How do blood vessels keep orientation during growth? Bautch and colleagues show that VEGF-induced proliferation of endothelial cells is regulated to occur only perpendicular to the plane of the existing vascular tube.
Formation of new blood vessels is a feature of both health and disease. Expansion of the vascular bed is essential for growth of the embryo. In the healthy adult, blood vessels are relatively quiescent, apart from the breakdown and formation of new vessels that occur in conjunction with the fertility cycle. However, during a growing number of diseases, blood vessel formation may accompany and aggravate the pathologic process. To learn about the detailed mechanism of how the vascular bed expands is of interest from a basic scientific point of view, but this knowledge also offers the possibility of developing selective stimulatory or inhibitory treatment of the vasculature. Formation of vessels occurs through a number of different mechanisms, for example, through sprouting angiogenesis, intussusception (splitting of a vessel), integration of circulating precursors, or arteriogenesis (expansion of a collateral arteriolae in conjunction with ischemia). The mechanism, which perhaps currently is the best understood, is sprouting angiogenesis, where an invasive tip of endothelial cells is formed from a pre-existing vessel. The tip expands into the surrounding tissue in a complex process involving matrix metalloprotein production, endothelial-cell proliferation, migration, and eventually differentiation, to form a new vessel lumen.
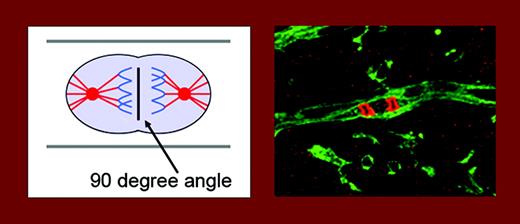
Endothelial-cell divisions are oriented at a 90° angle to the vessel long axis. The left panel is a schematic drawing. On the right, endothelial cells in stem-cell cultures are stained green (using isolectin) and DNA in mitotic cells is stained red (using phosphohistone 3). See the complete figures in the article beginning on page 1345.
Endothelial-cell divisions are oriented at a 90° angle to the vessel long axis. The left panel is a schematic drawing. On the right, endothelial cells in stem-cell cultures are stained green (using isolectin) and DNA in mitotic cells is stained red (using phosphohistone 3). See the complete figures in the article beginning on page 1345.
The focus of the study by Zeng and colleagues, in the current issue of Blood, is on the mechanism of the elongation of the vascular tube during sprouting angiogenesis; how are direction and width of the vascular tube maintained? The answer is that endothelial-cell divisions preferentially occur in a 90° angle to the vessel long axis, resulting in continuous lengthening of the vessel (see figure). This was true both in cultures of differentiating embryonic stem (ES) cells, devoid of blood flow, and in retinal vessels, exposed to flow. Of great interest, in a situation of gain-of-function of vascular endothelial growth factor (VEGF) in the ES-cell model, endothelial cells lost their ability to integrate the orientation of cell division with the morphogenetic process, eventually resulting in the formation of sheets of cells. It is well known that VEGF is a major growth factor for endothelial cells and is critical for development of the vasculature,1 but this work provides the first link between VEGF and orientation of endothelial division. Gene targeting has shown that loss of only one allele of Vegfa (the prototype member of the VEGF family) perturbs vascular development.2 Too much VEGF is also detrimental, as shown in the study from the Bautch group. It is interesting that one of the receptors for VEGF, denoted VEGF receptor-1 or Flt-1, occurs in a soluble form that binds and neutralizes VEGF, thereby allowing fine-tuned vessel stimulation. In accordance, when presented as a transgene in the ES-cell gain-of-function model, the soluble Flt-1 rescued the aberrant division orientation.
How does VEGF allow for integration of cell division with morphogenesis of the vascular tube? VEGF-A occurs in a spectrum of isoforms, and they differ in their ability to interact with coreceptors for VEGF, such as heparan sulfate proteoglycans (HSPGs) and neuropilin. It is known that the coreceptors are important for graded presentation of VEGF.3 It is an interesting possibility that quantitative or qualitative effects of coreceptors on VEGF signaling are essential in this intricate building and expansion of a 3-dimensional cellular tube.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal