Abstract
Transforming growth factor-β (TGF-β) and its signaling mediators play crucial roles in vascular formation. Our previous microarray analysis identified monocyte chemoattractant protein-1 (MCP-1) as a TGF-β target gene in endothelial cells (ECs). Here, we report that MCP-1 mediates the angiogenic effect of TGF-β by recruiting vascular smooth muscle cells (VSMCs) and mesenchymal cells toward ECs. By using a chick chorioallantoic membrane assay, we show that TGF-β promotes the formation of new blood vessels and this promotion is attenuated when MCP-1 activity is blocked by its neutralizing antibody. Wound healing and transwell assays established that MCP-1 functions as a chemoattractant to stimulate migration of VSMCs and mesenchymal 10T1/2 cells toward ECs. Furthermore, the conditioned media from TGF-β–treated ECs stimulate VSMC migration, and inhibition of MCP-1 activity attenuates TGF-β–induced VSMC migration toward ECs. Finally, we found that MCP-1 is a direct gene target of TGF-β via Smad3/4. Taken together, our findings suggest that MCP-1 mediates TGF-β–stimulated angiogenesis by enhancing migration of mural cells toward ECs and thus promoting the maturation of new blood vessels.
Introduction
Angiogenesis, a complex process for new blood vessel formation, involves several important aspects: degradation of the basement membrane by proteases, proliferation, and migration of endothelial cells (ECs), lumen formation, basement membrane resembling, recruitment of pericyte or vascular smooth muscle cells (VSMCs), vascular maturation, and finally blood flow.1-4 Many growth factors have been suggested to play pivotal roles in different aspects of this process. For instance, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) regulate many functions of ECs including proliferation, migration, extracellular proteolysis, and tube formation activity.5,6 Platelet-derived growth factor (PDGF) induces differentiation of mesenchymal cells toward pericytes and VSMCs and stimulates migration of these cells to the newly formed vessels.7-10
Transforming growth factor β (TGF-β) initiates its signals through heteromeric complexes of type II and type I serine/threonine kinase receptors. After phosphorylation by type II receptor in the receptor complex, the activated type I receptors, in turn, phosphorylate downstream receptor-regulated Smads (R-Smads: Smad2/3 for ALK5 and Smad1/5/8 for ALK1). Then the activated R-Smad proteins bind to Smad4, and together they move into the nucleus and regulate the transcription of target genes.11-13 One of angiogenic factors is TGF-β.3,14,15 The importance of TGF-β in establishing and maintaining the vascular system has been convincingly documented by genetic studies. Targeted disruption of TGF-β1 and its receptor genes in mice led to embryonic lethality due to defective vascular development.16-20 TGF-β maintains EC quiescence and induces vessel maturation, promotes basement membrane deposition, and enhances the interactions between ECs and mural cells.3,14,21 In addition, TGF-β regulates angiogenesis by influencing the expression and the activities of other angiogenic factors. For instance, both VEGF and PDGF are the direct target genes of TGF-β via Smad proteins.22-24
Monocyte chemoattractant protein-1 (MCP-1), a member of the CC chemokine family, was identified as a potent chemotactic factor for monocytes, macrophages, memory T lymphocytes, and natural killer (NK) cells.25 MCP-1 is well conserved among humans, mice, and rabbits.26 It binds to the CC chemokine receptor 2 (CCR2), which is also highly conserved. For example, rabbit CCR2 shares 80% identity to human CCR2b and can bind and respond to mouse MCP-1 and human MCP-1.27 MCP-1 is expressed in ECs, VSMCs, monocytes, and fibroblasts.28 Furthermore, MCP-1 is abundantly produced in a variety of inflammatory diseases such as atherosclerosis and rheumatoid arthritis and plays an important role in atherogenesis and in intimal hyperplasia.25
MCP-1 is highly produced by several tumors and contributes directly to tumor angiogenesis by a mechanism independent of monocyte recruitment.26 MCP-1 modulates angiogenesis by inducing EC migration and EC sprouting from aortal rings in the absence of an inflammatory response. In addition, recent studies have revealed that MCP-1 participates in VEGF-mediated angiogenesis and increases vascular permeability29 and up-regulates VEGF expression.30 Other chemokines have been also suggested to promote angiogenesis. For instance, the chemokine growth-regulated oncogene-α (GRO-α) has been recently shown to be required for thrombin-induced tumor angiogenesis.31
Our genome-wide analysis of TGF-β–modulated genes in ECs has identified MCP-1 as an inducible gene by TGF-β and its type I receptor ALK5.32 By using a chick chorioallantoic membrane (CAM) assay, we demonstrated that TGF-β promotes the formation of new blood vessels and this promotion is attenuated by anti-MCP-1–neutralizing antibody. Our results further showed that MCP-1 participates in TGF-β–induced migration of VSMCs and mesenchymal 10T1/2 cells toward ECs. Together with the findings that MCP-1 is a direct gene target of TGF-β and Smad3/4 can bind to the MCP-1 promoter and increase its activity, our results suggest that MCP-1 mediates the angiogenic effect of TGF-β by recruiting VSMCs and mesenchymal cells toward ECs.
Materials and methods
Cell culture and reagents
Human microvessel endothelial cells (HMVECs) were maintained according to the manufacturer's instruction (Clonetics, Walkersville, MD). Human umbilical cord vein endothelial cells (HUVECs) were cultured in M199 medium containing basic FGF (R&D Systems, Minneapolis, MN), 20% FBS (Hyclone, Logan, UT). Rabbit VSMCs were purchased from Beijing Union Medical College and grown in DMEM containing 10% FBS. 10T1/2 cells were cultured in MEM containing 10% FBS. TGF-β1, anti–TGF-β (AF-101-NA), and anti–human MCP-1 (AF-279-NA) neutralizing antibodies were from R&D Systems, anti-GFP antibody from Santa Cruz Biotechnology (Santa Cruz, CA), MCP-1 and RS-102895 from Sigma (St Louis, MO), and PDGF-BB from PeproTech, Rocky Hill, NJ). Recombinant adenoviruses expressing TGF-β receptors in a tetracycline-off mode were described previously.32
Constructs
The −1040-bp to +16-bp fragments of the MCP-1 promoter (GenBank: AF519531) were amplified by polymerase chain reaction (PCR) from the HeLa cell genome and subcloned into the SmaI site of pGL3-basic vector (Promega, Madison, WI) to generate a luciferase reporter. Wild-type −793 to −521, −1040 to −769, and the mutated −793 to −521 fragments of MCP-1 promoter were subcloned into MLP-pGL3-basic vector in the MluI and BglII sites. The reporter 540-Luc containing −540 bp to +16 bp of the MCP-1 promoter was a gift from Dr John Shyy (University of California, San Diego).28 All the sequences were confirmed by DNA sequencing.
RT-PCR and real-time PCR
After ECs were infected with recombinant viruses of 100 multiplicity of infection to express active receptors, total RNAs were extracted and reverse transcriptase-polymerase chain reaction (RT-PCR) was carried out as described previously.32 For real-time PCR, 4 μL cDNA was amplified in a 25-μL PCR volume containing 1.25 μL of 20 × SYBR green I (OPE, Shanghai, China). The reaction was performed with Mx3000P real-time PCR system (Stratagene, La Jolla, CA). After 3 minutes of denaturation at 94°C, PCR was performed at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 40 seconds for 35 cycles. Expression of GAPDH was used to serve as loading control. The primers used were 5′-CACTCACTCCACAACCCAAGA-3′ (MCP-1 forward), 5′-CAAAGACCCTCAAAACATCCC-3′ (MCP-1 reverse) and 5′-CATCACTGCCACCCAGAAGA-3′ (GAPDH forward), 5′-GCTGTAGCCAAATTCGTTGT -3′ (GAPDH reverse). The CCR2 primers were 5′-GTTCGTCTGCGGCTTCGTG-3′ (rabbit CCR2 forward) and 5′-GGTGACCGTCCTGGCTTTT-3′ (rabbit CCR2 reverse); 5′-TTATGCCGTGACTGAACC-3′ (mouse CCR2 forward) and 5′-CGTCCCAAATGAGAAAGA-3′ (mouse CCR2 reverse).
CAM assay
Fertilized eggs were incubated at 37°C, 60% to 70% humidity for 10 days and candled at the blunt end of the egg to identify the air sac and prominent blood vessels. A small square burr window (0.5 × 1.5 cm) was made on the air sac to expose CAM. Sterile 0.25 × 0.25-cm2 filter paper soaked with TGF-β1 (5 ng/mL) or together with neutralization antibodies was applied onto the surfaces of the growing CAMs. Physiologic saline was used as a negative control. Windows were sealed with tape and the eggs were incubated for another 3 days. Then CAMs were fixed with methanol/acetone (1:1, vol/vol) for 15 minutes and the number of blood vessels around the filter papers within 1 mm were counted with microscopy.
Cell migration assays.
Wound healing assay. The confluent cell monolayer in 12-well plate was wounded by manually scraping the cells with a blue pipette tip. The cells were treated with MCP-1 or PDGF in serum-free medium or with the conditioned medium of the endothelial cells pretreated with or without TGF-β1 (5 ng/mL). Cell migration into the wound surface was monitored by microscopy at various times. Quantitation was done by measuring the distance of the wound edge of the migrating cells from the start point to the migrated point from 3 independent experiments.
Chemotaxis assay.
The chemotaxis assay was performed in transwell plates of 6.5-mm diameter with polycarbonate membrane filters containing 8-μm pores (Corning, Corning, NY). Growth medium (2% FBS) containing PDGF or MCP-1 was added to the lower wells of the chambers and aliquots of 2 × 104 cells in 300 μL medium without FBS were seeded into the upper wells of the cell inserts. After 16 to 20 hours, the nonmigrating cells were removed from the upper surface of the membrane. The cells on the lower surface of the membrane were fixed with ice-cold methanol and then stained with crystal violet. The number of migrated cells was counted with microscopy.
EMSA
Electrophoretic mobility shift assays (EMSAs) were performed as previously described 33 with some modifications. Purified Smad protein (1 μg) and 32P-radiolabeled oligonucleotide probe (10 ng) were incubated at room temperature for 30 minutes in 10 μL reaction buffer (4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5). In competition experiments, 20-fold excess unlabeled oligonucleotide was included with the radiolabeled probe. The mixtures were resolved in 4% or 6% nondenaturing polyacrylamide gel containing 2.5% glycerol in 0.5 × TBE buffer. The DNA-protein complexes were visualized by autoradiography. The oligonucleotide probes were A, 5′-TGTCCAAGTCTGAAACCCT-3′; B, 5′-TGTAAAGGTCTGAGAATAT-3′; C, 5′-TTCCTGGCAGAGTAAGCAC-3′; D, 5′-GAATTTACAGAGTTAATAT-3′; and A mutant, 5′-TGTCCAAGTTTGAAACCCT-3′.
Luciferase assay
HepG2 cells were transfected with the indicated constructs and the internal control pRenilla-TK vector using the calcium phosphate precipitation method. Cells were serum-starved for 8 hours before being treated with TGF-β1 (1.25 ng/mL) and luciferase activity was quantified about 16 hours later using the Dual Luciferase Assay (Promega). Each experiment was performed in triplicate and the data represent the mean ± SD of 3 independent experiments after normalized to Renilla activity.
ChIP assay
The chromatin immunoprecipitation (ChIP) assay was carried out essentially as described.34 HMVECs at 80% to 90% confluence were treated with 5 ng/mL TGF-β1 for 2 hours. The cells were treated with 1% formaldehyde for 10 minutes followed by sonication. Immunoprecipitation was performed with anti-Smad4 antibody. The eluted DNA was extracted and subjected to PCR with the following primers: MCP-1 promoter region, 5′-AGTTGCCTGATCTATAACATG-3′ (forward, −793 to −773), 5′-GCTCATTCAAATGCAGAATAG -3′ (reverse, −543 to −523); MCP-1 downstream region, 5′-ACCAGGCATAGCCTATTCAGAGTC-3′ (forward, 501-524), 5′-AGGCAGGAGAAGAGAAAGAGCTC-3′ (reverse, 755-777).
Imaging processing and statistics
The CAM images were taken using a Leica MZ16 microscope (Leica, Heidelberg, Germany) equipped with a Spot Insight 3.2.0 color camera (Diagnostic Instruments, Sterling Heights, MI). The cell migration images were visualized using a Leica DMIL microscope (Leica) equipped with a Leica DC200 camera. All the images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Images were obtained using a 5×/0.12 numerical aperture Planapo objective lens. Student t tests were performed to assess the significance of treatments versus controls.
Results
TGF-β–induced expression of MCP-1 in ECs
Our microarray analysis revealed that MCP-1 is up-regulated by TGF-β and active ALK5 in HMVECs.32 To confirm the microarray result, we carried out semiquantitative RT-PCR and real-time PCR to verify TGF-β induction of MCP-1 expression. MCP-1 expression was quantitated and normalized to GAPDH. As shown in Figure 1, MCP-1 expression was up-regulated by TGF-β and the active form of ALK5, ALK5(AAD), in HMVECs (Figure 1A) as well as in HUVECs (Figure 1B). The same result was obtained in HMVECs with real-time PCR (Figure 1C).
MCP-1 expression is up-regulated by TGF-β and active ALK5. (A-C) ECs were treated with or without TGF-β1 (5 ng/mL) for 2 hours before being harvested for RNA extraction. To express receptors, ECs were infected with recombinant adenovirus expressing active forms of TGF-β type I receptor ALK1(AAD) or ALK5(AAD) in the presence of 2 μg/mL tetracycline for 16 hours and then incubated in growth medium without tetracycline for 10 hours to turn on receptor expression. Total RNAs were extracted and RT-PCR (A-B) and real-time PCR (C) were performed. MCP-1 expression was quantitated and normalized to GAPDH and relative value expressed in histograms. Three independent experiments have been performed and a representative is shown. Error bars indicate standard deviation (SD). (D) The culture medium of HMVECs after different treatments was immunoprecipitated with anti–MCP-1 antibody. Anti–MCP-1 immunoblotting was carried out. (E) Lysates from different type cells were subjected to anti-ALK5 immunoblotting. Tubulin served as a loading control.
MCP-1 expression is up-regulated by TGF-β and active ALK5. (A-C) ECs were treated with or without TGF-β1 (5 ng/mL) for 2 hours before being harvested for RNA extraction. To express receptors, ECs were infected with recombinant adenovirus expressing active forms of TGF-β type I receptor ALK1(AAD) or ALK5(AAD) in the presence of 2 μg/mL tetracycline for 16 hours and then incubated in growth medium without tetracycline for 10 hours to turn on receptor expression. Total RNAs were extracted and RT-PCR (A-B) and real-time PCR (C) were performed. MCP-1 expression was quantitated and normalized to GAPDH and relative value expressed in histograms. Three independent experiments have been performed and a representative is shown. Error bars indicate standard deviation (SD). (D) The culture medium of HMVECs after different treatments was immunoprecipitated with anti–MCP-1 antibody. Anti–MCP-1 immunoblotting was carried out. (E) Lysates from different type cells were subjected to anti-ALK5 immunoblotting. Tubulin served as a loading control.
We next checked the MCP-1 protein level after TGF-β treatment. Because the MCP-1 is a secreted protein, the culture media of HMVECs with different treatments were subjected to anti–MCP-1 immunoprecipitation and innmuoblotting. As shown in Figure 1D, TGF-β stimulated MCP-1 protein secretion, whereas SB431542, an ALK5 kinase inhibitor,35 blocked the basal MCP-1 secretion, indicating that the autocrine TGF-β may play an important role in MCP-1 basal secretion. Consistent with the role of ALK5 in mediating TGF-β stimulatory effect on MCP-1 expression in ECs, ALK5 was expressed in HUVECs, HMVECs, and HepG2 and Hep3B cells (Figure 1E).
MCP-1 mediates TGF-β–induced angiogenesis
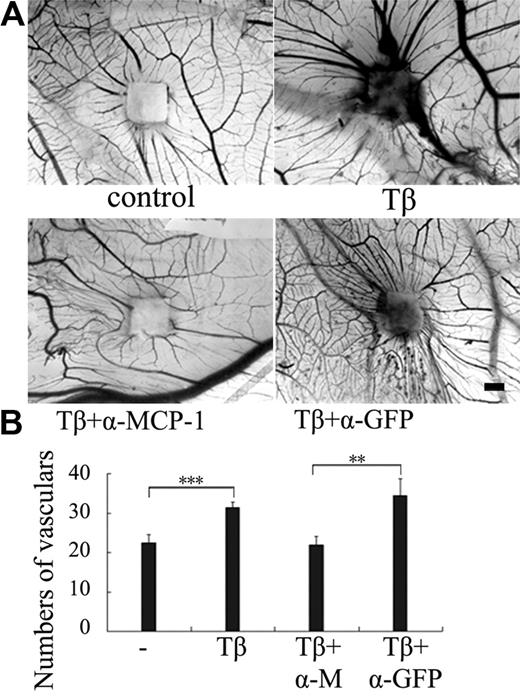
Because TGF-β is an important angiogenic factor and induces MCP-1 expression in ECs, we then attempted to investigate the role of MCP-1 in TGF-β–induced angiogenesis. One of the well-established in vivo angiogenesis models is to examine new blood vessel formation in the CAM of developing chick embryos. To study the effect of TGF-β and MCP-1 on angiogenesis, small pieces of filter papers soaked with TGF-β1 alone or together with anti-MCP-1–neutralizing antibody were applied onto the CAM surfaces. After incubation for 3 days, the CAMs were fixed and the numbers of blood vessels around the filter papers within 1 mm were counted. As shown in Figure 2, TGF-β1 apparently induced the radial formation of new vessels, consistent with the note that TGF-β functions as an angiogenic factor. Anti–MCP-1 antibody significantly interfered with the inducible effect of TGF-β on vessel formation, whereas anti-GFP antibody showed no effect. These data suggest that MCP-1 is involved in TGF-β–induced angiogenesis.
MCP-1 mediates TGF-β–induced angiogenesis. (A) The CAM assay was used to examine angiogenesis. Sterile 0.25 × 0.25-cm2 filter papers soaked with TGF-β1 (5 ng/mL) or together with anti-MCP-1–neutralizing antibody or anti-GFP antibody (200 ng/mL) were applied onto the surfaces of the growing CAMs. Physiologic saline was used as negative control. After incubation for 3 days, the CAMs were fixed and the photos were taken by microscopy. The area with increased opacity indicates an inflammatory response. Bar indicates 1 mm. (B) Quantitation of the newly formed blood vessels (n = 8). Eight to 10 eggs were used for each data point. Significant differences were measured by mean vascular numbers between control and TGF-β–treated CAM (***P < .001) and between α-MCP-1 and α-GFP antibody treatment (**P < .01). Error bars indicate SD.
MCP-1 mediates TGF-β–induced angiogenesis. (A) The CAM assay was used to examine angiogenesis. Sterile 0.25 × 0.25-cm2 filter papers soaked with TGF-β1 (5 ng/mL) or together with anti-MCP-1–neutralizing antibody or anti-GFP antibody (200 ng/mL) were applied onto the surfaces of the growing CAMs. Physiologic saline was used as negative control. After incubation for 3 days, the CAMs were fixed and the photos were taken by microscopy. The area with increased opacity indicates an inflammatory response. Bar indicates 1 mm. (B) Quantitation of the newly formed blood vessels (n = 8). Eight to 10 eggs were used for each data point. Significant differences were measured by mean vascular numbers between control and TGF-β–treated CAM (***P < .001) and between α-MCP-1 and α-GFP antibody treatment (**P < .01). Error bars indicate SD.
MCP-1 induces VSMCs migration via CCR2
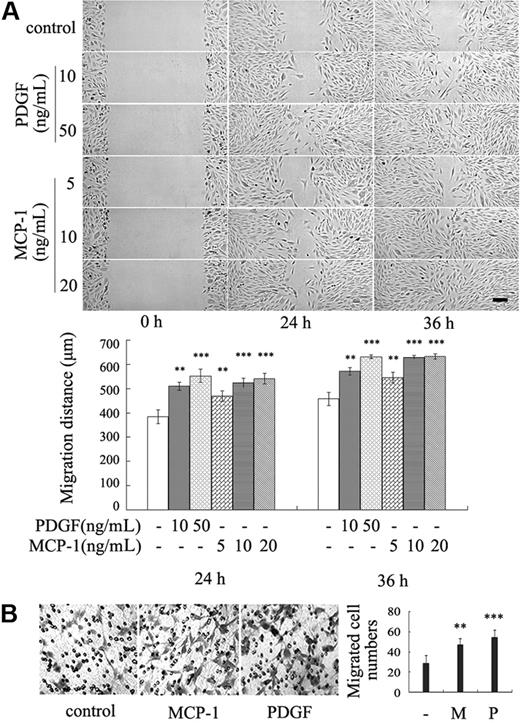
The recruitment of VSMCs and pericytes to the newly formed endothelial tubes is a key step of blood vessel maturation. Because MCP-1 is a chemoattractant factor of several cell types, we hypothesized that MCP-1 functions to recruit mural cells onto endothelial tubes. To test this hypothesis, wound healing assay was performed to assess whether MCP-1 promotes migration of VSMCs. Consistent with the previous reports,7 PDGF-BB stimulated VSMC migration (Figure 3A). Similarly, MCP-1 apparently enhanced cell migration at the concentration of 10 ng/mL (Figure 3A). At 36 hours after treatment with these growth factors, the wounds were healed completely, whereas the cells with mock treatment remained creviced. Similar MCP-1–enhanced migration was observed with mesenchymal precursor 10T1/2 cells (data not shown), which can differentiate to smooth muscle cells and pericytes.7
MCP-1 induces VSMC migration. (A) The confluent VSMC monolayers were wounded by scraping and treated with indicated concentration of MCP-1 or PDGF in serum-free medium. Cell migration to the wound surface was monitored from 0 to 36 hours. The migrated distance of the wound edge was quantitated (n = 3) (bottom panel). Bar represents 200 μm. (B) Chemotaxis assay was carried out with transwell culture chambers. Growth medium containing 10 ng/mL MCP-1 or PDGF was added to the lower wells of the chambers, and 2 × 104 VSMCs were seeded into the upper wells. After 20 hours, the cells migrating to the lower surface of the membrane were examined (original magnification ×100). Five different areas of migrated cells were counted for each data point (n = 5) (right panel). The asterisk indicates a significant difference (**P < .01, ***P < .001). Error bars indicate SD.
MCP-1 induces VSMC migration. (A) The confluent VSMC monolayers were wounded by scraping and treated with indicated concentration of MCP-1 or PDGF in serum-free medium. Cell migration to the wound surface was monitored from 0 to 36 hours. The migrated distance of the wound edge was quantitated (n = 3) (bottom panel). Bar represents 200 μm. (B) Chemotaxis assay was carried out with transwell culture chambers. Growth medium containing 10 ng/mL MCP-1 or PDGF was added to the lower wells of the chambers, and 2 × 104 VSMCs were seeded into the upper wells. After 20 hours, the cells migrating to the lower surface of the membrane were examined (original magnification ×100). Five different areas of migrated cells were counted for each data point (n = 5) (right panel). The asterisk indicates a significant difference (**P < .01, ***P < .001). Error bars indicate SD.
To confirm these results, a chemotaxis assay was carried out. VSMCs were seeded into the upper wells of transwell chambers, and MCP-1 or PDGF was added to the lower wells of the chambers. The cells migrating to the lower surface of the membrane were counted. Like PDGF, MCP-1 dramatically increased chemotactic migration of VSMCs (Figure 3B) and 10T1/2 cells (data not shown). Taken together, these results indicate that MCP-1 is a chemoattractant for VSMCs.
MCP-1 acts through its receptor CCR2. Because both VSMCs and 10T1/2 cells respond to MCP-1, we reasoned that these cells express CCR2. RT-PCR analysis revealed that CCR2 was expressed in VSMCs and 10T1/2 cells (Figure 4A), consistent with the previous reports that this receptor is expressed in smooth muscle cells.36 We next examined whether CCR2 mediates MCP-1–induced migration of VSMCs. As shown in Figure 4B, RS-102895, a potent CCR2 antagonist,37 effectively inhibited the MCP-1–induced migration of VSMCs. These results suggested that MCP-1 induces VSMC migration via its receptor CCR2.
Inhibition of CCR2 activity blocks MCP-1–induced VSMC migration. (A) MCP-1 receptor CCR2 was expressed in VSMCs and 10T1/2 cells. Total RNAs were isolated for RT-PCR using primers for rabbit and mouse CCR2, respectively, or GAPDH (as control). The data are representative of 3 individual experiments. Negative control (NC) had no cDNA templates. (B) The confluent VSMC monolayers were wounded by scraping and treated with MCP-1 (10 ng/mL) or together with RS-102895 (300 nM) in serum-free medium. DMSO was the solvent of RS-102895. Cell migration to the wound surface was monitored from 0 to 36 hours. A representative was shown (bar represents 200 μm) and the migrated distance of the wound edge quantitated (bottom panel). Asterisks indicate a significant difference of migrated distance compared with the control (*P < .05, **P < .01; n = 3). Error bars indicate SD.
Inhibition of CCR2 activity blocks MCP-1–induced VSMC migration. (A) MCP-1 receptor CCR2 was expressed in VSMCs and 10T1/2 cells. Total RNAs were isolated for RT-PCR using primers for rabbit and mouse CCR2, respectively, or GAPDH (as control). The data are representative of 3 individual experiments. Negative control (NC) had no cDNA templates. (B) The confluent VSMC monolayers were wounded by scraping and treated with MCP-1 (10 ng/mL) or together with RS-102895 (300 nM) in serum-free medium. DMSO was the solvent of RS-102895. Cell migration to the wound surface was monitored from 0 to 36 hours. A representative was shown (bar represents 200 μm) and the migrated distance of the wound edge quantitated (bottom panel). Asterisks indicate a significant difference of migrated distance compared with the control (*P < .05, **P < .01; n = 3). Error bars indicate SD.
MCP-1 mediates TGF-β–induced VSMC recruitment toward endothelial cells
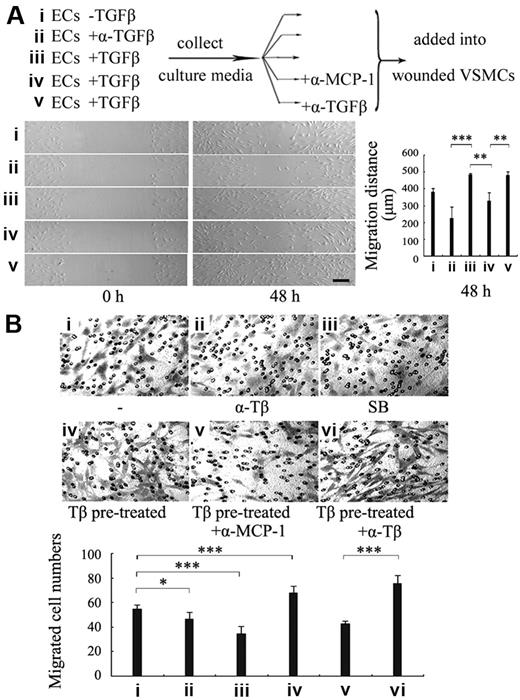
Because TGF-β enhances MCP-1 production from ECs and MCP-1 induces VSMC migration, we further tested whether MCP-1 can mediate the TGF-β effect on VSMC recruitment toward ECs. The conditioned media (CM) from the HUVECs treated with TGF-β or anti-TGF-β–neutralizing antibody for 24 hours were examined for their ability to induce VSMC migration by wound healing assay. As shown in Figure 5A, the CM from the TGF-β–treated ECs stimulated VSMC migration (Figure 5Aiii), whereas the medium from anti–TGF-β antibody-treated ECs considerably inhibited VSMC migration (Figure 5Aii). To study whether MCP-1 mediates the stimulatory effect of TGF-β on VSMC migration, anti-MCP-1–neutralizing antibody was added into the CM from TGF-β–treated ECs. Anti–TGF-β antibody was used to rule out the direct influence of TGF-β on VMSC migration. Blockade of MCP-1 activity by anti–MCP-1 antibody greatly reduced TGF-β–promoted VMSC migration, whereas anti–TGF-β antibody had no effect (Figure 5Aiv-v), suggesting that it is MCP-1 but not TGF-β that directly induced VSMC migration. Similar results were also obtained with 10T1/2 cells.
MCP-1 mediates TGF-β effect on VSMC recruitment onto endothelial cells. (A) The conditioned medium (CM) of ECs treated with TGF-β1 stimulated VSMCs migration via MCP-1. The CM from HUVECs treated with none (i), anti–TGF-β antibody (ii), or with TGF-β1 (iii) for 24 hours was added into VSMCs in wound healing assay. Aliquots of the CM from the TGF-β1–treated ECs were added into VSMCs together with anti–MCP-1 antibodies (iv) or anti–TGF-β antibody (v). A representative was shown (bar represents 200 μm) and the migrated distance of the wound edge quantitated (n = 3) (bottom panel). (B) Blockage of MCP-1 activity impaired VSMC recruitment induced by TGF-β–treated ECs. HUVECs were first seeded to the lower wells of transwell chambers and pretreated with mock (i-iii) or TGF-β1 (iv-vi) for 24 hours. Then anti–TGF-β antibody (ii,vi), SB431542 (10 μM) (iii), or anti–MCP-1 antibody (v) was added into the lower wells 1 hour before VSMCs were seeded in the upper wells. After 20 hours, the cells migrating to the lower surface of the membrane were fixed, stained, and examined (original magnification ×100). Five different areas of migrated cells were counted for each data point. Asterisks indicate a significant difference compared with the control (*P < .05, ***P < .001; n = 5). Error bars indicate SD.
MCP-1 mediates TGF-β effect on VSMC recruitment onto endothelial cells. (A) The conditioned medium (CM) of ECs treated with TGF-β1 stimulated VSMCs migration via MCP-1. The CM from HUVECs treated with none (i), anti–TGF-β antibody (ii), or with TGF-β1 (iii) for 24 hours was added into VSMCs in wound healing assay. Aliquots of the CM from the TGF-β1–treated ECs were added into VSMCs together with anti–MCP-1 antibodies (iv) or anti–TGF-β antibody (v). A representative was shown (bar represents 200 μm) and the migrated distance of the wound edge quantitated (n = 3) (bottom panel). (B) Blockage of MCP-1 activity impaired VSMC recruitment induced by TGF-β–treated ECs. HUVECs were first seeded to the lower wells of transwell chambers and pretreated with mock (i-iii) or TGF-β1 (iv-vi) for 24 hours. Then anti–TGF-β antibody (ii,vi), SB431542 (10 μM) (iii), or anti–MCP-1 antibody (v) was added into the lower wells 1 hour before VSMCs were seeded in the upper wells. After 20 hours, the cells migrating to the lower surface of the membrane were fixed, stained, and examined (original magnification ×100). Five different areas of migrated cells were counted for each data point. Asterisks indicate a significant difference compared with the control (*P < .05, ***P < .001; n = 5). Error bars indicate SD.
To directly examine the involvement of MCP-1 in EC-induced VSMC recruitment, HUVECs were seeded to the lower wells of transwell chambers and pretreated with or without TGF-β1, anti-TGF-β–neutralizing antibody or the ALK5 inhibitor SB431542 for 24 hours, and then VSMCs were seeded in the upper wells. Migration of VSMCs toward the lower wells was examined. Inhibition of endogenous TGF-β activity by the neutralizing antibody or ALK5 kinase activity by SB431542 in HUVECs attenuated VSMC migration to a certain extent (Figure 5Bii-iii), whereas the migration ability of VSMCs was enhanced in the wells where ECs were pretreated with TGF-β1 (Figure 5Biv). When anti-MCP-1–neutralizing antibody was added following TGF-β1 treatment, VSMC migration was dramatically reduced (Figure 5Bv). However, addition of the anti–TGF-β antibody after TGF-β treatment did not affect EC-induced VSMC migration (Figure 5Bvi). Similar data were acquired with 10T1/2 cells (data not shown). These results together strongly suggest that TGF-β up-regulates MCP-1 production from ECs through its receptor ALK5 and MCP-1, in turn, induces VSMC recruitment toward ECs.
TGF-β induced MCP-1 expression via ALK5 and Smad3/Smad4
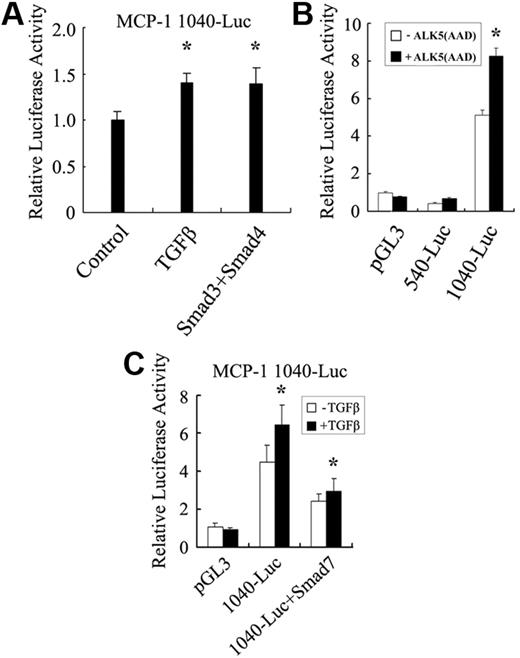
Both gene chip and RT-PCR analyses revealed that MCP-1 is up-regulated by TGF-β. To dissect the mechanism of TGF-β–regulated MCP-1 transcription, the MCP-1 promoter covering a 1040-bp fragment upstream of the start codon ATG was cloned from the HMVEC gDNA to generate the luciferase reporter 1040-Luc. As shown in Figure 6A, TGF-β1 and Smad3/Smad4 consistently, albeit moderately, stimulated luciferase expression driven by this promoter in HMVECs.
TGF-β stimulates MCP-1 promoter activity. (A) TGF-β and Smad3/Smad4 enhanced the activity of the MCP1 promoter in HMVECs. HMVECs were transfected with MCP-1 1040-luciferase or cotransfected with Smad3 and Smad4. After being serum-starved for 24 hours, the cells were treated with or without TGF-β1 for another 20 hours prior to being harvested for luciferase activity measurement. (B) The −1040-bp to −540-bp region of the MCP-1 promoter was important to respond to TGF-β stimulation. HepG2 cells were transfected with various constructs as indicated. The cells were then harvested for luciferase measurement 40 hours later. (C) Smad7 inhibited the TGF-β–induced expression of the 1040-luciferase reporter. HepG2 cells were transfected with reporters together with or without Smad7. Luciferase assay was performed similarly as in panel A. The asterisk indicates a significant difference compared with the control (*P < .05; n = 3). Error bars indicate SD.
TGF-β stimulates MCP-1 promoter activity. (A) TGF-β and Smad3/Smad4 enhanced the activity of the MCP1 promoter in HMVECs. HMVECs were transfected with MCP-1 1040-luciferase or cotransfected with Smad3 and Smad4. After being serum-starved for 24 hours, the cells were treated with or without TGF-β1 for another 20 hours prior to being harvested for luciferase activity measurement. (B) The −1040-bp to −540-bp region of the MCP-1 promoter was important to respond to TGF-β stimulation. HepG2 cells were transfected with various constructs as indicated. The cells were then harvested for luciferase measurement 40 hours later. (C) Smad7 inhibited the TGF-β–induced expression of the 1040-luciferase reporter. HepG2 cells were transfected with reporters together with or without Smad7. Luciferase assay was performed similarly as in panel A. The asterisk indicates a significant difference compared with the control (*P < .05; n = 3). Error bars indicate SD.
Because of the low transfection efficiency of ECs, we then analyzed the MCP-1 promoter activity in TGF-β–responsive human hepatoma HepG2 cells. Consistent with the results obtained in ECs, the higher basal activity of the −1040-bp MCP-1 promoter was further elevated by the ectopic expression of ALK5(AAD), whereas the −540-bp fragment had no response to ALK5(AAD) (Figure 6B), suggesting the fragment between −540 bp and −1040 bp contains TGF-β–responsive elements.
To verify that the TGF-β–induced expression of 1040-luciferase is Smad-dependent, Smad7, an inhibitory Smad,11,12 was cotransfected with the reporter. Overexpression of Smad7 led to a 50% decrease of the TGF-β–induced expression of 1040-luciferase (Figure 6C), indicating a possible involvement of Smad proteins in MCP-1 transcription.
Smad3 and Smad4 directly bind to the MCP-1 promoter
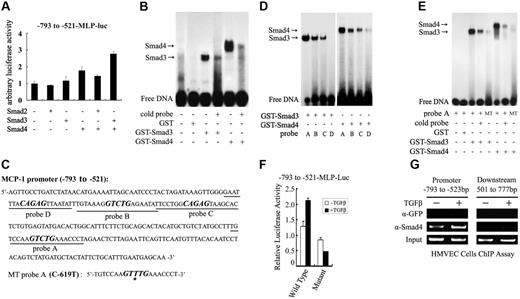
Subsequently, we assessed the ability of the downstream mediators of the TGF-β/ALK5 signaling pathway, Smad2, Smad3, and Smad4, in regulating MCP-1 promoter activity in HepG2 cells. Ectopic expression of Smad4 alone or Smad3 and Smad4 together enhanced the transcriptional activity of the −1040 bp MCP-1 promoter (data not shown). We further showed that Smad3 and Smad4 increased the transcriptional activity of the MCP-1 promoter fragment containing −793 bp to −521 bp (Figure 7A), but not that of the fragment covering −1040 bp to −769 bp (data not shown).
Smad3 and Smad4 directly bind to and activate the MCP-1 promoter. (A) Smad3 and Smad4 enhanced the expression of the luciferase reporter under the control of the MCP-1 promoter region containing −793 to −521 bp. The reporter −793 to −521-MLP-Luc was cotransfected into HepG2 cells with various Smad proteins as indicated. The luciferase assay was performed similarly as in Figure 6. Error bars indicate SD. (B) Smad3 and Smad4 directly bound to the −793-bp to −521-bp fragment as shown by EMSA. Purified GST, GST-Smad3, or GST-Smad4 and 32P-radiolabeled −793-bp to −521-bp fragment were incubated in the presence or absence of 20-fold excess unlabeled probe (cold probe). The DNA-protein complex was analyzed with a polyacrylamide gel and autoradiography. (C) There are 4 potential SBEs (bold and italic) in the −793-bp to −521-bp region. The SBE in probe A was mutated with C-619 replaced by T to generate MT probe A. (D) Smad3 and Smad4 bound to probe A with a high affinity. EMSA was performed similarly as in panel B. (E) Mutation in the SBE box in probe A abolished Smad binding. (F) Smad binding was essential for the TGF-β–enhanced MCP-1 promoter activity. The reporters containing wild-type or mutant (as in the MT probe A) −793-bp to −521-bp fragment were transfected to HepG2 cells. After being treated with TGF-β1 for 16 hours later, the cells were harvested for luciferase measurement. Error bars indicate SD. (G) TGF-β enhanced the binding of endogenous Smad4 to the MCP-1 promoter. HMVECs were treated with TGF-β1 for 2 hours and subjected to ChIP with anti-Smad4 antibody. The Smad4-bound DNA was MCP-1 amplified by PCR with the primers for the promoter region −793 bp to −523 bp or for the 501 to 777 bp region downstream of ATG.
Smad3 and Smad4 directly bind to and activate the MCP-1 promoter. (A) Smad3 and Smad4 enhanced the expression of the luciferase reporter under the control of the MCP-1 promoter region containing −793 to −521 bp. The reporter −793 to −521-MLP-Luc was cotransfected into HepG2 cells with various Smad proteins as indicated. The luciferase assay was performed similarly as in Figure 6. Error bars indicate SD. (B) Smad3 and Smad4 directly bound to the −793-bp to −521-bp fragment as shown by EMSA. Purified GST, GST-Smad3, or GST-Smad4 and 32P-radiolabeled −793-bp to −521-bp fragment were incubated in the presence or absence of 20-fold excess unlabeled probe (cold probe). The DNA-protein complex was analyzed with a polyacrylamide gel and autoradiography. (C) There are 4 potential SBEs (bold and italic) in the −793-bp to −521-bp region. The SBE in probe A was mutated with C-619 replaced by T to generate MT probe A. (D) Smad3 and Smad4 bound to probe A with a high affinity. EMSA was performed similarly as in panel B. (E) Mutation in the SBE box in probe A abolished Smad binding. (F) Smad binding was essential for the TGF-β–enhanced MCP-1 promoter activity. The reporters containing wild-type or mutant (as in the MT probe A) −793-bp to −521-bp fragment were transfected to HepG2 cells. After being treated with TGF-β1 for 16 hours later, the cells were harvested for luciferase measurement. Error bars indicate SD. (G) TGF-β enhanced the binding of endogenous Smad4 to the MCP-1 promoter. HMVECs were treated with TGF-β1 for 2 hours and subjected to ChIP with anti-Smad4 antibody. The Smad4-bound DNA was MCP-1 amplified by PCR with the primers for the promoter region −793 bp to −523 bp or for the 501 to 777 bp region downstream of ATG.
Smad proteins regulate gene expression through their physical interaction with the promoters of the target genes. These data suggested that TGF-β induces MCP-1 expression via Smad3/4 and the TGF-β–responsive elements are located in the fragment −793 bp to −521 bp of the MCP-1 promoter. To examine whether this fragment contains Smad-binding sites, we performed EMSAs using purified GST-Smad proteins. Four fragments of MCP-1 promoter (+16 to −1040) were prepared by PCR and radiolabeled with 32P-ATP. Only the −793-bp to −521-p fragment, but not other fragments, could bind Smad3 and Smad4 (Figure 7B-C and data not shown). This binding is specific as the DNA-Smad complex was disrupted by the excess unradiolabeled DNA probe.
Smad3 and Smad4 can specifically recognize the DNA sequence CAGAC, Smad-binding element (SBE),38-40 and there are 4 CAGAC or CAGAC-like boxes in the −793- to −521-bp fragment in both sense and antisense sequences (Figure 7C). To explore the binding ability of Smad3 and Smad4 to these sites, 4 oligonucleotide probes (probe A, B, C, and D) containing the potential Smad-binding sites were synthesized. EMSA studies revealed that Smad3 and Smad4 bound with the highest affinity to probe A, which contains the GTCTG box (Figure 7D), but not to the mutant with one nucleotide replacement in this box (GTTTG) (Figure 7E). To further study the importance of this binding, the same mutation was introduced into the −793-bp to −521-bp fragment. This mutation clearly decreased the basal level of the promoter activity and abolished its ability to respond to TGF-β stimulation (Figure 7F), suggesting that Smad3/4 bind to this box and mediate TGF-β–induced MCP-1 expression.
Next, we carried out ChIP assays to verify the in vivo binding of Smad4 to the MCP-1 promoter. As shown in Figure 7G, endogenous Smad4 bound to the −793-bp to −523-bp region of the MCP-1 promoter in the basal state in HMVECs and this binding was apparently increased by TGF-β treatment. In contrast, Smad4 did not bind to the 501-bp to 777-bp region downstream of the start codon ATG, and the control immunoprecipitation with anti-GFP antibody did not pull down the MCP-1 promoter, indicating the binding of Smad4 to the MCP-1 promoter is specific. Taken together, these results indicate that Smad3/4 mediate the TGF-β–induced expression of MCP-1.
Discussion
TGF-β is a pleiotropic growth factor and plays a pivotal role in establishment and maintenance of the vascular system, but the molecular mechanism remains largely unclear. Here we provide compelling evidence that MCP-1 mediates the angiogenic effect of TGF-β by promoting the recruitment of mural cells to ECs. We demonstrated that TGF-β treatment enhanced the ability of ECs to recruit VSMCs and mesenchymal precursor 10T1/2 cells in both wound healing and transwell assays. The chemotactic response of VSMCs and 10T1/2 cells to ECs was attenuated by inhibiting MCP-1 activity. Furthermore, blockade of MCP-1 activity also reduced TGF-β–mediated angiogenesis in CAM assay. These data strongly suggest that MCP-1 plays an important role in TGF-β–promoted angiogenesis.
The recruitment of pericytes and VSMCs toward endothelial tubes has a key part in angiogenesis. These mural cells stabilize nascent endothelial tubes and therefore are essential for the formation of functional vascular vessels.41 TGF-β signaling has been suggested to play a vital role in this process as demonstrated by targeted gene disruption of the TGF-β–signaling components. Mice with targeted mutations in the genes encoding TGF-β1, its type I receptor ALK5 and its type II receptor TβRII exhibit similar phenotypes: embryonic death due to defective vascular development.16-18,42 The major vascular defects include deficiency in vascular smooth muscle differentiation and lack of recruitment of mural cells although there is establishment of the endothelial tubes. Therefore, TGF-β may be essential for differentiation of mesenchymal cells to VSMCs and their recruitment to endothelial tubes. One of the factors that has been suggested to mediate TGF-β–induced differentiation and recruitment of mural cells is PDGF. It has been documented that PDGF induces the proliferation, migration, and differentiation of mural cells7,9,10 and is up-regulated by TGF-β.22 PDGF-B and PDGF-β receptor deficiency in mice leads to embryonic lethal and cardiovascular, renal, placental, and hematologic disorders.43 The endothelium-specific ablation of PDGF-B results in impaired recruitment of pericytes to endothelial tubes.44 However, in contrast to the embryonic lethality of PDGF-B-null mutants, the endothelium-specific mutants survive into adulthood, implicating that PDGF is not the only factor required for the recruitment of mural cells to ECs.
Accumulating evidence suggested that MCP-1 can function as an angiogenic factor. MCP-1 induces blood vessel formation in vivo as assessed by CAM and matrigel plug assays.26,30 MCP-1 was suggested to induce chemotaxis of endothelial cells26 or up-regulate the expression of hypoxia-inducible factor 1α, which in turn induces VEGF-A.30 The angiogenic effect of MCP-1 may contribute to tumor progression.26,45 Here, we demonstrate that MCP-1 can induce recruitment of VSMCs toward to ECs, consistent with the previous report that MCP-1 can enhance VSMC migration.46 It is noteworthy that MCP-1–deficient mice exhibited defects in recruitment of monocytes and macrophages but did not display detectable impairments regarding vascularization.47,48 A convincing explanation is the compensatory functions of other angiogenic factors such as PDGF.
Our microarray analysis revealed that MCP-1 expression is enhanced in HMVECs by TGF-β treatment and overexpression of active ALK5.32 Indeed, we found that ALK5 protein is expressed in both HMVECs and HUVECs, which agrees with the finding that both ALK1 and ALK5 are expressed in endothelial cells,49 but contrasts to a recent study showing that ALK5 is lacking in developing endothelium.42 Using RT-PCR analysis, we confirmed that MCP-1 expression is up-regulated in TGF-β–treated HMVECs and HUVECs. Concomitantly, the reporter driven by the MCP-1 promoter was consistently stimulated by TGF-β, Smad3, and Smad4 in both HMVECs and HepG2 cells. It is unclear why the responsiveness of the MCP-1 promoter to TGF-β was low. It could be that the fragment (−1040 bp to +16 bp) only is a part of the TGF-β–responsive region. We further showed that Smad3 and Smad4 can specifically bind to the MCP-1 promoter by EMSA. In agreement with the function of Smad proteins in mediating TGF-β–induced MCP-1 expression, ChIP assay revealed that TGF-β enhanced the binding of endogenous Smad4 to the MCP-1 promoter. Smad proteins regulate transcription in collaboration with other cofactors.12 At this moment, it is unclear what other factors work with Smad3 and Smad4 to modulate MCP-1 expression. Interestingly, TGF-β was reported to inhibit MCP-1 expression in macrophages via an antagonistic effect of Smad3 on AP-1 activity.50 This discrepancy may be due to cell type-specific regulation of MCP-1 by TGF-β. Nonetheless, our findings clearly demonstrated that MCP-1 is a direct target gene of TGF-β in ECs.
In summary, the present study unraveled a novel pathway for TGF-β to promote angiogenesis. TGF-β stimulates MCP-1 production from ECs and Smad3 and Smad4 mediate this stimulation by directly binding to the MCP-1 promoter. The secreted MCP-1 in turn induces recruitment of VSMCs toward ECs and thus facilitates the maturation of nascent blood vessels. Although our results indicated MCP-1–induced VSMC migration is mediated by CCR2, a G protein-coupled receptor, the detailed molecular basis awaits further investigation.
Authorship
Contribution: J.M. and Q.W. performed most of the experiments; T.F. performed the ChIP assay; J.-D.J.H. provided help in data analysis and discussion; and Y.-G.C. was involved in experiment design, data analysis and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
J.M. and Q.W. contributed equally to this work
Correspondence: Ye-Guang Chen, Department of Biological Sciences and Biotechnology, Tsinghua University, Beijing 100084, China; e-mail: ygchen@tsinghua.edu.cn.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC) 30430360, 30428002, and 973 Program (2004CB720002, 2006CB0F0200) (Y.G.C.), and NSFC 30500083 (Q.W.), and partially by a grant from the Bugher Foundation (New York, NY). Y.G.C. is a Chueng Kong Scholar. We are grateful to Dr John Shyy for MCP-1 540bp-luciferase plasmid.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal