src tyrosine kinases, endothelial nitric oxide synthase (eNOS) is subject to negative regulation by caveolin-1 and its scaffolding domain.52-54 Thus, through generation of nitric oxide (NO), eNOS may also be complicit in the permeability changes resulting from cellular loss of caveolin-1. This hypothesis is lent credence by reports describing that capillaries from caveolin-1–deficient animals exhibit hyperpermeability along with constitutive activation of eNOS activity20 and that the ability of cavtratin to reverse hyperpermeability reflects eNOS inhibition.21,29 Offering further support for possible eNOS involvement in caveolin-1 effects, Schubert et al20 showed that microvascular hyperpermeability of caveolin-1–deficient mice could be rescued by the NOS inhibitor l-NAME. However, eNOS's role as a downstream effector of caveolin-1–regulated endothelial permeability might depend on the level of NO produced, because constitutive eNOS-derived NO has been shown to stabilize endothelial junctional integrity.55
There is also suggestive evidence that caveolin-1 might at least partially regulate some endothelial junctional proteins through controlling their degradation by matrix metalloproteinase-9 (MMP-9). Interestingly, cavtratin has been shown to markedly reduce secretion of MMP-9,56 a gelatinase activity expressed by BMECs.57-59 MMP-9 can also degrade occludin in retinal pigment epithelial cells,60 which perform barrier functions analogous to BMECs. VE-cadherin61 and the tight junction–associated protein claudin-162 show sensitivity to MMP-9 activity as well.
In light of the results presented here—highlighting functional coupling of caveolin-1 loss with both reduced endothelial barrier integrity and heightened CCL2 stimulation of monocytic transmigration across BMECs—it is interesting that Millan et al63 recently reported reduced transcellular migration of lymphocytes across human umbilical vein endothelial monolayers subjected to siRNA-mediated caveolin-1 down-regulation. Notably, only transcellular migration, which may proceed via caveolae-forming channels within endothelial cells,64,65 was affected in this latter study. Total transendothelial migration, including that achieved through both transcellular and paracellular routes, was undisturbed. This leaves open the possibility that an increase in paracellular movement, as witnessed here, might have balanced the diminished transcellular migration. Such results are thus aligned with our observations.
To the best of our knowledge, this is the first report to establish that reduction in caveolin-1 level causes loss of endothelial junction–associated proteins—a loss functionally coupled to heightened passage of both soluble and cellular elements across a model of the BBB. These results herald the prospect that caveolin-1 is a critical determinant of inflammatory responses in the CNS and perhaps at peripheral sites as well. Such a possibility could allow for therapeutic targeting of caveolin-1 expression to control inflammatory disease.
Authorship
Contribution: L.S., S.G., and J.S.P. designed the research; L.S. and S.G. performed the research and data analysis; and J.S.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
L.S. and S.G. contributed equally to this study.
Correspondence: Joel S. Pachter, Blood-Brain Barrier Laboratory, Department of Pharmacology, University of Connecticut Health Center, 263 Farmington Ave, Farmington, CT 06030; e-mail: pachter@nso1.uchc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants RO-1-MH54718 from the National Institutes of Health and RG-2633 and PP-1215 from the National Multiple Sclerosis Society (J.S.P.).
We are grateful to Drs David Dorsky and Arvind Chhabra for their assistance with CsCl2 purification of adenovirus.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal