Abstract
FTY720 is a potent immunomodulator drug that inhibits the egress of lymphocytes from secondary lymphoid tissues and thymus. FTY720 is phosphorylated in vivo by sphingosine kinase 2 to FTY720-phosphate, which acts as a potent sphingosine-1-phosphate (S1P) receptor agonist. However, in contrast to S1P, FTY720 has no effect on mast-cell degranulation, yet significantly reduces antigen-induced secretion of PGD2 and cysteinyl-leukotriene. Unexpectedly, this effect of FTY720 was independent of its phosphorylation and S1P receptor functions. The rate-limiting step in the biosynthesis of all eicosanoids is the phospholipase A2 (PLA2)–mediated release of arachidonic acid from glycerol phospholipids. Although FTY720 also reduced arachidonic acid release in response to antigen, it had no effect on translocation of cPLA2 or ERK1/2 activation, suggesting that it does not interfere with FcϵRI-mediated events leading to cPLA2 activation. Remarkably, however, FTY720 drastically inhibited recombinant cPLA2α activity, whereas FTY720-phosphate, sphingosine, or S1P had no effect. This study has uncovered a unique action of FTY720 as an inhibitor of cPLA2α and hence on production of all eicosanoids. Our results have important implications for the potential therapeutic mechanism of action of FTY720 in eicosanoid-driven inflammatory disorders such as asthma and multiple sclerosis.
Introduction
FTY720 is a novel immunosuppressive agent that was derived from myriocin, a sphingosine-like fungal metabolite.1 FTY720 potently inhibits a variety of experimental autoimmune disorders, such as type I diabetes2 and arthritis,3 but clinically, its greatest potential for humans appears to be in the prevention of renal graft rejection and treatment of multiple sclerosis (MS) where it is currently in phase 3 clinical trials.4,5 The immunosuppressive properties of FTY720 stem from its ability to prevent T cells' egress from secondary lymphoid organs and back into circulation, sequestering them away from the transplanted graft,6-8 or preventing their entry into the central nervous system.9 FTY720 is phosphorylated by sphingosine kinase 2 (SphK2),10-12 and a large body of evidence suggests that the phosphorylated drug (FTY720-P), an analog of sphingosine-1-phosphate (S1P), is the biologically active form. FTY720-P can bind to all of the known S1P receptors, except S1P2,6,13,14 and has been shown to regulate S1P1 actions that are crucial for lymphocyte migration and trafficking.15-18 Nevertheless, the exact mechanism of action of this prodrug is still a matter of debate.19-21 S1P is also an important chemoattractant in the immune system, modulating T-cell responses to chemokines (reviewed in Cyster13 and Rosen and Goetzl18 ).
FTY720 inhibits airway inflammation, induction of bronchial hyperresponsiveness, and lymphocyte and eosinophil infiltration in an actively Ag-sensitized murine asthma model,22 suggesting that it might have therapeutic benefits in asthma. Given that S1P and its receptors may play a crucial role in asthma,23 and are required for mast-cell degranulation and arrival at sites of inflammation,24,25 it was of interest to examine the effects of FTY720 on mast-cell functions.
Mast cells are bone marrow–derived, tissue-resident effector cells of immune responses that play a central role in the triggering mechanisms of allergic reactions, such as asthma.26 They orchestrate the recruitment of inflammatory cells and perpetuate inflammation through their ability to release a wide array of inflammatory mediators, including histamine and other preformed mediators, de novo–synthesized arachidonic acid (AA) metabolites (leukotrienes and prostaglandins), and numerous proinflammatory cytokines and chemokines. Several studies have shown that S1P regulates mast-cell functions.24,27-30 Although the biologic effects of FTY720 are generally attributed to its actions as an S1P mimetic after its phosphorylation, we found that in contrast to S1P, FTY720 has no effect on mast-cell degranulation. Unexpectedly, FTY720 drastically inhibited secretion of the AA-derived eicosanoids, prostaglandin D2 (PGD2) and cysteinyl-leukotrienes (CysLTs), independently of its phosphorylation and S1P receptor functions. Remarkably, we have uncovered a unique action of FTY720 as an inhibitor of group IVA cytosolic PLA2 (cPLA2α), the rate-limiting step in eicosanoid formation. Our study has important implications for the potential therapeutic mechanism of action of this potent immunosuppressive drug.
Materials and methods
Reagents
S1P was obtained from Biomol (Plymouth Meeting, PA). SEW2871 was from Avanti Polar Lipids (Alabaster, AL). FTY720 and FTY720-P were kindly provided by Dr Volker Brinkmann (Novartis, Basel, Switzerland). Anti–DNP IgE was a generous gift from Dr Juan Rivera (NIH). Monoclonal anti–phospho-ERK1/2 was obtained from Cell Signaling (Beverly, MA), and polyclonal goat anti-cPLA2 was from Santa Cruz Biotechnologies (Santa Cruz, CA). Goat antimouse-HRP and donkey anti–goat HRP secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). 1-palmitoyl-2-[14C]-arachidonyl-sn-glycerol-phosphocholine and [3H]AA were from Perkin Elmer (Wellesley, MA). 1-palmitoyl-2-[14C]palmitoyl-sn-glycero-3-phosphocholine and [3H]-oleic acid were purchased from New England Nuclear (Boston, MA).
Cell culture
RBL-2H3 mast cells (CRL-2256; American Type Culture Collection, Manassas, VA) were grown as monolayer cultures in Eagle minimal essential medium (EMEM; Biosource, Camarillo, CA) supplemented with 15% heat-inactivated fetal bovine serum. LAD2 human mast cells (obtained from A. S. Kirshenbaum and D. D. Metcalfe, NIH, Bethesda, MD) were cultured in StemPro-34 medium (Invitrogen, Carlsbad, CA) supplemented with 100 ng/mL recombinant human stem-cell factor (SCF; Peprotech, Rocky Hill, NJ) as described.31
Measurement of PGD2, CysLT, and thromboxane secretion
RBL-2H3 or LAD2 mast cells were sensitized with 1 μg/mL mouse anti–DNP IgE overnight and washed once to remove unbound IgE; fresh medium was then added without and with lipids (S1P, FTY720, or FTY720-P) for various times as indicated in figure legends, and then cells were stimulated with DNP-HSA (Ag, 10 ng/mL) for 15 minutes. Supernatants were collected and PGD2, thromboxane, and CysLT were measured with enzyme immunosorbent assay (EIA) kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Degranulation assay
Degranulation of mast cells was measured by assaying β-hexosaminidase release as described.24 Net degranulation is expressed as the percentage of total cellular β-hexosaminidase released to the medium after Ag stimulation minus that released spontaneously. Spontaneous release was always 5% or less.
Arachidonic acid release
Mast cells were labeled overnight with 1 μCi (0.037 MBq)/mL [3H]AA during sensitization with IgE, washed, treated without or with lipids, and stimulated with Ag as described in “Measurement of PGD2, CysLT, and thromboxane secretion.” Culture medium was centrifuged to remove any cells, and [3H]AA released into the medium was determined by scintillation counting. Total cellular [3H]AA was determined by lysing nonstimulated cells with 0.2% Triton X-100. AA release is expressed as percentage of total [3H]AA.
Western blotting
Mast cells were lysed in buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1% Triton X-100, and 1:500 protease inhibitor cocktail (Sigma, St Louis, MO). Protein was measured by the Bradford method (Bio-Rad, Hercules, CA) and equal amounts were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transblotted to nitrocellulose, blocked with 5% BSA for 1 hour, and incubated overnight with primary antibodies (1:1000, anti–phospho-ERK1/2) followed by 1:10 000 diluted HRP-conjugated secondary antibody in 5% BSA for 2 hours. Immunoreactive signals were detected by enhanced chemiluminescence with SuperSignal West Pico Chemiluminescent substrate (Pierce Chemical, Rockford, IL).
cPLA2 translocation
Activated mast cells were suspended at 4°C in buffer containing 20 mM Hepes, pH 7.4, 1 mM EGTA, 0.34 M sucrose, and 1:500 diluted protease inhibitor cocktail (Sigma), and probe sonicated. Lysates were centrifuged at 100 000g for 30 minutes and equal amounts of proteins from membrane (25 μg) and cytosol (5 μg) fractions were separated on 8% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-cPLA2 antibody.
Measurement of cPLA2 activity
HEK 293 cells were transfected with pcDNA3.1/Zeo containing group IVA cPLA2 (obtained from Dr Michael Gelb, University of Washington) using Fugene (Roche, Indianapolis, IN) according to the manufacturer's instructions. cPLA2 activity in lysates from HEK 293 cells transiently expressing cPLA2 and mast-cell lysates was measured using a sonicated liposome assay.32 Briefly, cells were lysed in buffer containing 10 mM Hepes, pH 7.4, 0.34 M sucrose, and 1:500 diluted protease inhibitor cocktail. Aliquots were incubated at 37°C with liposomes containing a 2:1 molar ratio of 1-palmitoyl-2-[14C]-arachidonyl-sn-glycerol-phosphatidylcholine and dioleoylglycerol in buffer containing 1 mM CaC12, 2 mM 2-mercaptoethanol, 150 mM NaC1, 50 mM Hepes, pH 7.4, and 1 mg/mL BSA. FTY720 or other sphingoid analogs were added separately. Released [14C]-AA was extracted as described33 and quantified by scintillation counting.
Expression and activity of recombinant cPLA2α
Human cPLA2 was expressed in Sf9 cells with a His6 tag using a baculovirus expression system and purified as previously described.34 Briefly, Sf9 cells were infected with high-titer recombinant baculovirus at a multiplicity of infection of 10. After 72 hours, cells were lysed and cleared lysate was bound to nickel nitrilotriacetic acid–agarose. The agarose beads were washed and eluted with a step gradient of imidazole exactly as described.34 Recombinant cPLA2α activity was measured in a phosphatidylcholine-mixed micellar assay in a buffer composed of 80 mM HEPES, pH 7.5, 150 mM NaCl, 10 μM Ca2+, and 1 mM dithiothreitol with 0.3 mM [14C]-labeled palmitoyl-2-arachidonoylphosphatidylcholine, 2 mM Triton X-100, and 26% glycerol, as described.34
Measurement of sPLA2 and iPLA2 activities
HEK 293 cells transiently transfected with the cDNA-encoding human group V sPLA2 (kind gift from Dr Michael Gelb, University of Washington) or with group VIA calcium-independent iPLA2. sPLA2 activity was measured in culture supernatants using [3H]-oleic acid–labeled E coli membranes as a substrate as described previously.35 The percent hydrolysis was determined from the percent of total input radioactivity released into the supernatants. iPLA2 activity was determined with L-α-dipalmitoyl[2-palmitoyl-1-14C]phosphatidylcholine dipalmitoylphosphatidylcholine in 400 mM Triton X-100 as described.36 All phospholipase assays were performed under conditions where substrate hydrolysis was linear with respect to protein and time.
Statistical analysis
Experiments were repeated at least 3 times and statistics were performed using GraphPad InStat 3.06 (San Diego, CA). Differences between groups were determined with the Student t test, or a one-way analysis of variance (ANOVA) with a Tukey posthoc; P values less than .05 were considered significant.
Results
FTY720 has no effect on degranulation but inhibits PGD2 secretion by mast cells
Similar to its effect on murine CPII mast cells28 and bone marrow–derived mast cells,24 S1P induced degranulation of LAD2 human mast cells, as determined by β-hexosaminidase release, albeit to a lesser extent than antigen (Ag) triggering (Figure 1A). Although the immunosuppressive effects of the prodrug FTY720 are mediated by its phosphorylated form, neither FTY720 nor FTY720-P had significant degranulating activity (Figure 1A-B). Even pretreatment with FTY720 prior to Ag stimulation did not affect degranulation induced by cross-linking of the high-affinity receptor for IgE (FcϵRI) (Figure 1B).
S1P but not FTY720 induces degranulation of human mast cells. (A) LAD2 mast cells were treated with vehicle, S1P (100 nM), phospho-FTY720 (100 nM), or ionomycin (1 μM) for 2 hours or sensitized overnight without anti–DNP IgE (1 μg/mL), washed, and then treated with Ag (1, 10, 100 ng/mL) for 2 hours. (B) LAD2 cells were sensitized overnight with anti–DNP IgE, washed, and treated with FTY720 (1 μM) for 2 hours before stimulation with Ag (10 ng/mL) or FTY720 (1 μM) for 15 minutes. Degranulation was determined by hexosaminidase release. Data are means ± SD of quadruplicate determinations. Similar results were obtained in 5 independent experiments.
S1P but not FTY720 induces degranulation of human mast cells. (A) LAD2 mast cells were treated with vehicle, S1P (100 nM), phospho-FTY720 (100 nM), or ionomycin (1 μM) for 2 hours or sensitized overnight without anti–DNP IgE (1 μg/mL), washed, and then treated with Ag (1, 10, 100 ng/mL) for 2 hours. (B) LAD2 cells were sensitized overnight with anti–DNP IgE, washed, and treated with FTY720 (1 μM) for 2 hours before stimulation with Ag (10 ng/mL) or FTY720 (1 μM) for 15 minutes. Degranulation was determined by hexosaminidase release. Data are means ± SD of quadruplicate determinations. Similar results were obtained in 5 independent experiments.
PGD2 is the major eicosanoid produced in activated mast cells by the cyclooxygenase (COX) pathway. Although recent studies suggest that S1P regulates prostaglandin synthesis by up-regulating COX-2,37-39 treatment of mast cells for 2 hours with S1P, FTY720, or FTY720-P did not induce PGD2 secretion (Figure 2A). In sharp contrast, cross-linking of FcϵR1 with Ag or treatment with ceramide-1-phosphate (C1P), a newly discovered allosteric activator of cPLA2α,34 markedly stimulated PGD2 secretion (Figure 2A). However, pretreatment of RBL-2H3 mast cells with FTY720 for 2 hours decreased Ag-induced PGD2 secretion in a dose-dependent manner (Figure 2B-C), and significant inhibition was observed at a concentration of FTY720 as low as 1 nM (Figure 2C). FTY720 at a concentration as low as 10 nM also inhibited PGD2 secretion from LAD2 human mast cells stimulated by Ag (Figure 2D). A significant inhibitory effect was found within 15 minutes of treatment with FTY720, and inhibition gradually increased thereafter. FTY720 at high concentrations (> 5 μM) can induce apoptosis of some cancer cell lines.40 However, growth and viability of either RBL-2H3 or LAD2 mast cells were not compromised by the presence of FTY720 (Figure 2B,D inserts).
FTY720 attenuates Ag-induced secretion of PGD2. (A) RBL-2H3 cells (5 × 104) were treated with vehicle, S1P (1 μM), FTY720 (1 μM), C1P (2.5 μM), or FTY720-P (1 μM) for 2 hours or sensitized overnight without or with anti–DNP IgE (1 μg/mL), washed, and then treated with Ag (10 ng/mL) for 15 minutes. PGD2 release was measured by enzyme-linked immunosorbent assay (ELISA) and data are means ± SD of quadruplicate determinations. (B) RBL-2H3 cells were sensitized overnight with anti–DNP IgE (1 μg/mL), treated for 2 hours with FTY720 (0.1 or 1 μM, ▪), and then stimulated with Ag (10 ng/mL) for 15 minutes, and PGD2 release was determined by ELISA. Data are expressed as means ± SEM (n = 4). (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪) and cell viability was measured with the WST-1 assay. Data are the means of A450 ± SD of triplicate determinations. (C) Sensitized RBL-2H3 cells were treated with the indicated concentrations of FTY720 for 2 hours, and then stimulated with Ag for 15 minutes, and PGD2 release was determined. Data are expressed as percent inhibition. (D) LAD2 human mast cells were sensitized overnight with anti–DNP IgE, pretreated with the indicated concentrations of FTY720 for 2 hours, and then stimulated without or with Ag (10 ng/mL) for 15 minutes, and secreted PGD2 was determined. (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪), and cell viability was measured. (B-D) *P < .05; **P < .01. (E) Sensitized RBL-2H3 cells were treated with vehicle, S1P (1 μM), or Ag (10 ng/mL) for the indicated times, and equal amounts of lysate proteins were immunoblotted with anti–COX-2 antibody. Blots were stripped and reprobed with antitubulin as a loading control. (F) Sensitized LAD2 cells were treated with vehicle, 1 μM each of S1P, FTY720, or FTY720-P, or Ag (10 ng/mL) for the indicated times, and proteins were immunoblotted with anti–COX-2 antibody or antitubulin. Similar results were obtained in 3 independent experiments.
FTY720 attenuates Ag-induced secretion of PGD2. (A) RBL-2H3 cells (5 × 104) were treated with vehicle, S1P (1 μM), FTY720 (1 μM), C1P (2.5 μM), or FTY720-P (1 μM) for 2 hours or sensitized overnight without or with anti–DNP IgE (1 μg/mL), washed, and then treated with Ag (10 ng/mL) for 15 minutes. PGD2 release was measured by enzyme-linked immunosorbent assay (ELISA) and data are means ± SD of quadruplicate determinations. (B) RBL-2H3 cells were sensitized overnight with anti–DNP IgE (1 μg/mL), treated for 2 hours with FTY720 (0.1 or 1 μM, ▪), and then stimulated with Ag (10 ng/mL) for 15 minutes, and PGD2 release was determined by ELISA. Data are expressed as means ± SEM (n = 4). (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪) and cell viability was measured with the WST-1 assay. Data are the means of A450 ± SD of triplicate determinations. (C) Sensitized RBL-2H3 cells were treated with the indicated concentrations of FTY720 for 2 hours, and then stimulated with Ag for 15 minutes, and PGD2 release was determined. Data are expressed as percent inhibition. (D) LAD2 human mast cells were sensitized overnight with anti–DNP IgE, pretreated with the indicated concentrations of FTY720 for 2 hours, and then stimulated without or with Ag (10 ng/mL) for 15 minutes, and secreted PGD2 was determined. (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪), and cell viability was measured. (B-D) *P < .05; **P < .01. (E) Sensitized RBL-2H3 cells were treated with vehicle, S1P (1 μM), or Ag (10 ng/mL) for the indicated times, and equal amounts of lysate proteins were immunoblotted with anti–COX-2 antibody. Blots were stripped and reprobed with antitubulin as a loading control. (F) Sensitized LAD2 cells were treated with vehicle, 1 μM each of S1P, FTY720, or FTY720-P, or Ag (10 ng/mL) for the indicated times, and proteins were immunoblotted with anti–COX-2 antibody or antitubulin. Similar results were obtained in 3 independent experiments.
FTY720 inhibits prostaglandin and thromboxane secretion independently of S1P receptors
Because recent studies implicate S1P in regulation of COX-2 expression in A549 human lung cancer cells,41 fibroblasts,37,38 and smooth muscle cells,39 we examined whether S1P can up-regulate COX-2 expression in mast cells. In agreement with previous results,42 Ag triggering increased COX-2 expression in RBL-2H3 cells, yet S1P failed to increase COX-2 even after 5 hours (Figure 2E). Similarly, in LAD2 cells, incubation for 2 or 4 hours with S1P, FTY720, or FTY720-P had little or no effect, although Ag also induced a significant increase in COX-2 expression in these cells (Figure 2F).
If the suppression of eicosanoid secretion by FTY720 is mediated by FTY720-P, as are its effects on lymphocyte sequestration and trafficking,6,13,14 similar, if not greater, inhibition would be expected when cells are treated with FTY720-P. However, as shown in Figure 3A, 1 μM FTY720-P had a much smaller effect on PGD2 secretion than FTY720. Similarly, dihydro-S1P, which lacks the double bond in the sphingoid base and binds to all of the S1P receptors, and the S1P1 selective agonist, SEW2871, had only slight inhibitory effects compared with FTY720. Although sphingosine is structurally similar to FTY720, it decreased PGD2 secretion by only less than 10%, compared with 55% inhibition by FTY720 (Figure 3A). Moreover, at a concentration of 100 nM, which is above the Kd values for S1P binding to S1P receptors, FTY720 inhibited PGD2 secretion by approximately 25%, whereas S1P was ineffective (Figure 3B). In agreement with other studies,43 following FcϵRI cross-linking, mast cells also secreted TXA2, but at much lower levels than PGD2 (Figure 3C). Once again, FTY720, but not S1P, potently blocked Ag-induced thromboxane release (Figure 3C).
Effect of S1P receptor agonists on prostaglandin and thromboxane secretion. (A,C) Sensitized RBL-2H3 cells (5 × 104) were treated without or with 1 μM each of FTY720, FTY720-P, S1P, SEW2871, or sphingosine (A) or 100 nM FTY720 or S1P (B) for 2 hours, and then stimulated with Ag for 15 minutes. PGD2 secretion was measured and results were expressed as percent inhibition of Ag-induced release ± SEM (n = 4). (C) Sensitized RBL-2H3 cells were treated without or with 1 μM FTY720 or S1P for 2 hours, then stimulated with Ag for 15 minutes, and secreted thromboxane was measured by an ELISA for TXB2. Data are means ± SD of quadruplicate determinations. (D) RBL-2H3 cells (2 × 106) were serum-starved for 8 hours and then stimulated with 1 μM S1P, FTY720, or FTY720-P for the indicated times. Equal amounts of lysate proteins were immunoblotted with anti–phospho-ERK1/2. Blots were stripped and reprobed with antitubulin as a loading control. (E) Sensitized RBL-2H3 cells were treated with S1P or FTY720 (1 μM) for the indicated times prior to stimulation with Ag. PGD2 secretion was measured, and results were expressed as percent inhibition of Ag-induced release ± SEM.
Effect of S1P receptor agonists on prostaglandin and thromboxane secretion. (A,C) Sensitized RBL-2H3 cells (5 × 104) were treated without or with 1 μM each of FTY720, FTY720-P, S1P, SEW2871, or sphingosine (A) or 100 nM FTY720 or S1P (B) for 2 hours, and then stimulated with Ag for 15 minutes. PGD2 secretion was measured and results were expressed as percent inhibition of Ag-induced release ± SEM (n = 4). (C) Sensitized RBL-2H3 cells were treated without or with 1 μM FTY720 or S1P for 2 hours, then stimulated with Ag for 15 minutes, and secreted thromboxane was measured by an ELISA for TXB2. Data are means ± SD of quadruplicate determinations. (D) RBL-2H3 cells (2 × 106) were serum-starved for 8 hours and then stimulated with 1 μM S1P, FTY720, or FTY720-P for the indicated times. Equal amounts of lysate proteins were immunoblotted with anti–phospho-ERK1/2. Blots were stripped and reprobed with antitubulin as a loading control. (E) Sensitized RBL-2H3 cells were treated with S1P or FTY720 (1 μM) for the indicated times prior to stimulation with Ag. PGD2 secretion was measured, and results were expressed as percent inhibition of Ag-induced release ± SEM.
Because these results imply that FTY720 may act independently of S1P receptors, it was important to establish that these mast cells can respond to FTY720-P and S1P. In accordance with previous results,44 S1P and FTY720-P rapidly and transiently activated ERK1/2, as determined with phospho-specific antibodies (Figure 3D), suggesting that the lack of effect of S1P and FTY720-P was not due to impaired receptor signaling. In comparison, FTY720-induced ERK1/2 activation was delayed, detectable only after 15 minutes (Figure 3D). The slower kinetics are likely due to its phosphorylation by SphK2 at the plasma membrane to FTY720-P, which then is responsible for the S1P receptor signaling.
Because external S1P rapidly activates its cell-surface receptors (Figure 3D), it was of interest to examine whether such rapid activation of S1P receptors might be involved in inhibition of Ag-induced PGD2 secretion. However, no effects on PGD2 secretion were observed when S1P or FTY720-P was added simultaneously with Ag (data not shown) or when mast cells were treated for 5 or 15 minutes with S1P prior to stimulation with Ag (Figure 3E). Moreover, in agreement with the lack of effect on COX-2 expression (Figure 2E), even prolonged incubation for 2 or 4 hours had no significant effect on PGD2 secretion nor did it significantly affect Ag-induced PGD2 secretion from mast cells (Figure 3E).
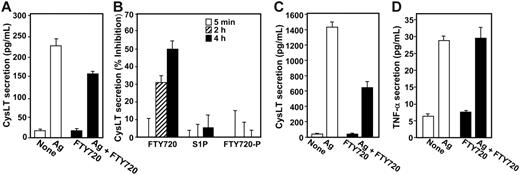
FTY720 inhibits secretion of cysteinyl leukotrienes but has no effect on secretion of TNF-α
To examine whether the inhibitory effect of FTY720 was specific for prostanoids or also affected other eicosanoids, we measured secretion of cysteinyl leukotrienes (CysLTs) generated by the metabolism of AA through the 5-lipoxygenase pathway. FTY720 also inhibited Ag-induced CysLT secretion by both RBL-2H3 (Figure 4A). and LAD2 mast cells (Figure 4C). Once again, in contrast to FTY720, neither S1P nor FTY720-P had a significant effect on CysLT secretion, after incubation for as short a period as 5 minutes or as long as 4 hours (Figure 4B). It should be noted that the kinetics of the effects of FTY720 on CysLT and PGD2 secretion may be different since AA produced by cPLA2 is converted by 2 different enzymes (5-lipoxygenase and COX) with different rates to make these products. Moreover, much more PGD2 is produced by RBL-2H3 mast cells than CysLT after antigenic stimulation, and differences at early times might also be attributed to detection sensitivity differences.
FTY720 inhibits CysLT secretion by mast cells. (A-B) RBL-2H3 mast cells were sensitized overnight with anti–DNP IgE (1 μg/mL), treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated with Ag (10 ng/mL) for 15 minutes. Secreted CysLT was measured by ELISA, and data were expressed as means ± SEM (n = 4). (B) Sensitized RBL-2H3 cells were pretreated without or with 1 μM S1P, FTY720, or FTY720-P for the indicated times and then stimulated with Ag for 15 minutes. CysLT secretion was measured, and results were expressed as percent inhibition of Ag-induced release ± SD. (C) Sensitized LAD2 mast cells were treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated with Ag (10 ng/mL) for 15 minutes. Secreted CysLT was measured by ELISA. (D) FTY720 has no effect on TNF-α formation. RBL-2H3 mast cells were sensitized overnight with anti–DNP IgE, treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated without or with Ag (10 ng/mL) for an additional 16 hours. TNF-α secretion was determined by ELISA. Data are means ± SD of quadruplicate determinations. Similar results were obtained in 3 independent experiments.
FTY720 inhibits CysLT secretion by mast cells. (A-B) RBL-2H3 mast cells were sensitized overnight with anti–DNP IgE (1 μg/mL), treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated with Ag (10 ng/mL) for 15 minutes. Secreted CysLT was measured by ELISA, and data were expressed as means ± SEM (n = 4). (B) Sensitized RBL-2H3 cells were pretreated without or with 1 μM S1P, FTY720, or FTY720-P for the indicated times and then stimulated with Ag for 15 minutes. CysLT secretion was measured, and results were expressed as percent inhibition of Ag-induced release ± SD. (C) Sensitized LAD2 mast cells were treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated with Ag (10 ng/mL) for 15 minutes. Secreted CysLT was measured by ELISA. (D) FTY720 has no effect on TNF-α formation. RBL-2H3 mast cells were sensitized overnight with anti–DNP IgE, treated without or with FTY720 (1 μM, ▪) for 2 hours, and then stimulated without or with Ag (10 ng/mL) for an additional 16 hours. TNF-α secretion was determined by ELISA. Data are means ± SD of quadruplicate determinations. Similar results were obtained in 3 independent experiments.
Of importance, FTY720 had no significant effect on secretion of TNF-α (Figure 4D), which when released, elicits a general inflammatory response mainly through enhanced release of proinflammatory/chemotactic mediators and up-regulation of adhesion molecules leading to recruitment of eosinophils and neutrophils.45 Taken together, these data indicate that FTY720 inhibits eicosanoid production in mast cells independently of S1P receptors.
To investigate whether the effects of FTY720 were specific for activated mast cells, we used RAW264.7 macrophages, which produce another prostaglandin, PGE2, in response to stimulation with lipopolysaccharide (LPS), a critical signal for the innate immune response to Gram-negative bacteria. In these cells, even low concentrations of FTY720 potently inhibited LPS-induced secretion of PGE2 (Figure 5A), suggesting that inhibition of prostaglandin production might be a general property of FTY720. Moreover, FTY720 also suppressed the rather high constitutive production of PGE2 in these cells.
FTY720 inhibits release of prostanoids and arachidonic acid independent of CDLA2 translocation and ERK activation. (A) Effect of FTY720 on LPS-stimulated PGE2 production in RAW264.7 cells. RAW264.7 cells were treated for 2 hours with the indicated concentrations of FTY720, stimulated without (□) or with LPS (1 μg/mL, ▪) for 1 hour, and PGE2 secretion was determined by ELISA. Data are means ± SD of triplicate determinations. (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪), and cell viability was measured. (B) FTY720 inhibits Ag-induced arachidonic acid release from mast cells. RBL-2H3 mast cells (5 × 104) were sensitized with anti–DNP IgE (1 μg/mL) in the presence of 1 μCi (0.037 MBq)/mL [3H]AA for 18 hours, washed, and incubated further in the absence or presence of FTY720 (1 μM) in 0.5% BSA-EMEM. Cells were then stimulated without or with Ag (10 ng/mL) or ionomycin (5 μM) for 15 minutes, and [3H]AA release was measured by scintillation counting. Data are means ± SD of quadruplicate determinations expressed as percent of total AA released. (A) *P < .05; **P < .01. (C) Lack of effect of FTY720 on Ag-induced translocation of cPLA2α. Sensitized RBL-2H3 cells were treated without (vehicle) or with 1 μM FTY720 for 18 hours and then stimulated with Ag for the indicated times. Membranes were isolated from cell lysates by centrifugation at 100 000g, and equal amounts of proteins were analyzed by immunoblotting with anti-cPLA2. Blots were stripped and reprobed with anti–αV-integrin to demonstrate equal loading. (D-E) FTY720 does not inhibit Ag-induced ERK1/2 activation. RBL-2H3 (D) or LAD2 (E) mast cells were sensitized with IgE, treated with FTY720 (1 μM) for 2 hours (D) or 18 hours (E), and then stimulated with Ag (10 ng/mL) for the indicated times. Cells were lysed and phospho-ERK1/2 activation was determined by immunoblotting. Blots were stripped and reprobed with anti–β-tubulin to demonstrate equal loading. Similar results were obtained in 3 independent experiments.
FTY720 inhibits release of prostanoids and arachidonic acid independent of CDLA2 translocation and ERK activation. (A) Effect of FTY720 on LPS-stimulated PGE2 production in RAW264.7 cells. RAW264.7 cells were treated for 2 hours with the indicated concentrations of FTY720, stimulated without (□) or with LPS (1 μg/mL, ▪) for 1 hour, and PGE2 secretion was determined by ELISA. Data are means ± SD of triplicate determinations. (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪), and cell viability was measured. (B) FTY720 inhibits Ag-induced arachidonic acid release from mast cells. RBL-2H3 mast cells (5 × 104) were sensitized with anti–DNP IgE (1 μg/mL) in the presence of 1 μCi (0.037 MBq)/mL [3H]AA for 18 hours, washed, and incubated further in the absence or presence of FTY720 (1 μM) in 0.5% BSA-EMEM. Cells were then stimulated without or with Ag (10 ng/mL) or ionomycin (5 μM) for 15 minutes, and [3H]AA release was measured by scintillation counting. Data are means ± SD of quadruplicate determinations expressed as percent of total AA released. (A) *P < .05; **P < .01. (C) Lack of effect of FTY720 on Ag-induced translocation of cPLA2α. Sensitized RBL-2H3 cells were treated without (vehicle) or with 1 μM FTY720 for 18 hours and then stimulated with Ag for the indicated times. Membranes were isolated from cell lysates by centrifugation at 100 000g, and equal amounts of proteins were analyzed by immunoblotting with anti-cPLA2. Blots were stripped and reprobed with anti–αV-integrin to demonstrate equal loading. (D-E) FTY720 does not inhibit Ag-induced ERK1/2 activation. RBL-2H3 (D) or LAD2 (E) mast cells were sensitized with IgE, treated with FTY720 (1 μM) for 2 hours (D) or 18 hours (E), and then stimulated with Ag (10 ng/mL) for the indicated times. Cells were lysed and phospho-ERK1/2 activation was determined by immunoblotting. Blots were stripped and reprobed with anti–β-tubulin to demonstrate equal loading. Similar results were obtained in 3 independent experiments.
Effects of FTY720 on arachidonic acid release and cPLA2 activation
The observation that FTY720 inhibits secretion of prostaglandins, thromboxanes, and leukotrienes points to a common target. Since the first, and rate-limiting, step in all eicosanoid production is the release of AA from glycerol phospholipids, we next examined the effect of FTY720 on secretion of AA from mast cells. Indeed, preincubation of RBL-2H3 cells with FTY720 significantly inhibited Ag-induced AA release (Figure 5B). FTY720 also inhibited AA release induced by the calcium ionophore ionomycin (Figure 5B). It is well known that one of the major phospholipases that regulates eicosanoid synthesis in response to FcϵRI triggering is group IVA cytosolic phospholipase A2 (cPLA2α). cPLA2α activation appears to be a multifaceted process, and its translocation from the cytosol to perinuclear membranes where its substrate is located is a crucial step in its activation. However, although as expected, Ag induced translocation of cPLA2α to perinuclear membranes within 2 minutes, as revealed by Western blotting of isolated membranes, FTY720 did not inhibit this translocation (Figure 5C). Even after a prolonged preincubation with FTY720 of 18 hours, which totally blocked eicosanoid formation, there were no evident differences in the time course or extent of cPLA2α translocation (Figure 5C). These data also indicate that FTY720 does not reduce cPLA2α expression, suggesting that its effects are most likely mediated at the level of enzymatic activity.
Because phosphorylation of cPLA2α by ERK1/2 enhances its enzymatic activity,46 and FTY720 inhibits ERK1/2 activation in several types of cells,40 it was of interest to determine whether the inhibitory effect of FTY720 on cPLA2 activity was due to inhibition of ERK1/2 activation. However, pretreatment with FTY720 for 2 or 18 hours did not prevent Ag-induced ERK1/2 phosphorylation in either RBL-2H3 or LAD2 mast cells (Figure 5D-E).
Since FTY720 did not inhibit cPLA2 translocation, we determined whether it inhibits cPLA2 activity. In vitro, cPLA2 is activated by calcium; however, addition of salt at physiologic concentrations also activates it, and thus, its catalytic activity becomes independent of calcium.47 As shown in Figure 6A, in the presence of saturating [Ca2+] and 150 mM NaCl, FTY720 significantly inhibited cPLA2 activity in mast-cell lysates. Moreover, pretreatment of RBL-2H3 or HEK 293 cells with FTY720 also significantly reduced cPLA2 activity, suggesting that FTY720 inhibits cellular activity of cPLA2 in vivo (Figure 6B). To further substantiate these results, the effect of FTY720 on cPLA2 activity in lysates from HEK 293 cells overexpressing human cPLA2α was determined. FTY720 also dramatically inhibited ectopically expressed cPLA2α activity in a dose-dependent manner (Figure 6C). At equimolar concentrations of cPLA2 substrate and FTY720, there was a significant inhibition of cPLA2 activity of about 50%, which increased to 65% when the ratio of FTY720 to substrate was 2:1 (Figure 6C). sPLA2 and iPLA2 are other PLA2 isoforms that liberate AA for the eicosanoid synthesis pathways. Group V sPLA2 in particular has been implicated in eicosanoid production by mast cells.48 However, FTY720 did not inhibit the in vitro activity of ectopically expressed sPLA2 (Figure 6D) or iPLA2 (Figure 6E). Taken together, these results suggest that inhibition of cellular eicosanoid production by FTY720 is probably due to inhibition of cPLA2.
FTY720 inhibits cPLA2 activity in vitro. cPLA2 activity was determined in lysates from RBL-2H3 cells (A) or in lysates from HEK 293 cells overexpressing cPLA2 (C) using a liposome assay with 1-palmitoyl-2-[14C]-arachidonyl-sn-glycerol-phosphatidylcholine (400 pmol) as substrate. Increasing concentrations of FTY720 were added separately. Data are expressed as picomole AA released per minute per milligram and are means ± SD of duplicate determinations. (B) RBL-2H3 or HEK 293 cells were pretreated without (□) or with FTY720 (▪) for 18 hours, washed extensively, and lysed. cPLA2 activity was determined in lysates. *P < .05. (D) sPLA2 activity was determined in lysates from HEK 293 cells overexpressing sPLA2 as described.35 Data are expressed as percent hydrolysis of [3H]-oleate–labeled lipids. (E) iPLA2 activity was determined with L-α-dipalmitoyl[2-palmitoyl-1-14C]phosphatidylcholine as substrate in liposomes containing dipalmitoylphosphatidylcholine and Triton X-100 as described.36 Similar results were obtained in 3 independent experiments. Data are expressed as the means ± SD of duplicate determinations (D,E).
FTY720 inhibits cPLA2 activity in vitro. cPLA2 activity was determined in lysates from RBL-2H3 cells (A) or in lysates from HEK 293 cells overexpressing cPLA2 (C) using a liposome assay with 1-palmitoyl-2-[14C]-arachidonyl-sn-glycerol-phosphatidylcholine (400 pmol) as substrate. Increasing concentrations of FTY720 were added separately. Data are expressed as picomole AA released per minute per milligram and are means ± SD of duplicate determinations. (B) RBL-2H3 or HEK 293 cells were pretreated without (□) or with FTY720 (▪) for 18 hours, washed extensively, and lysed. cPLA2 activity was determined in lysates. *P < .05. (D) sPLA2 activity was determined in lysates from HEK 293 cells overexpressing sPLA2 as described.35 Data are expressed as percent hydrolysis of [3H]-oleate–labeled lipids. (E) iPLA2 activity was determined with L-α-dipalmitoyl[2-palmitoyl-1-14C]phosphatidylcholine as substrate in liposomes containing dipalmitoylphosphatidylcholine and Triton X-100 as described.36 Similar results were obtained in 3 independent experiments. Data are expressed as the means ± SD of duplicate determinations (D,E).
Although our results show that FTY720 inhibits cPLA2 activity in whole-cell lysates, they do not show whether FTY720 directly inhibits activity or acts indirectly by inhibiting or interfering with a cofactor in cell lysates that might be required for cPLA2 activity. To further address this question, the effect of FTY720 on purified recombinant cPLA2α was determined in a mixed micelle assay that provides a homogeneous, physically-defined system. The mixed micelle assay also has several advantages compared with liposome assays in that Triton X-100 mixed micelles provide an inert surface for cPLA2α containing an average of 140 molecules/micelle with an Mr of 95 000; the size of the micelles is relatively independent of ionic strength and temperature within the physiologic range and there are no pure Triton X-100 micelles present; finally, cPLA2α activity is linear for at least 60 minutes in the mixed micelle assay compared with only a few minutes in the vesicle assay.34 Using this assay, 4 mol% FTY720 markedly inhibited recombinant cPLA2α activity (Figure 7A). Moreover, significant inhibition was observed with as little as 0.5 mol% FTY720 and maximal inhibition was observed at approximately 3 mol%, with an IC50 of 1.1 mol% (Figure 7B). Taking into account the ratio of 2 molecules of cPLA2α per micelle in the reaction,34 these results suggest that one molecule of FTY720 interacts with one molecule of cPLA2α to achieve full inhibition.
FTY720 reduces the enzymatic activity of recombinant cPLA2α. (A) Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of 4 mol% FTY720 or C1P, or 2 mol% of each in a mixed micelle assay. Data are means ± SD of duplicate determinations expressed as nanomole AA released per minute per milligram. (B) Stoichiometry of cPLA2 inhibition by FTY720. Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of increasing mol% FTY720 (100 × [FTY720]/[Triton X-100 + PC + FTY720]). The PC concentration was fixed at 15 mol% (100 × [PC]/[Triton X-100 + PC + FTY720]). Data are means ± SD of duplicate determinations expressed as nanomole AA produced per minute per milligram. Nonlinear regression analysis was performed (R2 = 0.999). The IC50 value for FTY720 inhibition of cPLA2α activity was 1.1 ± 0.03 mol%, and the Hill coefficient was 2.3 ± 0.1. (C) Lack of effect of sphingoid bases on cPLA2α activity. Recombinant cPLA2 (0.5 μg) activity was determined in the absence or presence of 4 mol% FTY720, FTY720-P, sphingosine, or S1P. Data are expressed as fold change in cPLA2α activity compared with control activity (none) and are means ± SD of triplicate determinations. Similar results were obtained in 3 independent experiments.
FTY720 reduces the enzymatic activity of recombinant cPLA2α. (A) Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of 4 mol% FTY720 or C1P, or 2 mol% of each in a mixed micelle assay. Data are means ± SD of duplicate determinations expressed as nanomole AA released per minute per milligram. (B) Stoichiometry of cPLA2 inhibition by FTY720. Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of increasing mol% FTY720 (100 × [FTY720]/[Triton X-100 + PC + FTY720]). The PC concentration was fixed at 15 mol% (100 × [PC]/[Triton X-100 + PC + FTY720]). Data are means ± SD of duplicate determinations expressed as nanomole AA produced per minute per milligram. Nonlinear regression analysis was performed (R2 = 0.999). The IC50 value for FTY720 inhibition of cPLA2α activity was 1.1 ± 0.03 mol%, and the Hill coefficient was 2.3 ± 0.1. (C) Lack of effect of sphingoid bases on cPLA2α activity. Recombinant cPLA2 (0.5 μg) activity was determined in the absence or presence of 4 mol% FTY720, FTY720-P, sphingosine, or S1P. Data are expressed as fold change in cPLA2α activity compared with control activity (none) and are means ± SD of triplicate determinations. Similar results were obtained in 3 independent experiments.
It has previously been shown that another bioactive sphingolipid metabolite, C1P, is a potent allosteric activator of cPLA2α.34 Of interest, FTY720 suppressed not only basal cPLA2α activity but also C1P-stimulated activity (Figure 7A). To further examine the specificity of FTY720, we compared its effects on recombinant cPLA2α with those of structurally-related compounds. In accord with a previous report,34 S1P did not stimulate or inhibit cPLA2 activity (Figure 7C). Moreover, FTY720-P, which had no effect on eicosanoid synthesis in mast cells (Figure 3A), also had no effect on cPLA2 activity (Figure 7C). Moreover, sphingosine, which is structurally similar to FTY720, also did not significantly affect cPLA2 activity (Figure 7C). Collectively, our data suggest that FTY720 is a direct specific inhibitor of cPLA2α.
Discussion
Eicosanoids are produced from AA that is released from cell-membrane glycerophospholipids by PLA2, and are converted to leukotrienes by 5-lipoxygenase or to prostanoids by cyclooxygenases. Both CysLTs and PGD2 are involved in asthma pathogenesis, and their levels are increased in airways of patients with asthma.49 CysLT increases vascular permeability and stimulates mucus secretion and smooth-muscle-cell contraction, and CysLT receptor antagonists are currently in clinical use for the treatment of asthma.50 PGD2 increases vascular permeability and has been linked to infiltration of eosinophils and Th2 lymphocytes into the airway submucosa in asthma, which augments the inflammatory response.50,51 PGD2 can also enhance production of Th2-type cytokines, thereby promoting growth and differentiation of mast cells, inducing IgE production by B cells, and, like CysLTs, up-regulating their own production by mast cells.52,53 Anti-IgE challenge of human mast cells also results in the generation of another metabolite, thromboxane A2 (TXA2), that increases microvascular leakage, impairs mucociliary clearance, and induces bronchoconstriction and airway hyperresponsiveness.43,51
The immunosuppressive effects of FTY720 have largely been attributed to its phosphorylation by SphK2 to FTY720-P, a mimetic of S1P. Previous studies demonstrated that FTY720 not only inhibits the Th2-cell–mediated eosinophilic inflammation but also is active in a relevant asthma model using active sensitization and challenge to allergen, which besides allergen-specific Th2 cells also involves allergen-specific IgE and subsequent allergen-mediated mast-cell degranulation events.22 Moreover, FTY720 inhibited the infiltration of both Th2 cells and eosinophils into bronchial tissue, reduced the levels of Th2-related cytokines and goblet-cell hyperplasia, and almost completely blocked the hyperresponsiveness to methacholine.22
Unexpectedly, we discovered that FTY720, but not FTY720-P, strongly inhibited mast-cell secretion of PGD2, CysLT, and thromboxanes, but not other polypeptide mediators, such as TNF-α or IL-6 (data not shown). Like FTY720-P, other S1P receptor agonists, including S1P, dihydro-S1P, and SEW2871, did not inhibit eicosanoid secretion, demonstrating that the inhibitory effects of FTY720 are S1P receptor independent, highlighting a novel mechanism of action for FTY720. In contrast to our results, it has been suggested that FTY720 stimulates the LTC4 transporter Abcc1 and CysLT-dependent T-cell chemotaxis to lymph nodes.54 However, FTY720-induced Abcc1 activity was measured by the efflux of the fluorescent dye Fluo-3, and no direct evidence was presented that FTY720 increased secretion of CysLT.
The common first, and rate-limiting, enzyme in eicosanoid production is PLA2. The PLA2 family includes many groups, most of which also include several subgroups.55 Of these, the 85-kDa group IV cPLA2 plays important roles in the early and late eicosanoid generation.56,57 Indeed, cPLA2-deficient mice revealed an essential role for cPLA2 not only in the immediate but also in delayed phase of eicosanoid production, and these mice show decreased allergic symptoms and a loss of bronchial hyperreactivity to methacholine.56 Moreover, inhibition of cPLA2 by a small molecule inhibitor (MAFP)57 significantly alleviated airway hyperresponsiveness in the OVA-sensitized mouse model.58 Although earlier studies suggested that sPLA2 may have a role in asthma,59 LY333013, a potent inhibitor of sPLA2, had no effect on the responses of atopic asthmatics to inhaled allergen.60 Because sPLA2 and iPLA2 have other important physiologic functions, such as maintaining homeostatic phospholipid levels in cellular membranes,33,55 to inhibit the consequences of eicosanoid production after allergen exposure it is important to develop drugs that specifically target cPLA2. At present, there are no clinically useful specific inhibitors of cPLA2. The main obstacles to their development are insufficient oral bioavailability, low affinity and potency in vivo, and insufficient isoenzyme selectivity. Thus, FTY720 might be a drug that overcomes these obstacles since it is highly effective after oral administration and our results show that it inhibits cPLA2 activity in vitro, but has no effect on sPLA2 or iPLA2, and also inhibits production of AA-derived eicosanoids by mast cells. These results thus have important implications for its potential use in treatment of asthma.
FTY720 has therapeutic effects in humans at very low doses. Although it is considered that FTY720 is a prodrug that is active only after phosphorylation, it should be noted that plasma concentrations not only of FTY720-P but also of FTY720 reach steady-state levels of 30 nM, both in humans and in experimental animal models.61-63 This is within the range of concentrations that significantly inhibit cPLA2. Of importance, the half-life for clearance of FTY720 in humans is nearly 8 days,61 and we observed that nanomolar concentrations of FTY720 markedly inhibited eicosanoid release from mast cells and macrophages within a very short period of treatment (15 minutes to 2 hours). Moreover, FTY720 stoichiometrically interacts with recombinant cPLA2α to achieve full inhibition of its enzymatic activity. Collectively, our results suggest that FTY720 is a potent, direct inhibitor of cPLA2.
Previous studies administering FTY720 by gavage showed reductions in airway hyperresponsiveness in a murine model.22 This was most likely due to its conversion to FTY720-P, which then systemically decreased circulating lymphocytes, thereby preventing increases in lung Th2 cells and producing immunosuppression.13,16-18 However, it is not likely that Th2 inhibition will have an impact on allergic asthma. Because we have uncovered a novel action of FTY720 itself as an inhibitor of cPLA2α independently of its conversion to FTY720-P, instillation of FTY720 into the lungs should minimize systemic effects and decrease potential side effects. Due to the central role that different eicosanoids play in the initiation and maintenance of airway inflammation, inhibition of cPLA2 has important implications for the potential therapeutic mechanism of action of this potent drug in asthma and other eicosanoid-driven inflammatory responses.
In recent years, up-regulation of COX-2 and PGE2 secretion have been implicated in the progression of some malignancies, such as lung, colon, and breast cancer.64 As COX-2 selective inhibitors have shown some efficacy in several animal cancer models,64 FTY720 might be useful as an antitumor agent. Indeed, FTY720 was recently shown to inhibit angiogenesis and tumor vascularization.65 Specific cPLA2 inhibitors would clearly not have the same nonspecific effects as COX-2 inhibitors and might have beneficial effects by decreasing availability of AA. Since other eicosanoids, such as leukotrienes and thromboxanes, also regulate inflammatory responses, cPLA2-specific inhibitors such as FTY720 could have the added benefit of decreasing all eicosanoid synthesis by limiting AA release in response to inflammatory mediators.
In preclinical studies, FTY720 treatment almost completely protected against disease in the experimental autoimmune encephalomyelitis (EAE) animal model for MS.9,66 Encouraging recent data from the extension of a phase 2 study to 12 months suggest significant effects of oral FTY720 for the treatment of patients with relapsing MS, an inflammatory demyelinating disease of the central nervous system (CNS) that results in motor and sensory deficits. Although MS and its animal model, EAE, are thought to be T-cell–mediated diseases, the mechanisms underlying the lesions in the CNS are not fully understood. Recent studies have demonstrated that cPLA2 is a central mediator in evoking the complex pathologic changes seen in EAE.67 One of the metabolic products of this enzyme, AA, is proinflammatory, and elevated levels of prostaglandins and leukotrienes have been found in the cerebrospinal fluid of MS patients.68 The other metabolite, lysophosphatidylcholine, induces myelin breakdown, demyelination, and chemokine/cytokine expression. cPLA2 is highly expressed in EAE lesions and blocking this enzyme with the AA analog, arachidonyl trifluromethyl ketone, led to a remarkable reduction in the onset and progression of EAE by reducing inflammation, axonal damage, and the clinical severity and preventing further remissions in animals showing a relapsing-remitting form of EAE.67 Moreover, cPLA2α-deficient mice are resistant to EAE.69 Thus, specific cPLA2α inhibition by FTY720 might be an optimal treatment for MS, as this would prevent both inflammation and axonal damage including demyelination. Development of nonphosphorylatable analogs of FTY720 that still target cPLA2 but would not affect lymphocyte trafficking and homing through S1P receptors could also lead to new potent and specific therapeutics.
Authorship
Contribution: S.G.P. made the discovery, performed research, and wrote the first draft; C.A.O. performed research; R.G. and P.S. performed research; S.E.B. contributed vital reagents and analyzed data; C.E.C. contributed vital reagents and analyzed data; S.M. analyzed data and wrote the paper; S.S. directed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah Spiegel, Department of Biochemistry, Virginia Commonwealth University School of Medicine, Richmond, VA 23298-0614; e-mail: sspiegel@vcu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants R01AIS0094 and in part by R37 GM043880 (S.S.), RO1 HL072925 (C.E.C.), and National Science Foundation grant 0212213 (S.E.B.). S.M. was supported by National Institute of Mental Health (NIMH) Intramural Research Program. Research was carried out in space renovated with NIH-1C06RR17393 grant to Virginia Commonwealth University (VCU).




![Figure 5. FTY720 inhibits release of prostanoids and arachidonic acid independent of CDLA2 translocation and ERK activation. (A) Effect of FTY720 on LPS-stimulated PGE2 production in RAW264.7 cells. RAW264.7 cells were treated for 2 hours with the indicated concentrations of FTY720, stimulated without (□) or with LPS (1 μg/mL, ▪) for 1 hour, and PGE2 secretion was determined by ELISA. Data are means ± SD of triplicate determinations. (Inset) Duplicate cultures were treated without (□) or with 1 μM FTY720 (▪), and cell viability was measured. (B) FTY720 inhibits Ag-induced arachidonic acid release from mast cells. RBL-2H3 mast cells (5 × 104) were sensitized with anti–DNP IgE (1 μg/mL) in the presence of 1 μCi (0.037 MBq)/mL [3H]AA for 18 hours, washed, and incubated further in the absence or presence of FTY720 (1 μM) in 0.5% BSA-EMEM. Cells were then stimulated without or with Ag (10 ng/mL) or ionomycin (5 μM) for 15 minutes, and [3H]AA release was measured by scintillation counting. Data are means ± SD of quadruplicate determinations expressed as percent of total AA released. (A) *P < .05; **P < .01. (C) Lack of effect of FTY720 on Ag-induced translocation of cPLA2α. Sensitized RBL-2H3 cells were treated without (vehicle) or with 1 μM FTY720 for 18 hours and then stimulated with Ag for the indicated times. Membranes were isolated from cell lysates by centrifugation at 100 000g, and equal amounts of proteins were analyzed by immunoblotting with anti-cPLA2. Blots were stripped and reprobed with anti–αV-integrin to demonstrate equal loading. (D-E) FTY720 does not inhibit Ag-induced ERK1/2 activation. RBL-2H3 (D) or LAD2 (E) mast cells were sensitized with IgE, treated with FTY720 (1 μM) for 2 hours (D) or 18 hours (E), and then stimulated with Ag (10 ng/mL) for the indicated times. Cells were lysed and phospho-ERK1/2 activation was determined by immunoblotting. Blots were stripped and reprobed with anti–β-tubulin to demonstrate equal loading. Similar results were obtained in 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/3/10.1182_blood-2006-03-011437/5/m_zh80030707430005.jpeg?Expires=1767698713&Signature=QR8VUm3yHkwgr9YPylpnPLwc~CpB64meTGGZcmx6~gOFuzisbEAXB0kjQeWAlRmLKpRm7DBc8cWAyQBghMcw40aFPnnFjAqqLyiDb01Qa85a9c-Y~BrrtWrMzkmIQwwgtU56ZDzhygUzLr~DT3oLJ2-1eGwHp~E4WyMzMvCL5CnnTPYzbTnLdYxnhUtVYCGZIDGG9H5uXupqduw7fIh8nJXfpxCn3ARkLyqZPoK1sr3pfTfCRN7D4YG0aqTdPJOwOJW-Jn2fd6wgIHHqUgCxYHZeVpKeHKQff6z1~LEQnLQrNOsxacwokidY04SuVNIEmj6KD0pjPN82IobwNQlb1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. FTY720 inhibits cPLA2 activity in vitro. cPLA2 activity was determined in lysates from RBL-2H3 cells (A) or in lysates from HEK 293 cells overexpressing cPLA2 (C) using a liposome assay with 1-palmitoyl-2-[14C]-arachidonyl-sn-glycerol-phosphatidylcholine (400 pmol) as substrate. Increasing concentrations of FTY720 were added separately. Data are expressed as picomole AA released per minute per milligram and are means ± SD of duplicate determinations. (B) RBL-2H3 or HEK 293 cells were pretreated without (□) or with FTY720 (▪) for 18 hours, washed extensively, and lysed. cPLA2 activity was determined in lysates. *P < .05. (D) sPLA2 activity was determined in lysates from HEK 293 cells overexpressing sPLA2 as described.35 Data are expressed as percent hydrolysis of [3H]-oleate–labeled lipids. (E) iPLA2 activity was determined with L-α-dipalmitoyl[2-palmitoyl-1-14C]phosphatidylcholine as substrate in liposomes containing dipalmitoylphosphatidylcholine and Triton X-100 as described.36 Similar results were obtained in 3 independent experiments. Data are expressed as the means ± SD of duplicate determinations (D,E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/3/10.1182_blood-2006-03-011437/5/m_zh80030707430006.jpeg?Expires=1767698713&Signature=cCNR15Q9Qivmygme9hOrQTIlBH1EbWWVQAT0cGkB292MjpnXtzS2Yahu10yqb~1NJ~aZMbwlKuOWPOxNsmz0A0~uheRIE3jHP8-eg4hvI4lGBctmX44iYMadWFY05OWtezrJDZKDP8YgcAowrAmD-1Mg7jXzSBRuWx4I0ETODMdRWvPf5cqAUiGjJ~Hfl~j6~OUVR7KzeWO3ocEfVBkqQg4fUN6NQH9KHuLssjmjXprv2t3rgXKn8GUI2UxakkPKTTesaG-wFBOEwwMYeK5CnM9SNuaW40KjfzQtPY8jIR114YV9s-cghqFk4nicxmpwsF-Pc4uPY9doJznOqXxa0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. FTY720 reduces the enzymatic activity of recombinant cPLA2α. (A) Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of 4 mol% FTY720 or C1P, or 2 mol% of each in a mixed micelle assay. Data are means ± SD of duplicate determinations expressed as nanomole AA released per minute per milligram. (B) Stoichiometry of cPLA2 inhibition by FTY720. Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of increasing mol% FTY720 (100 × [FTY720]/[Triton X-100 + PC + FTY720]). The PC concentration was fixed at 15 mol% (100 × [PC]/[Triton X-100 + PC + FTY720]). Data are means ± SD of duplicate determinations expressed as nanomole AA produced per minute per milligram. Nonlinear regression analysis was performed (R2 = 0.999). The IC50 value for FTY720 inhibition of cPLA2α activity was 1.1 ± 0.03 mol%, and the Hill coefficient was 2.3 ± 0.1. (C) Lack of effect of sphingoid bases on cPLA2α activity. Recombinant cPLA2 (0.5 μg) activity was determined in the absence or presence of 4 mol% FTY720, FTY720-P, sphingosine, or S1P. Data are expressed as fold change in cPLA2α activity compared with control activity (none) and are means ± SD of triplicate determinations. Similar results were obtained in 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/3/10.1182_blood-2006-03-011437/5/m_zh80030707430007.jpeg?Expires=1767698713&Signature=pptcfauZcRNimdCpYpVS9I6xqBG3EGoVnwxTLt4L379Yjw6pOD3y52wI5L92Wsjo4ECUFtJmDPcJ32Eh1Ny~1PBME7-ncOYyBSE5T7oLju281hFYUSGDis9fS4SSvEMiNs5w8A29C2Rl9TXeiNY-OHT4xDzWbm52pG2bAKMcEIR5t8D5BCsqAWnKb-oiOISEmRcjCImL61BOvoAzF28OwvReLgqWz8NCQHeOeiEX9s8-C8KWVatVN4VM13WFyIGMg9HfTStvGdiG7Hvl9qikBUl6SFEtw2lqvhBU6s~iaCSw8Db9O2MltRlQh8bzXXWDFUHTGBDebI9-nQTUwHV3jA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal