Abstract
The mechanisms by which tumors metastasize to sentinel and distant lymph nodes, and beyond, are poorly understood. We developed transgenic mice that overexpress vascular endothelial growth factor-C (VEGF-C) and green fluorescent protein specifically in the skin and studied the effects of chemically-induced skin carcinogenesis in this model. We found that in contrast to VEGF-A, VEGF-C does not increase the growth of primary tumors, but instead induces expansion of lymphatic networks within sentinel lymph nodes, even before the onset of metastasis. Once the metastatic cells arrived at the sentinel lymph nodes, the extent of lymphangiogenesis at these sites increased. Of importance, in mice with metastasis-containing sentinel lymph nodes, tumors that expressed VEGF-C were more likely to metastasize to additional organs, such as distal lymph nodes and lungs. No metastases were observed in distant organs in the absence of lymph node metastases. These findings indicate an important role of VEGF-C–induced lymph node lymphangiogenesis in the promotion of cancer metastasis beyond the sentinel lymph nodes. VEGF-C is therefore a good target to slow or even prevent the onset of metastasis.
Introduction
Metastasis—the spread of cancer cells from the primary neoplasm to other tissues and organs—usually first occurs via the sentinel lymph nodes. The extent of lymph node metastasis is therefore an important determinant in the staging and the prognosis of most human malignancies, and often guides therapeutic decisions. Although the clinical significance of tumor metastasis to the lymph nodes is well documented, little is known about the molecular mechanisms by which tumors spread to the lymphatic vessels, to the sentinel and distal lymph nodes, and beyond.
In contrast to the extensive characterization of the molecular control of tumor angiogenesis—the growth of new blood vessels from pre-existing tumor-associated vessels—research into the role of the lymphatic system in cancer metastasis has been hampered by the lack of specific markers that distinguish lymphatic vessels from blood vessels, and also by the lack of known lymphatic-specific growth factors.1,2 However, researchers have recently identified novel lymphatic-specific markers such as lymphatic vascular endothelial-cell hyaluronan receptor-1 (LYVE-1)3 and Prox1—a homeobox transcription factor that induces lymphatic lineage-specific differentiation and that is essential for the embryonic development of the lymphatic system.4-6 Furthermore, vascular endothelial growth factor-C (VEGF-C), a member of the VEGF family of angiogenic factors, specifically activates VEGF receptor-3 (VEGFR-3), which is expressed on the lymphatic endothelium.7 Transgenic overexpression of VEGF-C in the skin of mice promotes cutaneous lymphangiogenesis.8 In VEGFC–null mice, early lymphatic endothelial cells fail to migrate away from cardinal veins and to form lymphatic vessels.9 These findings indicate that VEGF-C is an important regulator of lymphangiogenesis.
Studies have recently shown that VEGF-C, and the related factor VEGF-D, also promote tumor-associated lymphatic vessel growth (a process called tumor lymphangiogenesis) in xenotransplantation and transgenic mouse models of cancer, resulting in metastasis of tumors to sentinel lymph nodes.10-12 Moreover, transgenic expression of a VEGFR-3 decoy receptor inhibited metastasis of VEGF-C–expressing tumor xenotransplants to sentinel lymph nodes.13,14 Of importance, recent studies of human tumor samples have shown that the level of lymphangiogenesis, as well as the level of VEGF-C expression, is associated with a primary tumor's risk of metastasis to sentinel lymph nodes.15 Several other studies have also associated VEGF-C expression levels with risk of tumor metastasis.16 VEGF-C is therefore an important mediator of tumor metastasis to sentinel lymph nodes. Recent studies showed that in human melanomas, the extent of tumor lymphangiogenesis predicted the likelihood of metastasis to distant organs and patient survival.17 However, it is not clear whether VEGF-C—and tumor lymphangiogenesis—actually promotes tumor metastasis to distant sites, or simply serves as an indicator of tumor invasiveness and aggressiveness.
To directly investigate the effects of VEGF-C–induced lymphangiogenesis on distant metastasis, we studied the effects of its overexpression on skin tumor growth in transgenic mice. We previously created mice that express green fluorescent protein (GFP) specifically in the skin, under the transcriptional control of the keratin 14 promoter.18 We crossed these mice with those that specifically express VEGF-C in the skin, under the control of the same promoter.8 The offspring of this cross were subjected to a chemically-induced multistep regimen of skin carcinogenesis. Using this approach, we have been able to study the effects of VEGF-C on skin tumor formation and progression, and to track the spread of GFP-labeled skin tumor cells.
We found that in contrast to skin-specific expression of VEGF-A,18 expression of VEGF-C does not increase the rate of primary tumor growth. However, VEGF-C–expressing primary tumors of the skin formed expanded lymphatic networks within sentinel lymph nodes, even before metastasis occurred. Tumors of VEGFC transgenic mice also spread to the sentinel lymph nodes with increased frequency. Tumors that had spread to the sentinel lymph nodes of VEGFC transgenic mice were also more likely to continue metastasizing, to distal lymph nodes and to the lungs, than controls. These findings identify VEGF-C–induced lymph node lymphangiogenesis as a novel mechanism by which tumor cells mediate their metastatic spread to the lymph nodes, and then on to other organs. Lymph node lymphangiogenesis therefore represents a novel target for the treatment or the prevention of metastasis.
Materials and methods
Chemically induced skin carcinogenesis
To initiate tumor growth, 50 μg 7,12-dimethylbenzanthracene (DMBA; Sigma, St Louis, MO) was applied topically to the shaved back skin of 8-week-old female K14/GFP/VEGFC transgenic mice (n = 31) and of K14/GFP transgenic mice (n = 32). K14/GFP/VEGFC transgenic mice express the full-length coding sequence of human VEGF-C under control of the epidermis-specific keratin-14 promoter.8 This was followed by weekly topical application of 5 μg of the tumor promoter phorbol 12-myristate 13-acetate (PMA; Sigma) over a 20-week period, as described.19 Raised lesions of a minimum diameter of 1 mm that had been present for at least 1 week were recorded as tumors. Mice were killed 35 weeks after the initiation of treatment, or at 8 weeks after the first diagnosis of squamous-cell carcinoma (SCC). The ratio of malignant conversion was calculated for each group of mice as the total number of SCCs divided by the total number of large papillomas, expressed as a percentage. The 2-sided unpaired Student t test was used to analyze differences in the number of tumors per mouse between VEGFC transgenic and control mice. Regional and distal lymph node involvement was evaluated macroscopically and by fluorescence microscopy analysis of 100-μm step sections, as described.18 Lung metastases were also evaluated from 100-μm step sections. Metastasis data were analyzed by the Mann-Whitney test. All animal experiments were approved by the Massachusetts General Hospital Subcommittee on Animal Research and Care.
Immunofluorescence, in situ hybridization, and enzyme-linked immunosorbent assay (ELISA) analysis
Tumors or skin samples were fixed for 2 hours in 4% paraformaldehyde and were embedded in paraffin or in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA) and snap-frozen. All axillary and inguinal lymph nodes isolated from SCC-bearing mice were examined for the presence of metastases. The presence of metastases was confirmed by fluorescence microscopic analysis of GFP expression in 5 100-μm thick serial sections for each lymph node. Immunostaining was performed on 6-μm paraffin sections, or on cryostat sections, as previously described,18 using a rat mAb against CD31 (BD Biosciences/Pharmingen, San Diego, CA), a rat mAb against mouse VEGFR-3 (eBioscience, San Diego, CA), a rat monoclonal and a rabbit polyclonal antibody against mouse LYVE-1 (a kind gift of Dr David Jackson), and a rabbit anti-Prox1 antibody (Covance, Berkeley, CA). Secondary antibodies were labeled with AlexaFluor 488 or 594 (Molecular Probes, Eugene, OR). Before immunostaining was performed, frozen sections were photobleached by exposure to ultraviolet-B irradiation. Nuclei were counterstained with Hoechst bisbenzimide (Molecular Probes).
To quantify cell proliferation, mice were injected with 5-bromo-2′-deoxyuridine (BrdU, 50 mg/kg; Sigma) 2 hours before they were killed, and a fluorescein isothiocyanate (FITC)–conjugated mouse mAb against BrdU (BD Biosciences, Franklin Lakes, NJ) was used to detect proliferating cells. In situ hybridization was performed as described.20 Antisense and sense single-stranded 35S-labeled RNA probes against VEGFA, VEGFC, and VEGFD were prepared as previously described.21,22 Human VEGF-C and murine VEGF-A protein levels were quantified by ELISA (Bender MedSystems, Vienna, Austria for VEGF-C; R&D Systems, Minneapolis, MN for VEGF-A) of skin or tumor lysates, as described23 (n = 5 per genotype). The VEGF-C ELISA detects all human VEGF-C forms containing the VEGF homology domain, including full-length VEGF-C and the proteolytically processed mature form. Statistical analysis was performed using the unpaired Student t test.
Computer-assisted morphometric vessel analyses
Representative LYVE-1 and CD31 double-stained sections, obtained from biopsies of PMA-treated skin (n = 7), small papillomas (1-3 mm in diameter; n = 6), large papillomas (> 3 mm in diameter; n = 6), and SCCs (n = 7) were analyzed for each genotype, using a Nikon E-600 microscope (Nikon, Tokyo, Japan) equipped with a Pan Fluor 10×/0.30 numerical aperture (NA) (Figures 3, 4, 6G-H) or a Pan Fluor 20×/0.50 NA (Nikon) (Figure 2, 6A-F, I, J). Images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI), and computer-assisted morphometric analyses of blood vessels and lymphatic vessels were performed as described,24 using IP-LAB software (Scanalytics, Billerica, MA). Statistical analyses were performed using the 2-sided, unpaired Student t test.
Flow cytometry analysis
Tissue samples of SCCs and of sentinel lymph nodes were gently minced in phosphate-buffered saline (PBS), followed by incubation with 0.25% trypsin (Invitrogen, Carlsbad, CA) for 10 minutes at 37°C. Single-cell suspensions were prepared as described,18 and were passed through 40-μm pore size cell strainers (BD Biosciences), washed in PBS, and stained with a PE-labeled mouse anti-CD45 antibody (BD Biosciences/Pharmingen) and with propidium iodide. The stained cells (> 10 000 cells per sample) were subjected to flow cytometry analysis (FACS) with a FACScan and a FACSCalibur instrument (BD Biosciences). GFP expression was detected under the “FITC” setting.25
Quantitative real-time RT-PCR
Total RNA was isolated from mouse skin or from SCC samples (n = 5 per tissue and genotype) using Trizol reagent (Sigma). Taqman-based real-time reverse transcriptase–polymerase chain reaction (RT-PCR) experiments to detect mouse VEGFD and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed as described.23 GAPDH transcripts were simultaneously measured in all reactions as internal controls. Total RNA was treated with RNase-free RQ-DNase (Promega, Madison, WI) before analysis, and 50 ng total RNA was used for each reaction. Data were normalized based on the expression levels of GAPDH.
Results
Targeted overexpression of VEGF-C does not promote skin carcinogenesis
To directly investigate the effects of VEGF-C on tumor formation and progression, we crossed our previously established line of K14/GFP mice18 with previously established and characterized heterozygous K14/VEGFC transgenic mice.8 The resulting offspring of this cross included both K14/GFP mice (referred to as control mice) and K14/GFP/VEGFC transgenic mice (referred to as VEGFC transgenic mice). These mice were subjected to a standard chemically-induced skin carcinogenesis regimen.
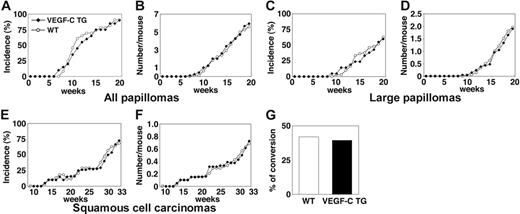
VEGFC transgenic mice formed skin papillomas within an average latency period of 12 weeks after the first application of PMA, compared with 11 weeks for control mice (Figure 1A). After 20 weeks of PMA treatment, VEGFC transgenic mice had developed 5.8 papillomas per mouse, compared with 5.6 papillomas per control mouse (P = .827; Figure 1B). The development of large papillomas was also comparable in both genotypes. In both control and VEGFC transgenic mice, the average latency period for the development of large papillomas (of a diameter > 3 mm) was 19 weeks (Figure 1C). The average number of large papillomas formed was also comparable between transgenic and control mice (Figure 1D; P = .780). The average latency period for the development of malignant SCCs was 30 weeks in both VEGFC transgenic mice and in controls (Figure 1E). VEGF-C overexpression resulted in formation of 0.72 SCCs per mouse, compared with 0.68 SCCs per control mouse (P = .812; Figure 1F). Moreover, no significant differences were observed in the tumor morphology or in the ratio of malignant conversion of large papillomas to SCCs (Figure 1G). No papillomas or carcinomas formed in the control or VEGF-C transgenic mice treated with DMBA or with PMA alone (data not shown). Together, these results reveal that VEGF-C overexpression does not affect the rate or size of skin tumor formation in response to exposure to chemical carcinogens.
Transgenic overexpression of VEGF-C does not affect the number or frequency of papillomas and squamous-cell carcinomas that form in mice. (A) Incidence of skin papilloma formation, over time (weeks), in VEGF-C transgenic mice (n = 31; ▪), compared with control mice (n = 32; ○). Incidence is expressed as the percentage of mice with detectable papillomas (> 1 mm) during the 20 weeks of topical PMA application. (B) No significant increase in the frequency (average number of papillomas per mouse) of papilloma formation was observed in VEGF-C transgenic mice. (C-D) No differences were observed in incidence or number of large papillomas more than 3 mm that formed in VEGF-C transgenic mice, compared with control mice, over the 20-week period of carcinogen application. (E-F) When mice were observed for an extended time period (33 weeks), squamous-cell carcinomas (SCCs) developed in both control and VEGF-C transgenic mice with the same incidence and numbers. (G) A comparable percentage of large papillomas underwent malignant conversion into SCCs in VEGF-C transgenic mice and control mice.
Transgenic overexpression of VEGF-C does not affect the number or frequency of papillomas and squamous-cell carcinomas that form in mice. (A) Incidence of skin papilloma formation, over time (weeks), in VEGF-C transgenic mice (n = 31; ▪), compared with control mice (n = 32; ○). Incidence is expressed as the percentage of mice with detectable papillomas (> 1 mm) during the 20 weeks of topical PMA application. (B) No significant increase in the frequency (average number of papillomas per mouse) of papilloma formation was observed in VEGF-C transgenic mice. (C-D) No differences were observed in incidence or number of large papillomas more than 3 mm that formed in VEGF-C transgenic mice, compared with control mice, over the 20-week period of carcinogen application. (E-F) When mice were observed for an extended time period (33 weeks), squamous-cell carcinomas (SCCs) developed in both control and VEGF-C transgenic mice with the same incidence and numbers. (G) A comparable percentage of large papillomas underwent malignant conversion into SCCs in VEGF-C transgenic mice and control mice.
Expression of transgenic VEGF-C is maintained throughout multistep carcinogenesis
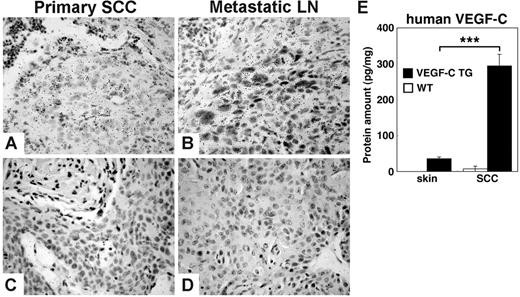
Although size and number of papillomas that formed in control and VEGFC transgenic mice were comparable, tumor cells of the primary SCCs that formed in the VEGFC transgenic mice expressed high levels of VEGFC mRNA, as revealed by in situ hybridization (Figure 2A). Strong VEGF-C mRNA expression was also maintained by metastatic tumor cells within lymph nodes (Figure 2B). Furthermore, ELISA analysis of tissue lysates indicated that human VEGF-C protein levels were increased in SCCs (294.2 ± 32.4 pg/mg), compared with normal skin of VEGFC transgenic mice (35.6 ± 4.9 pg/mg; P < .001) (Figure 2E). These findings are in accordance with the enhanced expression of keratin-14 in SCCs compared with normal skin and, therefore, the enhanced activity of the keratin-14 promoter that drives VEGF-C expression in these transgenic mice. No detectable protein levels of human VEGF-C were observed in lysates of SCCs or in normal skin of control mice. ELISA analysis of mouse VEGF-A protein revealed comparable levels in papillomas of control mice (346.1 ± 115.1 pg/mg) and of VEGFC transgenic mice (300.1 ± 50.2 pg/mg; P = .724). Little or no expression of VEGFD mRNA was observed in any tissues by in situ hybridization, and no major differences of VEGF-D mRNA expression levels between the 2 genotypes were detected by real-time RT-PCR (data not shown).
Transgenic VEGF-C expression is maintained during skin carcinogenesis. In situ hybridization demonstrates strong expression of human VEGFC mRNA in squamous-cell carcinomas (SCCs) of VEGF-C transgenic mice. (A) In primary tumors, SCC cells express high levels of VEGFC mRNA, as indicated by hybridization to an antisense human VEGFC probe. (B) In SCC metastases that form in sentinel lymph nodes (LNs), this expression of VEGF-C mRNA is maintained. (C-D) A human VEGF-C sense control probe did not hybridize with tumor samples. (A-D) Scale bars represent 100 μm. (E) ELISA analysis of human VEGF-C protein expression in skin and tumor lysates (n = 5 per group) revealed significant increases in the levels of VEGF-C protein in SCCs that form in VEGF-C transgenic mice, compared with the normal skin of these mice. No human VEGF was detected in skin or tumor lysates of control mice. Data are expressed as mean ± SD; ***P < .001.
Transgenic VEGF-C expression is maintained during skin carcinogenesis. In situ hybridization demonstrates strong expression of human VEGFC mRNA in squamous-cell carcinomas (SCCs) of VEGF-C transgenic mice. (A) In primary tumors, SCC cells express high levels of VEGFC mRNA, as indicated by hybridization to an antisense human VEGFC probe. (B) In SCC metastases that form in sentinel lymph nodes (LNs), this expression of VEGF-C mRNA is maintained. (C-D) A human VEGF-C sense control probe did not hybridize with tumor samples. (A-D) Scale bars represent 100 μm. (E) ELISA analysis of human VEGF-C protein expression in skin and tumor lysates (n = 5 per group) revealed significant increases in the levels of VEGF-C protein in SCCs that form in VEGF-C transgenic mice, compared with the normal skin of these mice. No human VEGF was detected in skin or tumor lysates of control mice. Data are expressed as mean ± SD; ***P < .001.
Pronounced tumor lymphangiogenesis in VEGF-C transgenic mice
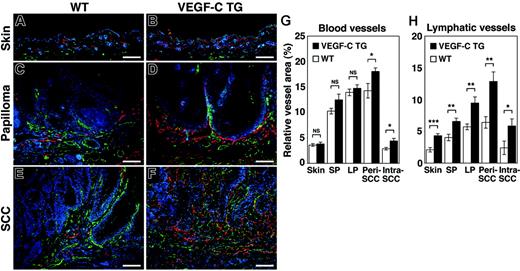
The effects of chronic VEGF-C overexpression were investigated on the tumor-associated formation of lymphatic and blood vessels, which can be detected with antibodies against LYVE-1 and CD31, respectively. An increased density of LYVE-1–positive lymphatic vessels was observed in the normal skin of VEGF-C transgenic mice, compared with control mice (Figure 3A-B). The benign papillomas that developed in VEGFC transgenic mice also had larger numbers of greatly enlarged peritumoral lymphatic vessels. Furthermore, peritumoral and intratumoral lymphangiogenesis was more pronounced in the SCCs of VEGFC transgenic mice, compared with tumors of control mice (Figure 3C-F).
Increased tumor lymphangiogenesis and angiogenesis in VEGF-C transgenic mice. Immunofluorescence analyses of CD31 (green) and LYVE-1 (red) expression in normal skin (A-B), early papillomas (C-D), and SCCs (E-F) of control (WT; A,C,E) and VEGF-C transgenic (VEGFC TG; B,D,F) mice revealed increased vascularization of papillomas and SCCs in VEGF-C transgenic mice and in control mice, compared with their PMA-treated skin. Tumor lymphangiogenesis was more prominent in VEGFC transgenic mice (D,F) than in control mice (C,E), with increased numbers of enlarged lymphatic vessels (red). Slight increases in the amount of tumor angiogenesis (green) in SCCs of VEGFC transgenic mice (F) were also observed, compared with that of control mice (E). Nuclei are labeled blue (Hoechst stain). (A-F) Scale bars represent 200 μm. (G-H) Computer-assisted morphometric analysis of normal cutaneous vessels and of tumor-associated lymphatic and blood vessels was performed. A significant increase in the relative area occupied by blood vessels in the peritumoral area of SCCs (Peri SCC), as well as within SCCs (Intra SCC), was observed in VEGFC transgenic mice (TG; ▪), compared with that of the control mice (WT, □) (G). A significant increase of the relative area occupied by lymphatic vessels was observed in the VEGFC transgenic mice, throughout all stages of skin carcinogenesis (H). Skin indicates PMA-treated normal skin (n = 7); SP, small papillomas (1-3 mm; n = 6); LP, large papillomas (> 3 mm; n = 6); and SCC, squamous-cell carcinoma (n = 7). Data are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001; NS = not significant.
Increased tumor lymphangiogenesis and angiogenesis in VEGF-C transgenic mice. Immunofluorescence analyses of CD31 (green) and LYVE-1 (red) expression in normal skin (A-B), early papillomas (C-D), and SCCs (E-F) of control (WT; A,C,E) and VEGF-C transgenic (VEGFC TG; B,D,F) mice revealed increased vascularization of papillomas and SCCs in VEGF-C transgenic mice and in control mice, compared with their PMA-treated skin. Tumor lymphangiogenesis was more prominent in VEGFC transgenic mice (D,F) than in control mice (C,E), with increased numbers of enlarged lymphatic vessels (red). Slight increases in the amount of tumor angiogenesis (green) in SCCs of VEGFC transgenic mice (F) were also observed, compared with that of control mice (E). Nuclei are labeled blue (Hoechst stain). (A-F) Scale bars represent 200 μm. (G-H) Computer-assisted morphometric analysis of normal cutaneous vessels and of tumor-associated lymphatic and blood vessels was performed. A significant increase in the relative area occupied by blood vessels in the peritumoral area of SCCs (Peri SCC), as well as within SCCs (Intra SCC), was observed in VEGFC transgenic mice (TG; ▪), compared with that of the control mice (WT, □) (G). A significant increase of the relative area occupied by lymphatic vessels was observed in the VEGFC transgenic mice, throughout all stages of skin carcinogenesis (H). Skin indicates PMA-treated normal skin (n = 7); SP, small papillomas (1-3 mm; n = 6); LP, large papillomas (> 3 mm; n = 6); and SCC, squamous-cell carcinoma (n = 7). Data are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001; NS = not significant.
In contrast, the “angiogenic switch” from vascular quiescence to up-regulation of angiogenesis, demonstrated by CD31 staining, was detected at the early stages of epithelial tumorigenesis both in control and VEGFC transgenic mice (Figure 3C and D, respectively). The extent of tumor angiogenesis, in both small and large papillomas, was almost indistinguishable between the 2 genotypes, although there was a slight increase in blood vessel formation in the SCCs of VEGF-C transgenic mice (Figure 3E-F).
Computer-assisted morphometric analysis confirmed that the relative area occupied by lymphatic vessels (LYVE-1 positive) was strongly increased in VEGFC transgenic mice, compared with control mice, throughout the successive stages of skin carcinogenesis (Figure 3H). The number of both peritumoral (P = .003) and intratumoral lymphatic vessels (P = .049) was increased more than 2-fold in the SCCs of VEGF-C transgenic mice. In contrast, the relative area occupied by CD31+/LYVE-1–negative vessels (blood vessels) in the normal skin and in benign papillomas was comparable between control and VEGFC transgenic mice (Figure 3G)—there was only a slight increase in blood vessel formation in the peritumoral and intratumoral areas of SCCs in VEGF-C transgenic mice (Figure 3G; P = .030 and P = .017, respectively). These results indicate that the VEGF-C promotes tumor-associated lymphangiogenesis in a chemically-induced model of skin carcinogenesis, with only minor effects on blood vessel formation (angiogenesis).
Up-regulation of VEGFR-3 on tumor-associated lymphatic vessels
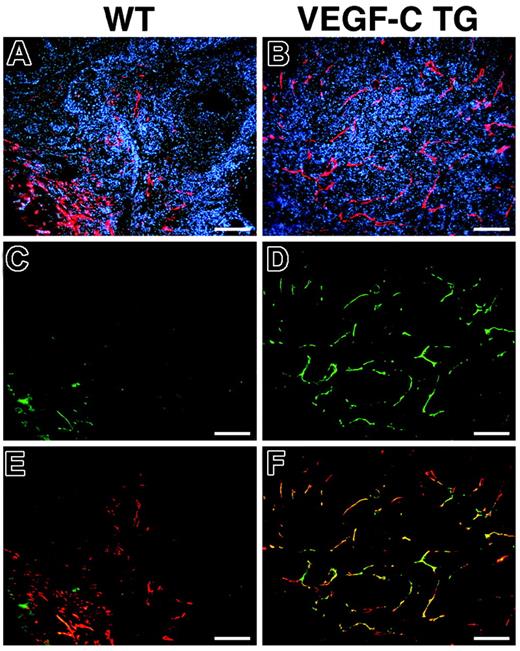
We investigated the expression pattern of the VEGF-C receptor VEGFR-3 in the lymphatic endothelium during skin carcinogenesis. Double-immunofluorescence analysis of VEGFR-3 and LYVE-1 expression confirmed an increased number of enlarged lymphatic vessels in the SCCs of VEGFC transgenic mice, compared with control mice (Figure 4A and B). All of the LYVE-1–positive tumor-associated lymphatic vessels also strongly expressed VEGFR-3 in SCCs of VEGF-C transgenic mice (Figure 4B,D,F), whereas lymphatic vessels only weakly expressed VEGFR-3 in the SCCs of control mice (Figure 4A,C,E), similar to normal lymphatics of adult organs. These results indicate that tumor-cell–derived VEGF-C promotes VEGFR-3 expression on lymphatic vessels, contributing to tumor-associated lymphangiogenesis.
Increased expression of VEGFR-3 in tumor-associated lymphatic vessels of VEGFC transgenic mice. Immunofluorescence analysis of LYVE-1 (red) and VEGFR-3 (green) expression was performed in SCC samples from control (A,C,E) and VEGFC transgenic (TG; B,D,F) mice. Based on LYVE-1 expression, the SCCs of VEGFC transgenic mice demonstrated prominent lymphangiogenesis (B), whereas tumor-associated lymphatic vessels were less pronounced in SCCs of control mice (A). Expression of the VEGF-C receptor (VEGFR-3) was strongly up-regulated in the tumor-associated lymphatic vessels of VEGF-C–overexpressing mice (D). Merging of images revealed that VEGFR-3 expression completely colocalized with that of LYVE-1 (F, yellow to orange), whereas VEGFR-3 was only weakly expressed in the tumor-associated lymphatic vessels of control mice (C,E). (A-F) Scale bars represent 200 μm.
Increased expression of VEGFR-3 in tumor-associated lymphatic vessels of VEGFC transgenic mice. Immunofluorescence analysis of LYVE-1 (red) and VEGFR-3 (green) expression was performed in SCC samples from control (A,C,E) and VEGFC transgenic (TG; B,D,F) mice. Based on LYVE-1 expression, the SCCs of VEGFC transgenic mice demonstrated prominent lymphangiogenesis (B), whereas tumor-associated lymphatic vessels were less pronounced in SCCs of control mice (A). Expression of the VEGF-C receptor (VEGFR-3) was strongly up-regulated in the tumor-associated lymphatic vessels of VEGF-C–overexpressing mice (D). Merging of images revealed that VEGFR-3 expression completely colocalized with that of LYVE-1 (F, yellow to orange), whereas VEGFR-3 was only weakly expressed in the tumor-associated lymphatic vessels of control mice (C,E). (A-F) Scale bars represent 200 μm.
Increased sentinel lymph node metastasis in VEGFC transgenic mice
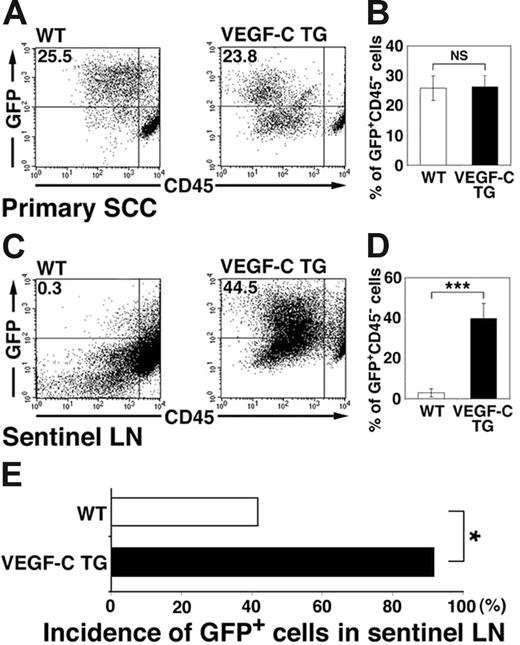
We next investigated whether the increased levels of tumor lymphangiogenesis observed in the SCCs of VEGFC transgenic mice were associated with increases in tumor metastasis to sentinel lymph nodes. We performed flow cytometry analysis of primary SCCs and of sentinel lymph nodes 8 weeks after the first diagnosis of SCC, to quantify lymph node metastasis by GFP-expressing tumor cells. These experiments revealed that 2.9% ± 2.0% of all sorted lymph node cells isolated from SCC-bearing control mice were GFP-positive metastatic tumor cells (Figure 5C-D). In contrast, VEGFC transgenic mice demonstrated a significant increase in the percentage of GFP-expressing metastatic tumor cells within the sentinel lymph nodes (39.8% ± 7.4%; P < .001; Figure 5C-D). The percentage of GFP-positive cells within primary SCCs of VEGFC transgenic mice (26.3% ± 3.8%; Figure 5A-B) was comparable with that of control mice (25.8% ± 4.1%). As assessed by fluorescence microscopy, GFP-expressing cells were found in the sentinel lymph nodes of 91.6% of SCC-bearing VEGF-C transgenic mice, but only of 41.6% of SCC-bearing control mice (Figure 5E; P = .038). Together, these results reveal that VEGF-C expression by primary SCCs potently promoted sentinel lymph node metastasis.
Increased tumor metastasis to sentinel lymph nodes in VEGFC transgenic mice. (A-D) Flow cytometry was used to calculate the percentage of GFP-expressing tumor cells in primary squamous-cell carcinomas (SCC; A-B) and in sentinel lymph nodes (LN; C-D) of control and VEGFC transgenic mice. Of all SCC-associated cells, 25.5% in the control GFP-transgenic mice and 23.8% in the VEGFC transgenic mice were observed to be GFP positive (A-B). Eight weeks after the first cutaneous SCCs were detected, the number and percentage of GFP-expressing tumor cells was significantly higher in metastases that formed in the sentinel lymph nodes of the VEGFC transgenic mice (44.5%) than of the control GFP-transgenic mice (0.3%; C). Data are expressed as mean ± SEM (n = 5 per group). (E) Fluorescence microscopy analysis revealed an increased incidence of sentinel lymph node metastasis in VEGFC transgenic mice, compared with control mice (n = 12). (B,D,E) ***P < .001; *P < .05; NS = no significance.
Increased tumor metastasis to sentinel lymph nodes in VEGFC transgenic mice. (A-D) Flow cytometry was used to calculate the percentage of GFP-expressing tumor cells in primary squamous-cell carcinomas (SCC; A-B) and in sentinel lymph nodes (LN; C-D) of control and VEGFC transgenic mice. Of all SCC-associated cells, 25.5% in the control GFP-transgenic mice and 23.8% in the VEGFC transgenic mice were observed to be GFP positive (A-B). Eight weeks after the first cutaneous SCCs were detected, the number and percentage of GFP-expressing tumor cells was significantly higher in metastases that formed in the sentinel lymph nodes of the VEGFC transgenic mice (44.5%) than of the control GFP-transgenic mice (0.3%; C). Data are expressed as mean ± SEM (n = 5 per group). (E) Fluorescence microscopy analysis revealed an increased incidence of sentinel lymph node metastasis in VEGFC transgenic mice, compared with control mice (n = 12). (B,D,E) ***P < .001; *P < .05; NS = no significance.
VEGF-C promotes lymphangiogenesis within sentinel lymph nodes
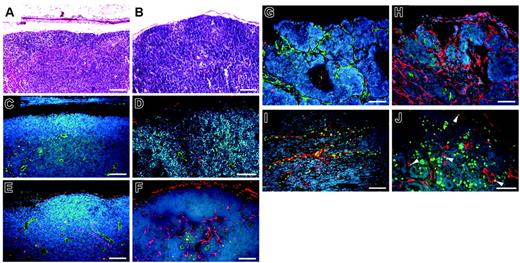
Immunofluorescence analysis of the lymph nodes of all genotypes of non–tumor-bearing mice used in this study revealed comparable numbers of blood vessels (CD31+) and of LYVE-1–positive sinusoids (Figure 6A-D). Of importance, however, increased numbers of LYVE-1–positive lymphatic vessels were found in the metastasis-containing lymph nodes of VEGFC transgenic mice (Figure 6H), compared with those of control mice (Figure 6G). The cells of the tumor-associated LYVE-1–positive vessels in VEGFC transgenic mice were undergoing proliferation, as revealed by BrdU staining (Figure 6J). All LYVE-1–positive vessels in VEGFC transgenic mice also expressed Prox1, confirming their lymphatic identity (Figure 6I). In situ hybridization studies confirmed that all metastatic tumors found in VEGFC transgenic mice expressed high levels of VEGFC mRNA (Figure 2B).
Prominent lymph node lymphangiogenesis in VEGFC transgenic mice. (A-B) Routine H&E stains of lymph nodes of non–tumor-bearing mice. (C-D) Double immunofluorescence staining of lymph nodes of non–tumor-bearing mice demonstrated a comparable pattern of CD31+/LYVE-1–negative blood vessels (green) and LYVE-1–positive sinusoids (red) in wild-type mice (C) and in VEGFC transgenic (TG; D) mice. Nonmetastatic sentinel lymph nodes of SCC-bearing VEGFC transgenic mice have increased numbers of enlarged LYVE-1–positive sinusoids (red; F), compared with control mice (E). An increased number of enlarged LYVE-1–positive lymphatic vessels (red) was also found in the metastatic sentinel lymph nodes of VEGFC transgenic mice (H), compared with control mice (G). The tumor-associated LYVE-1–positive vessels (red) in VEGFC transgenic mice showed high levels of BrdU staining in lymphatic endothelial cells (J; green), indicating active lymphatic proliferation (arrowheads) within sentinel lymph nodes. These cells also expressed Prox1 (I; green). Nuclei are stained blue (Hoechst stain). (A-F, I-J) Scale bars represent 100 μm; (G-H) scale bars represent 200 μm.
Prominent lymph node lymphangiogenesis in VEGFC transgenic mice. (A-B) Routine H&E stains of lymph nodes of non–tumor-bearing mice. (C-D) Double immunofluorescence staining of lymph nodes of non–tumor-bearing mice demonstrated a comparable pattern of CD31+/LYVE-1–negative blood vessels (green) and LYVE-1–positive sinusoids (red) in wild-type mice (C) and in VEGFC transgenic (TG; D) mice. Nonmetastatic sentinel lymph nodes of SCC-bearing VEGFC transgenic mice have increased numbers of enlarged LYVE-1–positive sinusoids (red; F), compared with control mice (E). An increased number of enlarged LYVE-1–positive lymphatic vessels (red) was also found in the metastatic sentinel lymph nodes of VEGFC transgenic mice (H), compared with control mice (G). The tumor-associated LYVE-1–positive vessels (red) in VEGFC transgenic mice showed high levels of BrdU staining in lymphatic endothelial cells (J; green), indicating active lymphatic proliferation (arrowheads) within sentinel lymph nodes. These cells also expressed Prox1 (I; green). Nuclei are stained blue (Hoechst stain). (A-F, I-J) Scale bars represent 100 μm; (G-H) scale bars represent 200 μm.
We next analyzed the nonmetastasis-containing lymph nodes of tumor-bearing mice of both genotypes. Surprisingly, we found increased numbers of enlarged LYVE-1–positive sinusoidal vessels in nonmetastasis-containing draining lymph nodes of tumor-bearing VEGFC transgenic mice (Figure 6F), compared with tumor-bearing control mice (Figure 6E). In contrast, no major difference was found in the number of CD31+ vessels within sentinel lymph nodes (Figure 6E-F). These intriguing findings reveal that VEGF-C secreted by primary skin tumors induces lymphangiogenesis within the draining lymph nodes even before the tumor has metastasized to these tissues, possibly facilitating future metastatic spread via the lymphatic system.
Sentinel lymph node lymphangiogenesis is associated with metastasis to distal sites
To investigate whether VEGF-C–induced lymphangiogenesis within the sentinel lymph nodes might facilitate further metastatic spread to distant lymph nodes and organs, we investigated the incidence of metastasis to distant lymph nodes and to the lungs in our experimental system. Of importance, the percentage of mice with sentinel lymph node metastases that also developed distant lymph node metastases was significantly higher in VEGFC transgenic mice (78.5%, 11 cases out of 14 sentinel lymph node–positive mice; P = .034) than in control mice (33.3%, 3 cases out of 9 sentinel lymph node–positive mice). Moreover, the incidence of lung metastases was also significantly higher in sentinel lymph node–positive VEGFC transgenic mice (64.3%; 9 cases out of 14 sentinel lymph node–positive mice: P = .045) than in control mice (33.3%; 3 cases out of 9 sentinel LN-positive mice). Of importance, all control mice with distant lymph node metastases and 9 out of 11 VEGFC transgenic mice with distant lymph node metastases also developed lung metastases. No lung metastases were found in mice without sentinel and distal lymph node metastasis. Together, these results reveal that VEGF-C not only induces tumor lymphangiogenesis, but also promotes tumor metastasis to regional as well as distal lymph nodes and organs, via promotion of lymphatic network expansion within sentinel lymph nodes.
Discussion
Our study identifies VEGF-C–induced lymph node lymphangiogenesis as a novel mediator of tumor metastasis from sentinel lymph nodes to distal lymph nodes and on to additional organs. Consistent transgenic overexpression of VEGF-C specifically in the skin of mice resulted in increased numbers of enlarged, actively proliferating, tumor-associated lymphatic vessels during multistep skin carcinogenesis. Tumor-expressed VEGF-C also promoted active expansion of the lymphatic network within draining sentinel lymph nodes—even before tumor metastasis occurred. This expansion in sentinel lymph node lymphangiogenesis was correlated with increased tumor metastasis to distant lymph nodes, and eventually to the lung.
VEGF-C appears to be specifically involved in the later stages of tumor progression, based on our observation that transgenic overexpression of VEGF-C in the skin does not affect primary tumor formation in a chemical model of carcinogenesis. This is in contrast to our recent findings that chronic transgenic overexpression of VEGF-A accelerated and increased development of benign papillomas and of malignant SCCs in an identical model.18 These intriguing differences might be, in part, explained by the different functions of these factors—VEGF-A is a potent inducer of early-stage tumor angiogenesis,18 whereas VEGF-C has no effect on blood vessel development during the early stages of skin carcinogenesis, and only minor effects on angiogenesis of late-stage SCCs, instead acting primarily as a lymphangiogenic factor. As a result, in spite of the fact that VEGFC transgenic mice develop more tumor metastases to lymph nodes and lungs, primary tumor morphology and invasiveness, as well as the ratio of malignant conversion of large papillomas into SCCs, are comparable between VEGFC transgenic mice and control mice.19 By manipulating the levels of VEGF-C in an experimental system of skin carcinogenesis, we have shown that VEGF-C–induced tumor lymphangiogenesis directly promotes tumor metastasis to the lymph nodes and on to distant organs, rather than just serving as a general indicator of the level of primary tumor invasiveness and aggressiveness.
The direct role of lymphangiogenesis in tumor progression has been questioned, since it has been proposed that tumor-associated lymphatic vessels might represent pre-existing lymphatics within or surrounding rapidly-invading tumor xenotransplants. We have used the lymphatic endothelium–specific hyaluronan receptor LYVE-13 as a marker to detect and quantify tumor-associated lymphatic vessels. LYVE-1 is also expressed by some nonlymphatic endothelial cells, such as those of liver sinusoids and of embryonic veins, so to increase specificity we also monitored expression of the transcription factor Prox1, which is specifically expressed by lymphatic vessels, but not by blood vessels, in normal and neoplastic tissues.1 Using these 2 markers, we have been able to carefully quantify lymphatic vessels associated with tumors and lymph node metastases. Through analysis of BrdU and LYVE-1 levels, our study reveals that active proliferation of lymphatic endothelial cells occurs in response to VEGF-C overexpression, and is associated with slowly growing, orthotopic cutaneous SCCs. Together, these results indicate that tumors can actively induce the growth of tumor-associated and lymph node–associated lymphatic vessels, and that VEGF-C mediates both of these processes.
One of the most intriguing findings of our study was that VEGF-C–overexpressing SCC cells maintained their lymphangiogenic activity after metastasizing to sentinel lymph nodes, maintaining the increased numbers of proliferating LYVE-1– and Prox1-positive lymphatic vessels at this location. These results indicate that VEGF-C not only promotes primary tumor lymphangiogenesis, but also induces lymph node lymphangiogenesis, which might facilitate further metastatic tumor spread throughout the lymphatic system and to distant organs. This model is supported by the observation that VEGFC transgenic mice were more likely than control mice to develop distant lymph node and lung metastases. This is in contrast to the VEGFA transgenic mice, which also develop lung metastases in the absence of distant lymph node metastases.18 These combined observations indicate that VEGF-C's ability to induce lymph node lymphangiogenesis promotes metastasis via distal lymph nodes, the thoracic duct, and the blood vascular system to distant organs, whereas VEGF-A might also promote metastasis via a non–lymph node–mediated pathway. In fact, in our recent studies in human cutaneous melanomas—which are characterized by predominant early metastasis via lymphatics to lymph nodes—we found that the degree of tumor-associated lymphangiogenesis and the level of VEGF-C—but not of VEGF-A—expression were correlated with lymph node metastasis and with reduced overall patient survival.15 At present, it remains unclear whether the intratumoral lymphatics within the sentinel lymph nodes are directly connected to the efferent lymphatic system of the sentinel lymph node. However, based upon previous studies with injection of ink or other tracers—and the rapid transport of these tracers through lymph nodes26 —there is a substantial amount of evidence that the afferent lymphatics are directly linked to the efferent lymphatics within lymph nodes. Moreover, the enhanced distant metastatic spread in the presence of tumor-induced lymph node lymphangiogenesis further suggests a link to efferent lymphatics.
The observation that both VEGF-C– and VEGF-A–overexpressing primary tumors induce lymphangiogenesis in sentinel lymph nodes before metastasizing to these tissues (this study and Hirakawa et al18 ) indicates that primary tumors can prepare their future metastatic site in advance of their arrival, partly by producing lymphangiogenic factors that mediate their transport. This concept is an important new twist to Paget's “seed-and-soil” hypothesis that proposes organ-specific characteristics as responsible for the preferential organ metastasis pattern of distinct tumors.27 Thus, tumors might actively modify the “soil” to make it more suitable for their further metastatic spread. This hypothesis is further supported by recent findings that attraction of bone marrow–derived hematopoietic progenitor cells to premetastatic sites was related to tumor-specific up-regulation of fibronectin in resident fibroblasts.28 It will be important to determine the exact mechanisms by which lymphangiogenesis induces tumor-cell migration, via the lymphatic vasculature, to the lymph nodes and other tissues. Recent studies have described the induction of lymph node lymphangiogenesis and subsequent dendritic-cell motility following adjuvant-induced skin inflammation.29 In this study, lymph node lymphangiogenesis appeared to promote migration of dendritic cells (even from uninflamed areas of skin that drained to the same lymph node) toward the draining lymph node. It will be interesting to determine whether lymph node lymphangiogenesis can also actively contribute to the recruitment of and motility of metastatic cancer cells.30
Our results indicate that VEGF-C might represent a novel target for treatment of patients with advanced forms of SCC or other cancers—blocking its activity could prevent metastasis to and from sentinel lymph nodes. Systemic inhibition of VEGF-C can be achieved by neutralizing anti–VEGF-C or anti–VEGFR-3 antibodies, or by administration of a soluble form of VEGFR-3 protein.13,14 Systemic blockade of VEGFR-3 has been recently shown to block tumor metastasis not only to lymph nodes, but also to lungs in experimental models of breast cancer.31,32 Treatment with a VEGF-C inhibitor could potentially prevent the systemic spread of an early- or even advanced-stage malignancy, by inhibiting lymphangiogenesis in tumors and lymph nodes. Additional studies are needed to determine the effects of long-term VEGF-C neutralization in multistep carcinogenesis and other models of lymph node–based metastasis. In addition to VEGF-C and VEGF-D, other newly-identified lymphangiogenesis factors, such as hepatocyte growth factor33 and VEGF-A,18 might also represent valuable targets for antilymphangiogenic therapies. Because we have recently also found metastasis-induced lymph node lymphangiogenesis in human cancer metastases,15 it is tempting to speculate that combination of antilymphangiogenic therapies with antiangiogenic therapies might provide synergistic benefits for the treatment of advanced human cancer.
Authorship
S.H. designed research, performed research, and wrote the paper; L.F.B. performed research and analyzed data; S.K. performed research and analyzed data; K.P. designed research and performed research; K.A. designed research and wrote the paper; M.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Detmar, Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology, ETH Zurich, Wolfgang-Pauli-Str 10, HCI H303, CH-8093 Zurich, Switzerland; e-mail: michael.detmar@pharma.ethz.ch.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH grants RO1 HL075183 (K.A.), CA69184, CA86410, and CA92644 (M.D.); American Cancer Society Research Project Grant 99-23901 (M.D.); the Susan G. Komen Foundation (M.D.); the EU lymphangiogenomics program (LSHG-CT-2004-503573LAG; K.A.); the Finnish Cancer Research Foundation (K.A.); Swiss National Science Foundation grant 3100A0-108207 (M.D.); Fonds für wissenschaftliche Förderung grant S9408-B11 (M.D.); the Krebsliga Zürich (M.D.); and Grant-in-Aid for Scientific Research on Priority Areas MEXT 18013037 (S.H.).
We thank M. Constant, L. Janes, M. Min, and L. Nguyen for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal