Abstract
Disturbances of blood flow at sites of atherosclerotic plaque rupture are one of the key pathogenic events promoting platelet activation and arterial thrombus formation. Shear effects of platelets have been extensively investigated in vitro; however, the mechanisms by which shear promotes platelet aggregation in vivo remain incompletely understood. By employing high-resolution imaging techniques to in vitro and in vivo thrombosis models, we demonstrate a unique mechanism initiating shear-dependent platelet aggregation involving aggregate formation between discoid platelets. These discoid platelet aggregates are initially unstable and result from the development of membrane tethers between coadhering platelets. Tether formation involves the adhesive function of GPIb/V/IX and integrin αIIbβ3, and conversion of discoid platelet aggregates into stable aggregates requires released ADP. The efficiency of this process is regulated by 3 independent variables, including the reactivity of the adhesive substrate, the level of shear flow, and the platelet density at the adhesive surface. These studies identify a new mechanism initiating platelet aggregation that is critically influenced by shear, physical proximity between translocating platelets, and membrane tether formation. Moreover, they provide a model to explain how the discoid morphology of platelets facilitates the maintenance of adhesive interactions with thrombogenic surfaces under high shear stress conditions.
Introduction
Excessive accumulation of platelets at sites of atherosclerotic plaque rupture is one of the key pathogenic events precipitating arterial thrombus formation, leading to acute myocardial infarction, sudden death, and ischemic stroke. This pathological process is responsible for more morbidity and mortality than any other disease process and, as a consequence, the platelet represents a major target for therapeutic intervention. Several factors contribute to the potent platelet-activating properties of ruptured plaques, including the high content of fibrillar collagens in the lesion,1-3 the presence of tissue factor,4 as well as the direct platelet-activating effects of high shear stress caused by arterial narrowing.5-8 Rheologic disturbances at sites of arterial stenosis are dynamic and complex and are highly influenced by the level of narrowing and altered geometry of the vascular lumen. Nonetheless, high shear is an inevitable consequence of progressive vascular occlusion, establishing a potentially hazardous cycle of further platelet activation and thrombus growth.
The mechanisms underlying platelet aggregation and thrombus formation have been extensively investigated and are highly influenced by the prevailing blood flow conditions. Under conditions of relatively low shear (0 to 1000 s−1), platelet aggregation is primarily mediated by soluble fibrinogen, which physically crosslinks platelets through engagement of integrin αIIbβ3.9-11 At progressively higher wall shear rates (1000 to 10 000 s−1) aggregation becomes more von Willebrand factor (VWF) dependent, with fibrinogen playing a supportive role in stabilizing formed aggregates.11 Immobilized VWF on the surface of platelets is indispensable for the initiation of shear-dependent platelet aggregation through its ability to rapidly engage glycoprotein Ib (GPIb). However, this adhesive interaction is rapidly reversible, leading to platelet translocation in the direction of flow. Based on current models, platelets become activated during this process, stimulating integrin αIIbβ3 engagement of VWF and/or fibrinogen, leading to stable platelet aggregation. Recent studies have defined a unique mechanism initiating platelet aggregation that primarily operates at pathological levels of shear (more than 10 000 s−1).12 This aggregation mechanism is exclusively mediated by VWF engagement of GPIb and does not require platelet activation or the ligand binding function of integrin αIIbβ3. While the pathophysiological significance of this new aggregation mechanism remains to be established, it nonetheless suggests that under certain experimental conditions the VWF-GPIb interaction can potentially sustain platelet interactions with thrombogenic surfaces independent of other adhesive interactions.
Central to current models of platelet adhesion under flow is the concept that platelets become activated during interaction with thrombogenic surfaces, stimulating integrin activation and the formation of additional adhesion bonds that serve to reduce cell translocation velocity and promote cell arrest. Such a model has been well established for leukocyte adhesion under flow.13 However, in the context of platelets, this model has potentially important implications for the mechanisms regulating surface translocation and aggregate formation. For example, in contrast to leukocytes, which have a spherical morphology well suited to rotational motion (rolling), platelets have a flat discoid morphology that does not support smooth rolling interactions. One possibility is that signals generated during surface translocation induce morphologic change sufficient to convert discoid platelets into spiny spheres, thereby facilitating a rolling phenotype. Such morphologic changes have previously been described during platelet adhesion to VWF under flow.14-16 An alternative possibility is that platelets principally translocate as flat discs through a rotational side-to-side flipping mechanism or through a sliding mechanism that maximizes surface contact (bond formation) with the adhesive surface. There is experimental evidence supporting the former mechanism15 ; however, the relationship between platelet morphologic change and stable adhesion has not been defined. The potential mechanisms regulating platelet adhesion under flow are further complicated by the finding that platelets form thin membrane tethers during surface translocation on VWF.12,17 Membrane tethers are pulled from the surface of discoid platelets under the influence of hemodynamic drag forces. As such, they are preferentially formed under high shear conditions where they provide mechanical advantage to VWF-GPIb adhesive bonds. However, the physiological significance of membrane tethers has yet to be defined, and it is currently unclear whether these structures primarily participate in platelet-vessel interactions or are also relevant to platelet aggregation.
In the present study we have used high-resolution imaging techniques to in vitro and in vivo thrombosis models to investigate the mechanisms regulating platelet aggregation under conditions of high shear stress. These studies demonstrate that platelets principally translocate on the surface of thrombi in vivo as flat, sliding discs and participate in the platelet aggregation process by forming transient adhesive contacts with other discoid platelets. In vitro perfusion studies suggest that the formation of discoid platelet aggregates involves the generation of membrane tethers between coadhering platelets via adhesive interactions mediated by GPIb and integrin αIIbβ3. We have identified 3 independent variables influencing the efficiency of conversion of reversible discoid platelet aggregates to stable aggregation: the reactivity of the thrombogenic surface, the level of shear stress, and the density of platelets at the adhesive surface. In combination, these factors have a major impact on the rate of platelet aggregation. Overall, our studies define a new mechanism of shear-dependent platelet aggregation that is relevant to thrombus development in vivo. Furthermore, our studies provide a model to explain how the discoid morphology of platelets facilitates the maintenance of adhesive interactions with thrombogenic surfaces under conditions of high shear stress.
Materials and methods
Materials
Human VWF was purified from plasma according to the method of Montgomery and Zimmerman.18 Human fibrinogen was purified from plasma according to the method of Jakobsen and Kierulf.19 Heparin (enoxaparin sodium) was from Aventis Pharma (Sydney, Australia). Hirudin (lepirudin) was from Pharmion (Melbourne, Australia). The c7E3 Fab (abciximab) was from Eli Lilly (Indianapolis, IN). Apyrase, A3P5P, and MRS2179 were from Sigma (St Louis, MO). ARC69931MX was from AstraZeneca (Sydney, Australia). Oregon green 488 BAPTA-1–AM, Fura red–AM, DiOC6, and NP-EGTA were from Molecular Probes (Eugene, OR). All other reagents were from sources previously described.17,20
Intravital studies
Approval was gained from the Monash University Animal Ethics Committee for all experiments involving animals. Intravital studies were performed according to a modified method of Denis et al21 and Kulkarni et al.22 Mesenteric arterioles (30 to 40 μm) of male Wistar rats were injured via photoactivation (550 nm, 10 to 30 seconds) of systemically administered rose bengal (5 mg/kg).23 The dynamics of platelet interactions with the damaged vessel wall and with growing platelet thrombi were viewed by differential interference contrast (DIC) microscopy using a Leica DMIRB microscope (Leica, Wetzlar, Germany; × 100 PL APO objective, NA 1.40 to 0.7) and recorded on video for off-line analysis using a CCD300 ETRCX camera (DAGE MTI, Michigan IN). Video was digitized using Pinnacle Systems DV500 Plus software (Pinnacle Systems, Mountain View, CA), and was edited using Adobe Premiere Pro version 1.5 (Adobe Systems, Chatsworth, Australia). Annotations and lines in Figure 1 were added using CorelDraw version 12 (Corel, Sydney, Australia).
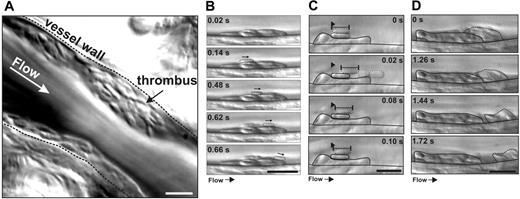
Dynamics of platelet thrombus formation in vivo. Mesenteric arterioles of rats were injured via photoactivation of systemically administered rose bengal, and platelet interactions with growing thrombi were visualized by DIC microscopy. (A) This single image (representative of 12) highlights the ability of this imaging technique to resolve single platelets during thrombus formation in vivo (see Video S2 for analysis of thrombus development in real time). (B) Representative example of a newly recruited platelet translocating over the thrombus surface with a sliding motion, maintaining maximal surface area contact (Video S2). (C) A single adherent platelet (dotted outline) extending and recoiling from a fixed point of contact over the surface of another stationary adherent platelet. Such behavior was often induced by transient contacts made with other translocating platelets (0.02 seconds, dotted outline) (Video S3). (D) Platelets within the superficial layer of thrombi were loosely packed such that platelets often detached from the thrombus surface in clusters (Video S2). White scale bar = 5 μM; black scale bar = 10 μM.
Dynamics of platelet thrombus formation in vivo. Mesenteric arterioles of rats were injured via photoactivation of systemically administered rose bengal, and platelet interactions with growing thrombi were visualized by DIC microscopy. (A) This single image (representative of 12) highlights the ability of this imaging technique to resolve single platelets during thrombus formation in vivo (see Video S2 for analysis of thrombus development in real time). (B) Representative example of a newly recruited platelet translocating over the thrombus surface with a sliding motion, maintaining maximal surface area contact (Video S2). (C) A single adherent platelet (dotted outline) extending and recoiling from a fixed point of contact over the surface of another stationary adherent platelet. Such behavior was often induced by transient contacts made with other translocating platelets (0.02 seconds, dotted outline) (Video S3). (D) Platelets within the superficial layer of thrombi were loosely packed such that platelets often detached from the thrombus surface in clusters (Video S2). White scale bar = 5 μM; black scale bar = 10 μM.
Preparation of washed platelets and red blood cells
Blood was collected from healthy volunteers who had given informed consent, with the approval of the Monash University Human Ethics Committee. Washed platelets were prepared as described previously.24 Platelets were suspended at a concentration of 300 × 106/mL in modified Tyrode buffer (12 mM NaHCO3, 10 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 5.5 mM d-glucose, 1 mM CaCl2, 0.5 mg/mL BSA) containing 0.02 U/mL apyrase. Red blood cells (RBCs) were prepared as described previously.25 To inhibit residual thrombin and secreted ADP, the packed RBC preparation was supplemented with 200 U/mL hirudin and 0.02 U/mL apyrase.
In vitro perfusion studies
Perfusion assays were performed according to a modified method of Cooke et al.26 Microcapillary tubes (VitroCom, Mountain Lakes, NJ) were coated with either purified VWF (50 μg/mL) or a mixture of VWF and fibrinogen (10 μg/mL VWF and 5, 10, 20, or 50 μg/mL fibrinogen) and then blocked with 5% human serum (supplemented with 50 μg/mL phenylmethylsulfonylfluoride) prior to experimentation. Either whole blood (anticoagulated with 400 U/mL hirudin) or washed platelets (150 × 106/mL) reconstituted with packed red blood cells (0.45 [45%] hematocrit) were perfused through microcapillary tubes at a shear rate of 600, 1800, or 5000 s−1. Where indicated, platelets were pretreated with c7E3 Fab (20 μg/mL), ARC69931MX (1 μM), A3P5P (1 mM), MRS2179 (100 μM), or PGE1 (0.5 μg/mL) prior to perfusion. Adherent platelets were visualized by DIC microscopy (× 100 PL APO objective, NA 1.40 to 0.7) on a Leica DMIRB microscope. Adhesion was monitored over a 250-second period and videorecorded for off-line analysis. At 15-, 30-, 45-, 60-, 90-, and 120-second time points, the number of adherent platelets within an optical field (dimensions, 70 μm × 90 μm) was counted. An aggregate was defined as a cluster of at least 5 platelets maintaining physical contact for at least 3 seconds. Aggregate formation was quantitated by counting the number of aggregates present within a field of view and recording the number of platelets within individual aggregates. An aggregate was classified as “unstable” if it was composed of reversibly adherent platelets. A “stable” aggregate was classified as one composed of irreversibly adherent platelets.
In some experiments, washed platelets reconstituted with RBCs were perfused over a mixed VWF/fibrinogen matrix for 15 seconds to allow a small number of platelets to adhere (approximately 6 to 7 platelets per 70 μm × 90 μm field). Modified Tyrode buffer was perfused for a further 2 minutes to allow these platelets to adhere irreversibly and begin to spread. Washed platelets pretreated with either c7E3 Fab (20 μg/mL) or cytochalasin D (5 μM) were reconstituted with RBCs as described and then perfused over the adherent platelets. DIC microscopy was used to visualize subsequent interactions with the preadhered platelets and video recorded as described in “Intravital studies.”
Platelet adhesion to monolayers
Blood was collected from healthy donors or individuals with Glanzmann thrombasthenia (1% αIIbβ3). Confluent platelet monolayers (more than 90% surface coverage) were obtained by allowing washed platelets (200 × 106/mL) to spread (30 minutes, 37°C) on glass microcapillary tubes. Anticoagulated whole blood (15 mM trisodium citrate, pH 7.4) was incubated with the fluorescent dye DiOC6 (1 μM, 10 minutes) and then perfused over preformed platelet monolayers at 1800 s−1 for 1 minute. The interaction of flowing platelets with preformed platelet monolayers was viewed in real time using fluorescence microscopy and recorded on video for off-line analysis. Video was digitized as described in “Intravital studies.” The number of platelets tethering to the surface of monolayers was analyzed frame by frame (25 frames per second) over the first 5 to 10 seconds of flow. In all studies, any cell forming an adhesion contact for more than 40 milliseconds was scored as an adherent platelet.
Analysis of cytosolic calcium flux under flow conditions
Changes in intracellular calcium levels were monitored according to previously published methods.27,28 Briefly, platelets in platelet-washing buffer (PWB) (1 × 109/mL) were loaded with Oregon green 488 BAPTA-AM (1 μM, emission wavelength 500 to 570 nm) and Fura red–AM (1.25 μM, emission wavelength 600 to 710 nm) for 30 minutes at 37°C. Calcium dye–loaded platelets were subsequently incubated in PWB with 10 μM NP-EGTA for 30 minutes at 37°C. NP-EGTA–treated platelets were washed once with PWB and resuspended in modified Tyrode buffer prior to experimentation. Platelets were combined with RBCs (to a final concentration of 150 × 106/mL or 20 × 106/mL) for perfusion studies as described in “In vitro perfusion studies.” To examine changes in calcium flux, sequential images of adherent platelets were captured at a scan rate of 0.586 frames per second for 200 frames. Real-time calcium flux was calculated based on a ratio of signal intensity between the 2 dye channels and converted to intracellular calcium concentrations as described previously.27 NP-EGTA uncaging was carried out following 18 seconds of reconstituted blood flow via exposure of platelets to a near-UV (300 to 400 nm) light source generated by a 100 W mercury lamp directed through the optical path of a Leica DMIRBE confocal microscope for an interval of 0.6 seconds. Control studies were carried out with unloaded control platelets and demonstrated that the brief UV exposure did not lead to photodynamic damage or activation of the platelets under flow. Images in Figure 6B show the fluorescence intensity from the Oregon green channel, generated using Leica software.
Scanning electron microscopy
Translocating platelets were fixed and prepared for scanning electron microscopy (SEM) imaging as described previously.29 Platelets were imaged on a Hitachi S570 scanning electron microscope (Tokyo, Japan) at 20 kV accelerating voltage, 3 mm working distance.
Statistical analysis and computer programs
Results
Analysis of platelet morphology during surface translocation in vivo
Platelets have been demonstrated to translocate on a VWF matrix in vitro as flat discs or as spiny spheres.14-16 To examine which of these morphologies is most relevant to thrombus growth in vivo, we established a high-magnification DIC imaging technique that has sufficient optical resolution to identify the morphology of individual platelets in the rat microcirculation (refer to Videos S1–S6 [available on the Blood website; see the Supplemental Materials link at the top of the online article], videos examining platelet thrombus formation in vivo, for full appreciation of the dynamics of platelet aggregation). Vascular injury of mesenteric arterioles was induced by photoactivation of systemically administered rose bengal, a technique that induces vascular injury leading to the development of small nonocclusive thrombi.22 Thrombi typically formed over a 1- to 5-minute period, and a characteristic feature of thrombus growth in this model is the high proportion of translocating platelets on the thrombus surface.22 As demonstrated in Figure 1A, most platelets (more than 95%) translocating on either the injured vessel wall or the surface of forming thrombi retained their discoid morphology (Video S1). While a small subset of platelets translocated in a flip-flop side-to-side motion, most typically translocated in a sliding motion, maintaining maximal surface contact with the thrombus surface (Figure 1B). Only a few translocating platelets (less than 1%) exhibited a spherical morphology. Furthermore, the platelets residing in the superficial layers of the thrombi retained their discoid shape and appeared to be loosely adherent. Sliding discoid platelets were a cardinal feature of thrombus development irrespective of the extent of vascular injury. While the optical resolution of the system was insufficient to enable clear visualization of individual membrane tethers, their presence was implied by the finding that many discoid platelets displayed free movement or even rotation around a single tethered point (Video S3). Furthermore, individual platelets were observed to extend and recoil from a single contact point (Figure 1C), a characteristic feature of membrane-tethered platelets17 (Video S3). The distance that the discoid body of an adherent platelet extended from the single attachment point progressively increased, reaching up to 3 μm (mean, 1.15 μm; range, 0.5 to 2.8 μm). As demonstrated in Figure 1C, despite no visible physical contact between their outer circumferential membranes, these platelets were clearly attached, as drag forces on downstream platelets were always transmitted to tethered upstream platelets (Figure 1C, 0.02 seconds), leading to coordinated propulsive and retractive platelet movement on the thrombus surface. As such, platelets in the superficial layers of forming thrombi were initially loosely tethered to each other such that under the influence of hemodynamic drag forces these cells readily detached from the thrombus surface, either as individual cells or small clusters of loose platelet aggregates (Figure 1D; Video S2). These findings indicate that most platelets retain their discoid morphology during initial aggregate formation in vivo. Furthermore, they suggest a potentially important role for membrane tethers in regulating the initial reversible interaction between platelets.
Membrane tethers regulate reversible aggregation of discoid platelets
To investigate the potential relationship between membrane tethers and reversible aggregation of discoid platelets, in vitro perfusion studies were performed on a VWF matrix. By performing perfusion studies using anticoagulated whole blood and imaging adherent platelets with high-resolution DIC microscopy, we were able to monitor in real time the morphology of platelets throughout the entire adhesion/aggregation process without the need for platelet isolation or fluorescent membrane labeling. As shown in Figure 2A-B, adhesion contacts between discoid platelets and the VWF matrix were initially mediated via thin membrane tethers and, similar to our in vivo findings, we observed that most of the initial platelet-platelet interactions occurred between discoid platelets and did not require platelet sphering during the translocation process. These initial platelet-platelet interactions were always reversible and as a consequence were associated with the development of unstable platelet aggregates (Video S4). High-magnification SEM imaging confirmed that the adhesion contacts between aggregating platelets were mediated through the development of thin membrane tethers (Figure 2C). Similar findings were observed with either whole blood or washed platelets, and under all conditions examined reversible aggregate formation between discoid platelets was dependent on the development of membrane tethers. These studies demonstrate that under physiologically relevant flow conditions, platelet aggregate formation on a VWF matrix is associated with the development of thin membrane tethers from the surface of discoid platelets.
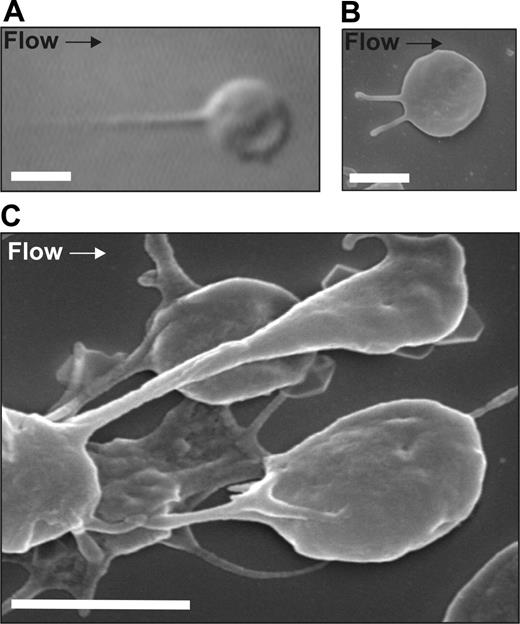
Formation of membrane tethers in vitro. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) at 1800 s−1. (A) DIC image and (B) SEM image of a single platelet forming membrane tethers with immobilized VWF. (C) SEM image of a forming discoid platelet aggregate. This image demonstrates that the adhesion contacts between aggregating platelets are mediated by membrane tethers. Platelets were fixed at 120 seconds and processed for SEM imaging as described in “Scanning electron microscopy.” Scale bar = 2 μm.
Formation of membrane tethers in vitro. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) at 1800 s−1. (A) DIC image and (B) SEM image of a single platelet forming membrane tethers with immobilized VWF. (C) SEM image of a forming discoid platelet aggregate. This image demonstrates that the adhesion contacts between aggregating platelets are mediated by membrane tethers. Platelets were fixed at 120 seconds and processed for SEM imaging as described in “Scanning electron microscopy.” Scale bar = 2 μm.
Effect of matrix reactivity on the reversible aggregation of discoid platelets
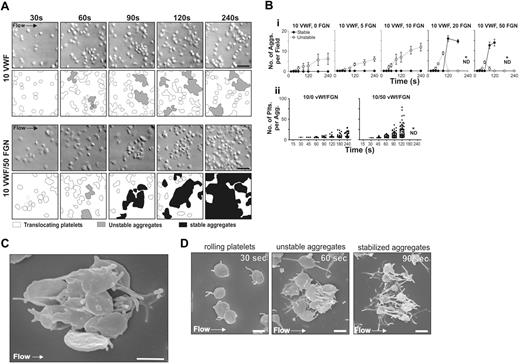
VWF is a relatively weak thrombogenic surface supporting limited platelet aggregation and thrombus growth.30 In vivo, platelets are typically exposed to a range of adhesive proteins at sites of vascular injury with VWF and fibrinogen playing the major role in supporting thrombus growth.31 Previous studies have demonstrated that a combined VWF/fibrinogen matrix is considerably more reactive than matrices prepared with either protein alone.30 To examine the effect of increasing surface reactivity on the dynamics of platelet aggregation, perfusion studies were performed on a mixed matrix composed of a fixed concentration of VWF and increasing concentrations of surface-adsorbed fibrinogen. As demonstrated in Figure 3A-B, perfusing washed platelets in the presence of RBCs over a purified VWF matrix resulted in the development of reversible discoid platelet aggregates with limited conversion to stable aggregates. In contrast, increasing the surface concentration of fibrinogen with VWF increased the reactivity of the matrix, leading to increased stable aggregate formation (Figure 3A-B; Videos S5–S6). As demonstrated in Figure 3Bi, the total number of unstable discoid aggregates was not dramatically altered by the presence of fibrinogen; however, above 10 μg/mL fibrinogen there was a sharp increase in the rate of conversion of unstable aggregates into stable aggregates. Increasing the reactivity of the matrix had 2 major effects on the aggregation process: It significantly shortened the time to formation of both reversible and irreversible aggregates and markedly increased the efficiency of platelet recruitment into stable aggregates, leading to a much greater number of platelets per aggregate at all time points examined (Figure 3Bii). Notably, at the highest concentrations of fibrinogen (50 μg/mL) used, the rapid growth of stable aggregates meant that the number of platelets within individual aggregates could not be accurately determined (Figure 3Bi). In addition, small aggregates often merged together, leading to a corresponding reduction in the total number of aggregates but an increase in aggregate size (Figure 3Bii). Scanning electron microscopy revealed that most platelets initially clustered around a central activated platelet through the formation of thin elongated membrane projections (Figures 2C and 3C). We confirmed that these projections were tethers, rather than filopodia, because they were not prevented by pretreating platelets with the inhibitor of actin polymerization, cytochalasin D (data not shown). Furthermore, SEM imaging confirmed that conversion of reversible aggregates into stable aggregates was associated with platelet sphering and the extension of multiple filopodial projections (Figure 3D). These studies define a major role for matrix reactivity in regulating the initial formation of discoid platelet aggregates and in promoting their conversion to stable aggregates.
Reversible aggregation of discoid platelets in vitro is promoted by increasing matrix reactivity. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and varying concentrations of fibrinogen (5, 10, 20, or 50 μg/mL) at 1800 s−1. (A) DIC images were taken at the indicated time points on a VWF (10 μg/mL) or VWF/fibrinogen matrix (10 μg/mL / 50 μg/mL, respectively). Schematic diagrams highlight nonaggregated platelets (white), reversible aggregates (gray), and stable aggregates (black). Scale bar = 10 μm. On VWF, aggregates remained reversible, while on VWF/fibrinogen reversible aggregates (60 seconds) eventually became stable (90 to 240 seconds). (B) Aggregate formation was quantified by determining the number (i) and size (ii) of aggregates within a visual field. (i) Graph showing the number of unstable (○, broken line) and stable (•, solid line) aggregates. Each point represents the mean ± SEM from at least 4 independent experiments. (ii) Each dot represents the number of platelets within an aggregate, showing data combined from 4 independent experiments (bar represents the median). *ND, quantitation was not performed because multiple small aggregates merged together into larger single aggregates. Scale bar = 10 μm. (C) SEM image demonstrating a typical reversible discoid platelet aggregate forming on a mixed VWF/fibrinogen matrix. Note that all platelets cluster around a central activated platelet through the development of thin membrane tethers. Platelets were fixed at 60 seconds. Scale bar = 2 μm. (D) SEM images demonstrating the sequential platelet morphologic changes associated with shear-dependent platelet aggregation. Platelets were perfused through VWF/fibrinogen-coated microcapillary tubes (10 μg/mL / 50 μg/mL, respectively) at 1800 s−1. These representative images demonstrate discoid platelets forming membrane tethers during surface translocation on the mixed matrix (30 seconds), a typical unstable aggregate composed of several discoid platelets clustered around a central partially spread platelet (60 seconds) and platelets that have undergone classical shape change (sphering and extension of multiple filopodia extensions) during stable aggregate formation (90 seconds). Scale bar = 2 μm.
Reversible aggregation of discoid platelets in vitro is promoted by increasing matrix reactivity. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and varying concentrations of fibrinogen (5, 10, 20, or 50 μg/mL) at 1800 s−1. (A) DIC images were taken at the indicated time points on a VWF (10 μg/mL) or VWF/fibrinogen matrix (10 μg/mL / 50 μg/mL, respectively). Schematic diagrams highlight nonaggregated platelets (white), reversible aggregates (gray), and stable aggregates (black). Scale bar = 10 μm. On VWF, aggregates remained reversible, while on VWF/fibrinogen reversible aggregates (60 seconds) eventually became stable (90 to 240 seconds). (B) Aggregate formation was quantified by determining the number (i) and size (ii) of aggregates within a visual field. (i) Graph showing the number of unstable (○, broken line) and stable (•, solid line) aggregates. Each point represents the mean ± SEM from at least 4 independent experiments. (ii) Each dot represents the number of platelets within an aggregate, showing data combined from 4 independent experiments (bar represents the median). *ND, quantitation was not performed because multiple small aggregates merged together into larger single aggregates. Scale bar = 10 μm. (C) SEM image demonstrating a typical reversible discoid platelet aggregate forming on a mixed VWF/fibrinogen matrix. Note that all platelets cluster around a central activated platelet through the development of thin membrane tethers. Platelets were fixed at 60 seconds. Scale bar = 2 μm. (D) SEM images demonstrating the sequential platelet morphologic changes associated with shear-dependent platelet aggregation. Platelets were perfused through VWF/fibrinogen-coated microcapillary tubes (10 μg/mL / 50 μg/mL, respectively) at 1800 s−1. These representative images demonstrate discoid platelets forming membrane tethers during surface translocation on the mixed matrix (30 seconds), a typical unstable aggregate composed of several discoid platelets clustered around a central partially spread platelet (60 seconds) and platelets that have undergone classical shape change (sphering and extension of multiple filopodia extensions) during stable aggregate formation (90 seconds). Scale bar = 2 μm.
Effect of shear on reversible platelet aggregation
Membrane tether formation in primary adherent platelets is regulated by shear,17 raising the possibility that reversible aggregate formation between discoid platelets may represent a shear-dependent phenomena. As demonstrated in Figure 4A, on a mixed VWF/fibrinogen substrate at a wall shear rate of 600 s−1 reversible aggregation between discoid platelets was negligible, with all stable aggregates developing from the recruitment of individual platelets to forming thrombi. Increasing the shear rates to 1800 and 5000 s−1 resulted in a progressive increase in the number of discoid platelet aggregates with conversion of these reversible aggregates to stable aggregates occurring within 50 to 70 seconds of initial adhesion (Figure 4B). These studies demonstrate that the formation of reversible aggregates between discoid platelets is a shear-dependent process, presumably mediated by the extension of membrane tethers.
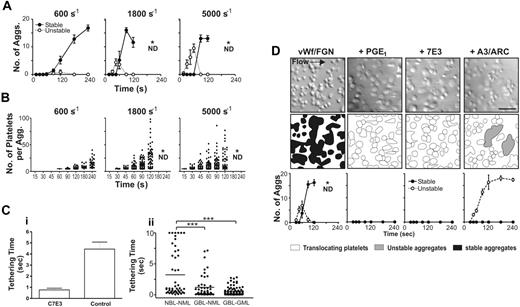
Formation of discoid platelet aggregates is shear dependent and requires platelet and integrin αIIbβ3 activation. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at wall shear rates of 600, 1800, or 5000 s−1. Aggregate formation was quantified by determining the number (A) and size (B) of aggregates within a 70 × 90 μm visual field at the indicated time points over a 240-second period. (A) An aggregate composed primarily of reversibly adherent platelets was classified as “unstable” (○, broken line), while a “stable” aggregate was classified as one composed of irreversibly adherent platelets (•, solid line). Each point represents the mean ± SEM from at least 5 independent experiments. (B) The number of platelets within individual aggregates was counted at the indicated time points, and results show data combined from 5 independent experiments. The bar represents the median. *ND, quantitation was not performed because multiple small aggregates merge together into larger single aggregates. (C) (i) Washed platelets reconstituted with RBCs were perfused over a mixed VWF/fibrinogen matrix for 15 seconds to allow a small number of platelets to adhere. Washed platelets, untreated (control) or pretreated with c7E3 Fab (7E3), were reconstituted with RBCs and then perfused over the adherent platelets. The time that translocating platelets interacted with the preadhered stationary platelets was analyzed (control n = 30, 7E3 n = 16). (ii) Blood was collected from healthy donors (NBL) or individuals with Glanzmann thrombasthenia (GBL) (less than 1% αIIbβ3 by immunoblot) and perfused over confluent platelet monolayers made from normal platelets (NML) or Glanzmann platelets (GML). The interaction of flowing platelets (prelabeled with the fluorescent dye DiOC6) with platelet monolayers was viewed in real time using fluorescence microscopy. The duration of adhesion contacts made by platelets tethering to the surface of monolayers was analyzed as described in “Platelet adhesion to monolayers.” Results show data combined from 3 independent experiments; n = 60; bar represents the mean (***P < .005). (D) Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at 1800 s−1 in the presence of the integrin αIIbβ3 antagonist c7E3 Fab, the activation inhibitor PGE1, or a combination of the P2Y1 and P2Y12 receptor antagonists, A3P5PS and ARC69931MX (A3/ARC). The DIC images shown were taken at 120 seconds of perfusion (scale bar = 10 μm), highlighting the complete inhibition of aggregate formation in the presence of PGE1 and the integrin αIIbβ3 antagonist. In contrast, when ADP receptors were inhibited, reversible aggregates formed but remained unstable (schematic diagrams indicate nonaggregated platelets, white; unstable aggregates, gray; and stable aggregates, black; scale bar = 10 μm). Each point represents the mean ± SEM from at least 4 independent experiments.
Formation of discoid platelet aggregates is shear dependent and requires platelet and integrin αIIbβ3 activation. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at wall shear rates of 600, 1800, or 5000 s−1. Aggregate formation was quantified by determining the number (A) and size (B) of aggregates within a 70 × 90 μm visual field at the indicated time points over a 240-second period. (A) An aggregate composed primarily of reversibly adherent platelets was classified as “unstable” (○, broken line), while a “stable” aggregate was classified as one composed of irreversibly adherent platelets (•, solid line). Each point represents the mean ± SEM from at least 5 independent experiments. (B) The number of platelets within individual aggregates was counted at the indicated time points, and results show data combined from 5 independent experiments. The bar represents the median. *ND, quantitation was not performed because multiple small aggregates merge together into larger single aggregates. (C) (i) Washed platelets reconstituted with RBCs were perfused over a mixed VWF/fibrinogen matrix for 15 seconds to allow a small number of platelets to adhere. Washed platelets, untreated (control) or pretreated with c7E3 Fab (7E3), were reconstituted with RBCs and then perfused over the adherent platelets. The time that translocating platelets interacted with the preadhered stationary platelets was analyzed (control n = 30, 7E3 n = 16). (ii) Blood was collected from healthy donors (NBL) or individuals with Glanzmann thrombasthenia (GBL) (less than 1% αIIbβ3 by immunoblot) and perfused over confluent platelet monolayers made from normal platelets (NML) or Glanzmann platelets (GML). The interaction of flowing platelets (prelabeled with the fluorescent dye DiOC6) with platelet monolayers was viewed in real time using fluorescence microscopy. The duration of adhesion contacts made by platelets tethering to the surface of monolayers was analyzed as described in “Platelet adhesion to monolayers.” Results show data combined from 3 independent experiments; n = 60; bar represents the mean (***P < .005). (D) Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at 1800 s−1 in the presence of the integrin αIIbβ3 antagonist c7E3 Fab, the activation inhibitor PGE1, or a combination of the P2Y1 and P2Y12 receptor antagonists, A3P5PS and ARC69931MX (A3/ARC). The DIC images shown were taken at 120 seconds of perfusion (scale bar = 10 μm), highlighting the complete inhibition of aggregate formation in the presence of PGE1 and the integrin αIIbβ3 antagonist. In contrast, when ADP receptors were inhibited, reversible aggregates formed but remained unstable (schematic diagrams indicate nonaggregated platelets, white; unstable aggregates, gray; and stable aggregates, black; scale bar = 10 μm). Each point represents the mean ± SEM from at least 4 independent experiments.
Role of integrin αIIbβ3 in regulating membrane tether formation and reversible aggregation of discoid platelets
Membrane tether formation can occur on an immobilized VWF matrix in the presence of integrin αIIbβ3 antagonists or on isolated VWF A1 domains,12,17 demonstrating that the VWF-GPIb interaction alone is sufficient to promote this process. To investigate whether the VWF-GPIb interaction was sufficient to support membrane tether anchorage and reversible aggregation of discoid platelets, platelet perfusion studies were performed in the presence of the integrin αIIbβ3 antagonist, c7E3 Fab. Blocking ligand binding to integrin αIIbβ3 completely eliminated membrane tether formation and the development of reversible discoid platelet aggregates. However, this treatment also eliminated stationary adhesion, and in SEM studies (Figure 3C) it was noted that discoid aggregates were often clustered around a central stationary activated platelet. The potential for c7E3 Fab-treated platelets to aggregate around an activated platelet was examined in a 2-stage perfusion assay in which an initial population of untreated washed platelets was allowed to firmly adhere to a VWF/fibrinogen substrate, followed by perfusion of a second population of c7E3 Fab-treated platelets. Under these conditions, translocating discoid platelets interacted only briefly (less than 3 seconds) with the immobilized platelets, preventing membrane tether formation and the development of reversible platelet aggregates (Figure 4C). Similarly, perfusion of c7E3-treated platelets over preformed spread platelet monolayers abolished membrane tether formation and aggregate formation (data not shown). These studies define a major role for integrin αIIbβ3 in supporting the reversible phase of platelet-platelet interactions necessary for membrane tether formation.
Previous studies have suggested a potentially important role for membrane tethers in regulating the transient “stop-phase” of platelet translocation on a VWF matrix.17 To investigate the impact of inhibiting ligand binding of integrin αIIbβ3 on the duration of platelet-platelet adhesion contacts under flow, c7E3-treated platelets were perfused over preformed spread platelet monolayers. Inhibiting ligand binding to integrin αIIbβ3 and the subsequent development of membrane tethers eliminated the stop-phase of surface translocation and dramatically reduced the duration of platelet-platelet adhesion contacts (data not shown). Similar findings were apparent using platelets congenitally deficient in integrin αIIbβ3 (Glanzmann thrombasthenic platelets) (data not shown), and in reciprocal flow experiments, in which normal and GT platelets were perfused over normal and GT monolayers (respectively and vice versa), integrin αIIbβ3 on the surface of both immobilized and flowing platelets contributed to the duration of platelet-platelet adhesion contacts under flow (Figure 4C).
It is known that integrin αIIbβ3 can engage fibrinogen independent of activation.32,33 Therefore, to determine whether reversible aggregate formation between discoid platelets was activation dependent, platelet perfusion studies were performed on a mixed VWF/fibrinogen matrix in the presence of the potent platelet activation inhibitor, PGE1. As shown in Figure 4D, platelet interaction with the matrix was unaffected by PGE1; however, the reversible phase of platelet aggregate formation was completely inhibited. Collectively, these studies define a key role for platelet activation and integrin αIIbβ3 bond formation in supporting the reversible phase of platelet-platelet interactions necessary for membrane tether formation.
Role of ADP in regulating the reversible aggregation of discoid platelets under high shear flow
The activation status of integrin αIIbβ3 during platelet adhesion to immobilized fibrinogen and VWF is partially regulated by ADP. To investigate the requirement for ADP secretion in regulating membrane tether formation and reversible aggregation, perfusion studies were performed in the presence of the ADP receptor antagonists A3P5P (P2Y1 antagonist) and ARC69931MX (P2Y12 antagonist). In contrast to c7E3 Fab, preventing ADP activation of platelets did not inhibit membrane tether formation or the initial reversible aggregation of discoid platelets (Figure 4D). Rather, inhibition of the purinergic receptors led to a progressive increase in the number of unstable aggregates, with most aggregates composed of a small number of loosely tethered platelets (typically fewer than 10 platelets per aggregate). The major effect of blocking the platelet-activating effects of ADP was to prevent the transition from reversible to stable adhesion, leading to unstable platelet aggregation. These studies indicate that ADP is not essential for membrane tether formation and reversible aggregation but is critical for the transition to stable aggregation.
Importance of platelet density in regulating the transition from reversible to stable aggregates
Our studies have indicated that surface reactivity and shear flow are 2 important variables regulating the formation and transition of membrane-tethered platelets into stable aggregates. To investigate whether physical proximity between translocating platelets also plays an important role in this process, perfusion studies were performed at various platelet concentrations. As demonstrated in Figure 5A, at very low platelet counts (5 × 106/mL) there was low surface density of translocating platelets with minimal platelet-platelet interactions and no aggregation. With progressive increases in platelet count there was a concentration-dependent increase in the number of reversible platelet aggregates that peaked at 50 × 106/mL and remained constant up to 800 × 106/mL (Video S6). The major effect of increased platelet count was to reduce the time required for formation of membrane-tethered reversible aggregates from 60 to 90 seconds at concentrations of 20 × 106/mL to 50 × 106/mL to 15 to 30 seconds at 800 × 106/mL. In addition, the lag time for stable aggregate formation was also considerably shortened from 120 seconds at 50 × 106/mL down to 45 seconds at 800 × 106/mL. Analysis of the total number of firmly adherent platelets in stable aggregates as a function of time revealed a steady concentration-dependent increase in firmly adherent platelets up to 150 × 106/mL, which increased markedly at 400 × 106/mL and 800 × 106/mL (Figure 5A). Significantly, a relatively modest increase in the density of platelets at the adhesive surface resulted in a disproportionate rise in the number of platelets incorporated into stable aggregates. For example, at the 60-second time point, the total number of platelets adherent at perfusion densities of 400 × 106/mL and 800 × 106/mL was about 250 and about 325 platelets per field of view, respectively (data not shown), while the number of platelets within stable aggregates was about 100 and about 300 platelets per field of view, respectively (Figure 5B). Thus, a 1.3-fold increase in the platelet surface density resulted in a 3-fold increase in those forming stable adhesive contacts. These studies demonstrate that platelet density has a major influence on aggregate formation, with higher platelet density correlating not only with an acceleration of the onset of unstable aggregate formation but also the transition to firmly adherent stable aggregates.
Aggregate formation is promoted by increasing platelet density. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at 1800 s−1. The platelet count was adjusted to 5 × 106/mL, 20 × 106/mL, 50 × 106/mL, 150 × 106/mL, 400 × 106/mL, or 800 × 106/mL. (A) Unstable and stable aggregate formation was quantified by determining the number of aggregates within a 70 × 90 μm visual field over a 240-second period. An aggregate composed primarily of reversibly adherent platelets was classified as “unstable,” while a “stable” aggregate was classified as one composed of irreversibly adherent platelets. (B) Total amount of platelets recruited into stable aggregates. Each point represents the mean ± SEM from 3 independent experiments. *ND, quantitation was not performed because multiple small aggregates merge together into larger single aggregates. (C) Data from panel A were used to determine the time for the initial nucleating platelet to adhere to the VWF/fibrinogen matrix. Data shown are representative of 5 independent experiments.
Aggregate formation is promoted by increasing platelet density. Washed platelets (150 × 106/mL) reconstituted with RBCs were perfused through microcapillary tubes coated with VWF (10 μg/mL) and fibrinogen (50 μg/mL) at 1800 s−1. The platelet count was adjusted to 5 × 106/mL, 20 × 106/mL, 50 × 106/mL, 150 × 106/mL, 400 × 106/mL, or 800 × 106/mL. (A) Unstable and stable aggregate formation was quantified by determining the number of aggregates within a 70 × 90 μm visual field over a 240-second period. An aggregate composed primarily of reversibly adherent platelets was classified as “unstable,” while a “stable” aggregate was classified as one composed of irreversibly adherent platelets. (B) Total amount of platelets recruited into stable aggregates. Each point represents the mean ± SEM from 3 independent experiments. *ND, quantitation was not performed because multiple small aggregates merge together into larger single aggregates. (C) Data from panel A were used to determine the time for the initial nucleating platelet to adhere to the VWF/fibrinogen matrix. Data shown are representative of 5 independent experiments.
Increased platelet density helps sustain platelet nucleating activity necessary for reversible aggregate formation
One of the most striking effects of increased platelet surface density was the enhancement of primary platelet adhesion on the mixed VWF/fibrinogen matrix, leading to the rapid formation of nucleation sites for subsequent platelet aggregation (Video S6). As demonstrated in Figure 5C, the time required for the formation of stable primary platelet adhesion correlated inversely with the platelet surface density such that, in the absence of platelet-platelet interaction, primary adhesion was delayed 4-fold to 6-fold. This effect of platelet surface density was also apparent on a purified VWF matrix, with no firm adhesion apparent in the absence of platelet-platelet interactions (data not shown). To gain insight into the biochemical mechanisms by which platelet-platelet interactions facilitate primary platelet adhesion, cytosolic calcium flux was examined, because there is a close correlation between cytosolic calcium levels and firm platelet adhesion.27 In general, platelet calcium responses can be broadly classified into low-, intermediate-, and high-range calcium responses, with formation of sustained adhesion contacts associated with sustained high-range calcium flux.27 As demonstrated in Figure 6A, at low platelet surface densities (platelet count of 5 × 106/mL), high-range calcium responses were typically transient in nature with the duration of the calcium response correlating with the time of stationary adhesion. In the absence of platelet-platelet interactions, no sustained calcium flux or stable adhesion was observed (Figure 6A). In contrast, at high platelet surface densities (platelet count of 150 × 106/mL), approximately 60% of high calcium responders exhibited a sustained calcium flux (Figure 6A), leading to the development of stable adhesion contacts (adhesion more than 30 seconds). These findings suggest that platelet-platelet interactions help promote sustained cytosolic calcium flux.
Increased platelet density helps sustain calcium flux in aggregating platelets. Washed platelets loaded with the caged calcium chelator NP-EGTA and calcium indicator dyes (see “Analysis of cytosolic calcium flux”) were reconstituted with RBCs (to a final concentration of 150 × 106/mL or 5 × 10 × 6 /mL) and perfused through microcapillary tubes coated with VWF (50 μg/mL) at 1800 s−1. A transient elevation of cytosolic calcium was induced in translocating platelets by uncaging with brief exposure to near UV light. (A) Proportion of high Δ[Ca2+]c platelets (displaying transient or sustained calcium oscillations at a platelet density of 5 × 106/mL or 150 × 106/mL [n = 3]). Notably, all of the cells were observed to undergo transient stationary adhesion events at a platelet density of 5 × 106/mL. (B) Single-channel Oregon green fluorescence images showing translocating platelets prior to uncaging (0 seconds) and the elevation in intracellular calcium levels following UV exposure (1.8 seconds). The left panels show a representative platelet uncaged at low density, where the platelet remains stationary for a period and then continues translocation when calcium levels decrease. The middle panels show a representative platelet at high density, where calcium levels remain elevated after uncaging and the platelet remains stationary and becomes a focal point for recruitment of other platelets into an unstable aggregate (highlighted in the higher-magnification panels on the right-hand side). (C) After uncaging, platelets remained stationary while calcium levels remained elevated. The duration of the stationary period was less in platelets at low density compared with those uncaged at high density (n = 50; bar represents the median; cutoff time was 48 seconds).
Increased platelet density helps sustain calcium flux in aggregating platelets. Washed platelets loaded with the caged calcium chelator NP-EGTA and calcium indicator dyes (see “Analysis of cytosolic calcium flux”) were reconstituted with RBCs (to a final concentration of 150 × 106/mL or 5 × 10 × 6 /mL) and perfused through microcapillary tubes coated with VWF (50 μg/mL) at 1800 s−1. A transient elevation of cytosolic calcium was induced in translocating platelets by uncaging with brief exposure to near UV light. (A) Proportion of high Δ[Ca2+]c platelets (displaying transient or sustained calcium oscillations at a platelet density of 5 × 106/mL or 150 × 106/mL [n = 3]). Notably, all of the cells were observed to undergo transient stationary adhesion events at a platelet density of 5 × 106/mL. (B) Single-channel Oregon green fluorescence images showing translocating platelets prior to uncaging (0 seconds) and the elevation in intracellular calcium levels following UV exposure (1.8 seconds). The left panels show a representative platelet uncaged at low density, where the platelet remains stationary for a period and then continues translocation when calcium levels decrease. The middle panels show a representative platelet at high density, where calcium levels remain elevated after uncaging and the platelet remains stationary and becomes a focal point for recruitment of other platelets into an unstable aggregate (highlighted in the higher-magnification panels on the right-hand side). (C) After uncaging, platelets remained stationary while calcium levels remained elevated. The duration of the stationary period was less in platelets at low density compared with those uncaged at high density (n = 50; bar represents the median; cutoff time was 48 seconds).
To investigate this phenomenon further, we performed perfusion studies on platelets loaded with the caged calcium chelator NP-EGTA. NP-EGTA is a caged calcium chelator that displays a marked increase in its Kd for Ca2+ upon photolysis with near UV light (300 to 400 nm). This reagent can effectively be used to transiently release a relatively large concentration of free Ca2+ in the cytosol within milliseconds of UV activation.34 As demonstrated in Figure 6B, at low platelet densities (platelet count of 5 × 106/mL) the induction of a transient calcium response in translocating platelets resulted in immediate platelet arrest on VWF; however, in all platelets the calcium signal was not sustained, leading to resumption of translocation in 100% of platelets. In contrast, at higher platelet densities (platelet count of 150 × 106/mL), induction of a transient calcium spike in a population of membrane-tethered reversible platelet aggregates triggered the onset of a sustained elevation in calcium levels that was sufficient to support prolonged adhesion of platelets and stable aggregation (Figure 6C). Notably, in the absence of uncaging, platelet-platelet interactions on the VWF matrix were not capable of inducing sustained calcium flux in the forming aggregates and did not lead to stable aggregate formation (Figure 3). Stable aggregate formation following uncaging was ADP dependent, because it was completely inhibited by apyrase (data not shown), suggesting that paracrine stimulation of platelets during initial aggregate formation is critical for sustaining cytosolic calcium responses necessary for stable primary platelet adhesion.
Discussion
It has long been assumed that thrombus development in vivo involves the sequential accrual of individual platelets onto the surface of thrombi, leading to platelet shape change, integrin αIIbβ3 activation, and firm adhesion.10,11,35-37 However, this concept has recently been challenged by the demonstration that under conditions of very high shear (more than 10 000 s−1), thrombus development can involve the initial formation and recruitment of reversible platelet aggregates onto the surface of forming thrombi through a process mediated solely through VWF-GPIb bonds independent of platelet activation.12 Whether a similar mechanism operates under more physiologically relevant shear conditions (1000 to 10 000 s−1) has not been defined. By using high-resolution imaging techniques to in vivo and in vitro thrombosis models, we have been able to identify the temporal sequence of events underlying platelet aggregation at physiological shear rates. We have demonstrated that the first phase of platelet aggregation involves shear-induced formation of membrane tethers between coadhering platelets. This initial phase of aggregation principally involves discoid platelets but is distinct from the mechanism described by Ruggeri and colleagues12 in that it requires platelet activation and the adhesive function of both GPIb and integrin αIIbβ3. The second phase of aggregation is associated with platelet shape change and irreversible platelet adhesion and is critically dependent on the release of ADP. We have demonstrated that the dynamics of this 2-stage aggregation process are critically influenced by 3 variables, including high shear stress, platelet surface density, and matrix reactivity, with the 2 former variables regulating reversible aggregate formation and the latter regulating the transition to stable aggregation. Overall, these studies define the mechanisms regulating platelet aggregation under physiologically relevant high shear flow and provide a mechanistic explanation as to how changes in matrix reactivity, in combination with high shear and platelet surface density, synergistically enhance platelet thrombus growth.
Rudolph Virchow described more than 150 years ago a triad of factors that contribute to pathological thrombosis, including changes in (1) the vessel wall, (2) blood flow, and (3) the constituents of blood.38 In the context of arterial thrombosis, it is well defined that high shear, in combination with enhanced subendothelial matrix reactivity and heightened platelet responsiveness, are major contributors to this process.39-41 While the latter 2 elements have a clearly defined role in thrombogenesis, the mechanisms by which high-shear forces promote platelet activation and aggregation remain less clearly defined. It is well established that shear enhances the adhesive and signaling function of GPIb27,42 and that shear-induced binding of VWF to GPIb is essential for occlusive thrombus formation; however, GPIb-derived signals are weak and only induce limited platelet activation in the absence of other costimuli.24,27,42 Consistent with this, exposing platelets to high shear (up to 10 000 s−1) during surface translocation on VWF does not promote rapid platelet activation or efficient thrombus growth.24 Similarly, exposing platelets in suspension to high shear (up to 10 000 s−1) in the presence of soluble VWF leads to relatively slow development of macroscopic platelet aggregates,43 suggesting that shear per se is a relatively weak thrombogenic stimulus. We have demonstrated here that one of the major effects of shear is to initiate the formation of reversible platelet aggregates by inducing the development of membrane tethers between coadhering platelets. On a weak thrombogenic surface such as VWF, this physical colocalization of platelets is inefficient at inducing platelet activation or stable aggregation independent of exogenous stimuli. As discussed in the following paragraphs, one of the major effects of shear may be to enhance the physical interaction between platelets, thereby increasing the probability of sustained platelet activation by soluble agonists.
Our studies demonstrate a central role for matrix reactivity in regulating the efficiency of conversion of membrane-tethered reversible aggregates into stable aggregates. It has been known for many years that highly thrombogenic surfaces, such as type I or III fibrillar collagens, are efficient at inducing primary platelet adhesion and subsequent aggregation.3,44 Notably, only the primary adherent layer of platelets is in direct physical contact with the collagen fibers, with all subsequent layers of platelets exposed to adhesive proteins expressed on the surface of aggregating platelets. The studies reported here suggest that the surface densities of VWF and fibrinogen are important in influencing the efficiency of stable platelet aggregation. These findings may have clinical relevance, because the concentrations of fibrinogen and VWF used in these experiments are well within pathophysiological levels and, furthermore, elevated plasma levels of either fibrinogen or VWF increase the risk of arterial thrombosis.45-49 Thus, it is tempting to speculate that increased surface adsorption of fibrinogen and VWF on the surface of developing thrombi, particularly under conditions of high shear stress, is one of the key mechanisms enhancing the conversion of discoid platelet aggregates into stable aggregates.
Our studies have defined an important role for platelet surface density in regulating the initial formation of reversible platelet aggregates, a not unexpected finding given that one of the important variables regulating the efficiency of the hemostatic process is the density of platelets at the adhesive surface.50 Notably, increased physical interaction between platelets enhanced platelet aggregate formation by sustaining cytosolic calcium levels in adherent platelets. This is ADP dependent and is likely to represent the phenomenon of intercellular calcium communication, a previously described mechanism propagating activating signals throughout a developing thrombus.20 Thus, shear-dependent tether formation and coclustering of platelets is likely to potentiate platelet aggregate formation by at least 2 mechanisms: by increasing the efficiency of activation between coadhering platelets and by sustaining activation in the primary nucleating platelet, thereby providing a feedback amplification mechanism to propagate aggregate development.
One of the most striking features of platelet thrombus development in vivo is the high proportion of reversible discoid platelet aggregates on the surface of thrombi. The shear-dependent clustering of discoid platelets may serve to facilitate paracrine platelet stimulation by limiting the “washout” effect of blood flow on generated soluble agonists. Such a mechanism may explain why most of the major soluble agonists regulating platelet activation, such as ADP, TXA2, and thrombin, are either released from the platelet themselves or generated in close proximity to the platelet surface. This is in marked contrast to leukocytes, in which many of the soluble chemokines and cytokines regulating leukocyte activation are generated from the vessel wall. Thus, through the formation of reversible platelet aggregates, platelets may have evolved a unique mechanism of facilitating localized and sustained platelet activation by soluble agonists in a high shear environment.
Our in vivo studies shed new light on the mechanisms regulating platelet translocation at sites of vascular injury. In contrast to leukocytes, which have a spherical morphology well suited to a rolling interaction with the vessel wall, the discoid morphology of nonstimulated platelets is less well adapted to rotational movement. The only data on platelet morphology during surface translocation have been derived from in vitro perfusion studies on a purified VWF matrix in which platelets undergo rotational movement as either flat discs (flipping platelets), following conversion to spiny spheres, or after adopting a smooth spherical morphology at very high shear rates (10 000 s−1).15 Rotational translocation of flat discs is relatively inefficient, because this morphology experiences marked fluctuations in tensile force during rotational movement leading to cell detachment from the adhesive surfaces,51 whereas adhesive bonds on spherical platelets experience more uniform force during surface translocation, producing a smoother, more sustained rolling interaction.15 Relative to rotating discs or rolling spheres, sliding flat discs experience minimal drag force and have much lower tensile stresses on adhesive bonds. A flat disc also enables maximal surface contact area with the adhesive surface, increasing the potential for multivalent adhesive interactions. Additionally, membrane tethers increase the moment arm of adhesive bonds, thereby providing mechanical advantage to adhesion contacts and reducing platelet detachment from the thrombus surface.17 Thus, the development of membrane tethers from the surface of flat, discoid platelets represents a unique mechanism of minimizing drag forces on adherent platelets, thereby enabling sustained platelet interaction with thrombogenic surfaces independent of substantial platelet activation.
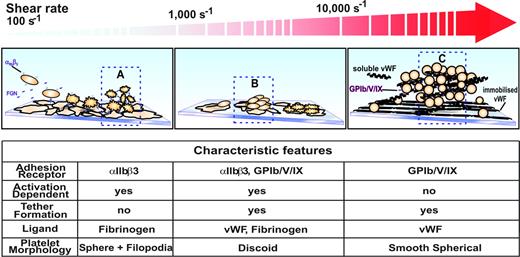
The studies presented here, in combination with previous reports,10,11,52 suggest that at least 3 distinct mechanisms can regulate the initiation of platelet aggregation, with the relative contribution of each mechanism dependent on the prevailing shear conditions. As demonstrated in Figure 7, the traditional model of platelet aggregation that operates under relatively low shear conditions (less than 1000 s−1) involves platelet stimulation by one or more soluble agonists that induces platelet shape change and the up-regulation in the affinity status of integrin αIIbβ3. Under relatively low flow conditions, activated integrin αIIbβ3 can engage fluid-phase fibrinogen, a dimeric molecule that physically bridges adjacent activated platelets. As demonstrated in detail in this report, under higher shear conditions (1000 to 10 000 s−1) the processes initiating aggregation are quite distinct, involving additional receptors, ligands, and membrane tethers. This mechanism is unique in that it involves integrin αIIbβ3 adhesive interactions between discoid platelets through an activation process independent of ADP. It is tempting to speculate that GPIb- and integrin αIIbβ3-derived signals help facilitate localized integrin αIIbβ3 activation, which is necessary for sustained adhesion and membrane tether formation. Consistent with such a possibility, we have demonstrated a progressive low-level increase in cytosolic calcium flux during platelet translocation and reversible aggregate formation. This calcium signal is ADP independent and occurs prior to the onset of shape change and the sustained oscillatory calcium flux that is necessary for irreversible aggregation (I. Goncalves and S.P.J., unpublished observations, January 2006). Recently, a third mechanism initiating platelet aggregation has been described. This mechanism operates at extremely high shear conditions (more than 10 000 s−1) such as those operating in severely stenosed arteries and requires both immobilized and soluble forms of VWF. In contrast to aggregation at physiological shear rates, aggregation does not require integrin αIIbβ3 or platelet activation and is exclusively mediated by VWF-GPIb adhesive bonds.12 These aggregates are most likely initiated through the development of membrane tethers; however, in contrast to our findings at physiological shear rates, these tethered aggregates develop between smooth spherical platelets, a unique platelet morphologic form that develops under pathological shear conditions.12,15 Taken together, these studies highlight the unique relationship operating between platelet adhesive ligands, receptors, and morphologic forms relevant to shear-dependent platelet aggregation. Furthermore, they raise the possibility that targeting processes selective to platelet aggregation at different shear rates may lead to the development of novel shear-specific inhibitors of platelet aggregation.
Model of platelet aggregation at various shear rates. (A) Platelet aggregation at shear rates below approximately 1000 s−1 is predominantly dependent on the activation of integrin αIIbβ3 receptors and the presence of its ligand fibrinogen. (B) At shear rates up to about 10 000 s−1 platelet aggregation is a 2-stage process. Platelets are initially captured to a growing aggregate via the GPIb/V/IX and integrin αIIbβ3 receptors binding to VWF and fibrinogen present at the aggregate surface. The formation of membrane tethers gives a mechanical advantage to adhesive bonds by reducing the level of force exerted on them.17,53 (C) At extreme shear rates well above 10 000 s−1 the aggregation of platelets is no longer dependent on the function of integrin αIIbβ3 receptors and tether formation. Instead, immobilized VWF combined with soluble multimeric VWF is capable of initiating large unstable aggregates of nonactivated platelets.12
Model of platelet aggregation at various shear rates. (A) Platelet aggregation at shear rates below approximately 1000 s−1 is predominantly dependent on the activation of integrin αIIbβ3 receptors and the presence of its ligand fibrinogen. (B) At shear rates up to about 10 000 s−1 platelet aggregation is a 2-stage process. Platelets are initially captured to a growing aggregate via the GPIb/V/IX and integrin αIIbβ3 receptors binding to VWF and fibrinogen present at the aggregate surface. The formation of membrane tethers gives a mechanical advantage to adhesive bonds by reducing the level of force exerted on them.17,53 (C) At extreme shear rates well above 10 000 s−1 the aggregation of platelets is no longer dependent on the function of integrin αIIbβ3 receptors and tether formation. Instead, immobilized VWF combined with soluble multimeric VWF is capable of initiating large unstable aggregates of nonactivated platelets.12
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: M.J.M. designed and performed most of the research and experiments and cowrote the paper with S.P.J.; E.W. collected and analyzed data and assisted in manuscript preparation; W.S.N. and S.G. collected data for Figures 1 and 7; S.M.D. collected data for Figure 1; and S.P.J. designed research, provided overall direction, and cowrote the paper with M.J.M.
This work was supported by a grant from the National Health and Medical Research Council of Australia.


![Figure 6. Increased platelet density helps sustain calcium flux in aggregating platelets. Washed platelets loaded with the caged calcium chelator NP-EGTA and calcium indicator dyes (see “Analysis of cytosolic calcium flux”) were reconstituted with RBCs (to a final concentration of 150 × 106/mL or 5 × 10 × 6/mL) and perfused through microcapillary tubes coated with VWF (50 μg/mL) at 1800 s−1. A transient elevation of cytosolic calcium was induced in translocating platelets by uncaging with brief exposure to near UV light. (A) Proportion of high Δ[Ca2+]c platelets (displaying transient or sustained calcium oscillations at a platelet density of 5 × 106/mL or 150 × 106/mL [n = 3]). Notably, all of the cells were observed to undergo transient stationary adhesion events at a platelet density of 5 × 106/mL. (B) Single-channel Oregon green fluorescence images showing translocating platelets prior to uncaging (0 seconds) and the elevation in intracellular calcium levels following UV exposure (1.8 seconds). The left panels show a representative platelet uncaged at low density, where the platelet remains stationary for a period and then continues translocation when calcium levels decrease. The middle panels show a representative platelet at high density, where calcium levels remain elevated after uncaging and the platelet remains stationary and becomes a focal point for recruitment of other platelets into an unstable aggregate (highlighted in the higher-magnification panels on the right-hand side). (C) After uncaging, platelets remained stationary while calcium levels remained elevated. The duration of the stationary period was less in platelets at low density compared with those uncaged at high density (n = 50; bar represents the median; cutoff time was 48 seconds).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-07-028282/4/m_zh80020706600006.jpeg?Expires=1769136930&Signature=JiBdc2GAr34FfXipnkeqOtGaJo7EdL3hWtqnZRYibyroTbDnwYVz12efruBBkdOlFc8KDGno~i0fB99dJuvQVM2S0Jyci8x-utmUr6BQrYxsxqcphSbInHQqvibtne0L5u20uN284v-uYU91Tjj6DIReIwQTpPHT~bkPZqp2kkMNnUNbgbGEudkRC-0hz2od1VX5I6-V7hl6j60rRQVv-chvh4DXC5CNQjc6rNxbN~v8~ZMF5nYFJKX1LQui5Iy8DMfpOjdh~aMU6-IL1nEDCgL4dkY5n0pSq3w37YaDvJHf3Y~lGXdeqEP-IptGl8Tdr7MwDLM-4jCGrmU38Gzw-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal