Abstract
Allograft transplantation requires chronic immunosuppression, but there is no effective strategy to evaluate the long-term maintenance of immunosuppression other than assessment of graft function. The ability to monitor naive alloreactive T cells would provide an alternative guide for drug therapy at early, preclinical stages of graft rejection and for evaluating tolerance-inducing protocols. To detect and quantify naive alloreactive T cells directly ex vivo, we used the unique ability of naive T cells to rapidly produce TNF-α but not IFN-γ. Naive alloreactive T cells were identified by the production of TNF-α after a 5-hour in vitro stimulation with alloantigen and were distinguished from effector/memory alloreactive T cells by the inability to produce IFN-γ. Moreover, naive alloreactive T cells were not detected in mice tolerized against specific alloantigens. The frequency of TNF-α–producing cells was predictive for rejection in an in vivo cytotoxicity assay and correlated with skin allograft rejection. Naive alloreactive T cells were also detected in humans, suggesting clinical relevance. We conclude that rapid production of TNF-α can be used to quantify naive alloreactive T cells, that it is abrogated after the induction of tolerance, and that it is a potential tool to predict allograft rejection.
Introduction
The ability to reject allografts is a hallmark of the immune system and is mediated by the vigorous immune response generated against MHC-disparate haplotypes. To prevent allograft rejection in patients undergoing transplantation, chronic immunosuppressive drug therapy is required.1-3 Clinical parameters of graft function currently guide drug choice and dosage during immunosuppressive therapy, but significant organ damage can occur prior to recognition of ongoing but subclinical graft rejection. The ability to identify and quantify alloreactive T cells prior to the appearance of clinical signs of graft rejection could improve patient management or the use of immunosuppressive drugs for maintaining allograft survival. A protocol to rapidly quantify naive and effector/memory alloreactive T cells could also be used to evaluate tolerance induction protocols as they enter clinical transplantation trials.4-6
T cells are an important component of allograft rejection, and alloreactive T cells represent a substantial proportion of the naive T-cell repertoire. Between 0.1% and 10% of naive T cells within an individual are estimated to be reactive with any unique allogeneic MHC haplotype.7-10 Assays that have been used to quantify alloreactive T cells, including limiting dilution analysis,11,12 enzyme-linked immunospot assays,13-16 and in vivo proliferation assays,10,13,15,17 require an extended in vitro culture period. However, these assays have not been translated effectively into the clinic and do not permit direct and rapid identification of naive alloreactive T cells.
Naive T cells are thought to acquire full functionality only after a programmed pathway of differentiation that is initiated by antigen recognition.18-22 The lack of measurable functions and the extremely low frequencies of naive T cells specific for any individual antigen have precluded the quantification of naive T cells by protocols that are commonly used to enumerate effector and memory T cells.23-25 We have recently observed that naive CD8+ T cells rapidly produce TNF-α but not IFN-γ following TCR engagement,26 and that this differential cytokine profile serves as a marker for naive T cells. The relatively high frequency of alloreactive T cells in a naive host makes this approach a potential methodology for identification and quantification of naive alloreactive T cells.
We demonstrate here that naive alloreactive T cells in mice and in humans are readily detectable by the rapid production of TNF-α after a short-term in vitro stimulation with alloantigen. The differential expression of TNF-α and IFN-γ can distinguish between naive and effector/memory alloreactive T cells, and moreover, the frequency of alloreactive CD8 T cells estimated by TNF-α production correlates with allograft survival.
Materials and methods
Mice
Male C57BL/6J (C57BL/6, H2b), BALB/cJ (H2d), and CBA/J (H2k) were purchased from the National Cancer Institute (Bethesda, MD) or from the Jackson Laboratory (Bar Harbor, ME) and used at 6 to 10 weeks of age. KB5.CBA TCR transgenic mice were obtained from Dr John Iacomini (Harvard Medical School, Boston, MA). The TCR transgene is expressed in CBA (H2k) mice by CD8+ T cells, and the transgenic TCR recognizes H2-Kb.27 B10.BR D10 TCR transgenic mice were obtained from the late Dr Charles Janeway (Yale University, New Haven, CT). The D10 TCR transgene was backcrossed 10 generations onto the CBA/J background and is maintained as a CBA.D10 stock. The D10 TCR transgene is expressed on the H2k background and directly recognizes the I-Ab MHC class II molecule.28 All animals were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Isolation of human PBMCs
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers, under signed informed consent in accordance with the Declaration of Helsinki and approval from the Institutional Review Board of the University of Massachusetts Medical School. PBMCs were collected in heparin and purified by Ficoll-Hypaque density centrifugation.
Preparation of LPS-treated splenocytes
Splenocytes were prepared from the indicated mouse strains (2 × 106 cells/mL in supplemented RPMI) and were treated with LPS (15 μg/mL) for 3 days in vitro. Following incubation, the cultures were washed 3 times with supplemented RPMI, γ-irradiated (20 Gy), and frozen at −70°C until used.
Intracellular cytokine staining
Cytokine-producing mouse T cells were detected using the Cytofix/Cytoperm Kit Plus (with GolgiPlug, BD Biosciences, Mountain View, CA), as described previously.29 Splenocytes (2 × 106 cells) or peripheral blood leukocytes (PBLs) from mice were incubated with 250 ng/mL anti–mouse CD3ϵ monoclonal antibody (mAb; 145-2C11, BD Biosciences) or with the indicated LPS-treated splenocytes populations (1 × 106 stimulator cells/sample) in the presence of 1 U/mL human recombinant IL-2 (BD Biosciences) and 1 μL/mL GolgiPlug at 37°C in the presence of 5% CO2 for 5 hours. Following the incubation, splenocytes were stained with mAb specific for CD8 (53-6.7), CD4 (RM4-5), and CD11a (M17/4). Samples were fixed and permeabilized with Cytofix/Cytoperm solution and stained with mAb specific for IFN-γ or TNF-α (XMG1.2 and MP6-XT22, respectively; BD Biosciences), or with an IgG1-isotype control (R3-34, BD Biosciences).
Human PBMCs were incubated in the presence of anti–human CD28, 5 μg/mL (CD28.2, BD Biosciences), for a total of 6 hours in AIM-V medium supplemented with 14% human AB serum (Nabi, Rockville, MD), 16% MLA-144 supernatant, 10 U/mL rIL-2 (BD Biosciences), 1% l-glutamine, 0.5% β-mercaptoethanol, and 1% HEPES. Anti–human CD3 antibody (HIT3a, BD Biosciences) was added to some cultures at 100 ng/mL to provide a polyclonal T-cell stimulation. To stimulate alloreactive T cells, PBMCs were incubated with CFSE-labeled (0.4 μM, Sigma, St Louis, MO) PBMCs derived from an allogeneic donor or with autologous CFSE-labeled PBMCs at a 1:1 ratio. After 2 hours GolgiPlug (1 μL/mL) was added to the cultures. After an addition 4 hours, samples were stained with mAb specific for CD8 (RPA-T8), CD4 (SK3), CD11a (HI111), and CD3 (UCHT1). Following treatment with Cytofix/Cytoperm solution, human PBMC samples were stained with mAb specific for IFN-γ and TNF-α (4S.B3 and MAb11, respectively; BD Biosciences). The samples were analyzed using a LSR-II (BD Biosciences). To evaluate cytokine production following allogeneic stimulation, samples were gated on the CFSE low T-cell populations. Samples were considered positive for TNF production if the values were more than 3 standard deviations over the mean of TNF produced by T cells from 12 donors after stimulation with autologous CFSE-labeled PBMCs (0.09% for CD8 and 0.08% for CD4). The presence of brefeldin A during the majority of the stimulation period would be expected to minimize the presentation of alloantigens by the indirect pathway, leading to this assay detecting predominantly T cells activated by the direct antigen presentation pathway.
Tolerance induction and skin transplantation
Recipient mice were treated with a donor-specific transfusion (DST) and anti-CD154 mAb, as described previously.30,31 Briefly, a DST consisting of 1 × 107 spleen cells was injected intravenously on day −7 relative to transplantation or intracellular cytokine analysis. Cohorts of mice also received intraperitoneal administration of 0.5 mg purified hamster anti–mouse anti-CD154 mAb (clone MR1, National Cell Culture Center, Minneapolis, MN) on days −7 and −4, 0, and +3, whereas other mice were untreated or received DST only. Mice received a skin allograft on day 0 as described.30 Thereafter, skin grafts were assessed 3 times weekly.30
Generation of KB5 TCR transgenic synchimeric CBA/J mice
In vivo cytotoxicity assay
The in vivo cytotoxicity assay was performed as previously described.34,35 Briefly, spleens were harvested from the indicated mouse strains and single-cell suspensions were prepared. Splenocytes were incubated with either 2 μM or 0.4 μM CFSE (Sigma) for 15 minutes at 37°C. Splenocytes were washed and the populations were combined at equal ratios. Cells (2 × 107) were adoptively transferred intravenously into naive recipient mice or into the indicated recipient mice. Spleens from recipient mice were harvested 20 hours later, and the survival of each transferred population was assessed by flow cytometry. To quantify alloreactive CD8+ T-cell cytotoxicity in the absence of natural killer (NK) cell killing, NK cells were depleted 1 day prior to adoptive transfer of CFSE-labeled cells using anti-NK1.1 antibody (PK136).36 Specific lysis was calculated using the following equation37 : 100 − ([(% target population in experimental/% syngeneic population in experimental) ÷ (% target population in NK1.1-depleted naive/% syngeneic population in NK1.1-depleted naive)] × 100). To determine specific lysis, the survival of allogeneic splenocytes in NK1.1-depleted naive mice was used as a baseline because optimal survival of these populations was found under these conditions.
Statistics
Statistical analyses were performed with InStat3 software (GraphPad Software, San Diego, CA). Three means were compared by one-way ANOVA and the Tukey-Kramer multiple comparison test, and means of 2 groups were compared by 2-tailed t tests. P values of less than .05 were considered to be statistically significant.
Results
Naive T cells produce TNF-α rapidly after TCR engagement by anti-CD3
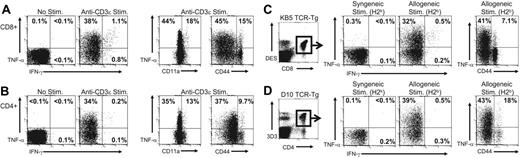
To examine the ability of naive T cells to produce cytokines rapidly following TCR engagement, splenocytes from C57BL/6 mice were stimulated with anti-CD3 mAb or left unstimulated for 5 hours, and CD8+ and CD4+ T cells were examined for intracellular TNF-α and IFN-γ expression. No TNF-α production was detected in unstimulated cultures (Figure 1A-B). In contrast, a large proportion of both CD8+ (Figure 1A) and CD4+ (Figure 1B) T cells from naive C57BL/6 mice produced TNF-α following anti-CD3 mAb stimulation (37% ± 1.8% and 33% ± 3.5%, n = 6, respectively) but not IFN-γ. T cells producing TNF-α had a naive phenotype because the majority of TNF-α+ cells were CD11alo and CD44lo (Figure 1A-B). These results demonstrate that naive CD4+ and CD8+ T cells rapidly produce TNF-α following TCR engagement, extending an observation that we have recently described only for CD8+ T cells.26
Naive T cells rapidly produce TNF-α but not IFN-γ after TCR engagement. (A-B) Splenocytes from naive C57BL/6 mice were stimulated for 5 hours with anti-CD3ϵ mAb and stained for cell surface markers and for intracellular TNF-α and IFN-γ, as described in “Materials and methods.” Samples were gated on CD8+ (A) or CD4+ (B) T cells and examined for expression of TNF-α or IFN-γ and for expression of CD11a and CD44. The values represent the percentage of T cells producing cytokine or the percentage of T cells that were either low or high for CD11a or CD44 that produced TNF-α. The data are representative of 5 experiments. (C-D) Splenocytes from (C) naive KB5 TCR CD8+ alloreactive transgenic mice or (D) naive D10 TCR CD4+ alloreactive transgenic mice were stimulated for 5 hours with either syngeneic (CBA, H2k) or allogeneic (C57BL/6, H2b) splenocytes and stained as described. For analysis, samples were gated on (C) DES+CD8+ cells or (D) 3D3+CD4+ cells. The values represent the percentage of TCR transgenic T cells positive for TNF-α or IFN-γ. The data are representative of 3 experiments.
Naive T cells rapidly produce TNF-α but not IFN-γ after TCR engagement. (A-B) Splenocytes from naive C57BL/6 mice were stimulated for 5 hours with anti-CD3ϵ mAb and stained for cell surface markers and for intracellular TNF-α and IFN-γ, as described in “Materials and methods.” Samples were gated on CD8+ (A) or CD4+ (B) T cells and examined for expression of TNF-α or IFN-γ and for expression of CD11a and CD44. The values represent the percentage of T cells producing cytokine or the percentage of T cells that were either low or high for CD11a or CD44 that produced TNF-α. The data are representative of 5 experiments. (C-D) Splenocytes from (C) naive KB5 TCR CD8+ alloreactive transgenic mice or (D) naive D10 TCR CD4+ alloreactive transgenic mice were stimulated for 5 hours with either syngeneic (CBA, H2k) or allogeneic (C57BL/6, H2b) splenocytes and stained as described. For analysis, samples were gated on (C) DES+CD8+ cells or (D) 3D3+CD4+ cells. The values represent the percentage of TCR transgenic T cells positive for TNF-α or IFN-γ. The data are representative of 3 experiments.
Identification of naive alloreactive CD8+ and CD4+ T cells by TNF-α production
The ability of naive T cells to produce TNF-α soon after anti-CD3 mAb stimulation suggests that production of this cytokine may be used as a marker to detect naive alloreactive T cells that will permit their identification directly ex vivo. To test this hypothesis, we first documented that defined alloreactive TCR transgenic T-cell populations would rapidly produce TNF-α on exposure to alloantigen. KB5 TCR transgenic CD8+ T cells that express a TCR that recognizes H2-Kb and D10 TCR transgenic CD4+ T cells that express a TCR that directly recognizes I-Ab were examined for their ability to produce TNF-α following stimulation with allogeneic targets.
Splenocytes from KB5 TCR transgenic (Figure 1C) and D10 TCR transgenic (Figure 1D) mice were stimulated for 5 hours with syngeneic (CBA, H2k) or allogeneic (C57BL/6, H2b) splenocytes, and TNF-α and IFN-γ production was assessed by intracellular staining. TCR transgenic T cells were identified using clonotypic antibodies (DES for KB5 CD8+ T cells and 3D3 for D10 CD4+ T cells). A large proportion of KB5 CD8+ T cells (31% ± 3.2%, n = 5; Figure 1C) and D10 CD4+ T cells (39% ± 2.4%, n = 5; Figure 1D) produced TNF-α but not IFN-γ following stimulation with allogeneic splenocytes (C57BL/6). In contrast, only very low levels of cytokines were produced by KB5 or D10 T cells following stimulation with syngeneic splenocytes (CBA). The majority of TNF-α–producing KB5 CD8+ T cells (40% ± 1.5% of CD44lo cells) and D10 CD4+ (42% ± 4.1% of CD44lo cells) T cells were naive, as determined by CD44 expression. These results demonstrate that naive alloreactive TCR transgenic T cells rapidly produce TNF-α following stimulation with the physiologically relevant level of alloantigen expressed by allogeneic splenocytes.
To determine if naive alloreactive CD8+ T cells in a wild-type (ie, nontransgenic) host could be identified by their rapid production of TNF-α following allo-stimulation, splenocytes from naive C57BL/6 mice were incubated in vitro with syngeneic (C57BL/6) or allogeneic (BALB/c, H2d or CBA, H2k) splenocytes. C57BL/6 T cells that were stimulated with either BALB/c or CBA splenocytes produced TNF-α (Figure 2A) but not IFN-γ (Figure 2B). The TNF-α–producing CD8+ T cells were CD11alo and CD44lo (data not shown), consistent with a naive phenotype. Stimulation with syngeneic splenocytes (C57BL/6) demonstrated that only very low background levels of TNF-α and IFN-γ are produced by unstimulated CD8+ T cells. Staining levels with isotype controls for cytokine mAb were always less than 0.1% on T cells stimulated with allogeneic splenocytes. The total number of naive alloreactive CD8+ T cells in a naive C57BL/6 mouse was estimated using the percentage of TNF-α–producing cells (Figure 2C), and the approximated frequency is consistent with previous estimations.1-5 Together these results demonstrate that for the first time naive alloreactive T cells can be identified directly ex vivo by their production of TNF-α in a short-term assay.
Rapid production of TNF-α by naive alloreactive CD8+ T cells in C57BL/6 mice. Splenocytes from naive C57BL/6 mice were stimulated with syngeneic (C57BL/6, H2b) or allogeneic (BALB/c, H2d or CBA, H2k) splenocytes, as described in “Materials and methods.” Splenocytes were then stained for cell surface markers and for intracellular (A) TNF-α or (B) IFN-γ. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for either cytokine. The total number of splenic alloreactive CD8+ T cells producing TNF-α in response to the indicated allo-stimulation is shown in panel C. The data are representative of 5 experiments. Error bars indicate SD.
Rapid production of TNF-α by naive alloreactive CD8+ T cells in C57BL/6 mice. Splenocytes from naive C57BL/6 mice were stimulated with syngeneic (C57BL/6, H2b) or allogeneic (BALB/c, H2d or CBA, H2k) splenocytes, as described in “Materials and methods.” Splenocytes were then stained for cell surface markers and for intracellular (A) TNF-α or (B) IFN-γ. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for either cytokine. The total number of splenic alloreactive CD8+ T cells producing TNF-α in response to the indicated allo-stimulation is shown in panel C. The data are representative of 5 experiments. Error bars indicate SD.
Differential production of TNF-α and IFN-γ distinguishes naive alloreactive CD8+ T cells from effector/memory T cells
Antigen-specific effector and memory CD8+ T cells can be identified by their numerous effector functions, including production of TNF-α and IFN-γ, and by the expression of high levels of the activation markers CD44 and CD11a. The production of TNF-α but not IFN-γ by antigen-specific naive CD8+ T cells should permit them to be distinguished from T cells that have had previous exposure to alloantigen.
Naive and effector/memory alloreactive CD8+ T cells were compared directly by evaluating CD8+ T cell responses in naive C57BL/6 mice or “primed” C57BL/6 mice that were inoculated with a BALB/c (H2d) DST 7 days earlier (Figure 3). Stimulation of splenocytes from naive C57BL/6 mice by BALB/c (Figure 3A) or CBA (H2k; Figure 3B) splenocytes led to rapid TNF-α production in CD11alo CD8+ T cells, as observed (Figure 2). In contrast, the CD8+ T cells from C57BL/6 mice primed with BALB/c DST that produced TNF-α following an in vitro stimulation with BALB/c splenocytes had an activated phenotype, being predominantly CD11ahi (Figure 3A). As expected, the H2k-specific CD8+ T cells from BALB/c-primed C57BL/6 mice maintained a naive phenotype (CD11alo), demonstrating the alloantigen specificity of the “priming” (Figure 3B). In addition, naive H2d-specific CD8+ T cells producing TNF-α did not synthesize IFN-γ to the H2d stimulation, whereas the majority of the H2d-specific CD8+ T cells from C57BL/6 mice primed with BALB/c DST did make IFN-γ (Figure 3C). These observations document that naive CD8+ T cells can be distinguished from effector CD8+ T cells by their rapid antigen-specific differential production of TNF-α and IFN-γ.
Naive, effector, and nonresponsive H2d-specific CD8+ T cells distinguished by differential expression of cytokines and activation markers. Splenocytes from naive C57BL/6 mice, from C57BL/6 mice that were injected with BALB/c splenocytes (BALB/c DST) 7 days previously, or from C57BL/6 mice that were tolerized with anti-CD154 and BALB/c DST were stimulated in vitro with either BALB/c (H2d) splenocytes (A) or CBA (H2k) splenocytes (B), as described in “Materials and methods.” Samples were then stained for cell surface markers and for intracellular cytokines. Samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for TNF-α. (C) H2d-specific CD8+ T cells producing TNF-α were evaluated for IFN-γ production and the values represent the percentage of TNF-α–producing CD8+ T cells that were positive for IFN-γ. The total number of CD8+ T cells producing TNF-α following stimulation with BALB/c (H2d) splenocytes is shown in panel D (n = 4). One-way ANOVA was used to compare naive C57BL/6 mice with mice treated with DST or with mice treated with DST and MR1 (*P < .05). The data are representative of 3 experiments. Error bars indicate SD.
Naive, effector, and nonresponsive H2d-specific CD8+ T cells distinguished by differential expression of cytokines and activation markers. Splenocytes from naive C57BL/6 mice, from C57BL/6 mice that were injected with BALB/c splenocytes (BALB/c DST) 7 days previously, or from C57BL/6 mice that were tolerized with anti-CD154 and BALB/c DST were stimulated in vitro with either BALB/c (H2d) splenocytes (A) or CBA (H2k) splenocytes (B), as described in “Materials and methods.” Samples were then stained for cell surface markers and for intracellular cytokines. Samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for TNF-α. (C) H2d-specific CD8+ T cells producing TNF-α were evaluated for IFN-γ production and the values represent the percentage of TNF-α–producing CD8+ T cells that were positive for IFN-γ. The total number of CD8+ T cells producing TNF-α following stimulation with BALB/c (H2d) splenocytes is shown in panel D (n = 4). One-way ANOVA was used to compare naive C57BL/6 mice with mice treated with DST or with mice treated with DST and MR1 (*P < .05). The data are representative of 3 experiments. Error bars indicate SD.
TNF-α and IFN-γ production can be used to monitor the efficacy of tolerance induction
The use of costimulation blockade allows for the prolonged survival of allogeneic grafts in the absence of chronic immunosuppression.31 Our laboratory's costimulatory blockade regimen consists of a single DST of splenocytes and a brief course of anti-CD154 mAb that induces nonresponsiveness in the T-cell compartment through both deletional and nondeletional mechanisms.30,31 Our observation that the rapid production of TNF-α permits identification of naive and effector alloreactive T cells may provide an approach for monitoring the efficacy of a tolerance induction regimen.
To test this, we quantified the ability of alloreactive CD8+ T cells to produce TNF-α and IFN-γ after costimulatory blockade. C57BL/6 mice were treated with a BALB/c DST and anti-CD154 mAb according to our standard protocol.32 The CD8+ T cells from mice treated with costimulation blockade produced only very low levels of TNF-α (Figure 3A,D; < 0.15% CD8+ T cells) or IFN-γ (< 0.05% CD8+ T cells) following stimulation with BALB/c splenocytes. TNF-α production by T cells in mice treated with costimulation blockade was significantly less than that observed by T cells from naive C57BL/6 mice (P < .05; Figure 3D). Naive CD8+ T cells readily produced TNF-α following stimulation with H2k alloantigens in C57BL/6 mice treated with a BALB/c DST and anti-CD154 mAb, again demonstrating the alloantigen specificity of the priming DST (Figure 3B). Similar results were obtained in reciprocal experiments in which C57BL/6 mice were primed with a CBA (H2k) DST or were tolerized to CBA alloantigens using our costimulation blockade protocol (Figure S1, available on the Blood website: see the Supplemental Figure link at the top of the online article). Together these results demonstrate that TNF-α and IFN-γ production can be used to distinguish between naive and effector alloreactive CD8+ T cells and that costimulation blockade induces an alloantigen-specific loss of TNF-α– and IFN-γ–producing CD8+ T cells.
Costimulation blockade leads to both deletion of alloreactive CD8+ T cells and to loss of function
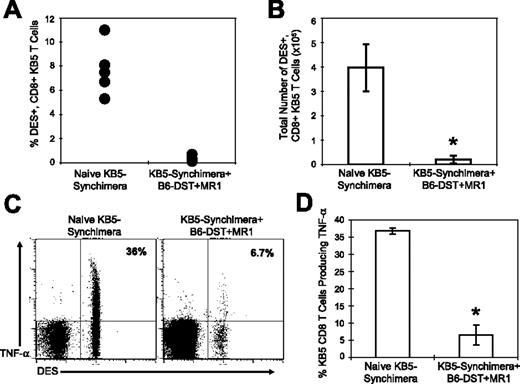
In the majority of C57BL/6 mice that were treated with costimulation blockade, TNF-α–producing, alloreactive CD8+ T cells specific to donor-specific alloantigen were greatly reduced in frequency. To determine if alloreactive CD8+ T cells were deleted or if the cells had lost their ability to produce TNF-α, we used KB5 synchimeric mice that circulate a trace population of alloreactive TCR transgenic CD8+ T cells that can be quantified using the DES clonotypic mAb. The frequency of DES+CD8+ T cells was measured in naive KB5-synchimeras and in synchimeric mice that were given C57BL/6 DST and anti-CD154 mAb costimulation blockade.
The percentage (Figure 4A) and total number (Figure 4B) of splenic DES+CD8+ KB5 T cells were significantly reduced in synchimeric mice given DST and anti-CD154 mAb as compared to levels observed in untreated synchimeric mice (P < .001). The few DES+CD8+ T cells that remained in mice treated with costimulation blockade were significantly impaired in their ability to produce TNF-α as compared to DES+CD8+ T cells in naive synchimeras (Figure 4C,D). The majority of DES+CD8+ T cells in mice given costimulation blockade expressed an activated phenotype (57% ± 8.9% CD44hi), whereas few DES+CD8+ T cells (0.8% ± 0.2% CD44hi) in naive synchimeric mice expressed an activated phenotype.38 These data indicate that costimulation blockade activates alloreactive T cells, leading to the deletion of a majority of alloreactive T cells, and that it impairs the ability of the remaining alloreactive population to produce TNF-α after allogeneic stimulation.
Costimulation blockade leads to the deletion of alloreactive CD8+ T cells and a loss in TNF-α production after allo-stimulation. Splenocytes from either naive KB5 bone marrow synchimeric mice or synchimeric mice treated with costimulation blockade were stained for cell surface makers, and the DES+CD8+ KB5 T cells are shown as a (A) percentage or (B) as total spleen cell number (n = 5). Splenocytes from the indicated group were stimulated for 5 hours with C57BL/6 (H2b) splenocytes and then stained for cell surface markers and for TNF-α. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+DES+ cells producing TNF-α (C-D, n = 5). Means were compared with a t test (*P < .001). The data are representative of 2 experiments. Error bars indicate SD.
Costimulation blockade leads to the deletion of alloreactive CD8+ T cells and a loss in TNF-α production after allo-stimulation. Splenocytes from either naive KB5 bone marrow synchimeric mice or synchimeric mice treated with costimulation blockade were stained for cell surface makers, and the DES+CD8+ KB5 T cells are shown as a (A) percentage or (B) as total spleen cell number (n = 5). Splenocytes from the indicated group were stimulated for 5 hours with C57BL/6 (H2b) splenocytes and then stained for cell surface markers and for TNF-α. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+DES+ cells producing TNF-α (C-D, n = 5). Means were compared with a t test (*P < .001). The data are representative of 2 experiments. Error bars indicate SD.
TNF-α production correlates with the rejection of skin allografts in mice treated with costimulation blockade
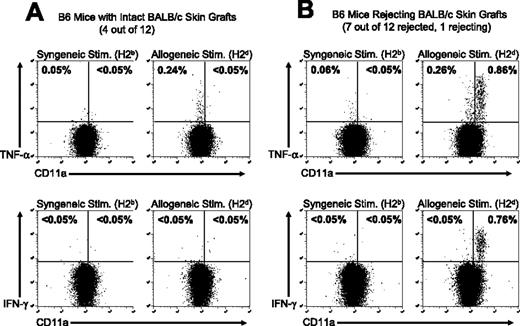
Treatment with costimulatory blockade prolongs the survival of allogeneic skin grafts, but these allografts are eventually rejected. We tested the hypothesis that the frequency of TNF-α–producing cells and their activation phenotype would correlate with skin allograft rejection in mice treated with costimulation blockade. TNF-α–producing cells were quantified in cohorts of mice that had intact long-term survival of skin allografts or in mice that had rejected the skin allograft. A group of 12 C57BL/6 mice were treated with BALB/c DST plus anti-CD154 mAb and given transplants of a BALB/c skin allograft according to our standard protocol.32 Skin allograft survival was monitored over the next 101 days, at which point 4 mice had surviving healthy BALB/c skin grafts, whereas 8 mice had either completely rejected the allograft or were in the process of rejecting the allograft (Figure 5). The ability of the alloreactive CD8+ T cells in each group to produce TNF-α and IFN-γ after in vitro stimulation with either syngeneic (C57BL/6) or allogeneic (BALB/c) splenocytes was determined.
The activation state of alloreactive T cells correlates with skin allograft outcome in mice treated with costimulation blockade. C57BL/6 mice were treated with anti-CD154 mAb and a BALB/c DST and engrafted with BALB/c skin as described in “Materials and methods.” At 101 days after transplantation, (A) 4 of 12 C57BL/6 mice retained intact BALB/c skin grafts and (B) 8 of 12 mice had completely rejected the allogeneic skin or were in the process of graft rejection. Splenocytes were harvested from the recipient mice 101 days after skin transplantation and stimulated in vitro with syngeneic C57BL/6 (H2b) or allogeneic BALB/c (H2d) splenocytes. Samples were then stained for cell surface markers and for TNF-α or IFN-γ. Samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for TNF-α or IFN-γ. Representative dot plots are shown.
The activation state of alloreactive T cells correlates with skin allograft outcome in mice treated with costimulation blockade. C57BL/6 mice were treated with anti-CD154 mAb and a BALB/c DST and engrafted with BALB/c skin as described in “Materials and methods.” At 101 days after transplantation, (A) 4 of 12 C57BL/6 mice retained intact BALB/c skin grafts and (B) 8 of 12 mice had completely rejected the allogeneic skin or were in the process of graft rejection. Splenocytes were harvested from the recipient mice 101 days after skin transplantation and stimulated in vitro with syngeneic C57BL/6 (H2b) or allogeneic BALB/c (H2d) splenocytes. Samples were then stained for cell surface markers and for TNF-α or IFN-γ. Samples were gated on CD8+ cells, and the values shown represent the percentage of CD8+ T cells staining positive for TNF-α or IFN-γ. Representative dot plots are shown.
In mice with intact skin allografts, a population of alloreactive T cells was detectable by TNF-α production, but these cells had a naive (CD11alo) phenotype and did not produce IFN-γ (Figure 5A). In contrast, CD8+ T cells from mice that had rejected their skin allografts had acquired an effector/memory phenotype (CD11ahi) and produced both TNF-α and IFN-γ in response to allo-stimulation (Figure 5B). The data shown are representative of the T-cell responses in each group. The data shown in Figures 3 and 4 demonstrate that early after the induction of tolerance there is a loss of alloreactive T cells in the host. However, the data in Figure 5 demonstrate that naive alloreactive CD8+ T cells (CD11alo, TNF-α but not IFN-γ production) repopulate recipient mice by 101 days after costimulation blockade and transplantation, but these alloreactive T cells have not been activated to acquire an effector/memory phenotype (CD11ahi, TNF-α and IFN-γ production). In mice that have rejected or are in the process of rejecting their skin allografts, the alloreactive T cells have acquired a functional, activated effector/memory phenotype.
Prediction of allograft rejection by TNF-α production
The ability to detect alloreactive T cells using TNF-α production should allow the assessment of tolerance induction protocols prior to allograft transplantation and may be used as a predictive tool for allograft rejection. To test this, we first quantified TNF-α production by CD8+ T cells in C57BL/6 mice that were treated with BALB/c DST and anti-CD154 mAb 6 days previously. The same mice were then analyzed for their ability to reject BALB/c splenocytes using an in vivo cytotoxicity assay 1 day after the cytokine analysis.34
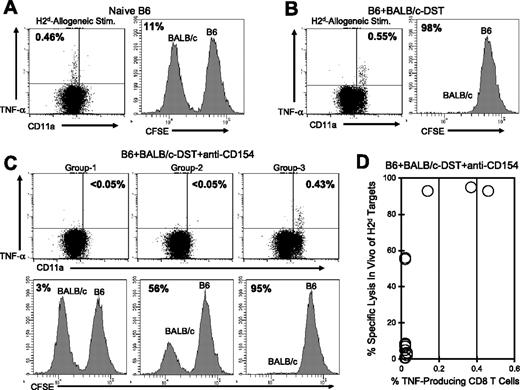
In naive NK cell-depleted C57BL/6 mice, H2d-specific CD8+ T cells were readily detectable by TNF-α production, and as expected, BALB/c (H2d) splenocytes were not rejected in the in vivo cytotoxicity assay (Figure 6A). CD8+ T cells with a naive phenotype have not yet acquired cytotoxic T-lymphocyte (CTL) activity and cannot reject allogeneic cells in this assay. In contrast, C57BL/6 mice that were injected 6 days earlier with BALB/c DST-generated effector H2d-specific CD8+ T cells (TNF-α+ and CD11ahi) and efficiently rejected BALB/c splenocytes (Figure 6B). In C57BL/6 mice that were treated with BALB/c DST and anti-CD154 mAb, 2 TNF-α production profiles and 3 distinct cytotoxicity patterns were observed (Figure 6C-D). Representative data are shown for each group (Figure 6C) and a summary of the data are shown in Figure 6D.
TNF-α production by alloreactive CD8+ T cells correlates with in vivo cytotoxic activity in mice treated with costimulation blockade. C57BL/6 mice were (A) left naive, (B) injected with BALB/c DST, or (C-D) treated with anti-CD154 mAb and a BALB/c DST. Six days later peripheral blood samples were stimulated in vitro with BALB/c (H2d) splenocytes and then stained for cell surface markers and for TNF-α. The values shown in the dot plots represent the percentage of CD8+ T cells producing TNF-α. One day after the cytokine assay, CFSE-labeled BALB/c (H2d) and C57BL/6 (H2b) splenocytes were transferred at equal ratios into the mice, and the survival of each population was examined 20 hours later. All recipient mice were treated with a mAb specific for NK1.1 to deplete NK cells. Survival of the BALB/c splenocytes is shown in representative histograms, and the values represent the percent specific lysis of BALB/c splenocytes. A summary of TNF-α production and percent specific lysis is shown in panel D (n = 15). The data are representative of 3 separate experiments.
TNF-α production by alloreactive CD8+ T cells correlates with in vivo cytotoxic activity in mice treated with costimulation blockade. C57BL/6 mice were (A) left naive, (B) injected with BALB/c DST, or (C-D) treated with anti-CD154 mAb and a BALB/c DST. Six days later peripheral blood samples were stimulated in vitro with BALB/c (H2d) splenocytes and then stained for cell surface markers and for TNF-α. The values shown in the dot plots represent the percentage of CD8+ T cells producing TNF-α. One day after the cytokine assay, CFSE-labeled BALB/c (H2d) and C57BL/6 (H2b) splenocytes were transferred at equal ratios into the mice, and the survival of each population was examined 20 hours later. All recipient mice were treated with a mAb specific for NK1.1 to deplete NK cells. Survival of the BALB/c splenocytes is shown in representative histograms, and the values represent the percent specific lysis of BALB/c splenocytes. A summary of TNF-α production and percent specific lysis is shown in panel D (n = 15). The data are representative of 3 separate experiments.
The majority of mice treated with DST plus anti-CD154 mAb (group 1; 10 of 15 mice) had no detectable H2d-specific CD8+ T cells as measured by TNF-α production and were unable to reject BALB/c splenocytes in the in vivo cytotoxicity assay, suggesting that these mice had lost donor-specific effector T-cell function. Group 2 (2 of 15 mice) had no detectable H2d-specific TNF-α–producing CD8+ T cells but displayed low levels of in vivo cytotoxicity, suggesting that deletion or inactivation of donor-specific alloreactive T cells was incomplete. The third group (3 of 15 mice) generated a population of TNF-α–producing H2d-specific CD8+ T cells that were activated (CD11ahi) and these mice effectively rejected the BALB/c splenocytes in the in vivo cytotoxicity assay. These data suggest that costimulation blockade in these 3 mice was ineffective and that the alloreactive CD8+ T cells were primed after injection of DST plus anti-CD154 mAb rather than rendered nonresponsive. Together, these results document that TNF-α production in response to allo-stimulation correlates closely with the generation of effector CD8+ CTL activity as assessed by an in vivo cytotoxicity assay.
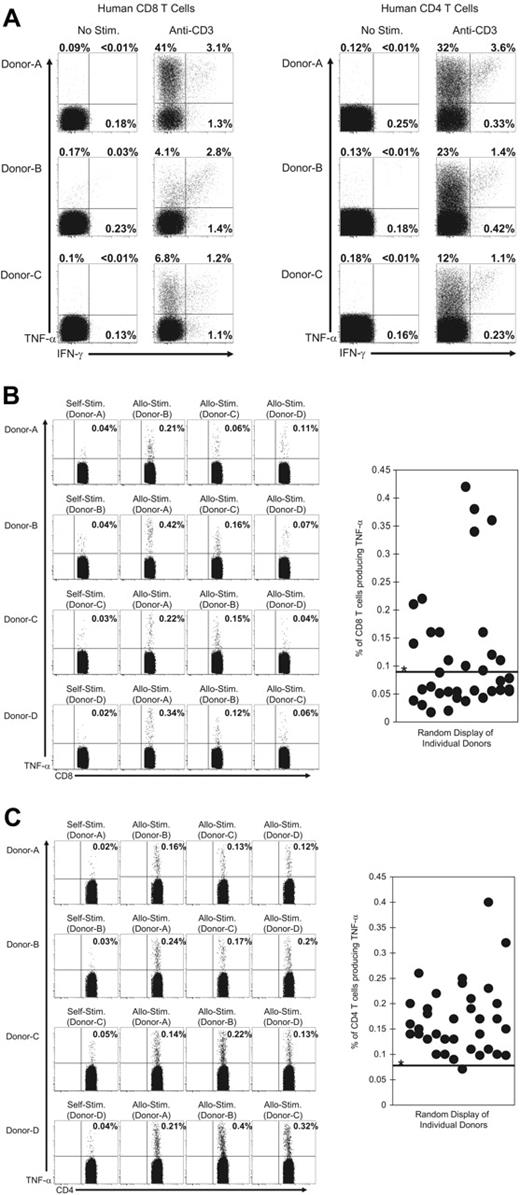
Detection of TNF-α production by alloreactive human T cells
We examined the ability of human T cells to rapidly produce TNF-α or IFN-γ following ex vivo TCR engagement (Figure 7). PBMCs were isolated from healthy human donors and then stimulated in vitro with anti–human CD3 mAb for 6 hours. Both CD8+ and CD4+ (Figure 7A) T cells produced TNF-α or IFN-γ (or both) following stimulation with anti-CD3 antibody, and T cells that produced only TNF-α were the dominant population. We next determined if alloreactive T cells from humans could be detected by TNF-α production. To test this, PBMCs from donors were mixed with allogeneic PBMCs or with autologous (syngeneic) PBMCs that had been labeled with CFSE. After a 6-hour incubation, TNF-α production by the CFSE low T-cell populations was examined by intracellular staining. Samples were gated on cells that were CD3+ and positive for either CD8 or CD4. Figure 7B-C shows representative results for CD8+ or CD4+ T cells derived from 4 donors that were either stimulated with autologous or allogeneic PBMCs. TNF-α–producing CD8+ and CD4+ T cells were detectable in a number of donor PBMCs. The percentage of CD8+ and CD4+ T cells producing TNF-α from all donor combinations (12 donors in 36 total combinations) is shown in Figure 7B-C. Alloreactive CD4+ T cells were detected in more donors relative to alloreactive CD8+ T cells. Taken together, these data suggest that the detection of TNF-α production will allow the identification of alloreactive T cells in humans and may be useful in screening potential transplant recipients for reactivity against specific donor tissues.
Human alloreactive T cells rapidly produce TNF-α after TCR engagement. PBMCs from human donors were stimulated with (A) a mAb specific for human CD3 or (B-C) with either allogeneic or autologous CFSE-labeled PBMCs as described in “Materials and methods.” After 6 hours samples were stained for cell surface markers and for TNF-α and IFN-γ. For T cells stimulated with anti-CD3 mAb (A), samples were gated on CD3+ cells and then on either CD8+ or CD4+ cells and analyzed for their expression of TNF-α or IFN-γ. The values represent the percentage of T cells that produced TNF-α or IFN-γ. For T cells stimulated with CFSE-labeled PBMCs, samples were gated on the CFSE low cells that were CD3+ cells and either (B) CD8+ or (C) CD4+. The values represent the percentage of T cells that produced TNF-α. Samples were considered positive for TNF-α production if the values were more than 3 standard deviations over the mean of TNF-α produced by T cells from 12 donors after stimulation with autologous CFSE-labeled PBMCs (*0.09% for CD8 and 0.08% for CD4). This threshold value is indicated by the horizontal line on the graphs. The data are representative of 3 experiments with a total of 12 donors in 36 combinations.
Human alloreactive T cells rapidly produce TNF-α after TCR engagement. PBMCs from human donors were stimulated with (A) a mAb specific for human CD3 or (B-C) with either allogeneic or autologous CFSE-labeled PBMCs as described in “Materials and methods.” After 6 hours samples were stained for cell surface markers and for TNF-α and IFN-γ. For T cells stimulated with anti-CD3 mAb (A), samples were gated on CD3+ cells and then on either CD8+ or CD4+ cells and analyzed for their expression of TNF-α or IFN-γ. The values represent the percentage of T cells that produced TNF-α or IFN-γ. For T cells stimulated with CFSE-labeled PBMCs, samples were gated on the CFSE low cells that were CD3+ cells and either (B) CD8+ or (C) CD4+. The values represent the percentage of T cells that produced TNF-α. Samples were considered positive for TNF-α production if the values were more than 3 standard deviations over the mean of TNF-α produced by T cells from 12 donors after stimulation with autologous CFSE-labeled PBMCs (*0.09% for CD8 and 0.08% for CD4). This threshold value is indicated by the horizontal line on the graphs. The data are representative of 3 experiments with a total of 12 donors in 36 combinations.
Discussion
The ability to identify and distinguish naive alloreactive T cells is important for monitoring immunosuppressive therapy in patients undergoing transplantation and for evaluation of tolerance induction protocols. In this report, we demonstrate that the unique ability of naive T cells to rapidly produce TNF-α but not IFN-γ after antigen stimulation allows the identification of naive alloreactive T cells directly ex vivo. Our results show that the frequency and activation state of alloreactive T cells can be rapidly determined ex vivo by simultaneously evaluating cytokine production and the expression of activation markers, and this information allowed the prediction of in vivo CTL activity against allogeneic tissues and correlated with allograft outcome in mice treated with costimulation blockade. Rapid production of TNF-α by naive alloreactive human T cells suggests that this approach may be applicable in the clinic.
Alloreactive T cells are actively involved in the rejection of tissue allografts,9 and the development of protocols to quantify T-cell reactivity against allogeneic grafts has been a primary focus for transplantation research.39 Most approaches for the detection of alloreactive T cells have used the production of IFN-γ to identify alloreactive T cells. However, only T cells that have been previously activated by antigenic stimulation rapidly produce IFN-γ when re-exposed to the antigen ex vivo.40 Interestingly, IFN-γ producing alloreactive T cells are detectable in hosts that have never been exposed to alloantigen, and the activation of these cells has been attributed to cross-reactivity to environmental antigens, including viral and bacterial infections.41-44 Importantly, the presence of IFN-γ–producing alloreactive T cells has been shown to correlate with the rejection of allogeneic tissues,14,16,45,46 but in these studies, the frequency of naive alloreactive T cells could not be determined. Our results demonstrate that TNF-α production by activated alloreactive T cells also correlates with the rejection of allogeneic tissues and has the additional advantage of identifying naive alloreactive T cells that would be overlooked in studies evaluating only IFN-γ production.
Current transplantation procedures require the chronic administration of immunosuppressive drugs to prevent the rejection of the allograft. An alternative to generalized immunosuppression is the induction of donor-specific tolerance, which will suppress alloreactive responses while allowing other components of the immune system to function normally.47 Costimulation blockade has been used successfully to promote the long-term survival of allogeneic tissues in mice and in nonhuman primates.31,48-55 We demonstrate here that TNF-α production by T cells can be used to measure the efficiency of tolerance induction at the time of transplantation and to identify hosts that may be at an increased risk for graft rejection. The production of TNF-α correlated with rejection of allogeneic tissues as measured by the in vivo cytotoxicity assay in mice treated with costimulation blockade. However, there were mice in which TNF-α was not detectable but some cytotoxic activity against allogeneic targets was observed in vivo. It will be important in future studies to determine if the TNF-α−, but in vivo cytotoxicity-positive hosts display impaired survival of allografts. In addition, it will be important to determine which type of allograft associates with TNF-α production and in vivo cytotoxicity, because treatments that are required for successful prolongation skin allografts are more robust than those required for prolongation of islet allografts.56
The ability of naive alloreactive T cells to produce TNF-α may have important consequences for the generation of responses against alloantigen in vivo. CD8+ T cells have been demonstrated to amplify the innate immune response against cardiac allografts within the first 24 hours after transplantation, indicating an early effector function for the alloreactive T cells.57 Whereas IFN-γ was implicated in amplifying the innate response to cardiac allografts, our results suggest that early TNF-α production may also contribute to this process. TNF-α production by naive alloreactive T cells may also play a role during the induction of tolerance with costimulation blockade, as the allogeneic DST would be expected to initially stimulate TNF-α production in the host alloreactive T-cell population. Further study of early T-cell functions that are activated during the initial stimulation with alloantigen will advance our understanding on the generation of the alloimmune response and may aid in the development of more efficient tolerization techniques.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: M.A.B. designed and performed research, analyzed data, and drafted the manuscript; J.M., T.G.M., T.P., and T.B.T. performed research; K.A.D. performed research and collected data; R.M.W. and D.L.G. designed research and drafted the manuscript; and A.A.R. drafted the manuscript.
We thank Drs Sung-Kwon Kim and Liisa K. Selin for helpful discussions and Jean Leif for excellent technical assistance.
This work was supported by National Institutes of Health research grants AI-17672, AR-35506, AI-46629, AI-42669; a fellowship from the Charles A. King Trust, Bank of America, Co-Trustee, Boston, MA (M.A.B.); Juvenile Diabetes Research Foundation grant 1-2002-396 (T.G.M.); and an institutional Diabetes Endocrinology Research Center (DERC) grant DK52530.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal