Abstract
Therapy of Hodgkin disease (HD) is designed to prolong progression-free survival and minimize toxicity. The best regimen to achieve this has not yet been defined. A total of 108 patients with newly diagnosed HD and adverse prognostic factors were prospectively studied between 1999 and 2004. They were assigned to therapy according to defined risk stratification. Patients were defined depending on the International Prognostic Score (IPS). Those with IPS of 3 or higher received 2 cycles of escalated therapy, including bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP [EB]). All others received 2 cycles of standard BEACOPP (SB). Subsequent therapy was prospectively assigned following 2 cycles according to results of early interim 67Ga or positron emission tomography/computed tomography (PET/CT). Following a positive interim scan, 4 cycles of EB were administered, whereas 4 cycles of SB were given to patients with a negative scan. The complete remission rate, the 5-year event-free survival (EFS), and overall survival (OS) rates were 97%, 85% and 90%, respectively. Relapse or progression occurred in 27% of patients with interim positive PET/CT versus 2.3% of negative scans (P < .02). Early interim fluorine-18 2-fluoro-2-deoxy-d-glucose (FDG)–PET/CT is a useful tool for adjustment of chemotherapy on an individual basis. Similar EFS and OS rates were observed for patients in both risk groups.
Introduction
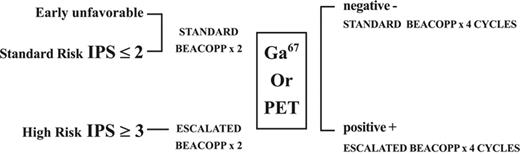
Although standard combination chemotherapy has been effective in treating patients with Hodgkin lymphoma, many patients with advanced disease ultimately have a relapse. The use of more intensive regimens, such as doxorubicin, vinblastine, nitrogen mustard, vincristine, bleomycin, etoposide, prednisone (BEACOPP), or Stanford V administered as initial therapy, has suggested improved complete remission (CR) rates over standard regimens.1,2 However, escalated BEACOPP (EB) is associated with an increased risk of secondary malignancies,3 implying that the risk-to-benefit ratio for intensifying therapy is not favorable for all patients. Developing a strategy that can identify patients requiring more intensive regimens as well as those who may do as well with less intensive regimens is of great importance and may enable maximizing dose intensity for patients with a more adverse prognosis or reducing the dose for those with lesser adverse features or for the early responders. In the present study, patients were assigned to their initial regimen based on risk categories (Table 1) and an attempt was made to prospectively adjust the therapy based on the response as determined by scintigraphy at the end of 2 cycles (Figure 1). Gallium 67 (67Ga)–single-photon emission computed tomography (SPECT) was performed until 2001 and fluorine-18 2-fluoro-2-deoxy-d-glucose–positron emission tomography/computed tomography (FDG-PET/CT) scanning has become routine since then.
Initial therapy based on pretherapy risk stratification
| . | Very low risk . | Intermediate risk . | High risk . | |
|---|---|---|---|---|
| Low risk . | Standard risk . | |||
| Characteristics | Early favorable; stage I or II; nonbulky; no B symptoms; fewer than 4 sites; age below 50 y; ESR below 50 mm/h; CHL histology | Early unfavorable; stage I or II; nonbulky; no B symptoms; 4 or more sites; age above 50 y; ESR greater than 50 mm/h | Stage I or II bulky or including B symptoms; any stage III or IV; IPS 0-2 | Stage I or II bulky or including B symptoms; any stage III or IV; IPS 3-7 |
| Therapy | ABVD (not a part of this study) | Standard BEACOPP (SB) | Standard BEACOPP (SB) | Escalated BEACOPP (EB) |
| . | Very low risk . | Intermediate risk . | High risk . | |
|---|---|---|---|---|
| Low risk . | Standard risk . | |||
| Characteristics | Early favorable; stage I or II; nonbulky; no B symptoms; fewer than 4 sites; age below 50 y; ESR below 50 mm/h; CHL histology | Early unfavorable; stage I or II; nonbulky; no B symptoms; 4 or more sites; age above 50 y; ESR greater than 50 mm/h | Stage I or II bulky or including B symptoms; any stage III or IV; IPS 0-2 | Stage I or II bulky or including B symptoms; any stage III or IV; IPS 3-7 |
| Therapy | ABVD (not a part of this study) | Standard BEACOPP (SB) | Standard BEACOPP (SB) | Escalated BEACOPP (EB) |
CHL indicates classic Hodgkin lymphoma.
Therapy planned for initial 2 cycles for each group of patients is indicated. Patients with early unfavorable and patients with advanced disease and low score chemotherapy regimen was started with standard BEACOPP. Only patients with advanced disease and score ≥3 were started with escalated BEACOPP. Further therapy was based on results of interim scanning. Early favorable patients were in the very low-risk group and were excluded from the study.
Treatment scheme. Initial 2 cycles were based on pretreatment evaluation. Only high-risk patients (IPS of ≥ 3) were started with escalated BEACOPP (EB). Patients with early unfavorable and those with an IPS less than 3 had 2 cycles of standard BEACOPP (SB). Following 2 cycles of chemotherapy, additional therapy was determined according to the results of the interim scintigraphy. Those with a persistently positive scintigraphy interpreted as residual disease were treated with 4 additional cycles of EB. Patients with a negative scintigraphy were treated with additional 4 cycles of SB.
Treatment scheme. Initial 2 cycles were based on pretreatment evaluation. Only high-risk patients (IPS of ≥ 3) were started with escalated BEACOPP (EB). Patients with early unfavorable and those with an IPS less than 3 had 2 cycles of standard BEACOPP (SB). Following 2 cycles of chemotherapy, additional therapy was determined according to the results of the interim scintigraphy. Those with a persistently positive scintigraphy interpreted as residual disease were treated with 4 additional cycles of EB. Patients with a negative scintigraphy were treated with additional 4 cycles of SB.
The aim of the study was to examine the event-free survival (EFS) and overall survival (OS) using risk-adapted BEACOPP.
Patients and methods
Patients
From 1999 to 2004, a prospective multicenter study was initiated for patients, aged 18 to 65 years, with classical Hodgkin disease (HD; Table 2). Individuals at the early stage of the disease and with favorable features were excluded from the study. Patients were eligible for the study if they had HD stage I-II with one or more unfavorable features: B symptoms, bulky disease, 4 or more sites of disease, age 50 years or older, erythrocyte sedimentation rate (ESR) 50 mm/h or higher, lymphocyte-depleted histology, “E” site, or stage III-IV disease. Patients with HD stage I-II and B or bulky, or stage III-IV were defined according to the International Prognostic Score (IPS).4 They were subdivided into 2 groups based on risk stratification as defined in Table 1. Low-risk patients with an early unfavorable disease and standard-risk patients with an IPS of 2 or less were treated with 2 cycles of standard BEACOPP (SB). High-risk patients were defined as patients with an IPS of 3 or higher. This decision was based on the observation made in the IPS study indicating that only 55% of these patients had freedom from progression in a 5-year follow-up, and only 70% of them were alive at 5 years from diagnosis.4 High-risk patients were treated with 2 cycles of EB (Table 1). After 2 cycles of chemotherapy, functional imaging results (67Ga scan or FDG-PET/CT) determined the subsequent therapeutic regimen (Figure 1). All patients underwent scintigraphy at diagnosis and early during treatment (that is, 67Ga scan, after the first or second cycle, or hybrid FDG-PET/CT after the second cycle). Results were available prior to the third cycle of therapy. Scintigraphy studies were interpreted in association with all other patient data.
Characteristics of the 108 patients
| Parameter . | Data . |
|---|---|
| No. men/no. women | 64/44 |
| Median age, y (range) | 33 (19-63) |
| B symptoms, yes/no, no. patients | 63/45 |
| Stage I/II, no. patients | 2/46 |
| Standard risk/high risk, no. patients | 32/4 |
| Stage III/IV, no. patients | 27/33 |
| Standard risk/high risk, no. patients | 24/35 |
| Score 0/1/2 points, no. patients | 3/13/40 |
| Score 3/4/5-7 points, no. patients | 27/5/7 |
| Parameter . | Data . |
|---|---|
| No. men/no. women | 64/44 |
| Median age, y (range) | 33 (19-63) |
| B symptoms, yes/no, no. patients | 63/45 |
| Stage I/II, no. patients | 2/46 |
| Standard risk/high risk, no. patients | 32/4 |
| Stage III/IV, no. patients | 27/33 |
| Standard risk/high risk, no. patients | 24/35 |
| Score 0/1/2 points, no. patients | 3/13/40 |
| Score 3/4/5-7 points, no. patients | 27/5/7 |
Pretherapy characteristics of patients with early unfavorable (n = 13) and advanced HD (n = 95) who were treated according to risk-stratified group, with interim reassessment.
Interim gallium or PET/CT scanning was considered as positive for lymphoma in the presence of any focus of increased uptake that could not be related to physiologic biodistribution of the tracer or to a known benign process. A decreased but residual uptake was also interpreted as positive. A negative study showed no foci of increased uptake unrelated to physiologic or benign tracer uptake. Further treatment after the first 2 cycles was based on scan results (Figure 1). Patients with resolution of the scintigraphy findings were subsequently treated with 4 additional cycles (cycles 3-6) of SB. Those with residual uptake, interpreted as a positive scan, had 4 additional cycles of EB for a total of 6 cycles. Patients had scans following chemotherapy and then every 6 months for 2 years and once during the third year. Radiation therapy at the end of chemotherapy was planned for patients with a bulky mediastinal mass of more than 10 cm on CT and those with residual scintigraphy uptake of a single lesion following 6 cycles of chemotherapy. This study was performed in accordance with the Declaration of Helsinki guidelines and all patients signed informed consent.
Chemotherapy was given as previously reported by Diehl et al.2 Cycles were repeated every 21 days.
Compared with SB, EB included increased doses of the following drugs: cyclophosphamide, doxorubicin, and etoposide (Table 3). G-CSF (filgrastim) was given to the patients who were treated with EB or to any patient who had febrile neutropenia or in whom treatment was delayed due to incomplete neutrophil recovery. The doses were reduced for patients requiring prolonged hospitalization for neutropenia and fever. Dose reduction due to toxicity following EB was graded from level I to level IV and was used as step-down if the preceding cycle was accompanied with febrile neutropenia of 5 days or longer.
Planned chemotherapy regimens
| Drug . | Administration, method (time) . | Standard BEACOPP doses . | Escalated BEACOPP doses . |
|---|---|---|---|
| Bleomycin | IV (d 8) | IU/m2/d | 10 IU/m2/d |
| Etoposide | IV (d 1-3) | 100 mg/m2/d | 200 mg/m2/d |
| Doxorubicin | IV (d 1) | 25 mg/m2/d | 35 mg/m2/d |
| Cyclophosphamide* | IV (d 1) | 650 mg/m2/d | 1250 mg/m2/d |
| Vincristine† | IV (d 8) | 1.4 mg/m2/d | 1.4 mg/m2/d |
| Procarbazine | PO (d 1-7) | 100 mg/m2/d | 100 mg/m2/d |
| Prednisone | PO (d 1-7) | 40 mg/m2/d | 40 mg/m2/d |
| Drug . | Administration, method (time) . | Standard BEACOPP doses . | Escalated BEACOPP doses . |
|---|---|---|---|
| Bleomycin | IV (d 8) | IU/m2/d | 10 IU/m2/d |
| Etoposide | IV (d 1-3) | 100 mg/m2/d | 200 mg/m2/d |
| Doxorubicin | IV (d 1) | 25 mg/m2/d | 35 mg/m2/d |
| Cyclophosphamide* | IV (d 1) | 650 mg/m2/d | 1250 mg/m2/d |
| Vincristine† | IV (d 8) | 1.4 mg/m2/d | 1.4 mg/m2/d |
| Procarbazine | PO (d 1-7) | 100 mg/m2/d | 100 mg/m2/d |
| Prednisone | PO (d 1-7) | 40 mg/m2/d | 40 mg/m2/d |
IV indicates intravenously; PO, orally.
MESNA, total dose is the same as that of cyclophosphamide, administered: 20% IV at hour 0; 40% PO at hour 2; 40% PO at hour 5.
Vincristine, up to 2 mg (maximal dose).
Adapted from Diehl et al.2
Statistical methods
The primary end point of the study was the EFS defined as the occurrence of one of the following: death from any cause, no CR achieved at the end of treatment, disease progression, or relapse. The secondary end point was death from any cause. All patients were analyzed and included in the intention-to-treat group. The trial was designed to test the hypotheses that risk-adapted BEACOPP, irrespective of the primary risk group, results in a similar EFS rate as the escalated BEACOPP arm observed in the HD9 of the German Hodgkin study group.2 A total of 108 patients will allow detecting a difference of about 10% with power of 80% and a 5% level of significance.
CR was defined as the disappearance of all disease manifestations, including radiographic findings, at the end of therapy. Primary resistant disease (PRD) was defined as persistence of the disease, either biopsy proven or the appearance of new findings during the first 3 months after completion of therapy. Major attempts were made to obtain tissue diagnosis prior to making the decision of therapy failure if uptake was only present at a single site at end of therapy. This included one thoracotomy and one core needle biopsy. Relapse was defined as the appearance of new symptoms or new findings on scintigraphy studies among patients who were in complete responses more than 3 months from the end of therapy. EFS was calculated from the diagnosis to the occurrence of any event. OS was measured from the diagnosis to death from any cause. EFS and OS were described using Kaplan-Meier curve5 and log-rank test. The χ2 test was used to compare proportions between different groups. Analysis was carried out using SPSS 11.5 (SPSS, Chicago, IL).
Results
Response to therapy
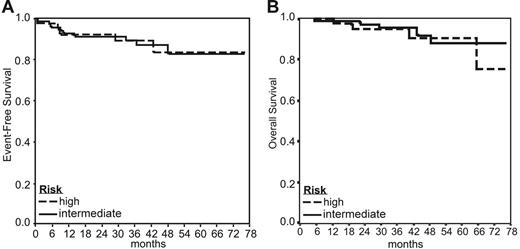
A total of 112 patients were enrolled in 4 medical centers; 108 with a follow-up of at least 12 months from the end of therapy were evaluated. Patient demographics are shown in Table 2. A total of 105 patients (97%) achieved CR. Three patients (3%) had PRD observed as disease progression in multiple sites. A biopsy was performed in only one of these patients to confirm the diagnosis of PRD. Nine patients (8%) experienced disease relapse 8 to 43 months after the beginning of therapy. Of the 12 patients with treatment failure one had early unfavorable disease, 7 had an IPS of 2 or lower and 4 had an IPS of 3 or higher. With a median follow-up of 47 months, the 5-year EFS for all patients is 85% (95% CI, 77%-92%) and the OS from diagnosis is 90% (95% CI, 84%-97%; Figure 2).
EFS and OS according to patient risk groups. (A) The 5-year EFS for all patients was 85%. The 5-year EFS rates for patients with early unfavorable and standard risk (intermediate risk) versus patients with high risk were 84% and 85%, respectively. (B) The 5-year OS for all patients was 90%. The 5-year OS rates for patients with early unfavorable and standard risk (intermediate risk) versus patients with high risk were 90% and 91%, respectively.
EFS and OS according to patient risk groups. (A) The 5-year EFS for all patients was 85%. The 5-year EFS rates for patients with early unfavorable and standard risk (intermediate risk) versus patients with high risk were 84% and 85%, respectively. (B) The 5-year OS for all patients was 90%. The 5-year OS rates for patients with early unfavorable and standard risk (intermediate risk) versus patients with high risk were 90% and 91%, respectively.
Six patients had protocol violations: 3 had therapy started with SB instead of EB and 3 had a first cycle of EB instead of SB. All patients were included into an intent-to-treat analysis.
Radiation therapy was given to 39 patients (36%), including 27 with a bulky mediastinal mass, 6 with residual uptake on the scan, and 6 for other reasons. Thirty-one patients (45%) in the intermediate-risk group had radiation therapy after chemotherapy to the involved field; only 8 patients (21%) in the high-risk group had radiation therapy (P < .02).
Sixty-nine patients were defined as early unfavorable or at standard risk and therapy was started with 2 cycles of SB. Those 2 groups were analyzed together as intermediate risk. 67Ga or FDG-PET/CT became negative in 31 (88%) of 35 and 27 (79%) of 34 patients, respectively (not significant [NS]). Patients with a negative scintigraphy were treated with a total of 6 cycles of SB. Eleven patients had residual pathologic uptake and chemotherapy cycles were intensified to EB in 10 of them. Two of these patients had disease progression. In this cohort of 69 patients, 2 (3%) had PRD and 6 (8%) subsequently had a relapse. The 5-year EFS and OS rates for this group were 84% (95% CI, 74%-94%) and 90% (95% CI, 81%-99%), respectively, with a median follow-up of 46 months (Figure 2).
Among the 39 high-risk patients (IPS of ≥ 3) the early 67Ga or FDG-PET/CT scan became negative in 15 (79%) of 19 and 16 (80%) of 20 studies, respectively (NS). The treatment was decreased to SB in 31 patients (79%). Overall only 7 patients had the EB continued for a total of 6 cycles. A single patient went off the protocol following the first cycle because of hepatic toxicity. One patient had PRD and 3 subsequently had relapses. The 5-year EFS and OS rates for this group of patients were 85% (95% CI, 73%-98%) and 91% (95% CI, 82%-100%), respectively, with a median follow-up of 49 months (Figure 2).
Fifty-four interim 67Ga scans and 54 PET/CT scans were performed. Progression was observed in 3 of 11 patients with interim positive PET/CT scans versus 1 of 43 patients with negative scans (P < .02). The negative predictive values of normal 67Ga scans and PET/CT scans were 85% and 98%, respectively (P < .001; Table 4). In this cohort, there were no patients in whom interim PET/CT was negative, and at the end of therapy this test appeared to be positive. Progression was observed in 4 of 19 patients with interim positive scintigraphy. Two had PRD with multiple new sites of uptake and 2 had late relapses. Biopsies were obtained for a single patient with PRD and for 2 who had a relapse. Fifteen patients with interim positive scintigraphy (7 67Ga scans, 8 FDG-PET/CT scans) did not progress. In 11 of the 15 patients (5 67Ga scans and 6 PET/CT scans), interim scanning demonstrated residual abnormal uptake in only a single site of disease, located in the mediastinum in 9 of these patients (3 67Ga scans, 6 PET/CT scans). Despite the escalation of therapy, 9 patients had positive PET at the end of chemotherapy. Seven of 9 positive studies at the end of therapy were not subsequently found to be diagnostic for disease relapse. All the 7 studies showed a single-site uptake and biopsies performed in 2 of these patients confirmed the absence of active disease. Subsequent follow-up scintigraphic studies became negative after 6 to 14 months of follow-up (with a median of 9 months).
Interim scintigraphy results according to study performed with either 67Ga SPECT or PET/CT, and according to pretreatment risk group
| . | Intermediate risk . | High risk . | All . |
|---|---|---|---|
| No. patients | 69 | 39 | 108 |
| No. negative early interim scintigraphy studies/no. performed scans (%)† | 58/69 (84) | 31/39 (79) | 89/108 (82) |
| Relapse and PRD, no./no. positive scintigraphy study (%)† | 2/11 | 2/8 | 4/19 (21) |
| 67Ga negative predictive value, no. (%) | 26/31 (83) | 13/15 (86) | 31/46 (85) |
| PET negative predictive value, no. (%) | 27/27 (100) | 15/16 (93) | 42/43 (98) |
| . | Intermediate risk . | High risk . | All . |
|---|---|---|---|
| No. patients | 69 | 39 | 108 |
| No. negative early interim scintigraphy studies/no. performed scans (%)† | 58/69 (84) | 31/39 (79) | 89/108 (82) |
| Relapse and PRD, no./no. positive scintigraphy study (%)† | 2/11 | 2/8 | 4/19 (21) |
| 67Ga negative predictive value, no. (%) | 26/31 (83) | 13/15 (86) | 31/46 (85) |
| PET negative predictive value, no. (%) | 27/27 (100) | 15/16 (93) | 42/43 (98) |
Positive predictive value was calculated as disease progression divided by positive studies. Negative predictive value was defined as the number of event-free patients divided by the number of negative studies.
Resolution of uptake in early interim analysis.
Positive predictive value.
§P < .001 for negative predictive value between PET/CT and Ga.
Low positive predictive values were observed. However, the true positive predictive value is, of course, unknown because the therapy was escalated based on the results of the interim scintigraphy and the low positive predictive value of PET/CT scanning may therefore be explained by the successful intervention by escalation chemotherapy dose followed by radiation therapy to the sites of disease with an uptake at the end of therapy.
There was only a slight reduction in the chemotherapy doses due to toxicity in both groups; 93% of patients received their therapy with less than a 2-week delay of the scheduled 6-cycle course and 90% received at least 90% of a planned chemotherapy dose for all cycles. Six patients received fewer than 6 cycles, due to early death (1), hepatic toxicity with subsequent exclusion from the protocol (l), regimen-related anaphylaxis (1), and refusal to continue therapy (3).
Toxicity
Toxicity following EB was significantly higher than following SB, which was demonstrated by a 40% rate of hospitalization per cycle versus 8% and a 40.5% grade IV leukopenia versus 8.7% (P < .001). The treatment dose was reduced in 22 of 135 cycles of EB and 23 of 493 cycles of SB. Fifty-three of 108 patients were hospitalized during the therapeutic course. Twenty-eight patients were hospitalized once, 15 twice, 4 thrice, and 6 four times during therapy. Thirty-eight percent of patients treated with 6 cycles of SB were hospitalized at least once versus 65% in all others (P < .003). When patients who received only SB cycles (59 patients) were compared to all other patients who received EB or SB plus EB the following toxicity was demonstrated: grade III-IV leukopenia 63% versus 90% (P < .002), grade III- IV thrombocytopenia 10% versus 37% (P < .001).
Three patients had hepatic toxicity grade III-IV following EB; the therapy was reduced to SB in 2 of them following the first cycle and it was completely discontinued in the third patient. Three other patients developed grade III cardiac toxicity following EB, resulting in therapy reduction to SB. One patient developed pericarditis after the second cycle of SB. All these patients recovered from their toxicity prior to their next cycle of chemotherapy. Three patients developed aseptic necrosis of the head of femur following therapy. Since 2002, the dose of prednisone was modified, prescribed for only 1 week. One patient suddenly died at home after 4 cycles of SB, presumably from a cardiac event. Four patients were diagnosed with a second malignancy during the follow-up period: 1 breast carcinoma, 2 non-Hodgkin lymphoma, and 1 with myelodysplasia following salvage therapy for PRD and high-dose chemotherapy with stem cells rescue.
Twenty-eight women younger than 40 years old (range, 19-37 years), with a median age of 26 years, were evaluated for their fertility status. Cyclic ovarian function resumed in 26 (92%) of 28 patients. Nine patients became pregnant.
Discussion
During the last decade several attempts were made to improve the failure-free survival of advanced-stage HD. Standard therapeutic regimens provided 5-year rates EFS of 72% to 81% for patients with a low IPS score (< 3) and of 64% to 69% for patients with a higher score.6 The use of a more intensive regimen (Stanford V), demonstrated a 5-year progression-free survival (PFS) of 94% for patients with a score of 0 to 2 compared with 75% for those with a score of 3 or more adverse prognostic factors.1 Diehl et al2 in a multicenter randomized trial showed that both the OS and the EFS may be improved using a more intensive therapy, such as EB. Major toxicities reported following 8 cycles of EB included multiple episodes of febrile neutropenia, increased risk for secondary leukemia, and early menopause for women in their fertility age.2,3,7 Such toxicity led to further studies where the dose was reduced. An interim analysis of the German Hodgkin Study Group randomized trial HD12 revealed no differences between 8 EB and 4 EB plus 4 SB or between the groups with or without radiotherapy.8 These findings suggest that only a fraction of patients needed a full course of EB. Many of the patients treated with EB could have achieved the same results with SB or Adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) and thus toxicity could have been spared. In the currently reported study, the cumulative chemotherapy given for 6 cycles only was lower than the 8 cycles given in the multicenter German Hodgkin Study Group,2 and only 7 patients (6%) were given 6 cycles of EB (Table 3). We used an individual patient risk-adapted approach for consideration of the appropriate therapy. Patients with advanced disease and IPS of 3 or higher were treated with 2 cycles of EB; all others received 2 cycles of SB. Patients were assessed following 2 treatment cycles and depending on scintigraphy results further therapy was given. This approach was based on several studies showing that while a positive interim 67Ga SPECT or PET scan correlated with a low PFS, negative results of these tests correlated with high PFS rates.9-14 Recently published reports of studies where no interim change of therapy was done,15,16 revealed a 5-year disease-free survival of only 0% to 38% in patients with a positive interim study. Recent studies have demonstrated the superiority of FDG-PET over 67Ga scanning in terms of specificity, sensitivity, and accuracy.17 The accuracy of early interim FDG-PET as an independent predictive factor of disease progression was recently evaluated in 85 patients with HD. The 5-year PFS was 91% for patients with an interim negative PET scan compared with 38% for those with an interim positive PET.15 Survival analysis showed strong associations between early PET negativity PFS (P < .001) and OS (P < .01). The hazard ratio for progression was 20.1 to 36 for interim positive FDG-PET study.15,16 Recently the Italian Lymphoma Study Group in a multicenter study demonstrated 97% EFS for the patients with a negative interim PET and a hazard ratio for progression of 55 for an interim positive study. Therapy was not modified according to PET results in all these patients.18 The findings of these trials are in accordance with current study hazard ratio of 12.9 (CI, 1.5-111.9) and strongly support the integration of interim PET as a decision point for adjustment of chemotherapy. Low positive predictive values of interim PET were observed. A similar low positive predictive value of 38% was demonstrated by Hutchings et al.15 In contrast, the Italian Lymphoma Study Group showed a positive predictive value of 90% for an interim positive PET. Thirteen of 20 patients had progression on therapy and 4 relapsed within 6 months. However, the true positive predictive value is, of course, unknown as the therapy was escalated based on the results of the interim scintigraphy and the low positive predictive value of PET/CT may therefore be explained by the successful intervention with escalation of chemotherapy dose followed by radiation therapy to the sites of disease with an uptake at the end of therapy. Therefore, such patients were in a higher risk of progression and thus had escalation of therapy. It is indeed possible that some patients were overtreated; however, these formed a minority.
Similar to the results by Hutchings et al,15,16 our findings show the negative predictive value of PET to be superior to its positive predictive value. Because inflammation may be interpreted as a pathologic uptake, a lower positive predictive value is expected. In the current study, the negative and positive predictive values of normal interim PET scans were 98% and 27% concomitantly. The low positive predictive value of PET/CT may be explained by inflammation interpreted as pathologic uptake. Another explanation might be a successful intervention by escalation of the chemotherapy dose followed by radiation therapy to the sites of disease with an uptake at the end of therapy. Seven of 9 positive studies at the end of therapy were not subsequently found to be diagnostic for disease relapse. All 7 of these studies showed a single site uptake and biopsies performed in 2 of these patients confirmed the absence of active disease. Subsequent follow-up scintigraphic studies became negative after 6 to 14 months of follow-up (with a median of 9 months). These findings suggest the possibility of a treatment-related inflammatory process as the cause of such uptake.
The current study was not randomized. Its primary aim was to obtain the optimal clinical response and minimize late toxicity through the reduction in the cumulative dose of the administered chemotherapy. A negative interim PET/CT result is reassuring and may suggest that dose reduction is safe. The findings obtained suggest that evaluation of a positive uptake of a single lesion on PET/CT is an interpretive dilemma and should be confirmed with a tissue biopsy prior to making decision regarding further salvage therapy.
Only one patient with primary resistant disease, who underwent high-dose chemotherapy together with stem cells rescue, developed secondary myelodysplastic syndrome that could be related to the salvage therapy. This is an encouraging finding; however, longer follow-up is needed for the assessment of such late myelotoxicity. Because only 39 (36%) of 108 patients underwent involved field radiotherapy, a low rate of secondary solid tumors is expected. The study cohort included 28 women younger than 38 years old; 92% of them retained cyclic ovarian function, suggesting that reduction of therapy may protect fertility in the majority of women compared with 32% to 66% amenorrhea following 8 cycles of SB or EB reported by Behringer et al.7
In summary, a risk-adapted treatment modality, using IPS for planning initial chemotherapy and response to chemotherapy, as reflected by early interim FDG-PET/CT scintigraphy, for subsequent therapy may be used to decrease the cumulative dose of potentially toxic and leukemogenic chemotherapy without affecting efficacy. Six cycles of chemotherapy are effective and further follow-up is required to evaluate the efficacy and late toxicity of this regimen.
Authorship
Contribution: E.J.D. designed research and wrote the paper; R.B-S. performed research; A.T. analyzed data; N.H. designed research; M.B-S., I.A., T.Z., M.K., O.G., and D.L. contributed vital data; J.M.R. wrote the paper; and R.E. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eldad J. Dann, Department of Hematology and Bone Marrow Transplantation, Rambam Medical Center, PO Box 9602, Haifa 31096, Israel; e-mail: e_dann@rambam.health.gov.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Sonia Kamenetsky for her devoted secretarial support at regular times and in war time. We would also like to thank Dorit Dottan for thoughtful data management and Dr David Avigan for critical reviewing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal