Abstract
p38 MAP kinase in human platelets is activated by platelet agonists including thrombin, thromboxane A2 (TxA2), ADP, and others. However, both upstream mechanisms of p38 MAP kinase activation, and their downstream sequelae, are presently controversial and essentially unclear. Certain studies report sequential activation of cGMP-dependent protein kinase (PKG) and p38/ERK pathways by platelet agonists, leading to integrin activation and secretion, whereas others establish an essential role of Src/ERK-mediated TxA2 generation for fibrinogen receptor activation in human platelets. Here, we show that ADP secreted from platelet-dense granules, and subsequent activation of P2Y12 receptors, as well as TxA2 release are important upstream mediators of p38 MAP kinase activation by thrombin. However, p38 MAP kinase activation did not significantly contribute to calcium mobilization, P-selectin expression, αIIbβ3 integrin activation, and aggregation of human platelets in response to thrombin. Finally, PKG activation did not stimulate, but rather inhibited, p38 MAP kinase in human platelets.
Introduction
In vivo, circulating platelets are continually exposed to both adhesive and/or activating factors (fibrinogen, ADP, von Willebrand factor [VWF], thrombin, TxA2, etc), as well as inhibitory factors such as endothelium-derived nitric oxide (NO), prostacyclin (PG-I2), and ADPase.1 Most of these activating and inhibitory molecules bind to specific platelet receptors and stimulate signaling pathways that promote or inhibit platelet adhesion, aggregation, and secretion. A central role among platelet-activating factors is played by ADP, which induces multiple platelet responses and potentiates platelet aggregation caused by other agonists.2,3 ADP is released from platelet-dense granules upon activation by agonists such as thrombin and collagen, and binds to purinergic receptors (P2Y1, P2Y12, P2X1) to reinforce platelet aggregation and thrombus formation.2,4-6
Recently, new mechanisms for agonist-induced platelet activation and secretion were proposed to involve sequential activation of cGMP-dependent protein kinase (PKG)/p38/integrin or Akt/eNOS/sGC/cGMP/PKG secretion, respectively,7-9 whereas others indicate an essential role of Src/ERK-mediated TxA2 generation for fibrinogen receptor activation in human platelets.10 We11,12 and others10,13 were unable to reproduce the reported ERK activation by PKG. However, both the upstream mechanisms of p38 activation, and their downstream effects, are presently controversial and unclear. Here, we show that ADP secreted from platelet-dense granules, and subsequent activation of P2Y12 receptors, as well as TxA2 secretion are important mediators upstream of thrombin-evoked p38 activation. Furthermore, PKG activation does not stimulate, but rather inhibits, thrombin-evoked p38 activation, which alone has no significant effect on platelet stimulation/aggregation.
Materials and methods
Analysis of P-selectin expression, αIIbβ3 activation, aggregation, and intracellular calcium measurement
Platelets were isolated from whole blood obtained from healthy volunteers as described previously.11 Approval for this study was obtained from the local ethics committee of the University of Würzburg, and informed consent was provided according to the Declaration of Helsinki. For the determination of P-selectin expression, washed platelets were stained with RPE-conjugated anti-CD62P antibody, and for αIIbβ3 activation, with FITC-PAC-1 antibody. Aggregation was carried out using a Biodata PAP-4 aggregometer (Alpha Laboratories, Eastleigh, United Kingdom) with platelet-rich plasma, and calcium transients were determined with the fluorescence indicator Fura-2, as previously described.4,11
Analysis of data
All experiments were performed at least in triplicate and data shown are mean ± SEM. Differences between groups were analyzed by analysis of variance (ANOVA) followed by Bonferroni test. P values less than .05 were considered statistically significant.
Results and discussion
The purpose of this study is to characterize the mechanisms and functional consequences of both PKG and p38 activation in human platelets, about which conflicting reports exist. Human platelet agonists (thrombin, TxA2, ADP, collagen) are well known to induce p38 activation,14 but their interdependence for producing this effect has not been established. Therefore, we investigated the role of ADP in thrombin-stimulated platelet p38 activation, P-selectin expression, and integrin activation. Apyrase dose-dependently inhibited thrombin-induced effects such as p38 activation, phosphorylation of Hsp27 (a distal target downstream of p38), P-selectin expression (Figure 1A), as well as integrin αIIbβ3 activation (data not shown). Therefore, secreted ADP is, at least partially, the mediator of these thrombin effects that were also inhibited by a P2Y12 receptor antagonist (AR-C69931MX) but not by a P2Y1 receptor antagonist (MRS21799). These data support the mediating role of secreted ADP and its P2Y12 receptor in these thrombin effects. Consistent with this conclusion, as well as with previously published data,15 a selective P2X1 receptor agonist (α, β-MeATP, 2.5 μM) failed to cause any p38 phosphorylation (data not shown). Preincubation of platelets with indomethacin also inhibited thrombin-stimulated p38 activation, Hsp27 phosphorylation, and P-selectin expression to a similar extent as that observed with AR-C69931MX (Figure 1A). Indomethacin and AR-C69931MX had additive inhibitory effects on thrombin-stimulated p38 activation, Hsp27 phosphorylation, and P-selectin expression. p38 was slightly activated by either ADP (20 μM) or a thromboxane receptor agonist U46619 (1 μM) used alone, whereas an ADP/U46619 combination activated p38 to a similar extent as low-dose thrombin (data not shown). These results indicate that activation of P2Y12 (but not P2Y1 or P2X1) and thromboxane receptors by secreted ADP and TxA2, respectively, plays a significant amplifying role in p38 activation by low-dose thrombin. However, potent platelet agonists (ie, high thrombin concentration, 0.01 U/mL) did not require the amplifying mechanisms of secondary ADP/TxA2 secretion for p38 activation, since AR-C69931MX or indomethacin had no inhibitory effects on the response of potent platelet agonists (data not shown). Similar observations have been made in the case of ERK activation.16
Mechanisms and functional consequences of p38 activation in thrombin-stimulated platelets. (A) Thrombin-induced p38 phosphorylation is mediated by ADP and TxA2 secretion. Washed human platelets (4 × 108/mL) were preincubated for 5 minutes with either buffer alone, apyrase (0.1, 1, and 2 U/mL), AR-C (50 and 100 nM), MRS (100 μM), indomethacin (Ind, 5 μM), or AR-C (50 nM) and indomethacin (5 μM) together, and then stimulated with thrombin (0.005 U/mL) for 1 minute. Samples were then analyzed for P-selectin expression (fluorescence-activated cell sorter [FACS]), and phosphorylation of VASP (serine 239), p38, and Hsp27 (VASP-P, p38-P, Hsp27-P) on Western blots using phosphospecific antibodies. Data for P-selectin (mean ± SEM) are shown as fold increase with respect to the effect of thrombin alone designated as 1 (*significantly different at P < .05 compared with thrombin; n = 5). p38 inhibitors had no significant effect on thrombin-induced P-selectin expression (B-C), aggregation (D), and intracellular calcium mobilization (E). (B-C) Washed human platelets were preincubated with either DMSO, SNP (10 μM), p38 inhibitors SB203580 (20 μM) and SB202190 (20 μM), or control compound SB202474 (20 μM) for 5 minutes. Platelets were then stimulated with thrombin (0.005 U/mL [B] or 0.01 U/mL [C]). Data, expressed in arbitrary units for P-selectin, represent mean ± SEM for 4 independent experiments, with thrombin-stimulated samples designated as 1 (*significantly different at P < .05 compared with thrombin). Also shown are effects on VASP-P (Ser239), p38-P, and Hsp27-P obtained in at least 3 independent experiments. (D) Washed platelets were assayed for thrombin (0.005 U/mL)–induced aggregation in a turbidometric aggregometer. Platelets were preincubated for 5 minutes with RGDS (1 mM), SNP (10 μM), p38 inhibitors (SB203580, SB202190, 20 μM), a control (inactive) compound (SB202474, 20 μM), or DMSO, and then stimulated with thrombin. (E) Under similar experimental conditions, the effect of p38 inhibitors and the control compound on intracellular calcium was analyzed in platelets activated by 0.005 U/mL thrombin. Representative aggregation and calcium tracings from 3 independent experiments are shown.
Mechanisms and functional consequences of p38 activation in thrombin-stimulated platelets. (A) Thrombin-induced p38 phosphorylation is mediated by ADP and TxA2 secretion. Washed human platelets (4 × 108/mL) were preincubated for 5 minutes with either buffer alone, apyrase (0.1, 1, and 2 U/mL), AR-C (50 and 100 nM), MRS (100 μM), indomethacin (Ind, 5 μM), or AR-C (50 nM) and indomethacin (5 μM) together, and then stimulated with thrombin (0.005 U/mL) for 1 minute. Samples were then analyzed for P-selectin expression (fluorescence-activated cell sorter [FACS]), and phosphorylation of VASP (serine 239), p38, and Hsp27 (VASP-P, p38-P, Hsp27-P) on Western blots using phosphospecific antibodies. Data for P-selectin (mean ± SEM) are shown as fold increase with respect to the effect of thrombin alone designated as 1 (*significantly different at P < .05 compared with thrombin; n = 5). p38 inhibitors had no significant effect on thrombin-induced P-selectin expression (B-C), aggregation (D), and intracellular calcium mobilization (E). (B-C) Washed human platelets were preincubated with either DMSO, SNP (10 μM), p38 inhibitors SB203580 (20 μM) and SB202190 (20 μM), or control compound SB202474 (20 μM) for 5 minutes. Platelets were then stimulated with thrombin (0.005 U/mL [B] or 0.01 U/mL [C]). Data, expressed in arbitrary units for P-selectin, represent mean ± SEM for 4 independent experiments, with thrombin-stimulated samples designated as 1 (*significantly different at P < .05 compared with thrombin). Also shown are effects on VASP-P (Ser239), p38-P, and Hsp27-P obtained in at least 3 independent experiments. (D) Washed platelets were assayed for thrombin (0.005 U/mL)–induced aggregation in a turbidometric aggregometer. Platelets were preincubated for 5 minutes with RGDS (1 mM), SNP (10 μM), p38 inhibitors (SB203580, SB202190, 20 μM), a control (inactive) compound (SB202474, 20 μM), or DMSO, and then stimulated with thrombin. (E) Under similar experimental conditions, the effect of p38 inhibitors and the control compound on intracellular calcium was analyzed in platelets activated by 0.005 U/mL thrombin. Representative aggregation and calcium tracings from 3 independent experiments are shown.
p38 has been shown to mediate platelet adhesion in static as well as flow conditions on low-collagen-density surfaces,17 and platelet aggregation induced by low collagen, U46619,18 and thrombin8 concentrations. However, these studies relied on the use of p38 inhibitors (SB202190, SB203580), but unfortunately did not use appropriate controls such as the inactive analog SB202474.19
In low-dose (0.005 U/mL) thrombin-stimulated platelets, neither of the p38 inhibitors (SB202190, SB203580) prevented phosphorylation of p38 itself, but they strongly inhibited phosphorylation of Hsp27, whereas the control compound (SB202474) had no effect on Hsp27 phosphorylation (Figure 1B-C). Surprisingly, p38 inhibitors under these conditions did not significantly inhibit P-selectin expression (Figure 1B-C) and integrin activation (data not shown). Furthermore, the cGMP elevating agent SNP inhibited P-selectin expression and p38 phosphorylation (Figure 1B). In contrast to some published data,8 p38 inhibitors caused only moderate, unspecific effects in our hands, since both p38 inhibitors as well as the inactive analog had very little if any effect even on low (0.005 U/mL) thrombin-induced platelet aggregation that was inhibited by the αIIbβ3 integrin antagonist RGDS (1 mM) and SNP (10 μM) (Figure 1D). Similar p38 inhibitor effects were observed with respect not only to thrombin-stimulated P-selectin expression (Figure 1B-C) and platelet aggregation (Figure 1D) but also to intracellular calcium levels (Figure 1E). These results are in agreement with data obtained with other, more selective, second-generation p38 inhibitors that did not affect human platelet aggregation caused by a wide range of agonists.20 Therefore, we conclude that p38 does not play a significant role in thrombin-induced activation and aggregation of human platelets.
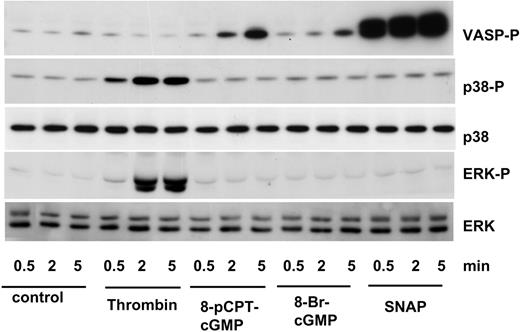
Recent investigations proposed the sequential activation of p38 and ERK pathways by PKG7,8,21 as a novel “roadway” to αIIbβ3 activation.22 These studies with human platelets again heavily relied on the use and functional effects of PKG inhibitors that (similar to p38 inhibitors) need to be used with caution and proper controls including nucleotides inactive with respect to PKG activation.11,23 Here, we performed experiments with 2 cGMP analogs (8-pCPT-cGMP and 8-Br-cGMP), and with a cGMP-elevating NO donor, SNAP, to investigate the role of cGMP and PKG on p38 and ERK activation (Figure 2). Incubation of platelets with 8-pCPT-cGMP, 8-Br-cGMP, or SNAP time-dependently increased VASP serine239 phosphorylation, a validated marker of PKG activation.11,23 However, neither cGMP analogs nor cGMP-elevating agents increased p38 or ERK phosphorylation (Figure 2) that was clearly observed with thrombin (0.01 U/mL) used as a positive control. Furthermore, SNP (10 μM) inhibited thrombin-stimulated p38 as well as Hsp27 phosphorylation in human platelets (Figure 1B), in agreement with earlier published data.24
p38 and ERK are not activated by cGMP-dependent protein kinase (PKG). Washed human platelets were incubated with thrombin (0.01 U/mL), 8-pCPT-cGMP (200 μM), 8-Br-cGMP (200 μM), or SNAP (100 μM) for 0.5, 2, or 5 minutes, and analyzed for VASP (VASP-P Ser239), p38 (p38-P), and ERK (ERK-P) phosphorylation by Western blotting. p38 and ERK proteins served as loading control. The blots shown are representative of 3 individual experiments.
p38 and ERK are not activated by cGMP-dependent protein kinase (PKG). Washed human platelets were incubated with thrombin (0.01 U/mL), 8-pCPT-cGMP (200 μM), 8-Br-cGMP (200 μM), or SNAP (100 μM) for 0.5, 2, or 5 minutes, and analyzed for VASP (VASP-P Ser239), p38 (p38-P), and ERK (ERK-P) phosphorylation by Western blotting. p38 and ERK proteins served as loading control. The blots shown are representative of 3 individual experiments.
In summary, here we demonstrate that p38 is not activated, but rather inhibited, by PKG activation. In thrombin-stimulated human platelets, secreted ADP and TXA2, but not cGMP/PKG, are important mediators upstream of p38 activation. Finally, p38 activation per se does not significantly contribute to thrombin-stimulated calcium mobilization, integrin activation, and aggregation of human platelets. Therefore, p38 appears to be involved in other platelet functions that remain to be elucidated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.J.B. performed platelet experiments and analysis and wrote initial paper drafts; S.G. is the PhD advisor of A.J.B., performed analysis of data, and was involved in paper writing; J.G. provided advice in design and interpretation of platelet calcium experiments; N.R. performed platelet experiments and analysis; S.R. provided advice and help in platelet experiments; and U.W. provided the initial plan of the work, was the PhD advisor of A.J.B., performed analysis of data, and wrote/finalized the paper.
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB688).

![Figure 1. Mechanisms and functional consequences of p38 activation in thrombin-stimulated platelets. (A) Thrombin-induced p38 phosphorylation is mediated by ADP and TxA2 secretion. Washed human platelets (4 × 108/mL) were preincubated for 5 minutes with either buffer alone, apyrase (0.1, 1, and 2 U/mL), AR-C (50 and 100 nM), MRS (100 μM), indomethacin (Ind, 5 μM), or AR-C (50 nM) and indomethacin (5 μM) together, and then stimulated with thrombin (0.005 U/mL) for 1 minute. Samples were then analyzed for P-selectin expression (fluorescence-activated cell sorter [FACS]), and phosphorylation of VASP (serine 239), p38, and Hsp27 (VASP-P, p38-P, Hsp27-P) on Western blots using phosphospecific antibodies. Data for P-selectin (mean ± SEM) are shown as fold increase with respect to the effect of thrombin alone designated as 1 (*significantly different at P < .05 compared with thrombin; n = 5). p38 inhibitors had no significant effect on thrombin-induced P-selectin expression (B-C), aggregation (D), and intracellular calcium mobilization (E). (B-C) Washed human platelets were preincubated with either DMSO, SNP (10 μM), p38 inhibitors SB203580 (20 μM) and SB202190 (20 μM), or control compound SB202474 (20 μM) for 5 minutes. Platelets were then stimulated with thrombin (0.005 U/mL [B] or 0.01 U/mL [C]). Data, expressed in arbitrary units for P-selectin, represent mean ± SEM for 4 independent experiments, with thrombin-stimulated samples designated as 1 (*significantly different at P < .05 compared with thrombin). Also shown are effects on VASP-P (Ser239), p38-P, and Hsp27-P obtained in at least 3 independent experiments. (D) Washed platelets were assayed for thrombin (0.005 U/mL)–induced aggregation in a turbidometric aggregometer. Platelets were preincubated for 5 minutes with RGDS (1 mM), SNP (10 μM), p38 inhibitors (SB203580, SB202190, 20 μM), a control (inactive) compound (SB202474, 20 μM), or DMSO, and then stimulated with thrombin. (E) Under similar experimental conditions, the effect of p38 inhibitors and the control compound on intracellular calcium was analyzed in platelets activated by 0.005 U/mL thrombin. Representative aggregation and calcium tracings from 3 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-07-038158/4/m_zh80020706430001.jpeg?Expires=1769136647&Signature=Ma~yJE4PUOOpnItOnTtveH53qYSIiGyc9Q6hz7SwE37elm0zk14-tRBuOziPXTnLhz-mhtGqII0pm0cTolDX~nd7Ms4HciJfnh-aOnXBIr7ytr9P50Rya2Cxt0AA8-QP6x1~aa5NxJ9nU~kjo1rKTmmfsqYjSHysuSPqrWe00FBorqLMB6IPeff44jMO2n6D7IaB~H3ZVqewVPyM-Uc2l6~nTGLSaPIuFxZT30r1-O9Vr31vIcFjYFACehXqq0pXWOUOpHIC3HvR5kgBi6eY6XSWe~4Np1FZ3CS8fHg6fzsz7fDIU6uuUjizB6vacCVBmy5iT8pQTouPQxmcg1Z7ew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal