Abstract
Recent studies have shown that endothelial protein C receptor (EPCR) polymorphisms and soluble EPCR levels are associated with thrombotic diseases. It is unknown whether membrane EPCR (mEPCR) heterozygosity and/or physiologically elevated sEPCR levels directly impact the hemostatic balance and the outcome of endotoxemia. In these studies, thrombin infusion experiments revealed that EPCR heterozygosity (Procr+/−) impaired protein C activation by approximately 30%. Infusion of factor Xa with phospholipid demonstrated that the Procr+/−genotype increased the coagulant response relative to wild-type mice. Challenge of the Procr+/− mice with lipopolysaccharide (LPS) did not significantly exaggerate their response compared with wild-type mice. We also generated mice in which one allele of full-length EPCR was replaced by sEPCR (Procrs/+). Compared with Procr+/− mice, Procrs/+ mice had 5-fold higher sEPCR and similar mEPCR levels. Procr+/− and Procrs/+ mice generated similar levels of activated protein C (APC) upon thrombin infusion. They also exhibited a similar coagulant response upon factor Xa/phospholipid infusion. Only supraphysiologic levels of sEPCR could influence protein C activation and exaggerate the coagulant response. In conclusion, mEPCR, but not physiologically elevated sEPCR, regulated protein C activation. Procr heterozygosity results in a mild increase of thrombosis tendency and little influence on the response to endotoxin.
Introduction
The protein C pathway serves as an “on-demand” anticoagulant system that controls thrombin generation by inhibiting factors Va and VIIIa. Thrombin not only activates platelets and clots fibrinogen, but also binds to thrombomodulin (TM) on the endothelium. The resulting complex rapidly activates protein C. Activated protein C (APC) binds to protein S and this complex then inactivates factors Va and VIIIa.1 Endothelial protein C receptor (EPCR) binds protein C and increases the rate of protein C activation on the endothelium.2 Primary protein C pathway defects increase the risk of venous thrombosis.3 Acquired protein C pathway defects are found in some patients with autoimmune diseases. Antibodies against TM have been found in patients with lupus and unexplained thrombosis.4 Recent studies have identified antibodies against EPCR in antiphospholipid syndrome and suggest that they may be a risk factor for fetal death5 and acute myocardial infarction in young women.6 The protein C pathway also performs anti-inflammatory functions.7-10 Administration of APC has been shown to reduce mortality in baboon sepsis models11 and is used clinically to treat patients with severe sepsis.12
In adult humans, EPCR is primarily localized on the endothelial cells of large blood vessels and is very low or absent from the microvascular endothelium of most tissues.13 A metalloprotease cleaves the entire extracellular domain of EPCR from the cell membrane.14 The resulting sEPCR retains its affinity for both protein C and APC. sEPCR inhibits protein C activation by competing with the membrane form of EPCR on the vessel wall.15 It also inhibits APC anticoagulant activity by blocking the interaction of APC with negatively charged membrane surfaces, an interaction that is necessary for efficient inactivation of factors Va and VIIIa.16 sEPCR levels are increased in patients with systemic inflammatory diseases.17 Hirudin can inhibit the elevation of sEPCR in a rodent endotoxemia model,18 implying that the increased sEPCR level is due to thrombin generation. Whether the sEPCR level increase is sufficient to impair protein C activation and increase the risk of thrombosis or aggravate severe sepsis remains unknown.
Notably, a dimorphism in exon 4 (A6936G) encodes an amino acid change (S219G) in the transmembrane region of EPCR. Previous studies19,20 showed that the S219G dimorphism increases EPCR shedding from the cell membrane in culture. It is possible that the S219G dimorphism will lead to not only higher sEPCR but also lower mEPCR in vivo. The relationship between the S219G dimorphism and the risk of thrombosis has been studied by several groups. One group found that the S219G dimorphism was overrepresented in patients with venous thrombosis relative to healthy subjects.21 Another group found that S219G homozygosity exhibited a 3-fold higher risk of coronary heart disease.20 However, 2 other groups did not find a correlation between S219G dimorphism and the risk of venous thrombosis.22,23 It is possible that such a dimorphism has only a mild effect on thrombotic risk, which could be influenced by genetic background and environmental factors. Under such circumstances, well-matched animal studies, especially in rodents that can be strictly controlled, are helpful in determining the contribution of decreased mEPCR and elevated sEPCR to the coagulation response.
In murine studies, gene deletion results in a hypercoagulable state that can lead to early embryonic death, but yields viable pups if the placental EPCR is not deleted.24 These pups grow to adulthood and show no overt thrombotic episodes. However, they are hypercoagulable, with more thrombi forming when challenged with a procoagulant stimulus (factor Xa plus phospholipids) and have an increased sensitivity to endotoxin. Overexpression of EPCR25 results in resistance to formation of thrombin in response to a procoagulant stimulus and decreased susceptibility to endotoxin-induced septic shock. Mice with severe EPCR deficiency have been reported to have no measurable influence on an arterial thrombosis model26 but have a more severe coagulation response to endotoxemia.8 It appears that protein C pathway deficiency contributes more prothrombotic effects in the venous and microvascular than in the arterial circulation,27 possibly related to rheologic differences.
In the study reported here, a knock-in mouse line (Procrs/+), which has increased sEPCR but similar mEPCR compared with Procr+/− mice, was also developed and studied. We conclude from these studies that it is the concentration of mEPCR, but not physiologically attainable elevated sEPCR, that plays a role in protein C activation. Procr heterozygosity did not demonstrably affect mortality in an endotoxemia mouse model. This heterozygosity contributes modestly to a hypercoagulable response.
Material and methods
Targeted mutagenesis of the murine Epcr locus
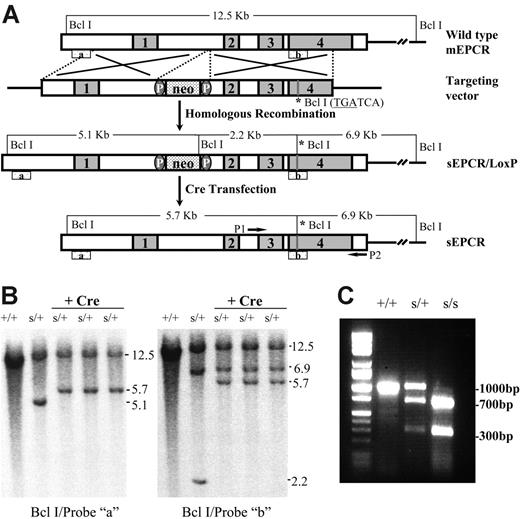
The Procrs/+ mice were generated by gene targeting using standard techniques.28 Briefly, to make a soluble EPCR form, mutagenesis was performed by introducing a BclI site (TGATCA) replacing Leu215 of the native protein with a stop codon that resulted in truncation immediately above the transmembrane domain. A neoR sequence flanked by 2 LoxP sites28 was introduced for clone selection (Figure 1A). Restriction enzyme mapping and sequencing of ligation junctions confirmed the correct structure of the targeting vector. The targeting vector was linearized and electroporated into AB2 129 mouse embryonic stem (ES) cells. Initially, polymerase chain reaction (PCR) was used to screen the G418-resistant clones. The PCR-positive ES clones were also confirmed by Southern blot using 2 different probes: a 5′-external probe (probe “a”) and a 3′-internal probe (probe “b”), after digestion of ES cell DNA with BclI (Figure 1B). To delete the neo gene, one ES cell clone was transiently transfected with the vector pBS-500/Cre. Thereafter, G418-sensitive clones were confirmed by Southern blotting (Figure 1B).
sEPCR gene targeting. (A) A schematic representation of the targeted Procr gene mutation (sEPCR: Leu215Stop) using the Cre/Lox system. Neomycin gene floxed by 2 loxP sites and a BclI restriction site (sEPCR: Leu215Stop) were incorporated in the Procr loci after homologous targeting. Subsequently, the neoR gene was deleted following transient transfection with a Cre expression vector. (B) Southern blot of ES clone DNA. After homologous targeting, the DNA was digested with BclI. The sEPCR mutant allele gave a 5.1-kb band, whereas the wild-type allele gave a 12.5-kb band with probe “a.” When the probe “b” was used, the mutant allele gave 6.9-kb and 2.2-kb bands due to a BclI site in the Neo gene and the integration of another BclI site in exon 4. After Cre transfection, the DNA was digested with BclI. The Procrs/+ clones showed 12.5-kb and 5.7-kb bands when hybridized with probe “a.” When the probe “b” was used, the mutant allele gave 6.9-kb and 5.7-kb bands, due to the integration of a BclI site in exon 4 of the Procr gene. (C) PCR genotyping. After PCR amplification, PCR products (1000 bp) were digested with BclI. For the Procrs/+ mutation, 1000-bp/300-bp and 700-bp bands were visualized. For Procrs/s embryos, only 700-bp and 300-bp bands were visualized.
sEPCR gene targeting. (A) A schematic representation of the targeted Procr gene mutation (sEPCR: Leu215Stop) using the Cre/Lox system. Neomycin gene floxed by 2 loxP sites and a BclI restriction site (sEPCR: Leu215Stop) were incorporated in the Procr loci after homologous targeting. Subsequently, the neoR gene was deleted following transient transfection with a Cre expression vector. (B) Southern blot of ES clone DNA. After homologous targeting, the DNA was digested with BclI. The sEPCR mutant allele gave a 5.1-kb band, whereas the wild-type allele gave a 12.5-kb band with probe “a.” When the probe “b” was used, the mutant allele gave 6.9-kb and 2.2-kb bands due to a BclI site in the Neo gene and the integration of another BclI site in exon 4. After Cre transfection, the DNA was digested with BclI. The Procrs/+ clones showed 12.5-kb and 5.7-kb bands when hybridized with probe “a.” When the probe “b” was used, the mutant allele gave 6.9-kb and 5.7-kb bands, due to the integration of a BclI site in exon 4 of the Procr gene. (C) PCR genotyping. After PCR amplification, PCR products (1000 bp) were digested with BclI. For the Procrs/+ mutation, 1000-bp/300-bp and 700-bp bands were visualized. For Procrs/s embryos, only 700-bp and 300-bp bands were visualized.
Generation, breeding, and genotyping of sEPCR mice
ES cells were microinjected at the Gene-Targeted Mouse Service of the University of Cincinnati. Tail genomic DNA was prepared with the DNeasy tissue kit (Qiagen, Valencia, CA) and genotyped by PCR. The 1.0-kb PCR product was generated by both wild-type and mutant alleles using the sense primer 5′-TTACAAGGGCTCCAGTCCCTCTC-3′, complementary to a region in intron 2 of the Procr gene and the antisense primer CCTTTGCAAACCCGTTACTTT GTT, complementary to a region in Epcr exon 4. The PCR products were then digested with BclI. BclI digestion did not alter the wild-type allele, whereas for the mutant allele, 700-bp and 300-bp digestion fragments appeared (Figure 1C). Procrs/+ mice were backcrossed into C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) until N10.
Mice expressing Cre-recombinase under the control of the embryo-specific meox-2 promoter (Meox2+/cre) were generated as described.29 ProcrLox/Lox and Procr+/− mice were generated as described24,28 and backcrossed into C57BL/6 mice until N10. Experimental designs and protocols were approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Challenge procedures
Under intraperitoneal avertin anesthesia, 8- to 12-week-old male mice were given injections of 15 U/kg per minute bovine thrombin through the carotid artery for 2 minutes and were killed immediately thereafter. Blood samples were collected by heart puncture. In a pair-controlled study, mice were administered factor Xa with phosphatidylcholine (40%) phosphatidylserine (20%) phosphatidylethanolamine (40%) vesicles (factor Xa/PCPSPE) by tail vein injection, without anesthesia. Factor Xa/PCPSPE was prepared using brain-derived phospholipids (Avanti Polar Lipids, Alabaster, AL) as described.30 The dose was 70.4 pmol/kg factor Xa and 108.3 nmol/kg PCPSPE. Mice were killed 10 minutes later by CO2 and blood samples were collected for assay. To measure the coagulation parameters, blood samples were collected in 0.38% sodium citrate. Part of each sample was immediately mixed with benzamidine hydrochloride (final concentration 0.01 M) for the APC assay. For LPS survival studies, mice were injected intraperitoneally in the evening with LPS (O111:B4; 50 mg/kg; Sigma, St Louis, MO) in sterile phosphate-buffered saline, and then the survival rate was measured 12 and 24 hours later. To analyze the coagulation and inflammatory response, a separate group of mice were challenged with LPS and blood samples were collected 4 and 8 hours later. The lungs were collected for myeloperoxidase (MPO) activity assay as described,25 4 hours after LPS challenge.
Monoclonal antibody (mAb) against murine TM was generated by standard methods. Briefly, the mAb 1701 (IgG1κ) was prepared by immunizing rats using recombinant soluble murine TM. The mAb was purified and passed through a Detoxi-Gel AffinityPak column (Pierce, Rockford, IL) to remove endotoxin. mAb 1701 (200 μg) was administrated intravenously 3 minutes before thrombin or factor Xa/PCPSPE infusion. Using cell-based protein C activation assays,2 compared with isotype control mAb, a TM mAb (1701) can impair protein C activation on TM-bearing murine thioglycollate-elicited peritoneal macrophages in culture at approximately 50% saturating concentration of the mAb (data not shown).
For sEPCR administration experiments, a soluble form of recombinant murine EPCR expressed in HEK293 cells18 was used.
Analytical procedures
Tissue and plasma EPCR levels were measured as described.18 Thrombin-antithrombin complex (TAT), APC, protein C and fibrinogen assays, blood cell count, and blood chemistry were performed as described.25 For mouse TM antigen assay, plates were coated with mAb 1699, blocked with 1% bovine serum albumin, and then incubated with diluted plasma samples. Plates were washed and bound TM was detected with a biotinylated goat anti–murine TM polyclonal Ab, followed by incubation with alkaline phosphatase–conjugated streptavidin. Color was developed with Blue-Phos substrate and measured at 650 nm. Serial dilutions of recombinant TM were used to generate a standard curve. The standard curve was linear (r = 0.99) from 1 to 128 ng mL−1.
Statistics
Results shown are the mean plus or minus the standard deviation (SD). The Student t test was used to compare values between groups. A P value of less than .05 was considered statistically significant.
Results
Procr heterozygosity reduces protein C activation
Resting Procr+/− mice appeared normal through life without thrombotic complications as determined by gross examination.28 Furthermore, the circulating TAT and platelet levels were similar between resting Procr+/− mice and their wild-type littermates. There are also similar TM levels between the resting Procr+/+ and Procr+/− mice in the circulation (13.8 ± 2.6 ng/mL vs 14.0 ± 2.1 ng/mL), in the liver (0.27 ± 0.06 mg/g vs 0.22 ± 0.07 mg/g, wet weight), in the lung (24.2 ± 3.3 mg/g vs 26.3 ± 3.5 mg/g, wet weight), and in the kidney (1.72 ± 0.47 mg/g vs 1.46 ± 0.53 mg/g, wet weight). Protein C antigen levels were higher in the circulation of Procr+/− mice compared with those of Procr+/+ mice (3.58 ± 0.20 μg/mL vs 3.16 ± 0.29 μg/mL; n = 6; P < .05). The ability of protein C to be activated was examined in Procr+/− mice. Upon thrombin infusion, the APC levels in Procr+/− mice were lower than those in wild-type mice (59 ± 9 ng/mL, n = 4 vs 86 ± 17 ng/mL, n = 5; P < .05). The APC levels in resting Procr+/− and Procr+/+ mice are too low to be detected (< 0.8 ng/mL).
Procr heterozygosity mildly enhances coagulation on challenge
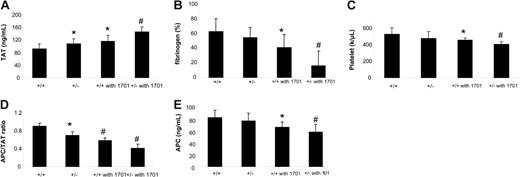
The EPCR level on the endothelium may play an important role in controlling the coagulant response to infusion of a procoagulant stimulant (factor Xa/PCPSPE). After factor Xa/PCPSPE infusion, there were mildly higher TAT levels and lower APC/TAT ratios present in Procr+/− mice compared with wild-type mice (Figure 2). In addition, Procr+/− mice exhibited a slight tendency to lower fibrinogen levels and platelet counts compared with wild-type mice, but these differences did not reach statistical significance.
Procr+/− genotype mildly enhances hemostatic parameters after factor Xa/PCPSPE challenge and has synergetic effects with TM antibody. The mice were challenged with factor Xa/PCPSPE, and the parameters were measured in the samples collected 10 minutes after challenge. (A) Levels of TAT complex in the plasma of Procr+/+ mice (n = 14), Procr+/− mice (n = 14), Procr +/+ mice with TM antibody (n = 5), and Procr +/− mice with TM antibody (n = 5). (B) Levels of circulating fibrinogen level. The fibrinogen levels are relative to the level of resting Procr+/+ mice (100%). (C) Platelet counts in the blood. (D) APC/TAT ratios in the plasma. (E) APC levels in the plasma. #P < .01, *P < .05, compared with Procr+/+ mice. Results are presented as mean ± SD.
Procr+/− genotype mildly enhances hemostatic parameters after factor Xa/PCPSPE challenge and has synergetic effects with TM antibody. The mice were challenged with factor Xa/PCPSPE, and the parameters were measured in the samples collected 10 minutes after challenge. (A) Levels of TAT complex in the plasma of Procr+/+ mice (n = 14), Procr+/− mice (n = 14), Procr +/+ mice with TM antibody (n = 5), and Procr +/− mice with TM antibody (n = 5). (B) Levels of circulating fibrinogen level. The fibrinogen levels are relative to the level of resting Procr+/+ mice (100%). (C) Platelet counts in the blood. (D) APC/TAT ratios in the plasma. (E) APC levels in the plasma. #P < .01, *P < .05, compared with Procr+/+ mice. Results are presented as mean ± SD.
Thrombotic tendencies in patients with EPCR S219G heterozygosity could be manifested primarily in those with compound defects in the protein C system. To simulate such a situation, we infused mAb 1701, a partial inhibitor of TM function, to determine whether this further increases the dependence on EPCR concentration upon coagulation stimulation. When infused with excess 1701 before thrombin administration, wild-type mice generated less APC compared with wild-type mice without 1701 (50 ± 8 ng/mL, n = 3 vs 86 ± 17 ng/mL, n = 5; P < .05). Procr+/− mice also generated less APC compared with Procr+/− mice without 1701 (30 ± 4 ng/mL vs 59 ± 9 ng/mL, n = 4, respectively; P < .05). In addition, wild-type mice injected with mAb 1701 exhibited a mildly enhanced coagulation response upon factor Xa/PCPSPE infusion (Figure 2) compared with mice without 1701. The isotype control mAb had no effects on the thrombin or factor Xa/PCPSPE infusion experiments (data not shown). Procr+/− mice with mAb 1701 had a further disturbed coagulation balance upon factor Xa/PCPSPE challenge, shown as higher TAT levels and lower platelet and fibrinogen levels, compared with Procr+/+ mice with TM mAb (Figure 2).
Physiologically elevated sEPCR in Procrs/+ does not impact protein C activation and coagulant response
A targeted mutation in the murine Procr gene (Leu215Stop) leads to the production of a soluble form of EPCR. When Procrs/+ mice were interbred, no Procrs/s progeny (0/135) were generated. In previous studies24 we have shown that as long as placental EPCR is maintained, it is possible to generate viable EPCR-null offspring. When male Procrs/+Meox2+/cre mice were crossed with female ProcrLox/Lox mice, Procrs/−Meox2+/cre mice were born at the expected Mendelian ratio (28:116; 24.1% vs expected 25%). Procrs/−Meox2+/cre mice contained one sEPCR allele with the other floxed allele deleted by cre-recombinase driven by the embryonic-specific Meox2 promoter at embryonic day (E) 6.5. Thus, as long as placental mEPCR was not deleted, embryos expressing only sEPCR were viable.
Adult Procrs/+ mice were of normal size and weight and appeared to have normal viability and fertility. Microscopic examination of the major organs of 2-month-old Procrs/+ mice did not indicate any abnormalities. Blood cell counts and chemistries were also normal. The TAT and platelet levels were similar between resting Procrs/+ and Procr+/+ mice. The soluble TM in the circulation and tissue TM antigen levels in liver and lung were also similar between the 2 groups (data not shown). The amount of plasma sEPCR in Procrs/+ mice was 2.7-fold higher than that detected in Procr+/+ mice (271 ± 57 ng/mL and 99 ± 21 ng/mL, n = 6, respectively). The amount of tissue EPCR in left lung extracts from Procrs/+ mice was approximately half of that detected in Procr+/+ mice (287 ± 46 ng/g and 506 ± 71 ng/g, n = 6, respectively).
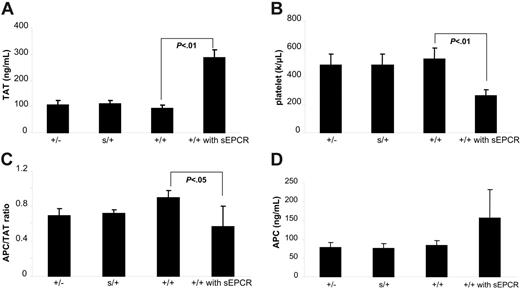
The generation of Procrs/+ mice facilitates the study of whether physiologically elevated sEPCR impacts protein C activation and thrombosis. Compared with Procr+/− mice, Procrs/+ mice had the same mEPCR but 5-fold higher sEPCR levels. Protein C activation upon thrombin infusion was compared in parallel between Procr+/− and Procrs/+ mice. Surprisingly, there was almost the same amount of APC generated between the 2 groups (59 ± 9 ng/mL vs 55 ± 10 ng/mL, n = 4, respectively; P = .64). This result indicates that physiologically elevated sEPCR does not influence protein C activation measurably, whereas mEPCR levels do. Since sEPCR can influence both protein C activation and APC functions, we also compared the coagulation parameters upon factor Xa/PCPSPE infusion between the 2 groups. Procrs/+ mice exhibited similar thrombin generation and platelet consumption compared with Procr+/− mice. The APC/TAT ratio was also similar in Procrs/+ mice compared with Procr+/− mice (Figure 3).
Supraphysiologic level but not physiologically elevated sEPCR can exaggerate the coagulant response to factor Xa/PCPSPE challenge. (A) Levels of TAT complex in the plasma of Procr+/− mice (n = 14), Procrs/+ mice (n = 6), Procr +/+ mice (n = 20), and Procr+/+ mice with 20 μg sEPCR (n = 4). The mice used for the experiments included Procr+/− mice and their Procr+/+ littermates, and Procrs/+ mice and their Procr+/+ littermates. The 2 groups of Procr+/+ mice exhibited almost the same results. Therefore, the results of Procr+/+ mice were combined together. (B) Platelet counts in the blood. (C) APC/TAT ratios in the plasma. (D) APC levels in the plasma. Results are presented as mean ± SD. Mice with supraphysiologic sEPCR exhibit a significantly higher TAT level, lower platelet count, and lower APC/TAT ratio compared with mice without sEPCR supplementation after factor Xa/PCPSPE challenge (P < .01).
Supraphysiologic level but not physiologically elevated sEPCR can exaggerate the coagulant response to factor Xa/PCPSPE challenge. (A) Levels of TAT complex in the plasma of Procr+/− mice (n = 14), Procrs/+ mice (n = 6), Procr +/+ mice (n = 20), and Procr+/+ mice with 20 μg sEPCR (n = 4). The mice used for the experiments included Procr+/− mice and their Procr+/+ littermates, and Procrs/+ mice and their Procr+/+ littermates. The 2 groups of Procr+/+ mice exhibited almost the same results. Therefore, the results of Procr+/+ mice were combined together. (B) Platelet counts in the blood. (C) APC/TAT ratios in the plasma. (D) APC levels in the plasma. Results are presented as mean ± SD. Mice with supraphysiologic sEPCR exhibit a significantly higher TAT level, lower platelet count, and lower APC/TAT ratio compared with mice without sEPCR supplementation after factor Xa/PCPSPE challenge (P < .01).
Supraphysiologic doses of sEPCR impair protein C activation and enhance coagulation
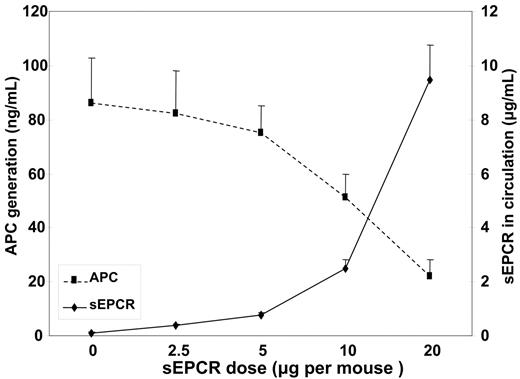
To obtain a supraphysiologic level of the soluble protein, we introduced purified recombinant sEPCR into the mice. Different doses of sEPCR were infused into wild-type C57BL/6J mice 3 minutes before thrombin infusion. The inhibition of APC generation (Figure 4) became significant only when the exogenous sEPCR dose reached 10 μg per mouse, which resulted in at least 2.5 μg/mL sEPCR in the circulation, much higher than that observed in Procrs/+ mice (0.27 μg/mL). This demonstrates the dose-dependent impairment of sEPCR on protein C activation, and the effective dose is far beyond the physiologically attainable level.
The plasma APC and sEPCR levels in mice upon thrombin infusion pretreated with different doses of sEPCR. Results are presented as mean ± SD. For each dose, n = 3.
The plasma APC and sEPCR levels in mice upon thrombin infusion pretreated with different doses of sEPCR. Results are presented as mean ± SD. For each dose, n = 3.
We also tested the impact of supraphysiologic exogenous sEPCR on coagulation. Wild-type mice were given 20 μg sEPCR, resulting in about 8 μg/mL sEPCR in the circulation. Three minutes after the administration of sEPCR, the mice were infused with factor Xa/PCPSPE. Compared with the mice without supplementation, these mice exhibited a severe coagulation disturbance displayed as high TAT levels and low platelet counts after factor Xa/PCPSPE challenge (Figure 3).
EPCR heterozygosity does not exaggerate the response to endotoxin
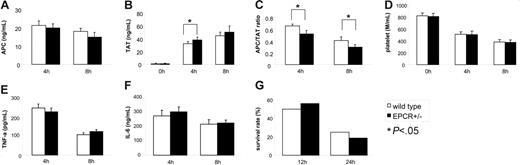
A low APC/TAT ratio on admission appears to be a predictor associated with an increase risk of death caused by sepsis,31 but it is unclear to what extent a patient's EPCR gene polymorphism affects the APC/TAT ratio on admission or the outcome of sepsis. Procrs/+ and Procr+/−mice, which have altered mEPCR and sEPCR levels and altered protein C activation competency, may serve as tools to study the effect of physiologic mEPCR and sEPCR on the response to endotoxin. Compared with Procr+/+ mice, Procr+/− mice had slightly lower APC/TAT ratios and higher TAT levels (Figure 5A-C) after LPS challenge, which indicated that Procr heterozygosity mildly disturbed the hemostatic balance. However, the mice did not exhibit a measurable survival disadvantage upon LPS challenge (Figure 5G). Procr+/− mice did not exhibit higher levels of TNF-α or IL-6 in the circulation after LPS challenge when compared with controls (Figure 5E-F). Compared with Procr+/− mice, Procrs/+ mice exhibited a similar survival rate, and similar levels of TNF-α, IL-6, APC, and TAT after LPS challenge (data not shown).
The response ofProcr+/−mice to LPS challenge. (A) At 4 and 8 hours after LPS challenge, levels of APC in the plasma of Procr+/− mice (n = 6 for each time point), ng/mL. (B) TAT, ng/mL. (C) APC/TAT. (D) Platelet counts, M/mL. (E) TNF-α, pg/mL. (F) IL-6, ng/mL. (G) The survival rates of Procr+/+ mice (n = 16) and Procr+/− mice (n = 16) are shown at 12 and 24 hours after LPS challenge. Results are presented as mean ± SD. *P < .05, compared with Procr+/+ mice.
The response ofProcr+/−mice to LPS challenge. (A) At 4 and 8 hours after LPS challenge, levels of APC in the plasma of Procr+/− mice (n = 6 for each time point), ng/mL. (B) TAT, ng/mL. (C) APC/TAT. (D) Platelet counts, M/mL. (E) TNF-α, pg/mL. (F) IL-6, ng/mL. (G) The survival rates of Procr+/+ mice (n = 16) and Procr+/− mice (n = 16) are shown at 12 and 24 hours after LPS challenge. Results are presented as mean ± SD. *P < .05, compared with Procr+/+ mice.
APC inhibits leukocyte migration into the lung in a human low-dose endotoxin study.32 Consistently, protein C–deficient mice exhibit more leukocytes in the organs after LPS challenge.9 However, Procr+/− mice did not appear to have increased leukocyte migration into the lungs compared with controls, at least as monitored by MPO activity. Soluble EPCR can bind to activated leukocytes through PR333 and may influence leukocyte migration. Using MPO activity as a marker of leukocyte migration, we found that, compared with Procr+/− mice, Procrs/+ mice also had comparable MPO activity in the lung after LPS challenge (data not shown).
Discussion
It has been reported that individuals with PROCR gene polymorphisms have altered sEPCR and APC levels, along with changed incidences of deep vein thrombosis (DVT).20,21 A dimorphism in the 3′ untranslated region (G7014C) is associated with elevated APC and a reduced risk of DVT.23 Another dimorphism in exon 4 (A6936G) is associated with elevated sEPCR levels and an increased risk of DVT.21 We felt that it would be interesting to test whether the higher sEPCR level influences the hypercoagulable state. In addition, we wished to determine whether PROCR heterozygosity would decrease APC generation or contribute to thrombosis. Here, we demonstrate for the first time that even reducing the mEPCR level by 50% results in impaired protein C activation in vivo.
Previous studies have demonstrated that the EPCR expression level on the endothelium plays an important role in controlling the coagulant response to a factor Xa/phospholipid challenge.24,25 Mice with EPCR overexpression on the endothelium activate more protein C and exhibit decreased coagulation in response to procoagulant stimuli.25 In contrast, total EPCR deletion exaggerates coagulation.24 However, the mEPCR levels in EPCR overexpression and EPCR-null mice are not observable in nature. We assumed that Procr+/− mice with half the mEPCR levels may mimic some human PROCR gene polymorphism conditions. Procr heterozygosity does reduce APC generation upon thrombin infusion to 70% of that of Procr+/+ mice. However, Procr+/− mice had only a mildly aggravated systemic coagulant response upon factor Xa/PCPSPE challenge.
Since thrombosis is usually seen as a result of accumulated risk factors, many common risk factors in humans do not result in thrombosis to a significant extent on their own. However, the combination of 2 or more such factors compounds the risk.34 Mice with either protein Z deficiency35 or tissue factor pathway inhibitor heterozygosity36 alone do not show overt thrombosis throughout their lives. However, when either of these mutations is combined with homozygous factor V Leiden, the thrombotic phenotype of the homozygous factor V Leiden is dramatically exaggerated. To mimic a compound deficiency, a TM deficiency was added to EPCR deficiency by use of a TM mAb. This TM mAb (1701) partially inhibits protein C activation when infused into wild-type animals. The response to factor Xa/PCPSPE challenge was only mildly exaggerated. When TM mAb (1701) is combined with the Procr+/− genotype, protein C activation was further inhibited and the coagulant response was exaggerated. Antibodies that inhibit APC anticoagulant activity are common in patients with antiphospholipid antibody and thrombotic diseases.4 This observation may explain the thrombotic phenotype in patients with the PROCR polymorphism, especially in autoimmune diseases.
Recently, the correlation of sEPCR level with the risk of DVT has been reported by several groups.20,21 These reports raised the intriguing question of whether physiologic levels of sEPCR have a direct effect on protein C activation and coagulation in vivo. To address this question, we generated Procrs/+ mice by gene targeting. The sEPCR level in Procrs/+ mice was 2.7-fold higher than that in wild-type mice, whereas the lung EPCR antigen level was only half that of wild-type mice. Interestingly, the heterozygous human PROCR gene S219G dimorphism is associated with a 2.5-fold higher plasma sEPCR level due to increased EPCR shedding.21,22 Therefore, the study of Procrs/+ mice may serve as a reasonable model for the functional study of the S219G dimorphism in humans.
Upon thrombin infusion, protein C activation was similar in Procrs/+ and Procr+/− mice. This indicates that although mEPCR heterozygosity reduces protein C activation, elevated sEPCR does not. This is most likely due to the fact that the sEPCR levels are only raised to 271 ng/mL (5.5 nM) whereas the protein C level (3.5 μg/mL; 56 nM) is in vast excess. From in vitro data,16 it is likely that one molecule of EPCR reacts with one molecule of protein C. Thus, 90% of the protein C is free and the sEPCR does compete with the membrane. This hypothesis is supported by the exogenous sEPCR supplementation experiment. Only when circulating sEPCR was above 2.5 μg/mL (51 nM), much higher than that observed in Procrs/+ mice (Figure 4), was APC generation measurably inhibited. Furthermore, upon factor Xa/PCPSPE infusion, Procrs/+ mice exhibited similar levels of thrombin generation and fibrinogen consumption compared with Procr+/− mice. Again, when wild-type mice were supplied with 20 μg sEPCR, which resulted in 8 μg/mL (163 nM) sEPCR in the circulation, these mice did exhibit enhanced thrombin formation and platelet consumption induced by factor Xa/PCPSPE infusion. Presumably, this level of sEPCR could inhibit protein C activation, and block APC anticoagulant function by inhibiting APC binding to the liposomes.
A logical question arises as to why the Procr+/− mice have lower levels of APC compared with the wild-type mice after thrombin infusion, yet have similar levels after Xa/PCPSPE infusion. A probable explanation is that APC or TAT levels are linked in the Xa infusion experiments. After Xa/PCPSPE infusion, Procrs/+ and Procr+/− mice generate slightly more thrombin than wild-type animals, which would increase protein C activation. Compared with wild-type mice, Procrs/+ and Procr+/− mice have a significantly lower APC/TAT ratio. Similar phenomena occurred in EPCR overexpression mice,25 which have large differences in APC/TAT ratios compared with wild-type mice after either factor Xa/PCPS or LPS infusion. However, the differences in APC level alone are not nearly as large.
Ample experimental and clinical evidence demonstrates the importance of the protein C system in sepsis. For example, mice heterozygous for protein C (Proc+/−) show aggravated responses in both endotoxemia9 and polymicrobial models.7 Here we demonstrated that Procr+/− mice had lower APC/TAT ratios after LPS challenge compared with Procr+/+ mice. Surprisingly, Procr+/− mice did not show a measurable survival disadvantage or enhanced inflammatory response, at least in the endotoxemia model that we explored.
Previous findings show that mice that overexpress EPCR on the endothelial cell have a 6-fold higher APC/TAT ratio than wild-type littermates, and are protected from endotoxemia.25 Mice with very low EPCR expression have an exaggerated response to endotoxemia.8 Therefore, it was surprising that Procr+/− mice did not show a measurable survival disadvantage. A likely explanation might be that the difference in APC/TAT ratio between Procr+/− and Procr+/+ mice is not sufficiently large. As shown in Figure 5, Procr+/− mice still had APC/TAT ratios 80% of that seen in controls.
Using the same LPS dose, it has been demonstrated9 that protein C heterozygous mice have a significant survival disadvantage, aggravated coagulation response (reflected as platelet consumption), and enhanced cytokine activation (reflected as higher TNF-α and IL-6 levels). Procr+/− mice did not have such significant phenotypes, indicating that protein C deficiency is more deleterious than EPCR deficiency. It is possible that sepsis is largely a disease of the microvasculature, where EPCR expression levels are low. Therefore, thrombin-TM alone could activate protein C and partially compensate for EPCR insufficiency during endotoxemia.
We conclude that (1) membrane Procr heterozygosity impairs protein C activation but is not a strong risk factor for thrombosis or survival of endotoxemia in this mouse model; and (2) elevated physiologically relevant levels of sEPCR cannot measurably inhibit protein C activation or enhance coagulation, whereas supraphysiologic levels of sEPCR can. It is important to recognize that although the murine model is uniquely useful at present for genetic manipulation, the possibility that comparable changes in humans might have qualitative or quantitative differences from those seen here cannot be ruled out. For instance, receptors for sEPCR may exist and may play a more important role in humans than mice. The sequence of the human membrane region in the S219G dimorphism, absent in the Procrs/+ animals, may affect signaling function of the EPCR in addition to making the receptor more sensitive to cleavage. In addition, it is also possible that the key regulatory proteins for the protein C system may be distributed on different cells, as is the case for thrombin signaling and protease-activated receptors.37
Authorship
Contribution: X.Z. and W.L. performed most of the experiments; J.-M.G. made the initial constructs used for gene modification; G.L.F. maintained the mouse husbandry and ES cell preparations; D.Q. helped TM antibody generation; N.L.E. and C.T.E. performed the overall design and interpretation of the experiments; and X.Z., W.L., N.L.E., and C.T.E. wrote the paper and all revisions.
Conflict-of-interest disclosure: Although one of the authors is employed by Berlex Biosciences, Berlex Biosciences has no commercial products that are discussed in the text of this article.
Correspondence: Charles T. Esmon, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104; e-mail: charles-esmon@omrf.ouhsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs L. Xia and Y. Hu for technical assistance. We thank T. Burnett, B. Carpenter, and M. Drake for help in protein purification. We thank M. Brady for help in mouse genotyping.
This work was supported by grant P50 HL54502 from the National Institutes of Health (C.T.E.). C.T.E. is an investigator of the Howard Hughes Medical Institute, and holds the Lloyd Noble Chair in Cardiovascular Research at the Oklahoma Medical Research Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal