Abstract
The interleukin-12 (IL-12) cytokine induces the differentiation of naive T cells to the T helper cell type 1 (Th1) phenotype and is integral to the pathogenesis of Th1-mediated immunologic disorders. A more recently discovered IL-12 family member, IL-23, shares the p40 protein subunit with IL-12 and plays a critical role in the generation of effector memory T cells and IL-17–producing T cells. We introduce a novel compound, STA-5326, that down-regulates both IL-12 p35 and IL-12/IL-23 p40 at the transcriptional level, and inhibits the production of both IL-12 and IL-23 cytokines. Oral administration of STA-5326 led to a suppression of the Th1 but not Th2 immune response in mice. In vivo studies using a CD4+CD45Rbhigh T-cell transfer severe combined immunodeficiency (SCID) mouse inflammatory bowel disease model demonstrated that oral administration of STA-5326 markedly reduced inflammatory histopathologic changes in the colon. A striking decrease in interferon-γ (IFN-γ) production was observed in ex vivo culture of lamina propria cells harvested from animals treated with STA-5326, indicating a down-regulation of the Th1 response by STA-5326. These results suggest that STA-5326 has potential for use in the treatment of Th1-related autoimmune or immunologic disorders. STA-5326 currently is being evaluated in phase 2 clinical trials in patients with Crohn disease and rheumatoid arthritis.
Introduction
An array of chronic inflammatory diseases, including Crohn disease, psoriasis, rheumatoid arthritis, and multiple sclerosis, cause widespread and severe health problems. These and other immune diseases can be considered to have a predominantly T helper cell type 1 (Th1) bias. Although injectable biologic treatments such as antibodies and soluble protein that block TNF-α have provided significant clinical benefit, there is still a high unmet medical need, particularly for a safe targeted drug that can be taken orally.
Interleukin-12 (IL-12) is a heterodimeric cytokine (p70) composed of 2 independently regulated protein subunits, p35 and p40. IL-12 plays a central role in the immune response by bridging innate resistance and antigen-specific adaptive immunity.1 It is produced from phagocytic cells and antigen-presenting cells, in particular macrophages and dendritic cells, upon stimulation with bacteria, bacterial products such as lipopolysaccharide (LPS), and intracellular parasites.2,3 The well-documented biologic functions of IL-12 are the induction of interferon-γ (IFN-γ) expression from T and natural killer (NK) cells4,5 and the differentiation of naive T cells toward a Th1-cell type.6,7 IL-12 also appears to be critical in the expansion and maintenance of the Th1 phenotype.8-10 IL-23, a more recently discovered member of the IL-12 family, also promotes Th1 response, but has distinct functions from IL-12. IL-23 is required for the generation of effector memory T cells.11 IL-23 is also needed for the generation of IL-17–producing T cells, which play a significant role in the inflammatory response.12 Through these activities, IL-23 plays a critical role in chronic inflammatory diseases.
Although the inflammatory effector function of Th1 is essential for the clearance of intracellular pathogens, the excessive production of proinflammatory cytokines leads to serious tissue damage typical of organ-specific autoimmunity,13,14 including type 1 diabetes,15 multiple sclerosis,16,17 rheumatoid arthritis,18,19 inflammatory bowel disease,20 psoriasis,21 and sepsis.22 IL-12/IL-23 has been validated as an attractive clinical target by a number of studies. Mice lacking the gene encoding the p40 subunit shared by IL-12 and IL-23 or lacking the IL-23–specific subunit p19 have proven the integral role of IL-12/IL-23 to the pathogenesis of these disorders, implying that blockade of the cytokines could be effective in the treatment of a variety of autoimmune diseases.21,23-28 Monoclonal antibodies to the IL-12/23 p40 subunit have been shown to be effective in human clinical trials in patients with Crohn disease and psoriasis.29,30
While antibodies against IL-12/IL-23 could provide significant medical benefit, a small-molecule IL-12/IL-23 inhibitor that could be administered orally would be highly desirable. Although some compounds weakly or nonselectively inhibit IL-12, there are no potent and selective small-molecule IL-12 inhibitors. To find an effective inhibitor against the IFN-γ–enhanced IL-12 production, we screened an 80 000-compound library using IFN-γ/LPS–stimulated human peripheral-blood mononuclear cells (PBMCs). A novel 1,3,5-triazine derivative that has not previously been ascribed any biologic activity was found through this screening, and its optimization eventually led to the finding of STA-5326. STA-5326 has now entered human clinical testing. After showing an acceptable safety profile in healthy volunteers, STA-5326 is now being tested in phase 2 clinical trials in patients with Crohn disease and rheumatoid arthritis. In this report, we introduce the biologic activity of the compound for the first time, presenting its potent and selective inhibitory activity against IL-12 and IL-23. STA-5326 suppresses a Th1 immune response in mice without significantly affecting Th2. Efficacy has been demonstrated in a mouse model of inflammatory bowel disease.
Materials and methods
Reagents
Staphylococcus aureus Cowan I (SAC) and lipopolysaccharide (LPS; Serratia marscencens) were obtained from Calbiochem (La Jolla, CA) and Sigma (St Louis, MO), respectively. Human and mouse recombinant IFN-γ were purchased from Boehringer Mannheim (Mannheim, Germany) and BD Biosciences Pharmingen (San Diego, CA), respectively. STA-5326 was synthesized in our laboratory.
In vitro assays
Human and monkey PBMCs were isolated using NycoPrep (Axis-Shield PoC AS, Oslo, Norway). Human monocytes were purified using RosetteSep (StemCell Technologies, Vancouver, BC, Canada), and dendritic cells were differentiated from monocytes by culture with granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/mL) and IL-4 (20 ng/mL) for 9 days. Purity of monocytes and dendritic cells was more than 70% CD14+ and CD1a+, respectively, by phenotypic analysis. Mouse PBMCs were isolated using OptiPrep (Sigma), and spleen cells were prepared using ACK lysing buffer (BioWhittaker, Walkersville, MD). Human PBMCs, THP-1 cells, and monkey PBMCs were primed with human IFN-γ (400 U/mL) for 22 hours and then stimulated with 1 μg/mL of LPS or 0.025% of SAC for 18 hours. To investigate requirement of de novo synthesis, cycloheximide (CHX) was added 2 hours or immediately prior to SAC. Human PBMCs were also stimulated by anti-CD3 (0.2 μg/mL) and anti-CD28 (1 μg/mL) antibodies, or ConA (1 μg/mL). Mouse cells were stimulated with a combination of mouse IFN-γ (100 ng/mL) with SAC (0.05%) for 22 hours. STA-5326 was prepared in DMSO; the final DMSO concentration was adjusted to 0.25% in all cultures, including the compound-free control.
IL-23 was detected using anti–IL-23 p19 polyclonal antibody (R&D Systems, Minneapolis, MN) and biotinylated goat anti–human p40 antibody (R&D Systems), and calculated using recombinant IL-23 (R&D Systems). Total p40 proteins were measured using p40 ELISA kit (R&D Systems). Monkey IL-12 p70 was measured using the human IL-12 p70 enzyme-linked immunosorbent assay (ELISA) kit from Cell Sciences (Canton, MA). IL-12 induced by SAC alone was measured using Quantikine HS ELISA Kit (R&D Systems). Other cytokines were measured using ELISA kits from Cell Sciences and R&D Systems, or Bio-Plex assays from Bio-Rad (Hercules, CA).
IL-12 promoter-driven luciferase assay
To construct the human IL-12 p35 and p40 promoter/luciferase reporter constructs, we generated p35 (−1.5 kb/+3 bp) and p40 (−1.3 kb/+56 bp) promoter fragments by polymerase chain reaction (PCR) of genomic DNA obtained from human PBMCs. The resulting PCR products were ligated upstream of the luciferase gene in pGL3-Basic vector (Promega, Madison, WI). All constructs were verified by DNA sequencing. RAW267.4 cells were transiently transfected using SuperFect Transfection Reagent (Qiagen, Valencia, CA). The cells were stimulated with IFN-γ (100 ng/mL) for 10 hours followed by LPS (1 μg/mL) in the presence or absence of test compound in duplicate for an additional 16 hours. Cells were cotransfected with a pCMVβ vector (BD Biosciences Clontech, Palo Alto, CA) for monitoring transfection efficiency. Luciferase and β-galactosidase activity were determined according to luciferase assay system (Promega) and luminescent β-gal detection system (BD Biosciences, Clontech). Luciferase activity was then normalized using the β-galactosidase value.
In vivo Th1 and Th2 response
To induce a Th1 response, C57BL/6 mice were given anti–IL-4 antibody on days 1 and 2 after sensitization with Mycobacterium tuberculosis in Freund adjuvant. Nine days later, lymph nodes were harvested. To induce a Th2 response, BALB/c mice were given anti–IFN-γ antibody on days 1 and 2 after sensitization with 150 μg of purified Ascaris powder and aluminum hydroxide in incomplete Freund adjuvant. Six days later, lymph nodes were harvested. In some Th1 and Th2 models, lymph node cells were counted from each mouse and tested for cytokine production individually. In the remainder of the studies, all lymph nodes from mice within a group were pooled for assessments of cell counts and cytokine production. Ex vivo cytokine production was assessed by incubation of harvested lymph node cells with coated anti-CD3 antibody and soluble anti-CD28 antibody (BD Biosciences Pharmingen) in a 96-well plate with 5 × 105 cells/well. Supernatants were then analyzed using a Bio-Plex kit (Bio-Rad).
CD4+CD45Rbhigh T-cell transfer SCID mouse inflammatory bowel disease
CD4+ T cells in spleen cells from female BALB/c mice were negatively selected using antibodies (BioSource, Camarillo, CA) against B220 (RA3-6B2), CD11b (M1/70), and CD8α (53-6.72), and labeled with FITC-conjugated anti-CD45RB (16A; BD Biosciences Pharmingen) and PE-conjugated anti-CD4 (CT-CD4; Caltag, Burlingame, CA). CD4+ CD45RBhigh cells were defined as the upper 40% of CD45Rb-staining CD4+ cells and were sorted by flow cytometry. Harvested cells were intraperitoneally injected into female C.B-17 SCID mice with 4 × 105 cells per mouse. STA-5326 and vehicle were orally administered once a day, 5 days per week, starting the day following the transfer.
Colon tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections (4 μm) from the ascending, transverse, and descending colon were stained with hematoxylin and eosin. Digital photomicrographs magnified at 40× and 200× of the original from the most affected areas were used for analysis. The extent of colonic inflammation was graded in a blind fashion on a scale of 0 to 3 in each of 4 histologic criteria: crypt elongation, inflammatory-cell infiltration, the number of crypt abscesses, and goblet-cell depletion. The controls were considered to be at baseline with a score of 0. Scores of 1, 2, and 3 represented the following: 2- to 3-fold, 4- to 6-fold, and more than 6-fold increase for crypt elongation; mild, moderate, and severe for inflammatory-cell infiltration; 1 to 2, 3 to 5, and more than 5 colonic crypt abscesses per section; and mild, moderate, and severe for goblet-cell depletion associated with epithelial hyperplasia or inflammatory infiltration. The total score for each animal was the sum of the average scores of ascending, transverse, and descending colon sections in all 4 categories.
Isolation of LPMCs and cytokine measurement
Freshly obtained colon was washed in Ca/Mg-free HBSS, and incubated twice in HBSS containing EDTA (0.75 mM), DTT (1 mM), and antibiotics (2.5 μg/mL amphotericin, 50 μg/mL gentamicin) at 37°C for 15 minutes. The tissue was digested in RPMI containing 0.5 mg/mL collagenase D, 0.01 mg/mL DNase I (Boehringer Manheim), and antibiotics at 37°C. Lamina propria (LP) cells were then layered on a 40% to 100% Percoll gradient (Pharmacia, Uppsala, Sweden), and lymphocyte-enriched populations were isolated at the 40% to 100% interface as lamina propia mononuclear cells (LPMCs). Cells were incubated with anti-CD3 and anti-CD28 antibody as described above, under “In vivo Th1 and Th2 response.” Supernatants were removed after 24 hours and assessed for IFN-γ using an ELISA kit (Endogen, Rockford, IL).
Statistical analysis
Results
Potent inhibition of IL-12 and IL-23 production by STA-5326
IL-12 plays an integral role in both innate and adaptive immune responses. IFN-γ is a strong and selective enhancer of IL-12 production, and the effect is evident only after extended treatment with IFN-γ for at least 8 hours prior to stimulation with LPS or SAC.31 This suggests that in Th1-mediated chronic autoimmune or immunologic disorders that are characterized by recurrently high levels of IFN-γ, production of the IL-12 family is mediated by IFN-γ. In order to discover effective IL-12 inhibitors, we carried out a phenotypic screening assay using IFN-γ/LPS–stimulated human PBMCs, and screened an 80 000-compound library. A novel compound, a 1,3,5-triazine derivative, was discovered through this screening. The lead optimization process produced and evaluated approximately 500 compounds and led to the discovery of STA-5326, a unique morpholinopyrimidine derivative. IL-12 production in cultures of IFN-γ/LPS–stimulated human PBMCs was strongly inhibited by STA-5326 with an IC50 of 10 nM (Table 1). The inhibitory activity was more pronounced in IFN-γ/SAC–stimulated human PBMCs, where STA-5326 completely inhibited IL-12 with an IC50 of 1 nM (Figure 1; Table 1). No decrease in cell viability was observed, even at a concentration of 10 μM (J.C., unpublished data, December 2001).
Inhibition of IL-12p70 by STA-5326
| Cell type . | Stimulus . | Baseline value, pg/mL . | IC50, nM . |
|---|---|---|---|
| Human PBMCs | IFN-γ/SAC | 200 | 1 |
| Human PBMCs | IFN-γ/LPS | 180 | 10 |
| Human PBMCs | SAC | 5 | 5 |
| Human monocytes | IFN-γ/SAC | 180 | 1 |
| Human dendritic cells | IFN-γ/SAC | 130 | 5 |
| Human THP-1 | IFN-γ/SAC | 60 | 10 |
| Mouse PBMCs | IFN-γ/SAC | 30 | 2 |
| Mouse spleen cells/PBMCs | IFN-γ/SAC | 250 | 20 |
| Cell type . | Stimulus . | Baseline value, pg/mL . | IC50, nM . |
|---|---|---|---|
| Human PBMCs | IFN-γ/SAC | 200 | 1 |
| Human PBMCs | IFN-γ/LPS | 180 | 10 |
| Human PBMCs | SAC | 5 | 5 |
| Human monocytes | IFN-γ/SAC | 180 | 1 |
| Human dendritic cells | IFN-γ/SAC | 130 | 5 |
| Human THP-1 | IFN-γ/SAC | 60 | 10 |
| Mouse PBMCs | IFN-γ/SAC | 30 | 2 |
| Mouse spleen cells/PBMCs | IFN-γ/SAC | 250 | 20 |
All IC50s shown in the table are representatives of at least 3 studies.
Inhibitory activity of STA-5326 on IL-12 p70, IL-23, and total p40 in human PBMCs. Human PBMCs were primed with IFN-γ and then stimulated with SAC in the presence of different concentrations of STA-5326. Supernatants were tested for IL-12 p70 (⋄), IL-23 (□), and total p40 (▵), and the levels in compound-free control were 15 pg/mL, 1.2 ng/mL, and 5.5 ng/mL, respectively. Results are shown as mean percentage of inhibition of 1 representative of 3 individual experiments. Compared with the level of corresponding compound-free control, P < .001 at concentrations 8 to 1000 nM, 8 to 1000 nM, and 1.6 to 1000 nM of STA-5326 for IL-12 p70, IL-23, and total p40, respectively.
Inhibitory activity of STA-5326 on IL-12 p70, IL-23, and total p40 in human PBMCs. Human PBMCs were primed with IFN-γ and then stimulated with SAC in the presence of different concentrations of STA-5326. Supernatants were tested for IL-12 p70 (⋄), IL-23 (□), and total p40 (▵), and the levels in compound-free control were 15 pg/mL, 1.2 ng/mL, and 5.5 ng/mL, respectively. Results are shown as mean percentage of inhibition of 1 representative of 3 individual experiments. Compared with the level of corresponding compound-free control, P < .001 at concentrations 8 to 1000 nM, 8 to 1000 nM, and 1.6 to 1000 nM of STA-5326 for IL-12 p70, IL-23, and total p40, respectively.
The production of IL-12 p70 heterodimer requires the simultaneous expression of the 2 individually regulated genes that encode the IL-12 p35 and p40 subunits. The p40 subunit is shared by a new IL-12 family member, IL-23, which was identified as playing a critical role in the inflammatory response. STA-5326 not only inhibited IL-12, but it also potently inhibited IL-23 and total p40 products in various forms as monomer, homodimer, and heterodimer in IFN-γ/SAC–stimulated human PBMCs (Figure 1).
The inhibitory activity is not limited to IFN-γ–primed signals, since STA-5326 similarly inhibited IL-12 production induced by SAC alone with an IC50 of approximately 5 nM. In addition, when STA-5326 was added during IFN-γ priming and washed away before addition of either SAC or LPS, no reduction in IL-12 was observed (Y. Wada, unpublished data, October 2001), indicating that STA-5326 acts on the signaling induced by SAC and LPS stimuli.
Inhibition of IL-12 production was also observed in primary human monocytes, monocyte-derived dendritic cells, and THP-1 cells, which are the only human monocytic cell line that produces IL-12 p70 in response to both LPS and SAC,32 demonstrating consistent inhibition of IL-12 in all mixed and purified human primary cell cultures, and in a pure cell line (Table 1).
Furthermore, the inhibitory activity of STA-5326 on IL-12 was shown in species other than human. STA-5326 demonstrated a strong inhibition in IL-12 induced from monkey PBMCs by SAC treatment following IFN-γ priming, with an IC50 of 2 nM (Table 1). In mouse spleen cells and PBMCs, although IFN-γ is required to induce IL-12 p70, priming of cells prior to LPS or SAC is not as essential as it is for human PBMCs. Mouse spleen cells and PBMCs were stimulated with a combination of IFN-γ with SAC in the presence or absence of STA-5326. A significant inhibition of IL-12 from mouse spleen cells and PBMCs by STA-5326 was observed with the estimated IC50 of 20 nM (Table 1).
Selectivity of STA-5326 in inhibition of cytokine production
The selectivity of cytokine inhibition is important particularly for the understanding of the mechanism of action and in vivo activity. STA-5326 inhibited IFN-γ production induced by either IFN-γ/SAC or SAC in human PBMCs, with an IC50 of approximately 20 nM, while the compound had no significant effect on the production when T cells were stimulated using anti-CD3 and anti-CD28 antibodies, suggesting that the T-cell–receptor–dependent production of IFN-γ that occurs independently of suppression of Th1 cells via inhibition of IL-12 is not directly inhibited by the compound (Table 2). A significant reduction in IL-6 production was observed in IFN-γ/SAC–stimulated human PBMCs, but not in IFN-γ/LPS–stimulated human PBMCs, IFN-γ/SAC-stimulated THP-1, and mouse spleen cells (Table 2; Y. Wada, unpublished data, 2001-2002). Similarly, IFN-γ/SAC–specific inhibition was observed in IL-10 production. STA-5326 showed some inhibition against IFN-γ/SAC–induced TNF-α and ConA-induced IL-5 from human PBMCs at high concentrations, but no suppressive effect against IL-1β, IL-2, IL-4, IL-8, and IL-18 in all cultures tested.
Cytokine inhibitory spectrum of STA-5326
| Cell type . | Stimulus . | Cytokine . | Baseline value, pg/mL . | IC50, nM . |
|---|---|---|---|---|
| Human PBMCs | IFN-γ/SAC | IL-12 p70 | 200 | 1 |
| Human PBMCs | IFN-γ/SAC | IL-23 | 1 000 | 10 |
| Human PBMCs | IFN-γ/SAC | IL-12/IL-23 p40 | 6 000 | 20 |
| Human PBMCs | IFN-γ/SAC | IFN-γ | 28 000* | 20 |
| Human PBMCs | IFN-γ/SAC | IL-6 | 25 000 | 100 |
| Human PBMCs | IFN-γ/SAC | IL-10 | 2 000 | 200 |
| Human PBMCs | IFN-γ/SAC | TNF-α | 56 000 | 1000 |
| Human PBMCs | IFN-γ/SAC | IL-1β | 25 000 | > 1000 |
| Human PBMCs | IFN-γ/SAC | IL-8 | 1 000 | > 1000 |
| Human PBMCs | IFN-γ/SAC | IL-18 | 300 | > 1000 |
| Human PBMCs | IFN-γ/LPS | IL-12 p70 | 180 | 10 |
| Human PBMCs | IFN-γ/LPS | IL-6 | 32 000 | > 1000 |
| Human PBMCs | IFN-γ/LPS | IL-10 | 2 000 | > 1000 |
| Human PBMCs | ConA | IL-5 | 100 | 100 |
| Human PBMCs | ConA | IL-4 | 20 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IFN-γ | 200 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IL-4 | 40 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IL-2 | 800 | > 1000 |
| Human THP-1 | IFN-γ/SAC | IL-12 p70 | 60 | 10 |
| Human THP-1 | IFN-γ/SAC | IL-6 | 9 000 | > 1000 |
| Cell type . | Stimulus . | Cytokine . | Baseline value, pg/mL . | IC50, nM . |
|---|---|---|---|---|
| Human PBMCs | IFN-γ/SAC | IL-12 p70 | 200 | 1 |
| Human PBMCs | IFN-γ/SAC | IL-23 | 1 000 | 10 |
| Human PBMCs | IFN-γ/SAC | IL-12/IL-23 p40 | 6 000 | 20 |
| Human PBMCs | IFN-γ/SAC | IFN-γ | 28 000* | 20 |
| Human PBMCs | IFN-γ/SAC | IL-6 | 25 000 | 100 |
| Human PBMCs | IFN-γ/SAC | IL-10 | 2 000 | 200 |
| Human PBMCs | IFN-γ/SAC | TNF-α | 56 000 | 1000 |
| Human PBMCs | IFN-γ/SAC | IL-1β | 25 000 | > 1000 |
| Human PBMCs | IFN-γ/SAC | IL-8 | 1 000 | > 1000 |
| Human PBMCs | IFN-γ/SAC | IL-18 | 300 | > 1000 |
| Human PBMCs | IFN-γ/LPS | IL-12 p70 | 180 | 10 |
| Human PBMCs | IFN-γ/LPS | IL-6 | 32 000 | > 1000 |
| Human PBMCs | IFN-γ/LPS | IL-10 | 2 000 | > 1000 |
| Human PBMCs | ConA | IL-5 | 100 | 100 |
| Human PBMCs | ConA | IL-4 | 20 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IFN-γ | 200 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IL-4 | 40 | > 1000 |
| Human PBMCs | αCD3/ αCD28 Ab | IL-2 | 800 | > 1000 |
| Human THP-1 | IFN-γ/SAC | IL-12 p70 | 60 | 10 |
| Human THP-1 | IFN-γ/SAC | IL-6 | 9 000 | > 1000 |
All IC50s shown in the table are representatives of at least 3 studies.
After subtraction of IFN-γ added as stimulus.
Effects of STA-5326 on IL-12 p35 and p40 promoter activities
Having shown that STA-5326 reduces production of both IL-12 p70 and p40 protein, a study of the p35 and p40 promoter activities was then undertaken to obtain more insight into the mechanism of action of this compound. The murine macrophage cell line RAW264.7 was transiently transfected with DNA constructs in which the p35 and p40 promoters directed expression of the luciferase reporter gene. Cells were then stimulated with murine recombinant IFN-γ, followed by LPS or SAC in the presence or absence of STA-5326 or STA-5392 (an inactive compound that is structurally related to STA-5326). The p35 and p40 promoter-driven luciferase activities were significantly induced after stimulation with IFN-γ/LPS or IFN-γ/SAC, and were completely suppressed by 100 nM STA-5326 (Figure 2). The closely structurally related inactive compound STA-5392 had no effect, even at 1 μM (Figure 2B). The inhibition of p35 and p40 promoter-mediated luciferase expression is in good correlation with the observed inhibition of p70 protein production. This result indicates that the suppression of IL-12 and IL-23 by STA-5326 occurs at a transcriptional level, and that STA-5326 inhibits the transcription of both IL-12 p35 and IL-12/IL-23 p40 genes.
IL-12 promoter-driven luciferase assay. Human IL-12 p40 (A) and p35 (B) promoter/luciferase reporter constructs were generated by ligating the human p40 (−1.3 kb/+56 bp) promoter and p35 (−1.5 kb/+3 bp) promoter fragments upstream of the luciferase gene in pGL3-Basic vector. RAW267.4 cells were transiently transfected and then stimulated with murine recombinant IFN-γ followed by LPS in the presence or absence of 100 nM STA-5326 or 1 μM STA-5392, a structurally related inactive compound in duplicate. Cells were cotransfected with pCMVβ, a vector in which the constitutively active CMV promoter drives expression of β-galactosidase, for the monitoring of transfection efficiency. The relative luciferase activity is the mean ratio of luciferase activity in the treated group relative to luciferase activity in the nontreated group after normalization with the β-galactosidase value.
IL-12 promoter-driven luciferase assay. Human IL-12 p40 (A) and p35 (B) promoter/luciferase reporter constructs were generated by ligating the human p40 (−1.3 kb/+56 bp) promoter and p35 (−1.5 kb/+3 bp) promoter fragments upstream of the luciferase gene in pGL3-Basic vector. RAW267.4 cells were transiently transfected and then stimulated with murine recombinant IFN-γ followed by LPS in the presence or absence of 100 nM STA-5326 or 1 μM STA-5392, a structurally related inactive compound in duplicate. Cells were cotransfected with pCMVβ, a vector in which the constitutively active CMV promoter drives expression of β-galactosidase, for the monitoring of transfection efficiency. The relative luciferase activity is the mean ratio of luciferase activity in the treated group relative to luciferase activity in the nontreated group after normalization with the β-galactosidase value.
The activity of STA-5326 does not require de novo protein synthesis
Corticosteroids,33-36 cyclic AMP,37 IFN-β,38 and IL-1039 exert their suppressive effects on cytokine production, including IL-12, by a mechanism that requires de novo protein synthesis. To determine whether de novo protein synthesis is involved in the inhibitory mechanism of STA-5326, the effect of a protein synthesis inhibitor, CHX, on the inhibition of IL-12 production by STA-5326 was assessed in comparison with the effect of CHX on IL-12 inhibition by IL-10. CHX at a concentration of 5 μg/mL reduced IL-12 production by more than half. STA-5326 remained potent in inhibiting IL-12 production in the presence of CHX and reduced IL-12 production to a greater degree than CHX treatment alone, indicating that CHX does not abrogate the inhibitory activity of STA-5326 (Figure 3). It is noted that the estimated IC50 of STA-5326 in the presence of CHX was reproducibly 2- to 3-fold lower than the IC50 in cultures without CHX. In the same experiment, IL-10 at a concentration of 10 ng/mL significantly reduced IL-12 production in the absence of CHX, but completely lost the inhibitory activity in the presence of CHX.
De novo synthesis-independent inhibitory activity of STA-5326 on IL-12 production. STA-5326 was added together with SAC to the culture of IFN-γ–primed human PBMCs in the presence (⋄) or absence (♦) of 5 μg/mL CHX. IL-10 was added 1 hour prior to SAC. Percentage of inhibition was calculated from the corresponding control. Results are shown as mean ± SD of 1 representative of 3 individual experiments.
De novo synthesis-independent inhibitory activity of STA-5326 on IL-12 production. STA-5326 was added together with SAC to the culture of IFN-γ–primed human PBMCs in the presence (⋄) or absence (♦) of 5 μg/mL CHX. IL-10 was added 1 hour prior to SAC. Percentage of inhibition was calculated from the corresponding control. Results are shown as mean ± SD of 1 representative of 3 individual experiments.
Selective suppression of the in vivo Th1 response by STA-5326
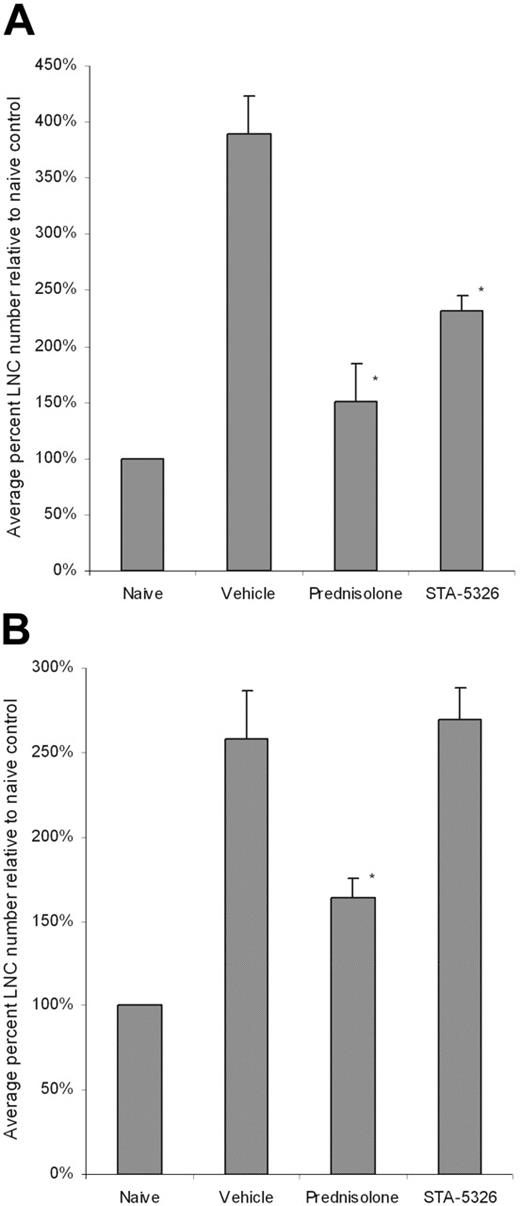
The potent and selective inhibition of in vitro IL-12 and IL-23 production by STA-5326 suggests that this compound should significantly suppress the Th1 response in vivo, but not the Th2 response. We evaluated the effect of STA-5326 on in vivo Th1 and Th2 mouse models that were induced by Mycobacterium tuberculosis with Freund adjuvant in C57BL/6 mice and Ascaris/aluminum hydroxide with incomplete Freund adjuvant in BALB/c mice, respectively. STA-5326, the steroid prednisolone as a positive control, or vehicle were orally given to mice from the day of immunization, and lymph node cells from each group were harvested for assessment of the effect on the induction of Th1 and Th2 responses in vivo. Figure 4 shows the average percentage of cell numbers relative to the naive control from 4 Th1 and 6 Th2 experiments, respectively. After immunization, lymph node cells from the vehicle control groups in these Th1 and Th2 models increased approximately 3-fold over the corresponding naive controls. A reduction was observed in animals treated with prednisolone in both models, indicating the indiscriminate inhibition of both Th1 and Th2 responses. In contrast, treatment with STA-5326 caused a significant reduction in cell number only in the Th1 model, with an average percentage of inhibition of 51% ± 8% relative to the vehicle control. STA-5326 treatment had no effect in the Th2 setting (Figure 4).
Percentage of LNC numbers relative to naive control after Th1 and Th2 exposures. C57BL/6 (A) and BALB/c (B) mice were sensitized with M tuberculosis and Ascaris plus aluminum hydroxide, respectively. Mice were treated with vehicle, prednisolone, or STA-5326 orally once a day. In some studies, lymph node cells (LNCs) were harvested and counted from each mouse, and cytokine production was tested individually. Otherwise, cells were pooled within each group for cell counting and ex vivo culture. Results shown are mean ± SEM percentage of LNC numbers relative to naive control from 4 individual Th1 experiments and 6 individual Th2 experiments (*P < .05 compared with the level of the vehicle group).
Percentage of LNC numbers relative to naive control after Th1 and Th2 exposures. C57BL/6 (A) and BALB/c (B) mice were sensitized with M tuberculosis and Ascaris plus aluminum hydroxide, respectively. Mice were treated with vehicle, prednisolone, or STA-5326 orally once a day. In some studies, lymph node cells (LNCs) were harvested and counted from each mouse, and cytokine production was tested individually. Otherwise, cells were pooled within each group for cell counting and ex vivo culture. Results shown are mean ± SEM percentage of LNC numbers relative to naive control from 4 individual Th1 experiments and 6 individual Th2 experiments (*P < .05 compared with the level of the vehicle group).
To examine the differentiation of T cells in mice, lymph node cells were then plated at equal cell numbers per well, and evaluated for anti-CD3/CD28 antibody-stimulated production of IFN-γ and IL-4, the cytokines that represent Th1 and Th2, respectively. Immunization of C57BL/6 mice with M tuberculosis effectively drove an in vivo Th1 response in the vehicle control with a marked increase in the production of IFN-γ (Figure 5). Alternatively, immunization of BALB/c mice with Ascaris/aluminum hydroxide gave rise to an in vivo Th2 response, and an increase in IL-4 was observed in the vehicle-treated group. Treatment with prednisolone blocked not only the elevation of IFN-γ in the Th1 model, but also IL-4 in the Th2 model moderately. In contrast, STA-5326 only inhibited IFN-γ in the Th1 model with an average 84% ± 10% reduction relative to the vehicle control group from 4 individual Th1 experiments. Interestingly, the production of IL-4, the Th2 cytokine, tended to be increased in the STA-5326–treated group, with an average 232% ± 91% increase relative to the vehicle control group from 6 individual Th2 experiments. These results clearly indicate that the effect of STA-5326 is stimulus dependent and selective against the Th1 response.
Cytokine profile of LNCs after Th1 and Th2 exposures. M tuberculosis–sensitized C57BL/6 (A) and Ascaris-sensitized BALB/c (B) mice were treated with vehicle, prednisolone, or STA-5326. Results are representative of 4 Th1 and 6 Th2 in vivo studies. In the studies, to calculate deviation, LNCs from sensitized mice were individually harvested and tested in triplicate from each mouse for cytokine production in response to immobilized anti-CD3ϵ antibody and soluble anti-CD28 antibody. Lymph nodes from naive mice in the Th1 study were pooled and tested for cytokine production in triplicate. IFN-γ (A) in the Th1 study and IL-4 (B) in the Th2 study were analyzed with supernatants collected at 24 hours and 48 hours, respectively. Each dot represents an individual mouse, and the bar drawn in each group represents the mean value. **P < .01 compared with the level of the vehicle group.
Cytokine profile of LNCs after Th1 and Th2 exposures. M tuberculosis–sensitized C57BL/6 (A) and Ascaris-sensitized BALB/c (B) mice were treated with vehicle, prednisolone, or STA-5326. Results are representative of 4 Th1 and 6 Th2 in vivo studies. In the studies, to calculate deviation, LNCs from sensitized mice were individually harvested and tested in triplicate from each mouse for cytokine production in response to immobilized anti-CD3ϵ antibody and soluble anti-CD28 antibody. Lymph nodes from naive mice in the Th1 study were pooled and tested for cytokine production in triplicate. IFN-γ (A) in the Th1 study and IL-4 (B) in the Th2 study were analyzed with supernatants collected at 24 hours and 48 hours, respectively. Each dot represents an individual mouse, and the bar drawn in each group represents the mean value. **P < .01 compared with the level of the vehicle group.
In vivo suppressive activity of STA-5326 in an inflammatory bowel disease animal model
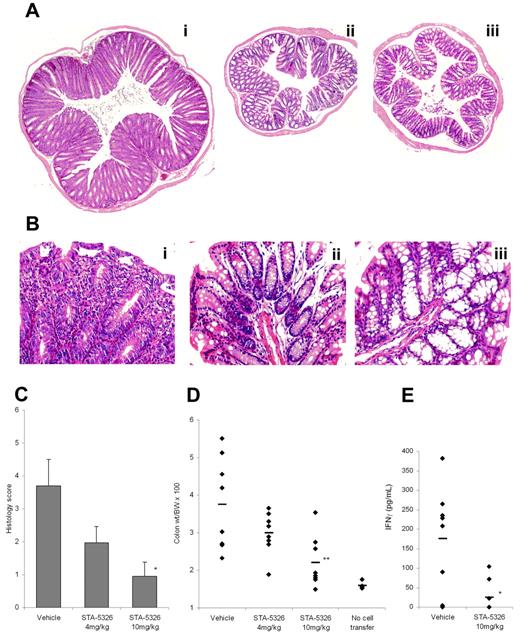
Recent human and animal studies suggest that the local immune response in Crohn disease is predominantly Th1, and IL-12 plays a critical role in the initiation and progression of the disease.40-42 To examine the potential of STA-5326 in the treatment of CD, we tested oral administration of this compound in the CD4+CD45Rbhigh T-cell transfer model. Histologic analysis showed inflammatory histopathologic changes in colons from vehicle-treated animals, such as overt epithelial hyperplasia, marked increase in crypt length, prominent infiltration of chronic inflammatory cells in mucosa and LP, including crypt microabscesses, marked reactive atypia, and various extents of depletion of goblet cells. These changes were significantly reduced in animals treated with oral administration of STA-5326, and the colons maintained a normal structure (Figure 6A-B). The histologic score clearly differentiated animals receiving STA-5326 from vehicle-treated animals, and the suppressive effect was dose dependent, with a substantial reduction at a dose of 4 mg/kg and stronger suppression at 10 mg/kg (Figure 6C). The calculated colon–to–body-weight ratio was consistent with the histologic score, showing dose-dependent attenuation by treatment with STA-5326 (Figure 6D). STA-5326 was effective not only when administered throughout the entire experiment, but also when administration was initiated on day 30 when disease was clearly measurable but not maximal (D.Z., J.C., unpublished data, January 2002). Furthermore, ex vivo analysis of cytokines from LPMCs harvested from mice in this study demonstrated that LP cells from the vehicle control produced an augmented level of IFN-γ and an undetectable level of IL-4. The production of IFN-γ was greatly diminished in animals with STA-5326, indicating that oral administration of STA-5326 down-regulated the in vivo Th1 response in this inflammatory bowel disease animal model (Figure 6E).
In vivo effects of STA-5326 in inflammatory bowel disease animal model. (A) Photomicrographs of representative H&E-stained colons in CD4+ CD45RBhigh T-cell–transferred SCID mice, acquired by Eclipse E800 microscope (Nikon, Tokyo, Japan). (Ai) vehicle control. (Aii) Mouse treated with STA-5326 at 10 mg/kg given orally. (Aiii) Normal control without cell transfer. The original magnification is equivalent to ×40 (4×/0.13 NA objective, 10× eyepiece) in all 3 photographs, this showing the greater colon size in the vehicle control colon relative to the STA-5326–treated colon and the control group without T-cell transfer. (B) Higher magnification (original, ×200; 20×/0.50 NA objective, 10× eyepiece) photomicrographs. (Bi) Vehicle control. (Bii) STA-5326. (Biii) Normal control. (C) Histologic scoring of the distal colon sections. The extent of colonic inflammation was graded on a scale of 0 to 3 in each of 4 criteria: crypt elongation, cell infiltration, depletion of goblet cells, and the number of crypt abscesses. Results shown are mean ± SEM histology scores for each group. The severity of STA-5326–treated mice was significantly reduced compared with the level of vehicle group (*P < .05). (D) Percentage of colon weight relative to body weight in CD4+ CD45RBhigh T-cell transfer SCID mice and normal control mice without cell transfer. Each dot represents an individual mouse, and the bar drawn in each group represents the mean value. **P < .01 compared with the level of the vehicle group. (E) IFN-γ produced from LPMCs after stimulation by anti-CD3/anti-CD28 antibodies. LP lymphocytes were isolated from freshly obtained colonic specimens, and stimulated with immobilized anti-CD3ϵ antibody and soluble anti-CD28 antibody. Culture supernatants were assayed for IFN-γ production. Each dot represents an individual mouse, and the bar drawn in each group represents mean value. *P < .05 compared with the level of the vehicle group.
In vivo effects of STA-5326 in inflammatory bowel disease animal model. (A) Photomicrographs of representative H&E-stained colons in CD4+ CD45RBhigh T-cell–transferred SCID mice, acquired by Eclipse E800 microscope (Nikon, Tokyo, Japan). (Ai) vehicle control. (Aii) Mouse treated with STA-5326 at 10 mg/kg given orally. (Aiii) Normal control without cell transfer. The original magnification is equivalent to ×40 (4×/0.13 NA objective, 10× eyepiece) in all 3 photographs, this showing the greater colon size in the vehicle control colon relative to the STA-5326–treated colon and the control group without T-cell transfer. (B) Higher magnification (original, ×200; 20×/0.50 NA objective, 10× eyepiece) photomicrographs. (Bi) Vehicle control. (Bii) STA-5326. (Biii) Normal control. (C) Histologic scoring of the distal colon sections. The extent of colonic inflammation was graded on a scale of 0 to 3 in each of 4 criteria: crypt elongation, cell infiltration, depletion of goblet cells, and the number of crypt abscesses. Results shown are mean ± SEM histology scores for each group. The severity of STA-5326–treated mice was significantly reduced compared with the level of vehicle group (*P < .05). (D) Percentage of colon weight relative to body weight in CD4+ CD45RBhigh T-cell transfer SCID mice and normal control mice without cell transfer. Each dot represents an individual mouse, and the bar drawn in each group represents the mean value. **P < .01 compared with the level of the vehicle group. (E) IFN-γ produced from LPMCs after stimulation by anti-CD3/anti-CD28 antibodies. LP lymphocytes were isolated from freshly obtained colonic specimens, and stimulated with immobilized anti-CD3ϵ antibody and soluble anti-CD28 antibody. Culture supernatants were assayed for IFN-γ production. Each dot represents an individual mouse, and the bar drawn in each group represents mean value. *P < .05 compared with the level of the vehicle group.
Discussion
We describe a potent and selective Th1 immunomodulator, STA-5326. The compound was developed from a novel triazine derivative identified through IL-12 inhibitor screening. Roughly 2000 derivatives have been generated, and the structural requirements for demonstration of the activity appear so rigorous that structurally related derivatives with small alterations from STA-5326 lose activity. Evaluation of a number of compounds in rat adjuvant arthritis models provided an excellent correlation between the in vitro IL-12 inhibitory activity and the in vivo efficacy (Y. Wu, unpublished data, 2001-2005).
STA-5326 suppressed IFN-γ/SAC–induced p35 and p40 promoter-driven luciferase expression in the murine macrophage cell line RAW264.7, indicating that the compound inhibits transcription of both p35 and p40 genes. The compound potently inhibited not only IL-12 but also IL-23, a new IL-12 family member that shares the p40 subunit and is believed to play a critical role in multiple sclerosis, psoriasis, and other inflammatory diseases.43,44 It is noted that although STA-5326 inhibits IL-23 and total p40 protein, the inhibitory activity was reproducibly less pronounced than the effect on IL-12 p70, while in the same experiment dexamethasone inhibited all proteins IL-12 p70, IL-23, and total p40 with equal potency. The observation was consistent with early Northern blot analysis using a precursor to STA-5326 in cultures of IFN-γ/SAC–stimulated human PBMCs, in which the expression of p35 mRNA was completely inhibited, whereas the expression of p40 mRNA was reduced significantly but incompletely at concentrations up to 1 μM (Kazuo Omi, unpublished data, 1999). STA-5326 also had less potency in inhibition of IL-23 and total p40 than IL-12 p70 in THP-1 cells (Y. Wada, unpublished data, 2004). These results suggest that to some extent STA-5326's suppressive activity favors p35, the IL-12 specific subunit.
STA-5326 inhibited IL-12 and IL-23 most effectively and consistently among the cytokines tested. In addition to the primary effect, substantial inhibition was also seen in IFN-γ, IL-6, and IL-10 production in cultures of IFN-γ/SAC–stimulated human PBMCs. Analysis of the mRNA levels showed that the decrease in IFN-γ, IL-6, and IL-10 mRNA in the presence of STA-5326 was observed 3 to 6 hours after the addition of SAC, which parallels the decrease in IL-12 p35 and p40, indicating the direct influence of STA-5326 on the production of these cytokines also. STA-5326 barely inhibited these cytokines under other stimulus conditions and in other cell types, which makes a clear distinction from dexamethasone, which strongly inhibits a wide variety of cytokines in all settings. In particular, dexamethasone inhibits IL-4 production while STA-5326 does not, which explains the differences in the activity of STA-5326 and prednisolone in response to Th2 induction in vivo. The inhibitory spectrum of STA-5326 will be further assessed under different stimuli in a variety of pure cell types.
The production of the IL-12 family is tightly controlled by a variety of modulators, and optimal induction of IL-12 requires a combination of TLR ligand stimulation plus IFN-γ. Although there are many small molecules that inhibit IL-12 production, including steroids,45,46 1,25-dihydroxyvitamin D3,47,48 acetyl salicylic acid (ASA),49 retinoids,50 thalidomide,51 and other agents which increase intracellular cAMP such as prostaglandin E2,52 β2 agonists,53 or phosphodiesterase inhibitors,54-56 most of these small-molecule IL-12 inhibitors fail to display their inhibitory activity when IL-12 production is strongly enhanced by IFN-γ. For example, pentoxifylline and thalidomide show no inhibitory activity against IFN-γ/SAC–induced IL-12 despite their significant inhibition of IL-12 induced by SAC alone.51,56 It is thus of note that STA-5326 markedly inhibits IFN-γ–augmented IL-12 production, and its activity is consistently higher than these known small-molecule IL-12 inhibitors, including dexamethasone, especially in cultures of IFN-γ/SAC–stimulated human PBMCs. STA-5326 has the potential to block the powerful feedback loop of IFN-γ production by IL-12 that leads to uncontrolled production of proinflammatory cytokines.31
Another feature that distinguishes STA-5326 and many other IL-12 inhibitors is the requirement of de novo protein synthesis. Corticosteroids, cyclic AMP, IFN-β, and IL-10 lose inhibitory activity in the presence of the protein synthesis inhibitor CHX, indicating that de novo protein synthesis is required for their inhibition of IL-12. In contrast, STA-5326 exerts inhibitory activity by a mechanism that does not require de novo protein synthesis. It is noteworthy that STA-5326 appeared to enhance the inhibitory activity in the presence of CHX, unlike the weak IL-12 inhibitor salbutamol, which remained effective in inhibiting IL-12 in the presence of CHX, but lost activity relative to its inhibitory activity in the absence of CHX.53 This may suggest that the target of STA-5326 plays a fundamental role in the induction of IL-12 that becomes more predominant in the absence of an inducible endogenous modulator(s).
The Th1 inhibitory effect of STA-5326 was clearly demonstrated in vivo following oral administration of STA-5326 in a mouse Th1 model induced by M tuberculosis inoculation with Freund adjuvant in C57BL/6 mice. STA-5326 blocked the increase in lymph-node cell numbers in response to the in vivo Th1 inducer and blocked anti-CD3/CD28 antibody–stimulated IFN-γ production. In contrast, no significant effect on the increase in lymph-node cell numbers, and an increase rather than a decrease in IL-4 production, was observed following Th2 induction. The results demonstrate that the treatment with STA-5326 results in selective suppression of the Th1 response, accompanying possible enhancement of the Th2 response.
In this report, we also demonstrate the in vivo efficacy of orally administered STA-5326 using the CD4+CD45Rbhigh T-cell transfer SCID inflammatory bowel disease animal model, in which IL-12 has been proven to play a pivotal role in the disease pathogenesis.21,57,58 While CTLA4-Ig,59 anti–IFN-γ monoclonal antibody,60 and rIL-1061 have been shown by others to prevent disease progression in this model, no significant efficacy was consistently observed with small-molecule drugs, including cyclosporine A59 and prednisolone (J.C., Y. Wada, unpublished data, 2001). STA-5326 markedly reduced the inflammatory changes compared with the vehicle control. The significant suppressive effect was observed even when treatment was initiated on day 30 after the transfer of CD4+CD45Rbhigh T cells, at which time disease was already established but not yet maximal. The striking decrease in IFN-γ production by treatment with STA-5326 provides evidence that STA-5326 significantly diminished the Th1 population, leading to the attenuation of disease progression. The efficacy of STA-5326 also has been observed in other Th1-mediated inflammatory diseases, such as collagen-induced arthritis, adjuvant arthritis, and 2-4-dinitrobenzene sulfonic acid (DNBS)-induced inflammatory bowel disease animal models (Y. Wu, unpublished data, 2001-2003).
STA-5326 is the first potent and selective IL-12/IL-23 inhibitor discovered through screening of a compound library. The unique action of the compound together with the in vivo efficacy in Th1-mediated animal disease models suggest that STA-5326 holds therapeutic promise for the treatment of chronic inflammatory diseases. Injection of monoclonal antibodies recognizing the p40 subunit shared by IL-12 and IL-23 has shown positive results in clinical trials in both patients with psoriasis and patients with Crohn disease. While the antibody acts by neutralizing the IL-12 and IL-23 proteins that already have been produced, STA-5326 acts by selectively shutting off transcription of the p35 and p40 genes. The investigation of the precise mechanism of transcriptional inhibition by STA-5326 is ongoing. STA-5326 now is in clinical evaluation in multiple studies. The compound was shown to be safe in healthy volunteers, and the inhibition of IL-12 production was demonstrated in an ex vivo assay (J.C., unpublished data, 2004). We have completed a phase 2 open-label clinical trial testing STA-5326 in patients with Crohn disease that shows preliminary signs of clinical response by this oral compound.62 STA-5326 is currently being tested in a blinded and placebo-controlled phase 2b Crohn disease study, and is being evaluated in a phase 2a study in rheumatoid arthritis and common variable immunodeficiency disease (CVID), a disease characterized by elevated IL-12 levels.
Authorship
Author contributions: R.L., D.Z., J.C., L.L., Y. Wu, J.Q., and V.B. participated in performing research; T.P., S.Z., and M.O. contributed vital material; D.Z. analyzed data; Y. Wu contributed vital analytical tools; Y. Wada, J.B., and M.O. designed the research; Y. Wada wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest statement: M.O. has a financial interest in, and Y. Wada, R.L., D.Z., J.C., T.P., S.Z., L.L.,Y.Wu, J.Q., V.B., and J.B. are employed by Synta Pharmaceuticals, whose potential product was studied in the present work.
Correspondence: Yumiko Wada, Synta Pharmaceuticals, 45 Hartwell Ave, Lexington, MA 02421; e-mail: ywada@syntapharma.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Beatrice A. Brunkhorst and Stephen D. Gillies for their contribution of the finding of the lead molecule, Drs Kazuo Omi and Hikaru Sonoda for providing fruitful discussion and technical support, and Dr Lan Bo Chen for his guidance.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal