Abstract
Homeostatic trafficking of lymphocytes through extralymphoid tissues has been recently observed, and a potential role in immune surveillance and the establishment of peripheral tolerance are considered. However, the mechanisms regulating lymphocyte recirculation through peripheral tissues under noninflammatory conditions are not well understood. Here, we demonstrate that the chemokine receptor CCR7 controls not only lymphocyte trafficking to and within secondary lymphoid organs but also homeostatic migration of T and B lymphocytes through nonlymphoid tissues. Lack of CCR7 results in a massive accumulation of lymphocytes in epithelial tissues. In particular, the gastrointestinal mucosal tissue of CCR7−/− mice is highly permissive for the formation of lymphoid aggregates, which develop into ectopic follicular structures with major topologic characteristics of lymph nodes. Flow cytometry analysis of CD4+ T cells derived from ectopic follicles revealed that CD44hiCD62Llo effector memory T cells predominate in the gastric lymphoid aggregates. In aged mice, lack of CCR7 induced age-dependent histomorphologic changes in the stomach with profound cystic hyperplasia and an increased rate of mucosal proliferation resembling Menetrier disease. Thus, CCR7 regulates the cellular organization of visceral tissue by governing life-long recirculation of naive and memory lymphocytes under homeostatic conditions.
Introduction
In the naive organism, lymphocytes home from their sites of lymphopoiesis to secondary lymphoid organs where they encounter antigen in specialized compartments, or they egress from lymph nodes and eventually recirculate.1 Previous studies have focused mainly on the signals that mediate lymphocyte homing and retention in B- and T-cell areas of secondary lymphoid organs, including selectin-mediated cell rolling, and spatially regulated expression and activation of chemokines and their cognate receptors.2
In contrast, very little is known about the molecular events regulating homeostatic recirculation of lymphocytes through nonlymphoid tissues. It remains to be shown whether the same molecular mechanisms which regulate homeostatic trafficking to and within lymphoid organs are also applicable to homeostatic lymphocyte recirculation through nonlymphoid tissues. In particular, memory T cells travel continuously from the blood into nonlymphoid tissues and from there back to local lymphoid organs by afferent lymphatics to maintain immune surveillance under homeostatic as well as inflammatory conditions.1 As to the physiologic relevance of extralymphatic routes, peripheral lymphocyte trafficking was found to induce neonatal tolerance to tissue antigens.3 The researchers showed that tolerance induction to low amounts of cutaneous antigens is facilitated by enhanced trafficking of naive T cells through peripheral tissues and not by cross-priming. Thus, rapid turnover of lymphocytes in extralymphoid tissues is required for the establishment of peripheral tolerance. Recently, Cose et al4 described that naive T cells enter nonlymphoid organs as part of their normal migratory pathway. They further suggested that naive T cells encounter tissue-specific or developmentally associated antigens or both within the tissues and that such an encounter may indeed play a role in the development of peripheral tolerance.
Studies with gene-targeted mice have established that the homeostatic chemokine receptors CCR7 and CXCR5 regulate trafficking and retention of lymphocytes and antigen-presenting dendritic cells (DCs) to and within secondary lymphoid organs.2 Their respective ligands, the chemokines CCL19, CCL21, and CXCL13, are constitutively expressed by resident stromal cells and by endothelial cells in lymphoid tissue, nonlymphoid tissue, and body cavities.5 Together with inflammatory cytokines belonging to the tumor necrosis factor/lymphotoxin family, homeostatic chemokines control lymphoid organ development and maintain lymphoid tissue microarchitecture.6-9 Consequently, severe abnormalities in B- and T-cell migration, lymphoid organogenesis, and adaptive immune responses have been observed in mice deficient for the chemokine receptors CCR7 or CXCR5.10-16 Homeostatic chemokines are also involved in the development of ectopic lymphoid-like structures which have been described frequently in chronic inflammatory processes and autoimmune diseases.17-19 More specifically, when expressed in the pancreatic islets of transgenic mice, CCL19, CCL21, and CXCL13 are capable of inducing the formation of lymph node–like structures.20,21
In this study, we addressed whether CCR7 expression is a prerequisite for homeostatic lymphocyte recirculation through nonlymphoid tissues under steady state conditions. Lack of CCR7 resulted in a massive accumulation of lymphocytes in various epithelial tissues of CCR7−/− mice. In the gastrointestinal tract, lymphocytes accumulated and formed functional lymphoid follicles, in which homeostatic chemokines such as CCL21 and CXCL13 were found to be expressed. In addition, aged CCR7-deficient mice exhibited a severe hypertrophic gastropathy reminiscent of Menetrier disease. These findings reveal that CCR7 is dispensable for the initial immigration of naive and memory lymphocytes into nonlymphoid tissues but is ultimately required for the maintenance of homeostatic lymphocyte recirculation.
Materials and methods
Mice
CXCR5−/− and CCR7−/− mice were backcrossed for at least 8 generations onto the C57BL/6 (CD45.2) background. Wt C57BL/6 45.1 congenic mice were obtained from Charles River Laboratories (Wilmington, MA). Animals were bred and kept under specific pathogen-free (SPF) conditions, and all animal studies were performed according to institutional and state guidelines.
Flow cytometry analysis
The following primary antibodies were used: allophycocyanin (APC)–labeled rat anti–mouse B220 (CD45R) and goat anti–mouse CD62L, phycoerythrin (PE)–labeled goat anti–mouse CD3, fluorescein isothiocyanate (FITC)–labeled goat anti–mouse CD4 and CD8 (Caltag, San Francisco, CA); FITC-labeled mouse anti–mouse CD45.1; biotin-labeled mouse anti–mouse CD45.2; PE-labeled goat anti–rat IgG, and APC-labeled goat anti–mouse CD44, and PerCP- or PE-labeled streptavidin (BD Biosciences, San Jose, CA) were used as secondary reagents. Data were acquired on a fluorescence-activated cell sorting (FACS) FACScalibur flow cytometer and analyzed with Cellquest software version 3.3 (BD Biosciences).
For immunohistology, the following primary antibodies were used: goat anti–mouse CD3ϵ (Santa Cruz Biotechnology, Santa Cruz, CA), biotin-labeled rat anti–mouse CD45R/B220, rat anti–mouse PNAd, CD11c-biotin, CD3ϵ-PE Cychrome 5, and B220-FITC (Becton Dickinson, Franklin Lakes, NY), rat anti–mouse follicular dendritic cells (FDCs; ImmunoKontact, Abingdon, United Kingdom); goat anti–mouse CCL21 and CXCL13 (R&D Diagnostics, Wiesbaden, Germany) biotin-labeled rat anti–mouse CD4 and CD8 (Caltag), rat anti–mouse F4/80, and rat anti 5′-bromo-2′-deoxyuridine (BrdU) (Serotec, Raleigh, NC). Biotinylated mouse antigoat and mouse antirat (Dianova, Hamburg, Germany) were used as secondary antibodies, followed by alkaline phosphatase-labeled streptavidin (Jackson ImmunoResearch Laboratories, Baltimore, PA). Streptavidin–Alexa Fluor 568 and 488 (Molecular Probes, Leiden, The Netherlands) were used to detect biotinylated primary antibody in immunofluorescence stainings.
Immunohistology
For paraffin sections, tissues were fixed in 4% phosphate-buffered paraformaldehyde, embedded in paraffin blocks, cut into 5-μm longitudinal sections, and stained with hematoxylin and eosin (HE). For frozen sections, tissues were embedded in Tissue Tek OCT compound (Sakura Finetek, Torrance, CA) and frozen on dry ice. Cryosections were cut to 5- to 8-μm thickness, air dried, and fixed for 10 minutes in −20°C acetone. For immunohistology, paraffin sections were deparaffinized and demasked by heat or trypsinization. Cryosections were rehydrated in 50 mM Tris-buffered saline, pH 7.6. Alkaline phosphatase activity was detected using the fuchsin substrate (DakoCytomation, Carpinteria, CA). All slides were mounted in Mowiol solution (11.7% wt/vol Mowiol, 29.4% wt/vol glycerine, 0.12 M Tris, pH 8.5). Images were acquired with a Zeiss Axiophot fluorescence microscope (Carl Zeiss, Jena, Germany) equipped with a 10×/25 eyepiece and a Plan Apochromat 10×/0.45 NA or 20×/0.8 NA objective, respectively. All images were obtained with an Axiocam HRc camera and further processed using AxioVision 3.1 software (all from Carl Zeiss). BrdU incorporation into DNA of proliferating cells was analyzed 18 hours after intraperitoneal injection of 800 μg BrdU in 200 μL PBS (Sigma, St Louis, MO).
Mucosal tissue mononuclear cell preparation
Tissue debris of murine stomach were incubated in RPMI 1640 medium, supplemented with 10% FCS and 6 mg/mL collagenase NB4 (Serva, Heidelberg, Germany) for 30 minutes at 37°C under vigorous agitation. Cells were separated from tissue debris by passing through a steel mesh, followed by purification through a Ficoll density gradient centrifugation.
Bone marrow transplantation
Bone marrow was transplanted into 8- to 12-week-old sex-matched CD45.1 C57BL/6 congenic mice (C57BL/6-Ly5.1; Charles River, Laboratories). Recipient mice were lethally irradiated with 6 Gy (600 rad). Subsequently, mice were intravenously reconstituted with 4 × 107 bone marrow cells from C57BL/6 wt (C57BL/6-Ly5.2) or C57BL/6 CCR7−/− (C57BL/6-Ly5.2) donor mice. After 8 weeks, reconstitution was controlled in the peripheral blood by flow cytometry. Stomach, gut, lung, and secondary lymphoid organs were harvested from mice that received a transplant with bone marrow.
Depletion of gut commensal microflora
For complete depletion of commensal bacterial growth, animals were given ampicillin (1 g/L), vancomycin (500 mg/L), and metronidazole (1g/L; Sigma) in drinking water ad libidum for 8 weeks as described.22
Bacterial culture
For detailed analyses of the bacterial flora, fecal matter was collected in sterile PBS and plated onto standard solid media (Oxoid, Hampshire, United Kingdom). Bacteria were grown at 36°C for 2 days under aerobic conditions or for 5 days under anaerobic conditions. Anaerobic bacteria were cultivated in gas pak jars (Merck, Darmstadt, Germany). Total counts of anaerobic and aerobic bacteria were determined on Columbia blood agar. Results were normalized for stool dry weights. To control for the colonization status of mice treated with antibiotics, individual stool samples were collected after 4 and 8 weeks; transferred into brain, heart, and thioglycolate broths; and incubated at 36°C. After 8 days, each broth was plated onto solid media for culture of aerobic and anaerobic bacteria as described. In some cases prolonged antibiotic treatment (8 weeks or longer) resulted in the emergence of the opportunistic bacteria Pseudomonas aeruginosa in the fecal matter, whereas nontreated CCR7−/− mice did not show any P aeruginosa growth at all.
Adoptive cell transfer
Donor splenocytes were biotinylated by incubation of 1 × 107 cells in 80 μg biotin-7-NHS/mL PBS (Boehringer Mannheim, Mannheim, Germany) for 15 minutes at room temperature. Untouched CD4 T cells and positively selected CD45R B cells were isolated by magnetic cell sorting (Miltenyi Biotec, Gergisch Gladbach, Germany) and also biotinylated. After washing, 2 to 3 × 107 biotinylated cells were injected intravenously in C57BL/6 wt or CCR7−/− mice. In some experiments, 2 × 107 biotinylated cells were mixed with 100 μg rat anti–mouse CD62L mAb (clone MEL-14) or 100 μg isotype IgG2a mAb prior to injection (BD). Recipient mice were killed at different time points (as indicated), and stomachs as well as control secondary lymphoid organs (spleen or inguinal LNs) were harvested.
Statistical analysis
Results are expressed as arithmetic means ± SEMs. Values of P less than .05 were considered statistically significant, as determined by the unpaired, 2-tailed Mann-Whitney test.
Results
Lack of CCR7 expression results in the accumulation of lymphocytes in epithelial tissues
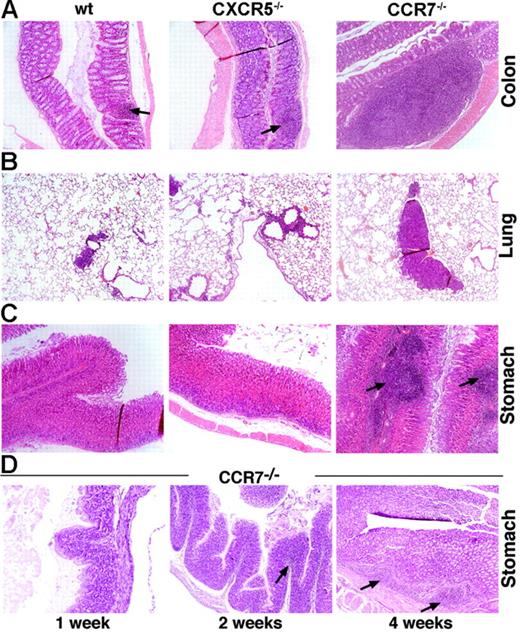
In this study, we investigated whether lymphocyte recirculation through nonlymphoid tissues is altered in the absence of CCR7 or CXCR5 expression. Immunohistologic analysis of various epithelial and parenchymateous tissues (ie, kidney, liver, lung, heart, colon, small intestine, skin, and stomach) of CXCR5- and CCR7-deficient mice in comparison to wild-type (wt) animals was carried out to assess abnormal morphology and cellularity of peripheral tissues. Within the mucosal tissue of the colon, stomach, and lung of CCR7−/− mice, numerous aberrant lymphoid infiltrates were observed, whereas CXCR5−/− and wt mice showed normal mucosal tissue morphology. In contrast, various parenchymateous tissues did not show any morphologic alterations or abnormal cellularity in all 3 mouse strains.
The colon of healthy adult wt mice normally harbors various numbers of lymphatic nodules located in the submucosa.23 In CCR7−/− mice, the colon (Figure 1A) exhibited a marked increase in lymphoid follicles, including parts of the intestine where they are usually not prominent, such as the lamina propria. In the respiratory tract, distinct bronchus-associated lymphoid tissue (BALT) was rarely observed in the lungs of healthy wt mice,23 whereas CCR7-deficient mice regularly formed lymphoid follicle-like structures of varying sizes (Figure 1B).
Lymphoid follicle-like aggregates are formed within the mucosal tissue of the gastrointestinal and respiratory tract of CCR7−/− mice. (A) Colons of wt and CXCR5−/− mice (representative lymphoid follicle indicated by an arrow) exhibited normal numbers of gut-associated lymphoid aggregates compared with CCR7−/− mice. In CCR7−/− animals, lymphoid aggregations were larger and more frequent. (B) Mucosal tissue of the lung exhibited enhanced lymphoid aggregates in CCR7−/− mice (depicted at the age of > 6 months) compared with wt and CXCR5−/− mice. (C) Mucosal tissue of the stomach of wt and CXCR5−/− mice showed no lymphoid infiltrates, whereas CCR7−/− mice harbored a large number of lymphoid follicular-like structures within the submucosa and lamina propria. (D) In the mucosal tissue of the stomach, aberrant lymphoid infiltrates were already detected at the age of 2 weeks in CCR7−/− mice (indicated by an arrow). At the age of 4 weeks, the onset of follicular-like aggregates was first observed (indicated by arrows). Data are from 1 representative mouse of 6 to 8 mice analyzed per group (A-D). Original magnification, ×100 (A-D); hematoxylin-eosin (HE) staining of paraffin-embedded tissue.
Lymphoid follicle-like aggregates are formed within the mucosal tissue of the gastrointestinal and respiratory tract of CCR7−/− mice. (A) Colons of wt and CXCR5−/− mice (representative lymphoid follicle indicated by an arrow) exhibited normal numbers of gut-associated lymphoid aggregates compared with CCR7−/− mice. In CCR7−/− animals, lymphoid aggregations were larger and more frequent. (B) Mucosal tissue of the lung exhibited enhanced lymphoid aggregates in CCR7−/− mice (depicted at the age of > 6 months) compared with wt and CXCR5−/− mice. (C) Mucosal tissue of the stomach of wt and CXCR5−/− mice showed no lymphoid infiltrates, whereas CCR7−/− mice harbored a large number of lymphoid follicular-like structures within the submucosa and lamina propria. (D) In the mucosal tissue of the stomach, aberrant lymphoid infiltrates were already detected at the age of 2 weeks in CCR7−/− mice (indicated by an arrow). At the age of 4 weeks, the onset of follicular-like aggregates was first observed (indicated by arrows). Data are from 1 representative mouse of 6 to 8 mice analyzed per group (A-D). Original magnification, ×100 (A-D); hematoxylin-eosin (HE) staining of paraffin-embedded tissue.
The most obvious phenotype, visible even by macroscopic inspection, was seen in the stomach of CCR7−/− mice (Figure 1C). Anatomically, the stomach is divided into a thin-walled left section (forestomach) and a thick-walled right section (glandular stomach). The luminal surface displays foveolae (gastric pits), and its base is perforated by several gastric glands of the lamina propria. Under noninflammatory conditions, wt and CXCR5−/− mice displayed no lymphoid structures in the stomach mucosa (Figure 1C). The aberrant lymphoid aggregates in CCR7−/− mice were mainly seen in the submucosa and lamina propria of the glandular stomach (Figure 1C).
To determine at what age lymphoid aggregates develop, we removed the stomachs of mice aged 1 week, 2 weeks, 4 weeks (Figure 1D), or 8 to 12 weeks (Figure 1C) and subjected them to immunohistochemical analysis. Diffuse lymphoid infiltrates were already detectable at the age of 2 weeks; at 4 weeks of age they became more prominent and the onset of follicle-like structures was observed (Figure 1D). At the age of 8 to 12 weeks, follicle-like structures developed in the submucosa and the lamina propria of all CCR7−/− mice analyzed (Figure 1C). These results suggest that the mucosal environment in CCR7−/− mice is highly permissive for lymphoid aggregate formation and maintenance.
Aberrant lymphoid aggregates display major characteristics of classical lymphoid follicles
To determine whether the observed lymphoid aggregates possessed key characteristics of classical lymphoid follicles, we characterized the cellular infiltrates by immunohistochemical staining. Both T (CD3+) and B (B220+) lymphocytes were present in the follicle-like structures; however, a clear-cut organization into separate T- and B-cell compartments reminiscent of lymph nodes was not observed (Figure 2A).
Cellular composition of lymphoid aggregates in the stomach mucosa of CCR7−/− mice. (A) The cellular composition of aggregated structures was assessed by immunohistochemical staining of paraffin or frozen sections. Consecutive sections were stained in red with antibodies against CD3, CD4, CD8, B220, FDC (indicated by an arrow), PNAd (indicated by an arrow), BrdU (indicated by an arrow), CCL21, or CXCL13. Original magnification, ×200 (with the exception of anti-BrdU, ×100). Representative sections are shown (> 60 sections obtained from 6 mice). (B) Frozen sections of aggregated structures were stained with anti-B220 (in green) and anti-CD3 (in red), and with anti-CD11c antibody (in red or green as indicated; representative CD11c+ cells are indicated by an arrow). Original magnification, ×200. Representative sections for each age group are shown (n = 3 mice for each group).
Cellular composition of lymphoid aggregates in the stomach mucosa of CCR7−/− mice. (A) The cellular composition of aggregated structures was assessed by immunohistochemical staining of paraffin or frozen sections. Consecutive sections were stained in red with antibodies against CD3, CD4, CD8, B220, FDC (indicated by an arrow), PNAd (indicated by an arrow), BrdU (indicated by an arrow), CCL21, or CXCL13. Original magnification, ×200 (with the exception of anti-BrdU, ×100). Representative sections are shown (> 60 sections obtained from 6 mice). (B) Frozen sections of aggregated structures were stained with anti-B220 (in green) and anti-CD3 (in red), and with anti-CD11c antibody (in red or green as indicated; representative CD11c+ cells are indicated by an arrow). Original magnification, ×200. Representative sections for each age group are shown (n = 3 mice for each group).
Further characterization of the CD3+ T-cell population revealed a predominance of CD4+ T cells. Mononuclear F4/80+ infiltrates of varying sizes were found exclusively in the areas adjacent to lymphoid follicle-like structures (data not shown). CD11c+ DCs were mostly found in the vicinity of T cells and sometimes in the peripheral areas of the lymphoid structures (Figure 2B). Interestingly, CD11c+ DCs were already detectable during the onset of follicle-like structures at 4 to 5 weeks of age, coincident with T and B cells (Figure 2B). Large follicular aggregates within the mucosal tissue of the stomach showed a significant number of BrdU+ proliferating cells and expression of CXCL13 and CCL21. Although a clear segregation into B- and T-cell areas was not seen, numerous FDCs were found in the large follicular aggregates (Figure 2A). Because FDCs are antigen-trapping cells, which are normally restricted to B-cell follicles within secondary lymphoid organs, the occurrence of FDCs suggested that functional, but aberrant, follicular structures had developed. In support of this conclusion, we detected specialized postcapillary peripheral-node addressin (PNAd)–positive vessels (Figure 2A), scattered throughout the lymphoid aggregates and the adjacent tissue. This links the lymphoid follicular structures to the peripheral blood system, providing similar access of recirculating cells as to lymph nodes.
Taken together, our results indicate that lack of CCR7 causes the development of lymphoid follicles with high endothelial venule (HEV)–like venules within the mucosal tissue of the gastrointestinal tract.
CD44hiCD62Llo effector memory T cells predominate in the lymphoid aggregates of CCR7−/− mice
We further quantitated the total number of lymphocytes prepared from the mucosal tissue of the stomach of CCR7−/− mice in comparison to those derived from wt animals. Because of the formation of aberrant follicular structures in the CCR7−/− mice, the total numbers of lymphocytes were significantly increased compared with those of wt mice. Eight- to 12-week-old CCR7−/− mice revealed 9.2 ± 2.9 × 103 total mucosal lymphocytes compared with 2.8 ± 1.1 × 103 total lymphocytes in 8- to 12-week-old wt animals (3.6-fold increase; n = 3). Aged CCR7−/− (> 12 months) animals showed an even higher increase in total lymphocytes compared with aged wt animals: 33.3 ± 13.1 × 103 total mucosal lymphocytes compared with 7.5 ± 3.0 × 103 total mucosal lymphocytes in the stomach of aged wt animals (4.4-fold increase; n = 4). Most interestingly, greater than 60% of all mucosal CD4+ T cells in 8- to 12-week-old CCR7−/− mice represented a CD44hiCD62Llo effector memory T cell (TEM) phenotype, less than 25% were of the central memory (TCM), and less than 15% were of the naive T-cell compartment compared with wt animals in which CD4+ T cells were distributed at a similar amount (about 30%) in all 3 subsets (Table 1; Figure 3). In aged mice, greater than 80% of all mucosal CD4+ T cells in CCR7−/− mice represented a CD44hiCD62Llo effector memory T-cell (TEM) phenotype and less than 10% were of the central memory (TCM) or naive T-cell compartment (Table 1; Figure 3). In comparison, mucosal CD4+ T cells in aged wt mice consisted of 50% to 60% CD44hiCD62Llo TEM cells, and about 20% belonged to each of the CD44hi/medCD62Lhi/med TCM and the CD44loCD62Lhi naive T-cell subset (Table 1; Figure 3). Thus, the formation of ectopic lymphoid follicles in the stomach of CCR7−/− mice led to an age-dependent increase in mucosal CD4+ T lymphocytes predominantly of the CD44hiCD62Llo TEM phenotype.
CD44hiCD62Llo effector memory T cells predominate in the mucosal tissue of CCR7−/− mice
| Genotype . | No. mice . | CD44loCD62Lhi naive CD4+ T cells, % . | CD44hi/medCD62Lhi/med CD4+ TCM cells, % . | CD44hiC62Llo CD4+ TEM cells, % . |
|---|---|---|---|---|
| 8-12 wk | ||||
| Wt | 3 | 31.8 ± 7.0 | 30.11 ± 1.9 | 34.14 ± 6.9 |
| CCR7−/− | 3 | 12.7 ± 3.6 | 24.28 ± 3.0 | 61.2 ± 1.0 |
| More than 12 mo | ||||
| Wt | 3 | 21.1 ± 3.0 | 17.7 ± 2.1 | 56.8 ± 5.7 |
| CCR7−/− | 5 | 3.3 ± 1.2 | 8.2 ± 1.4 | 85.3 ± 2.0 |
| Genotype . | No. mice . | CD44loCD62Lhi naive CD4+ T cells, % . | CD44hi/medCD62Lhi/med CD4+ TCM cells, % . | CD44hiC62Llo CD4+ TEM cells, % . |
|---|---|---|---|---|
| 8-12 wk | ||||
| Wt | 3 | 31.8 ± 7.0 | 30.11 ± 1.9 | 34.14 ± 6.9 |
| CCR7−/− | 3 | 12.7 ± 3.6 | 24.28 ± 3.0 | 61.2 ± 1.0 |
| More than 12 mo | ||||
| Wt | 3 | 21.1 ± 3.0 | 17.7 ± 2.1 | 56.8 ± 5.7 |
| CCR7−/− | 5 | 3.3 ± 1.2 | 8.2 ± 1.4 | 85.3 ± 2.0 |
CCR7-deficient mice show an age-dependent increase in mucosal CD4+ T lymphocytes predominantly of the CD44hiCD62Llo CD4+ TEM subtype. Flow cytometric analysis of CD4+-gated lymphocytes recovered from the mucosal tissue of the stomach of CCR7−/− compared with those of wt mice. Numbers indicate the percentages of CD44loCD62Lhi naive CD4+ T cells (top left quadrant), CD44hi/medCD62Lhi/med CD4+ TCM cells (top right quadrant), or CD44hiCD62Llo CD4+ TEM cells (bottom right quadrant). Data are from 2 representative wt and CCR7−/− mice of 3 to 5 mice per group.
CCR7-deficient mice show an age-dependent increase in mucosal CD4+ T lymphocytes predominantly of the CD44hiCD62Llo CD4+ TEM subtype. Flow cytometric analysis of CD4+-gated lymphocytes recovered from the mucosal tissue of the stomach of CCR7−/− compared with those of wt mice. Numbers indicate the percentages of CD44loCD62Lhi naive CD4+ T cells (top left quadrant), CD44hi/medCD62Lhi/med CD4+ TCM cells (top right quadrant), or CD44hiCD62Llo CD4+ TEM cells (bottom right quadrant). Data are from 2 representative wt and CCR7−/− mice of 3 to 5 mice per group.
Functional lymphoid follicles are formed in the gastrointestinal mucosal tissue of CCR7−/− mice
Do these histologic alterations in CCR7−/− mice lead to the formation of lymph node-like structures that are integrated into the lymphoid recirculation pathway by HEV-like structures?
To address this question, CCR7−/− mice were given intravenous injections of 3 × 107 biotinylated splenocytes derived from wt or CCR7−/− donor mice. When stomachs were removed from CCR7−/− recipient mice at different time points after adoptive transfer of biotinylated wt splenocytes, recirculated lymphocytes were observed up to 5 days after transfer within the lymphoid follicular aggregates and secondary lymphoid organs (Figure 4A-C). In contrast, when biotinylated CCR7−/− splenocytes were adoptively transferred into CCR7−/− animals, significantly less biotinylated lymphocytes were detectable within the lymphoid aggregates at the earlier time points [3 hours (data not shown) and 24 hours after transfer (Figure 4A-B)] compared with wt splenocytes. Previously, we had shown that CCR7-negative lymphocytes are hampered in entering lymph nodes by HEVs.10 In all likelihood, this property may also account for the lower numbers of biotinylated CCR7−/− donor lymphocytes within the ectopic lymphoid structures at the 24-hour time point (Figure 4B, lower right), suggesting that lymphocyte recirculation through ectopic follicles is an active process involving chemoattractant receptor signaling. Additionally, CD4+ T cells and B220+ B cells were purified from spleens of either wt or CCR7−/− mice and transferred separately into CCR7-deficient recipients. Recipient mice were killed 5 days after transfer, a time point at which CCR7−/− splenocytes have also been detected within the aberrant lymphoid aggregates. Both CD4+ and B220+ cells derived from wt or CCR7−/− mice, respectively, were detected at comparable numbers, suggesting that T and B lymphocytes are equally capable of entering mucosal follicular aggregates (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Next, we determined whether entry of lymphocytes from the blood stream into aberrant follicular aggregates requires interaction of CD62L on lymphocytes with peripheral node addressin-positive venules. As expected, blocking CD62L interaction inhibited the entry of lymphocytes into draining lymph nodes (Figure 4A), but surprisingly it had no significant effect on the entry of lymphocytes into lymphoid follicular aggregates of the stomach (Figure 4B-C).
Aberrant lymphoid aggregates of the stomach support recirculation of naive lymphocytes independently of CD62L expression. Biotinylated splenocytes (2-3 × 107) derived from wt or CCR7−/− mice were adoptively transferred without or together with anti-CD62L or IgG2a antibody (Ab) (as indicated) into adult CCR7−/− mice. Spleens and the draining iLNs contained biotinylated wt cells (transferred with or without IgG2a Ab) 3 hours (data not shown), 24 hours (A-B), and 5 days (C) after injection, as assessed by staining with Streptavidin-AP in red and flow cytometric analysis (A; representative FACS analyses for 3 to 5 experiments are shown). Comparable numbers of biotinylated wt cells ± IgG2a Ab, CCR7−/−, and wt cells + anti-CD62L Ab were found in the spleens, but significantly less CCR7−/− and wt cells + anti-CD62L Ab than wt cells ± IgG2a Ab could be retrieved from the draining LNs (A). Homing of biotinylated wt cells ± IgG2a Ab (red) in follicular aggregates in CCR7−/− mice was seen at all time points analyzed here (B; marked by arrows). CCR7−/− cells were observed at a significantly lower frequency at 3 hours (data not shown) and 24 hours (B; marked by arrows) after injection, whereas wt cells + anti-CD62L Ab were found in comparable numbers than were wt cells ± IgG2a Ab at 24 hours (B; marked by arrows) after injection. At 5 days after injection follicular aggregates contained comparable numbers of transferred biotinylated CCR7−/−, wt ± IgG2a antibody, and wt cells + anti-CD62L Ab (C) as visualized by Streptavidin-AP reactivity and HE-counterstaining in frozen sections. Original magnification, ×200. Data are representative of 3 to 5 mice in each group (B-C).
Aberrant lymphoid aggregates of the stomach support recirculation of naive lymphocytes independently of CD62L expression. Biotinylated splenocytes (2-3 × 107) derived from wt or CCR7−/− mice were adoptively transferred without or together with anti-CD62L or IgG2a antibody (Ab) (as indicated) into adult CCR7−/− mice. Spleens and the draining iLNs contained biotinylated wt cells (transferred with or without IgG2a Ab) 3 hours (data not shown), 24 hours (A-B), and 5 days (C) after injection, as assessed by staining with Streptavidin-AP in red and flow cytometric analysis (A; representative FACS analyses for 3 to 5 experiments are shown). Comparable numbers of biotinylated wt cells ± IgG2a Ab, CCR7−/−, and wt cells + anti-CD62L Ab were found in the spleens, but significantly less CCR7−/− and wt cells + anti-CD62L Ab than wt cells ± IgG2a Ab could be retrieved from the draining LNs (A). Homing of biotinylated wt cells ± IgG2a Ab (red) in follicular aggregates in CCR7−/− mice was seen at all time points analyzed here (B; marked by arrows). CCR7−/− cells were observed at a significantly lower frequency at 3 hours (data not shown) and 24 hours (B; marked by arrows) after injection, whereas wt cells + anti-CD62L Ab were found in comparable numbers than were wt cells ± IgG2a Ab at 24 hours (B; marked by arrows) after injection. At 5 days after injection follicular aggregates contained comparable numbers of transferred biotinylated CCR7−/−, wt ± IgG2a antibody, and wt cells + anti-CD62L Ab (C) as visualized by Streptavidin-AP reactivity and HE-counterstaining in frozen sections. Original magnification, ×200. Data are representative of 3 to 5 mice in each group (B-C).
We conclude that the recirculation kinetics of lymphocytes through mucosal lymphoid follicular aggregates is attenuated by CCR7 deficiency but independent of CD62L-mediated cell adhesion.
Formation of lymphoid aggregates is a direct consequence of CCR7 deficiency
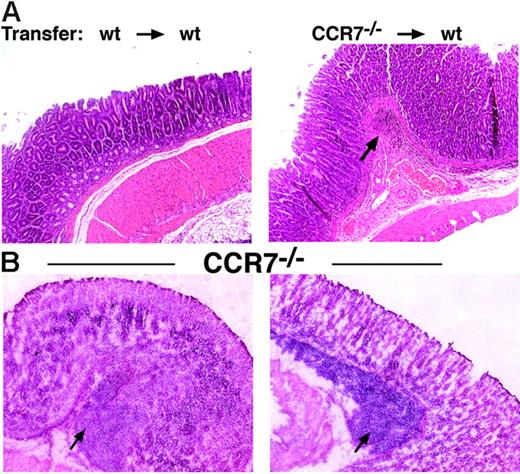
To rule out the possibility that lymphocytes are retained and recirculated in an abnormal fashion because of their development in an organism with disrupted lymphoid architecture, we generated bone marrow chimeras by transplanting bone marrow from CCR7-deficient mice (C57BL/6; CD45.2) into lethally irradiated congenic wt recipients (C57BL/6; CD45.1). After 8 weeks, organs and blood were removed and analyzed by immunohistology and flow cytometry. In all cases, the degree of donor chimerism in the recipient blood was greater than 95% at the time of analysis. Similar to CCR7−/− mice, wt recipients of CCR7-deficient bone marrow cells showed massive lymphoid infiltrates in the submucosa and lamina propia of the stomach (Figure 5A). In control mice that had received bone marrow from C57BL/6 (CD45.2) wt mice, no lymphoid infiltrates were observed (Figure 5A). These results indicate that lack of CCR7 expression on lymphocytes directly leads to disturbed homeostatic lymphocytic recirculation and, eventually, formation of lymphoid aggregates in nonpredestined sites of gastric mucosa.
The formation of aberrant lymphoid aggregates is a direct consequence of CCR7 deficiency and not due to inflammatory stimuli. (A) Stable bone marrow chimeras were generated, resulting in a donor chimerism greater than 95%. The phenotype of chimeric mice was analyzed 8 weeks after BM transplantation by hematoxylin staining in paraffin-embedded sections. Transfer of wt bone marrow (CD45.2) (4 × 107) into irradiated congenic wt recipients (CD45.1) did not elicit the formation of lymphoid infiltrates in the mucosal tissue of the stomach (left). Transfer of CCR7-deficient bone marrow cells into wt recipients resulted in the formation of lymphoid aggregates in the gastric mucosa (right). Data are representative of 4 to 6 mice in each group. Original magnification, ×100. (B) CCR7−/− mice raised under sterile conditions still developed aberrant lymphoid follicular-like structures in the gastric mucosa. Numerous aberrant lymphoid infiltrates were detected within the submucosa and lamina propria (indicated by an arrow) of CCR7−/− mice raised under vancomycin, metronidazole, and ampicillin treatment over 8 weeks. Histologic sections from 2 representative mice of 8 are shown. Magnification, ×100; HE staining of frozen tissue sections.
The formation of aberrant lymphoid aggregates is a direct consequence of CCR7 deficiency and not due to inflammatory stimuli. (A) Stable bone marrow chimeras were generated, resulting in a donor chimerism greater than 95%. The phenotype of chimeric mice was analyzed 8 weeks after BM transplantation by hematoxylin staining in paraffin-embedded sections. Transfer of wt bone marrow (CD45.2) (4 × 107) into irradiated congenic wt recipients (CD45.1) did not elicit the formation of lymphoid infiltrates in the mucosal tissue of the stomach (left). Transfer of CCR7-deficient bone marrow cells into wt recipients resulted in the formation of lymphoid aggregates in the gastric mucosa (right). Data are representative of 4 to 6 mice in each group. Original magnification, ×100. (B) CCR7−/− mice raised under sterile conditions still developed aberrant lymphoid follicular-like structures in the gastric mucosa. Numerous aberrant lymphoid infiltrates were detected within the submucosa and lamina propria (indicated by an arrow) of CCR7−/− mice raised under vancomycin, metronidazole, and ampicillin treatment over 8 weeks. Histologic sections from 2 representative mice of 8 are shown. Magnification, ×100; HE staining of frozen tissue sections.
Formation of lymphoid follicles is not due to inflammatory stimuli
Is it possible that the ectopic lymphoid follicular aggregates in the mucosal tissue of CCR7−/− mice are induced by inflammatory stimuli generated by the commensal microflora? To test this, CCR7−/− breeding pairs were depleted of all detectable commensals by a 4-week oral administration of vancomycin, metronidazole, and ampicillin (V/M/A). The progeny of those breeding pairs were also raised under V/M/A treatment over a period of 8 weeks. Four and 8 weeks after birth, the bacterial flora in the fecal matter were characterized. At 8 weeks of age, ectopic mucosal follicle formation was analyzed by histologic analysis. Although the mice were completely devoid of normal gut commensals in stool specimens, animals still developed ectopic lymphoid follicular structures in the stomach comparable to those of untreated mice (Figure 5B). Therefore, it seems unlikely that a specific group of commensal bacteria or inflammatory stimuli causes the formation of lymph node–like structures at a nonpredestined site.
Aged CCR7-deficient mice develop gastric histopathologic alterations resembling Menetrier disease
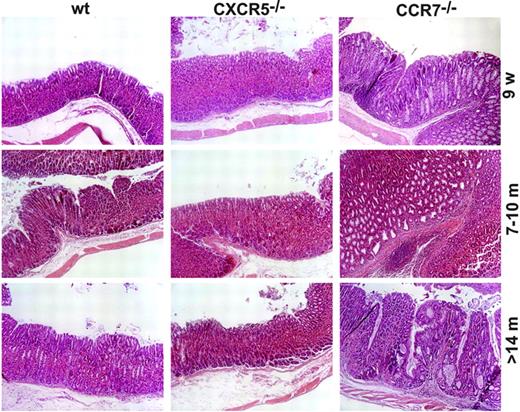
Lack of CCR7 resulted in a disturbed cellular homeostasis of gastric mucosa leading to formation of defined ectopic lymphoid follicular aggregates as early as 4 weeks of age. At the age of 8 to 10 weeks, lymphoid aggregates were already fully developed, containing proliferating cells and HEVs. These structures were maintained up to 18 months of age (last time point analyzed) (Figure 6).
Lack of CCR7 causes hypertrophic gastropathy that resembles Menetrier disease. Comparison of gastric mucosa histology between CCR7−/−, CXCR5−/−, and wt mice shows thickening (hypertrophy) of the mucosa of CCR7−/− mice between 9 weeks and 18 months of age. Progressive mucosal hyperplasia with dilated cysts lined with a single layer of cuboidal epithelium was regularly observed in the gastric fundic mucosa of aged CCR7-deficient mice (10 to 18 months). Wt and CXCR5−/− mice showed minimal or no pathomorphologic changes from 9 weeks to 18 months of age. One representative mouse is shown of 6 to 8 mice per group. Original magnification, ×100; HE staining in paraffin-embedded sections.
Lack of CCR7 causes hypertrophic gastropathy that resembles Menetrier disease. Comparison of gastric mucosa histology between CCR7−/−, CXCR5−/−, and wt mice shows thickening (hypertrophy) of the mucosa of CCR7−/− mice between 9 weeks and 18 months of age. Progressive mucosal hyperplasia with dilated cysts lined with a single layer of cuboidal epithelium was regularly observed in the gastric fundic mucosa of aged CCR7-deficient mice (10 to 18 months). Wt and CXCR5−/− mice showed minimal or no pathomorphologic changes from 9 weeks to 18 months of age. One representative mouse is shown of 6 to 8 mice per group. Original magnification, ×100; HE staining in paraffin-embedded sections.
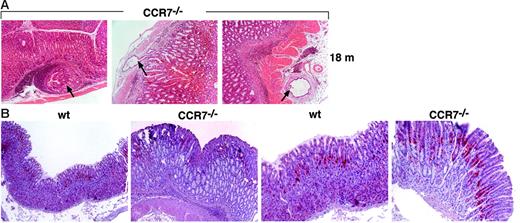
To determine long-term effects of follicular gastritis on gastric mucosal morphology, we examined the histology of stomach sections obtained from 8-week-old to 18-month-old wt and CCR7-deficient mice. In wt and CXCR5−/− mice, essentially no lymphatic tissue or major epithelial alterations developed up to 17 months after birth. A single cystically dilated gland was observed in the stomach of 1 of 10 wt animals (7 months of age) and in 2 of 9 CXCR5−/− mice (> 10 months of age). In contrast, stomachs of all CCR7-deficient mice examined showed markedly increased thickness of the mucous neck region starting at 7 to 9 weeks after birth. Profound hyperplasia with multiple cystically dilated glands was clearly observed in the fundic area of 12-, 14-, and 17-month-old mutant mice. Thus, the development of mucosal hyperplasia and cystic dilatation started at the age of 7 to 9 weeks, but full gastric pathomorphologic changes were not observed before the age of 12 months. In some aged CCR7−/− mice (> 14 months), the thickened fundic mucosa with cystic dilatation exhibited multiple diverticula beneath the muscularis mucosa, resembling gastritis cystica profunda (Figure 7A). The observed morphologic and histologic changes resemble those described in patients with hypertrophic gastropathy, such as Menetrier disease and hypertrophic lymphocytic gastritis (summarized in Table 2).24,25
Profound hyperplasia and increased rate of mucosal proliferation in CCR7−/− mice. (A) In some CCR7-deficient mice the thickened fundic mucosa exhibited multiple diverticula (marked by arrows) beneath the muscularis mucosa or even in the subserosa. Diverticula penetrating the muscularis mucosa were also observed. Three representative examples are shown for mice older than 18 months. Magnification, ×100. HE staining in paraffin-embedded sections. (B) Analysis of cellular proliferation in gastric mucosa from wt controls and in CCR7−/− mice. Determination of DNA synthesis rates was assessed by immunohistochemical detection of BrdU incorporation. Gastric mucosa from 10-week-old mice injected with BrdU was stained with anti-BrdU antibody (in red). Data are representative of 6 mice in each group. Original magnification, ×100 (left two panels); ×200 (right two panels). Hematoxylin counterstaining in paraffin-embedded sections.
Profound hyperplasia and increased rate of mucosal proliferation in CCR7−/− mice. (A) In some CCR7-deficient mice the thickened fundic mucosa exhibited multiple diverticula (marked by arrows) beneath the muscularis mucosa or even in the subserosa. Diverticula penetrating the muscularis mucosa were also observed. Three representative examples are shown for mice older than 18 months. Magnification, ×100. HE staining in paraffin-embedded sections. (B) Analysis of cellular proliferation in gastric mucosa from wt controls and in CCR7−/− mice. Determination of DNA synthesis rates was assessed by immunohistochemical detection of BrdU incorporation. Gastric mucosa from 10-week-old mice injected with BrdU was stained with anti-BrdU antibody (in red). Data are representative of 6 mice in each group. Original magnification, ×100 (left two panels); ×200 (right two panels). Hematoxylin counterstaining in paraffin-embedded sections.
Similarity between human Menetrier disease and gastric histomorphologic alterations in CCR7−/− mice
| . | CCR7−/− mice . | Menetrier disease . |
|---|---|---|
| Gastric wall thickening | + | + |
| Foveolar hyperplasia | + | + |
| Cyst formation | + | + |
| Lymphoid follicles | + | (+)* |
| Superficial edema | (+)† | + |
| Gastritis cystica profunda (glands extending in submucosa) | + | + |
| . | CCR7−/− mice . | Menetrier disease . |
|---|---|---|
| Gastric wall thickening | + | + |
| Foveolar hyperplasia | + | + |
| Cyst formation | + | + |
| Lymphoid follicles | + | (+)* |
| Superficial edema | (+)† | + |
| Gastritis cystica profunda (glands extending in submucosa) | + | + |
Variable degree of inflammation present with occasional lymphoid aggregates.
Variable degree of superficial edema present.
To determine whether lack of CCR7 leads to the accumulation of surface mucous cells through enhanced proliferation, rates of DNA synthesis were determined using immunohistologic localization of BrdU incorporation. In normal wt mice, the zone of maximal proliferation, the progenitor zone, is confined to a limited zone in the neck region of the gastric glands. In CCR7-deficient mice, a distortion in this pattern was evident. The progenitor zone in the hyperplastic regions of the stomach was markedly expanded (Figure 7B). Overall, there was a persistent increase in the rate of DNA synthesis in gastric glands of CCR7-deficient mice relative to wt or CXCR5-deficient mice. In conclusion, these observations suggest that the organization and integrity of the gastric mucosa is influenced by nonresident recirculating lymphocytes even under homeostatic, noninflammatory conditions.
Discussion
The present study extends our understanding of the mechanisms regulating homeostatic lymphocyte recirculation and the pathophysiologic consequences of disturbed peripheral lymphocyte trafficking through nonlymphoid tissues.
Governing continuous cell trafficking is a key function of chemokines and their receptors.2,26 Because homeostatic chemokines are expressed physiologically by lymphatic endothelium and vascular endothelium of nonlymphoid tissues among them gastric mucosa,27,28 it seems likely that these chemokines and their receptors are also involved in lymphocyte emigration from peripheral sites of lymphocyte infiltration.
When various epithelial and parenchymateous tissues of CCR7- and CXCR5-deficient mice were analyzed for abnormal cellularity and morphology, a striking phenotype was observed in CCR7- but not in CXCR5-deficient mice. Lack of CCR7 caused the appearance of multiple lymphoid follicular aggregates in the mucosal tissue of colon, stomach, and lung. The most profound phenotype was seen in the stomach of CCR7−/− mice. These mice exhibited diffuse lymphoid infiltrates in the mucosa and submucosa of the glandular stomach at the age of 2 to 4 weeks. At the age of 6 to 8 weeks, lymphocytic infiltrates were found to be organized into dispersed follicles mainly located in the lamina propria. With increasing age, scattered lymphoid follicles also extended to the submucosa, developed into confluent lymphoid follicles in the lamina propria, or both. The observed lymphoid follicular structures displayed major structural characteristics of lymph nodes; that is, they harbored B and T lymphocytes, DCs, and FDCs; harbored specialized PNAd+ vascular endothelium; expressed homeostatic chemokines; and executed organizing functions by attracting hematopoietic cells emigrating from the circulation. However, the unimpaired entry of adoptively transferred CD62L-blocked lymphocytes suggested either an additional migratory pathway or different functional structures of the ectopic vascular endothelium facilitating the interaction with other cognate ligands besides CD62L.
The developmental program leading to the formation of lymph nodes and ectopic lymph nodelike structures seems to be remarkably similar.29 Previous studies in transgenic mouse models have established that overexpression of homeostatic chemokines (ie, CCL21 and CXCL13) leads to the attraction of naive lymphocytes. Subsequently, these infiltrates consolidate into organized lymphoid structures composed primarily of T and B cells and a few DCs.21,30,31 In addition, cells derived from the local environment (ie, stroma and endothelial cells) are necessary for the development of a lymph node–like architecture, because they provide lymphotoxin and TNF-α,29 which support a follicular dendritic cell network with high expression of the homeostatic chemokines CCL21 and CXCL13.32,33
Here, we show that CCR7-deficient mice developed ectopic lymphoid follicles without an obvious T- or B-cell segregation. We conclude that CCL21/CCR7 signaling is ultimately required for the compartmentalization within tertiary lymphoid tissues.
What could account for the tissue-specific ability of CCR7-deficient lymphocytes to induce the formation of lymph node–like structures? Mucosal surfaces of the colon, stomach, and lung are thought to be the first line of defense against exogenous challenges, including microbes and soluble antigens.34 Because the mice are not kept under completely sterile conditions, one hypothesis would be that lymphocytic accumulation is induced under reactive inflammatory conditions. To rule out the possibility that the local environment in our animal facility, the commensal microflora, or both triggered the formation of ectopic lymphoid follicles, mice were treated continuously with antibiotics. Despite effective antibiotic treatment, CCR7−/− mice exhibited lymphoid follicular structures comparable in size and numbers to those of mutant mice housed under conventional conditions. We conclude that the formation of ectopic lymphoid follicles in CCR7-deficient mice represents an autonomous development and is not induced by a specific group of commensal bacteria or inflammatory stimuli. CXCR5-deficient mice and wt mice, which were housed under identical conditions, did not develop such ectopic follicles. This also points to an autonomous development of lymph nodelike structures in the CCR7-deficient mouse strain. Published data on the susceptibility of CCR7−/− mice to infections (ie, LCMV and Listeria monocytogenes15,16 ) showed no differences compared with wt mice, which suggests that CCR7−/− animals are not severely immunocompromised.
An alternative hypothesis for the formation of these aberrant lymphoid follicles would be that homeostatic chemokines are able to induce a permissive “endothelial” environment in which lymphocytes may be recruited through chemokines presented on the surface of the endothelium.31 Homeostatic chemokines could directly or indirectly induce expression of the requisite adhesion molecules (ie, MAdCAM-1 and PNAd), which could then facilitate initial adhesion and infiltration of lymphocytes. Differences in the recruiting properties of chemokines could depend on the ability of the endothelium of different tissues to present chemokines to circulating lymphocytes. Furthermore, ectopic expression of CCL21 in the pancreatic islets was described to cause infiltration by T cells, B cells, and DCs as well as to induce HEV development.20,31 Here, we show that the development of aberrant lymphoid follicles in CCR7−/− mice starts early with an accumulation of T cells, B cells, and DCs in the mucosal tissue of juvenile mice (4-5 weeks). On the basis of a recent publication,35 which shows that endothelial cell proliferation in lymph nodes is dependent on CD11c+ DCs, one might as well speculate about an early role of DCs in the formation of ectopic lymphoid follicles.
CCR7 is expressed not only by naive and central memory T cells but also by effector memory cells that infiltrate extralymphoid tissues.36-38 Here, we found that the majority of the CD4+ T cells isolated from the aberrant follicular structures of CCR7−/− mice were of a CD44hiCD62Llo effector memory phenotype. Because all animals have been analyzed under homeostatic conditions, we surmise that these CD44hiCD62Llo memory T cells represent a nonactivated intermediate differentiation stage between TCM and TEM cells, which have already down-regulated CD62L and up-regulated CD44 but still require CCR7 expression for continuous recirculation. Lack of CCR7 expression caused accumulation of these cells in peripheral tissues, suggesting that CCR7 expression is crucial for tissue exit and recirculation of the early stages of effector memory T cells. In this context, it was shown recently that T lymphocytes which were transferred subcutaneously into recipient mice require CCR7 for lymphocyte exit from the skin.39 We also used an adoptive transfer model to demonstrate that emigration of CCR7-deficient lymphocytes from the peritoneal cavity by lymphatics to the draining lymph nodes is disturbed (U.E.H. and M.L., unpublished data, March 2005). Both models provide some mechanistic insight into the control of lymphocyte exit from peripheral sites and entry into afferent lymphatics. Thus, disturbed lymphocyte egress might cause aberrant accumulation of lymphocytes at peripheral sites, as we observed in the peritoneal cavity14 and gastrointestinal tract of CCR7-deficient mice. In addition, lack of CCR7 might lead to a local predominance of CXCL12/CXCR signals, CXCL13/CXCR5 signals, or both because of an altered chemokine balance in the stomach, possibly leading to enhanced recruitment of lymphocytes to peripheral sites in which the ligands are constitutively expressed.
According to the recent results of Bromley et al,40 CCR7 also regulates effector T-lymphocyte transit and accumulation during inflammation in the asthmatic lung. Thus, one can propose a more generalized scheme in which CCR7 expression is a key player for T-lymphocyte exit from peripheral tissues during homeostasis as well as inflammation.
It has been generally accepted that infections or chronic autoimmune processes trigger pathogenic alterations in lymphoid and nonlymphoid sites. However, even in the absence of strong inflammatory stimuli, recirculation requires tight control, because we show that CCR7 deficiency causes severe changes in the cellular architecture of gastric mucosa. In many aspects these morphologic changes resemble the pathology of Menetrier disease in humans, which is characterized by a hypertrophic gastropathy.24,25 Thus far, similar phenotypes have been observed in Helicobacter infection–associated chronic gastritis models,41 in histamine H2-receptor–deficient mice,42,43 and in transgenic mice overexpressing transforming growth factor α.44
We surmise that the proliferative activity within the aberrant lymphoid follicles of CCR7−/− mice could trigger increased mucosal proliferation and hyperplastic gastric mucosa development.
The composition of the mucosal lymphoid aggregates with high numbers of effector memory T cells and the hypertrophic gastropathy could also point toward a selection of autoimmune T cells. However, so far no gross autoimmune disorders have been described in the CCR7−/− mice, and autoantibody levels against calf thymus DNA were unaltered in the CCR7−/− mice (U.E.H. and M.L., unpublished data, May 2004). Recently, it has been described that CCR7 is involved in the migration of developing thymocytes and thymic T-cell development.45-48 In this view, CCR7 deficiency might alter tolerance induction, and the observed phenotype might be indicative of an impaired development of tolerance against mucosal antigens.
In summary, aberrant formation of lymphoid follicular structures caused by CCR7 deficiency affects the microarchitecture and function in mucosal tissues. We envisage that CCR7 serves as a central regulator in peripheral lymphocyte recirculation and cellular homeostasis of the gastric mucosa.
Authorship
Contribution: U.E.H. designed, performed, and analyzed the research and wrote the paper;a.m. W. and M.M.H. performed the research; C.L. and H.S. analyzed the data; A.R. and M.L. designed the research and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Uta E. Höpken, Max-Delbrück-Center for Molecular Medicine, MDC, Berlin, 13125, Germany; e-mail: uhoepken@mdc-berlin.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Ariel H. Achtman and Gerd Müller for helpful suggestions and critical reading of the manuscript. We thank Gudrun F. Debes and Eugene C. Butcher for helpful discussions and Dagmar Breitfeld, Kerstin Gerlach, Kerstin Krüger, and Heike Schwede for expert technical assistance.
This work was supported by the Priority Program SFB 633 of the German Research Foundation (DFG).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal