Abstract
The paradoxical correlation between thrombosis and the lupus anticoagulant (LAC) effect is an enigmatic feature of the antiphospholipid (aPL) syndrome. The Dutch authors previously reported that thrombosis-related anti–β2-glycoprotein I (β2GPI) antibodies recognize domain I and cause LAC. The American authors reported that aPLs disrupt an anticoagulant annexin A5 (AnxA5) crystal shield. We investigated whether antidomain I antibodies correlate with disruption of AnxA5-anticoagulant activity. We studied a well-characterized group of 33 patients including subgroups with β2GPI-dependent LAC that recognize domain I (n = 11), with β2GPI-independent LAC (n = 12), and lacking LAC (n = 10). The effects on AnxA5-anticoagulant activity were determined with an AnxA5 resistance assay that measures coagulation times with and without AnxA5. Patients with β2GPI-dependent LAC (group A, all with thrombosis) had significantly lower AnxA5-anticoagulant ratios than those with β2GPI-independent LAC (group B, thrombosis n = 4; 157.8% versus 235.6%, P < .001) and those without LAC (group C, thrombosis n = 2; 157.8% versus 232.5%, P < .001). There was no difference in the ratios between groups B and C (P = .92). Plasmas with β2GPI-dependent LAC that recognize domain I displayed significantly increased AnxA5 resistance, suggesting that specifically anti-β2GPI antibodies compete with AnxA5 for anionic phospholipids. These results are consistent with a model in which aPL antibodies may promote thrombosis by interfering with the anticoagulant activity of AnxA5.
Introduction
The correlation between thrombosis in vivo and a prolonged clotting time in vitro is a striking feature of the antiphospholipid syndrome (APS).1 This paradox has intrigued clinicians and scientists for over 30 years but has not yielded a clear pathogenic mechanism that explains APS.2 One of the several pathogenic mechanisms that have been proposed for APS involves annexin A5 (AnxA5).
AnxA5 forms a 2-dimensional crystalline shield over phospholipid bilayers and has potent anticoagulant effects by inhibiting the binding (and activation) of coagulation factors.3-5 It was shown that antiphospholipid (aPL) antibodies were able to disturb the 2-dimensional crystallization of AnxA5 that was formed on a phospholipid bilayer.6 It was therefore suggested that aPL antibodies might interfere with the binding of AnxA5 and disrupt the integrity of this anticoagulant shield in vivo, thereby promoting a procoagulant state. This hypothesis was supported by that the finding that aPL antibodies could displace AnxA5 from vascular cells and trophoblasts, thereby accelerating the coagulation of plasma,9 and that this process could be observed on artificial phospholipids bilayers through atomic force microscopy (AFM).6 Based on the capacity of aPL antibodies to supplant AnxA5 from anionic phospholipids and reduce its anticoagulant activity, an assay was developed to detect the reduction of AnxA5 anticoagulant activity, that is, “AnxA5 resistance.”10 It was shown that the AnxA5 anticoagulant activity was reduced by plasma samples of patients with aPL antibodies and a history of thrombosis compared to patients with aPL antibodies without thrombosis or healthy volunteers.10 Although this difference proved to be significant, there was a significant overlap among the 3 groups.10 This might have been attributable to the heterogeneity of aPL antibodies used in that study.
This heterogeneity may be based on the fact that aPL antibodies recognize different phospholipid-binding plasma proteins such as β2-glycoprotein I (β2GPI), prothrombin, and AnxA5.1,10,11 β2GPI is believed to be the major antigenic target in APS. Of the various detection methods that have been empirically derived for diagnosing APS, for yet unknown reasons, it is the prolongation of phospholipid-dependent coagulation assays that correlates best with specificity for thrombosis in APS.12 This prolongation of coagulation time, known as the lupus anticoagulant (LAC) is caused by antibodies with various specificities.13 A subset of LAC caused by anti-β2GPI antibodies correlates best with thrombosis.11 Recently, it was shown that this population of anti-β2GPI antibodies specifically binds epitope G40-R43 on domain I of β2GPI.14 Other publications pointed to the involvement of other domains in antibody binding, for example, domain IV, but the association with APS-related clinical features has not been proven.15 aPL antibodies recognizing other plasma proteins, such as prothrombin, appear to be of little clinical value and may blur the diagnosis of patients at risk for APS.11
This study was conducted to investigate whether there might be a correlation of different subsets of aPL antibodies with their capacity to reduce the anticoagulant activity of AnxA5. For this purpose, we studied plasmas from small, well-defined groups of patients. Plasmas of these patients displayed an LAC caused by anti-β2GPI antibodies, an LAC caused by antibodies with other specificities, or no LAC at all. Plasmas from healthy controls were also analyzed for comparison.
Patients, materials, and methods
Patients
From a previously described, well-characterized patient population of 198 patients diagnosed with either systemic lupus erythematosus (SLE), lupuslike disease (LLD), or primary antiphospholipid syndrome (PAPS), we selected 33 patients.11,14 The patients were chosen from 3 different subgroups, based on their reactivity in an activated partial thromboplastin time (APTT)–based coagulation assay in which we were able to discriminate between an LAC caused by anti-β2GPI antibodies and an LAC caused by antibodies with other specificity (Table 1). Group A consisted of 11 patients (33%) who displayed an LAC caused by anti-β2GPI antibodies; all patients were diagnosed with APS, 8 patients suffered from secondary APS and 3 patients suffered from PAPS; all patients were positive for anti-β2GPI IgG antibodies with reactivity toward epitope G40-R43 on domain I of β2GPI. Group B consisted of 12 patients (36%) with an LAC independent of anti-β2GPI antibodies; 4 patients were diagnosed with secondary APS, 3 were positive for anti-β2GPI IgG antibodies, but none recognized G40-R43 on domain I of β2GPI. Group C consisted of 10 patients (30%) without LAC; one patient was diagnosed with secondary APS, one patient tested positively for anti-β2GPI IgG antibodies but did not recognize epitope G40-R43 on domain I of β2GPI. Patients were selected based on being representative for one of these 3 groups and on the amount of plasma available for further experiments. Patients with SLE (n = 24, 73%) met at least 4 criteria of the American College of Rheumatology (ACR) for the classification of SLE and patients with LLD (n = 6, 18%) met one to 3 of these criteria.13 Patients with PAPS (n = 3, 9%) have aPL antibodies (LAC or anticardiolipin [aCL] antibodies or both) and a history of thrombosis in the absence of any sign of another systemic autoimmune disease. Patients with secondary APS (n = 13, 39%) have APS in combination with another autoimmune disease. In addition to these patients, we also studied plasmas from healthy controls (group D), obtained from a commercial vendor (George King Bio-Medical, Overland Park, KS). This group consisted of 30 nonpregnant, disease-free women with a median age of 37 years (range, 21-55 years), who were on no medications at the time of blood sampling. The Institutional Review Board of the University Medical Center Utrecht approved this study, and informed consent was obtained from all patients according to the Declaration of Helsinki.
Characteristics of the 3 groups of patients based on their LAC reactivity
| Characteristic . | Group 1: β2GPI-dependent LAC . | Group 2: β2GPI-independent LAC . | Group 3: No LAC . |
|---|---|---|---|
| Total, no. | 11 | 12 | 10 |
| Females, no. | 10 | 11 | 10 |
| Median age, y | 31 | 35 | 30 |
| Primary APS, no. | 3 | 0 | 0 |
| Secondary APS, no. | 8 | 4 | 1 |
| Clinical symptoms | |||
| Thrombosis, no. | 11 | 4 | 2 |
| Arterial thrombosis, no. | 3 | 3 | 1 |
| Venous thrombosis, no. | 8 | 2 | 1 |
| Antibody distribution | |||
| Anti-β2GPI antibodies, no. | 11 | 3 | 1 |
| IgG, no. | 11 | 3 | 1 |
| IgM, no. | 4 | 0 | 0 |
| Antidomain 1 IgG antibodies, no. | 11 | 0 | 0 |
| Antiprothrombin antibodies, no. | 9 | 8 | 4 |
| IgG, no. | 8 | 5 | 3 |
| IgM, no. | 8 | 3 | 1 |
| aCL antibodies, no. | 10 | 7 | 3 |
| IgG, no. | 10 | 6 | 3 |
| IgM, no. | 8 | 3 | 2 |
| Characteristic . | Group 1: β2GPI-dependent LAC . | Group 2: β2GPI-independent LAC . | Group 3: No LAC . |
|---|---|---|---|
| Total, no. | 11 | 12 | 10 |
| Females, no. | 10 | 11 | 10 |
| Median age, y | 31 | 35 | 30 |
| Primary APS, no. | 3 | 0 | 0 |
| Secondary APS, no. | 8 | 4 | 1 |
| Clinical symptoms | |||
| Thrombosis, no. | 11 | 4 | 2 |
| Arterial thrombosis, no. | 3 | 3 | 1 |
| Venous thrombosis, no. | 8 | 2 | 1 |
| Antibody distribution | |||
| Anti-β2GPI antibodies, no. | 11 | 3 | 1 |
| IgG, no. | 11 | 3 | 1 |
| IgM, no. | 4 | 0 | 0 |
| Antidomain 1 IgG antibodies, no. | 11 | 0 | 0 |
| Antiprothrombin antibodies, no. | 9 | 8 | 4 |
| IgG, no. | 8 | 5 | 3 |
| IgM, no. | 8 | 3 | 1 |
| aCL antibodies, no. | 10 | 7 | 3 |
| IgG, no. | 10 | 6 | 3 |
| IgM, no. | 8 | 3 | 2 |
Numbers indicate absolute numbers of patients, unless otherwise indicated. Characteristics of the healthy controls are provided in “Patients, materials, and methods.”
Detection of aPL antibodies
LAC.
To determine LAC activity, an APTT and a dilute Russell viper venom time (DRVVT) were performed. For the APTT (PTT-LA, interassay variability 2.0%, Diagnostica Stago, Gennevilliers, France), 50 μL patient plasma was diluted 1:1 with normal pool plasma of 40 healthy volunteers and incubated with 50 μL APTT reagent. Coagulation was initiated by the addition of 50 μL CaCl2 (25 mM). As a control, samples were tested in an actin-FS–based APTT (APTT-FS, Dade Behring, Marburg, Germany), which is an LAC-insensitive assay. Patients were considered positive when the ratio PTT-LA/APTT-FS was more than 1.20.11 The DRVVT was performed according to the instructions of the manufacturer (interassay variability 3.2%, Gradipore, North Ryde, Australia) and considered positive when LAC screen/LAC confirm was more than 1.20. A patient was considered LAC positive if one of the 2 LAC assays was positive.
CL antibody ELISA.
The aCL antibodies were measured in an enzyme-linked immunosorbent assay (ELISA) as previously described.16 Nine IgG/IgM calibrators were used to report aCL levels as GPL or MPL units. Levels above 10 GPL or GPM units were considered positive.
Anti-β2GPI and antiprothrombin antibody ELISA.
Antibodies against β2GPI and prothrombin were measured in an ELISA as previously described.16 Plasma samples were regarded as positive when the absorbed value exceeded the cut-off value (mean + 3 SD of 40 healthy volunteers) and corrected for the absorption of standard positive plasma.
Antidomain I IgG ELISA.
As recently described, we measured anti-β2GPI IgG antibodies with reactivity toward epitope G40-R43 in domain I and anti-β2GPI IgG antibodies with other reactivity.14 In short, hydrophilic plates (Costar catalogue no. 9102; New York, NY) and hydrophobic plates (Costar catalogue no. 2595) were coated with domain I of β2GPI for 1 hour at 37°C. The plates were blocked with 4% BSA/0.1% Tween/TBS and subsequently incubated with patient plasma containing anti-β2GPI IgG antibodies. Bound IgG antibodies were detected by a goat anti–human IgG alkaline phosphatase-labeled antibody (diluted 1:1000, Biosource, Camarillo, CA). Anti-β2GPI IgG antibodies were regarded as antibodies with reactivity toward G40-R43 when these showed decreased affinity for domain I coated onto hydrophilic plates compared to that with domain I coated onto hydrophobic plates. Anti-β2GPI IgG antibodies did not recognize G40-R43 when they showed equal affinity for domain I on both plates. A ratio of more than 2 between the OD measured with the hydrophobic plate and the OD with the hydrophilic plate discriminates between anti-β2GPI IgG antibodies that recognize epitope G40-R43 and anti-β2GPI IgG antibodies with other specificity. This ratio is an indication for the relative amount of anti-β2GPI IgG antibodies that recognizes the positive charge (epitope G40-R43) on domain I (interassay variability 3.8%).
β2GPI-dependent LAC assay
To discriminate between a β2GPI-dependent LAC and a β2GPI-independent LAC we used an APTT-based clotting test (PTT-LA, Diagnostica Stago), as previously described.11 Briefly, 25 μL PTT-LA reagent, 50 μL patient plasma that was mixed 1:1 with normal pool plasma (of 40 healthy volunteers), and 25 μL of different concentrations (0, 25, 50, 100, 200 μM) of cardiolipin vesicles diluted in TBS were mixed and added to a KC-10 micro-coagulometer (Amelung, Lemgo, Germany). The cardiolipin vesicles were made as previously described.17 After 3 minutes of incubation at 37°C, coagulation was initiated by the addition of 50 μL CaCl2 and clotting time was measured. An LAC was considered β2GPI-dependent when the ratio of coagulation times of patient plasma and normal pool plasma was decreased by at least 0.05 with addition of cardiolipin vesicles at a concentration of 25 μM cardiolipin vesicles.11
AnxA5 resistance assay
To examine the effects of plasma samples on AnxA5 activity we used a 2-stage method as previously described, with minor modifications.9 EDTA (0.5 M) was added to recombinant human tissue factor (Innovin; Dade Behring, Newark, DE) to a final concentration of 10 mM, to neutralize the free calcium and allow for a 2-stage assay. The Innovin-EDTA was then mixed with APTT reagent-phospholipids (actin FSL; Dade Behring, Newark, DE) at a 1:1 ratio. Then, 200 μL of the Innovin-EDTA–actin FSL was incubated with 50 μL citrated test plasma for 5 minutes at room temperature. The mixture was centrifuged with a microcentrifuge (Eppendorf centrifuge 5417R, Brinkmann Instrument, Westbury, NY) for 15 minutes at 20 800g at 25°C. The pellets were washed once in HEPES buffer saline (HBS; 0.01 M HEPES, 0.14M NaCl, pH 7.5) and resuspended in 220 μL of the buffer. Then, 50 μL of the suspension was incubated with 50 μL pooled normal plasma at 37°C in a ST4 Coagulation Instrument (American Bioproducts, Parsipanny, NJ) for 30 seconds and 50 μL 0.02 M CaCl2 or 0.02 M CaCl2 containing AnxA5 (30 μg/mL) was then added. The coagulation times, in the presence and absence of AnxA5, were determined and the mean times of duplicate tests were recorded. The anticoagulant activity of AnxA5 was calculated as follows: AnxA5 anticoagulant ratio = (coagulation time in the presence of AnxA5/coagulation time in the absence of AnxA5) × 100%.
Study and statistical analysis
The 33 selected plasma samples were sent as coded samples to the Pathology Department of the Montefiore Medical Center (New York, NY) for AnxA5 resistance assay. The plasmas from the healthy controls were also coded and randomly interspersed among other coded samples. The thromboembolic events were diagnosed as previously described.11 Differences between groups were calculated with the Mann-Whitney test, by using SPSS (SPSS, Chicago, IL).
Results
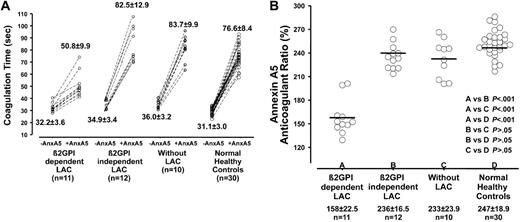
All 33 selected patient samples and the 30 healthy controls were tested for AnxA5 resistance (Figure 1A). We observed a shorter increase in coagulation time by the addition of AnxA5 in group A compared to group B, group C, and group D (18.6 seconds versus 47.6 seconds, 47.7 seconds, and 45.5 seconds, respectively). We calculated the AnxA5 anticoagulant ratio (coagulation time with AnxA5/coagulation time without AnxA5 × 100%). The results show that patient samples that displayed a β2GPI-dependent LAC (group A: mean, 157.8%; SD, 22.5%) had lower AnxA5 ratios than patient samples displaying a β2GPI-independent LAC (group B: mean, 235.6%; SD, 16.5%; P < .001), patient samples without LAC (group C: mean, 232.5%; SD, 23.9%; P < .001), or healthy controls (group D: mean, 246.5%; SD, 18.8; P < .001, Figure 1B). There was no significant difference in AnxA5 resistance between patients with a β2GPI-independent LAC, patients without LAC, or healthy controls (Figure 1B).
AnxA5 resistance in relation to aPL antibodies with different specificities. To determine the effects of different plasma samples on AnxA5 activity, a 2-stage PT reagent-phospholipid coagulation assay was used. The coagulation times, in the presence and absence of AnxA5 were measured; the anticoagulant activity of AnxA5 was calculated as follows: AnxA5 anticoagulant ratio = (coagulation time in the presence of AnxA5/coagulation time in the absence of AnxA5) × 100%. (A) The absolute coagulation times found with or without AnxA5 specified for the 4 groups. Horizontal bars indicate the mean ratio of the patients in different groups. (B) The AnxA5 anticoagulant ratio was significantly lower for patients who displayed a β2GPI-dependent LAC (group A: mean, 157%) compared to patients displaying a β2GPI-independent LAC (group B: mean, 235%; P < .001), patients without LAC (group C: mean, 232%; P < .001), or healthy controls (group D: mean, 247%: P < .001).
AnxA5 resistance in relation to aPL antibodies with different specificities. To determine the effects of different plasma samples on AnxA5 activity, a 2-stage PT reagent-phospholipid coagulation assay was used. The coagulation times, in the presence and absence of AnxA5 were measured; the anticoagulant activity of AnxA5 was calculated as follows: AnxA5 anticoagulant ratio = (coagulation time in the presence of AnxA5/coagulation time in the absence of AnxA5) × 100%. (A) The absolute coagulation times found with or without AnxA5 specified for the 4 groups. Horizontal bars indicate the mean ratio of the patients in different groups. (B) The AnxA5 anticoagulant ratio was significantly lower for patients who displayed a β2GPI-dependent LAC (group A: mean, 157%) compared to patients displaying a β2GPI-independent LAC (group B: mean, 235%; P < .001), patients without LAC (group C: mean, 232%; P < .001), or healthy controls (group D: mean, 247%: P < .001).
Because all patient with antidomain I antibodies had previous histories for thrombosis, we investigated whether thrombosis or antidomain I antibodies correlated with AnxA5 resistance. We found that there was a significant difference in AnxA5 resistance between patients with a history of thrombosis and patients without a history of thrombosis (AnxA5 anticoagulant ratio: mean 180.2% versus 239.0%, P < .001). This might indicate that thrombosis, and not antidomain I antibodies, correlates with AnxA5 resistance. But when all patients with antidomain I antibodies were excluded from the analysis there was no significant difference between patients with a history of thrombosis and patients without a history of thrombosis (AnxA5 anticoagulant ratio: mean 221.3% versus 239.0%, P > .05).
Discussion
Over the past several years a body of evidence has accumulated for a potential role for AnxA5 in APS-related pregnancy loss.7,8,19 It was shown that aPL antibodies are able to reduce the amount of AnxA5 on the surface of trophoblasts and artificial phospholipid membranes, which might result in a local prothrombotic state.6,7 Recently, it was shown by atomic force microscopy that aPL antibodies were able to disrupt an artificial 2-dimensional “anticoagulant” shield formed by AnxA5.6 This finding was translated into an assay to detect resistance to AnxA5 anticoagulant activity that correlated with thrombosis.9 In the present study, we found a clear correlation between the AnxA5 resistance assay and patients having thrombosis and LAC caused by anti-β2GPI antibodies that recognize domain I. We found that patients with a β2GPI-independent LAC and patients without LAC did not display AnxA5 resistance. One of the striking features of these results is that there was only a small overlap between patient samples with a β2GPI-dependent LAC and patient samples with a β2GPI-independent LAC or no LAC at all, indicating the detection of a distinct population of antibodies. It was already suggested that the disruption of an anticoagulant shield plays a role in pregnancy loss.9
Over the years, many efforts have been made to increase the specificity and sensitivity of assays for aPL antibodies to better detect patients at risk for thrombosis and pregnancy loss.18 In this study we found a strong correlation between 2 different assays that are both based on the functional activity of the aPL antibodies: β2GPI-dependent LAC and AnxA5 resistance. An interesting aspect that we noted with the AnxA5 resistance assay was the significantly shorter mean baseline clotting time in the group of patients with a β2GPI-dependent LAC compared to the other 2 autoimmune groups (β2GPI-independent LAC or no LAC at all). Although this difference was statistically significant, the large overlap among the 3 groups, and also the absence of difference compared to the healthy controls, makes this baseline clotting time unsuitable for use as a diagnostic marker, but of interest for further study. The statistical difference seen in the autoimmune groups is in accord with previous report of Pengo et al of a procoagulant effect on the clotting time by anti-β2GPI antibodies in a prothrombin time (PT) or DRVTT, only when the interaction of anti-β2GPI antibodies with β2GPI occurred in the absence of calcium.20
Both of the assays that we describe, the antidomain I of β2GPI and the AnxA5 resistance assay, detected a subpopulation of aPL antibodies that strongly correlated with thrombosis; thrombosis-related aPL antibodies recognize epitope G40-R43 on domain I, which causes dimerization of β2GPI resulting in: (1) the disturbance of the anticoagulant AnxA5 shield (AnxA5 resistance assay) and (2) binding to cardiolipin (β2GPI-dependent LAC). The fact that these assays detect the same population of aPL that highly correlates with thrombosis provides an interesting mechanism for thrombosis in APS, for which a model is depicted in Figure 2. An interesting aspect of this model is that optimal binding of AnxA5 occurs at supraphysiologic calcium concentrations, that is, 3 mM or more, in contrast to the binding of aPL-β2GPI complexes, whose affinities increase at lower calcium concentrations. It is, therefore, likely that aPL-β2GPI complexes are better able to compete against AnxA5 binding and reduce its anticoagulant effect, at physiologic concentrations of free ionized calcium, that is, 1.2 to 1.8 mM. It should be noted that there are several alternative proposed mechanisms for aPL-mediated thrombosis, including ones in which the binding of β2GPI by aPL antibodies triggers proadhesive and prothrombotic signaling events in endothelial cells and platelets.22-24
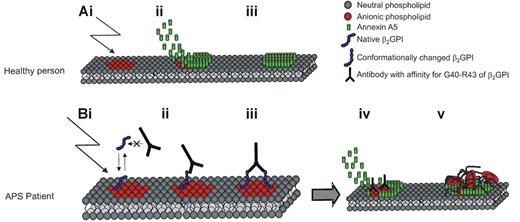
Model for a mechanism of anxA5 reduction on the surface of phospholipid membranes leading to thrombosis in APS. Panel Ai shows anionic phospholipids exposed at the surface of endothelial cells and platelets after activation/damage of or to the cells. Subsequently AnxA5 becomes positioned above the damaged site and thereby prevents further clotting (panels Aii and Aiii). Monomeric β2GPI does not have a sufficient affinity to compete with AnxA5 for binding. When damage occurs to the endothelial surface of an APS patient with antibodies against epitope G40-R43 on domain I of β2GPI, β2GPI initially binds the anionic phospholipids with a rather low affinity (panel Bi). However, following binding, β2GPI undergoes a conformational change that enables the antibody to bind the epitope g40-R43 of 2 β2GPI molecules (panels Bii and Biii). This dimerized β2GPI has a high affinity for the anionic phospholipids at the surface of the damaged cell.21 Panel Biv shows that due to the dimerization of β2GPI, it has a high enough affinity to compete with AnxA5 for the exposed anionic phospholipids. The reduced AnxA5 concentration on the damaged cell may lead to excessive clotting (panel Bv) and subsequently thrombosis.
Model for a mechanism of anxA5 reduction on the surface of phospholipid membranes leading to thrombosis in APS. Panel Ai shows anionic phospholipids exposed at the surface of endothelial cells and platelets after activation/damage of or to the cells. Subsequently AnxA5 becomes positioned above the damaged site and thereby prevents further clotting (panels Aii and Aiii). Monomeric β2GPI does not have a sufficient affinity to compete with AnxA5 for binding. When damage occurs to the endothelial surface of an APS patient with antibodies against epitope G40-R43 on domain I of β2GPI, β2GPI initially binds the anionic phospholipids with a rather low affinity (panel Bi). However, following binding, β2GPI undergoes a conformational change that enables the antibody to bind the epitope g40-R43 of 2 β2GPI molecules (panels Bii and Biii). This dimerized β2GPI has a high affinity for the anionic phospholipids at the surface of the damaged cell.21 Panel Biv shows that due to the dimerization of β2GPI, it has a high enough affinity to compete with AnxA5 for the exposed anionic phospholipids. The reduced AnxA5 concentration on the damaged cell may lead to excessive clotting (panel Bv) and subsequently thrombosis.
Several limitations of this study need to be addressed. Although the number of patients studied was relatively small, these 33 patients were clinically and serologically very well characterized. The study was retrospective; however, it was done in a single-blinded manner with coded samples. In the future, larger prospective trials will be needed to confirm these results. It will also be interesting to learn whether AnxA5 resistance is specific for APS or whether it may be a general mechanism involved in non–APS-related vascular occlusion.
It should also be noted that although several other authors have confirmed the phenomenon of antibody-mediated disruption of AnxA5, some other authors have presented dissenting views.23-29 The differences may be due to methodologies or, as indicated by the current work, might be attributable to heterogeneity of the antibodies, that is, the subset of aPL antibodies that do not recognize domain I of β2GPI are not associated with AnxA5 resistance.
In conclusion, in this study we describe 2 assays that detect the same population of aPL antibodies: LAC-causing anti-β2GPI antibodies, resistance to the anticoagulant activity of AnxA5, and a shorting of the clotting time. We were able to detect this distinct population of patients with 2 different assays that detect aPL antibodies on a functional basis. This population is highly correlated with thrombosis, indicating the detection of a pathogenic population of aPL antibodies. It also indicates a role for the reduction of AnxA5 at the vascular wall by LAC-causing anti-β2GPI antibodies, thereby shifting the hemostatic balance to a more procoagulant state.
Authorship
Contribution: B.d.L. designed and performed research and wrote the paper; X.-X.W. performed research, analyzed data, and wrote the paper; M.v.L. performed research; R.H.W.M.D. designed research and wrote the paper; J.H.R. and P.G.d.G. designed research, wrote the paper, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip. G. de Groot, Thrombosis and Haemostasis Laboratory, Department of Haematology, G03.647, University Medical Center Utrecht, PO Box 85500, 3584CX Utrecht, The Netherlands; e-mail: ph.g.degroot@azu.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal