Abstract
Homing and engraftment of hematopoietic stem cells (HSCs) to the bone marrow (BM) involve a complex interplay between chemokines, cytokines, and nonpeptide molecules. Extracellular nucleotides and their cognate P2 receptors are emerging as key factors of inflammation and related chemotactic responses. In this study, we investigated the activity of extracellular adenosine triphosphate (ATP) and uridine triphosphate (UTP) on CXCL12-stimulated CD34+ HSC chemotaxis. In vitro, UTP significantly improved HSC migration, inhibited cell membrane CXCR4 down-regulation by migrating CD34+ cells, and increased cell adhesion to fibronectin. In vivo, preincubation with UTP significantly enhanced the BM homing efficiency of human CD34+ cells in immunodeficient mice. Pertussis toxin blocked CXCL12- and UTP-dependent chemotactic responses, suggesting that G-protein alpha-subunits (Gαi) may provide a converging signal for CXCR4- and P2Y-activated transduction pathways. In addition, gene expression profiling of UTP- and CXCL12-treated CD34+ cells and in vitro inhibition assays demonstrated that Rho guanosine 5′-triphosphatase (GTPase) Rac2 and downstream effectors Rho GTPase–activated kinases 1 and 2 (ROCK1/2) are involved in UTP-promoted/CXCL12-dependent HSC migration. Our data suggest that UTP may physiologically modulate the homing of HSCs to the BM, in concert with CXCL12, via the activation of converging signaling pathways between CXCR4 and P2Y receptors, involving Gαi proteins and RhoGTPases.
Introduction
Homing of hematopoietic stem cells (HSCs) to the bone marrow (BM) is driven by chemotactic factors. The most powerful chemoattractant for HSCs is the α-chemokine CXCL12 (also named stromal cell–derived factor-1), which is constitutively secreted by BM stromal and endothelial cells.1-6 Its chemotactic activity is mediated by the CXCR4 receptor, a 7-spanning membrane receptor coupled to G-proteins. The activation of the CXCL12/CXCR4 axis induces intracellular calcium transients, actin polymerization, and activation of adhesion molecules important for transendothelial migration,1 BM homing,7 engraftment,8 and retention9 of HSCs in the BM microenvironment. Despite the prominent role of CXCL12, HSC homing does not depend exclusively upon the interaction of this chemokine with its cognate receptor, since the migration capacity of CXCR4null HSCs is impaired, but not completely abrogated.10 These findings suggest that other chemotactic factors may be involved in the modulation of HSC trafficking.
5′-nucleotide triphosphates, particularly adenosine triphosphate (ATP) and uridine triphosphate (UTP), play pivotal roles in subcellular metabolic functions. Furthermore, they are emerging as ubiquitous extracellular signaling molecules involved in a wide spectrum of biologic responses, such as cell proliferation, differentiation, cell death, and chemotaxis in different tissues, including the hematopoietic system.11 Their functions are mediated by P2 nucleotide receptors (P2Rs),11-13 further divided into P2X and P2Y subfamilies. In particular, P2YRs are 7-spanning membrane receptors, linked to G-proteins, which activate their signal transduction pathway via the activation of phospholipase C or activation/inhibition of adenylate cyclase.11
Extracellular nucleotides have been recently characterized as important chemotactic factors for different cell types,14-17 and they are emerging as important mediators of hematopoietic functions: P2R ligation by ATP and UTP stimulates HSCs, in vitro, in synergy with other cytokines, and preincubation with UTP expands the number of marrow-repopulating HSCs in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.18
In the present study, we investigated the capacity of extracellular nucleotides to affect the migration of human CD34+ HSCs in vitro and in vivo. We also explored potential signal transduction pathways involved in UTP-promoted HSC migration. Taken together, our data suggest that extracellular UTP, and to a lesser extent ATP, may play a physiological role in the modulation of HSC motility and homing to BM niches, in synergy with the chemotactic peptide CXCL12, via the activation of Rho guanosine triphosphatase (GTPase) transduction pathway.
Materials and methods
Reagents
Cell preparation and HSC purification
Healthy adult HSC donors (n = 35) received glycosylated recombinant human granulocyte colony-stimulating factor (rhG-CSF, lenograstim; Rhone-Poulenc Rorer, Milan, Italy), administrated subcutaneously at 10 μg/kg per day for 5 to 6 days. Peripheral blood (PB) stem cell collection was performed by leukapheresis on days 5 and 6, as previously described.18 The study protocol was approved by the Ethics Committee of the Azienda Ospedaliera–Universitága di Bologna, Policlinico S. Orsola-Malpighi. Each donor gave written informed consent in accordance with the Declaration of Helsinki.
PB mononucleate cells were enriched by Ficoll-Paque (Pharmacia, Uppsala, Sweden) and then processed by MiniMacs high-gradient magnetic separation column (Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain highly purified CD34+ cells (mean purity, 94% ± t5%).18
Cytoplasmic free Ca2+ measurements
Changes in the intracellular free Ca2+ concentration were measured with the fluorescent indicator fura-2/AM, in an LS50 Perkin Elmer fluorometer (Perkin Elmer, Beaconsfield, United Kingdom), as previously described.18
Chemotaxis assay
Chemotactic migration of HSCs was performed using transwell assays (diameter, 6.5 mm; pore size, 5 μm; Costar, Cambridge, MA) as previously described.8,20,21 Briefly, 100 μL chemotaxis buffer (X-VIVO 15; Cambrex Bio Science, Verviers, Belgium), containing 1 × 105 to 2 × 105 CD34+ cells, was added to the upper chamber, while 600 μL chemotaxis buffer, with or without CXCL12 (150 ng/mL), was added to the bottom chamber. UTP (10 μM), ATP (1 nM), or dinucleotide analogs (INS415 at 10 μM and INS45937 at 100 nM) were added to the upper or to the bottom chamber, in order to evaluate their priming or chemotactic activity, respectively. All these concentrations were selected after preliminary dose-response experiments (data not shown). After 4 hours of incubation (37°C, 5% CO2), migrating (bottom chamber) and nonmigrating (upper chamber) cells were harvested. The percentage of migration was calculated as follows: (number of migrating cells - spontaneous migrating cells) × 100/(1 × 105 to 2 × 105 cells). The percentage of spontaneously migrating cells was always less than 1%. Priming experiments were also conducted by incubating CD34+ cells for 60 minutes in the presence of UTP or ATP in serum-free conditions. Extracellular nucleotides were then washed out before migration assays.
Inhibition assays and toxin treatment
All toxins and inhibitory compounds were used at concentrations previously shown to be effective in dose-response experiments (data not shown). Inhibitory effects of Clostridium difficile Toxin B (ToxB; Sigma-Aldrich, Milan, Italy) and pertussis toxin (PTX; Sigma-Aldrich) were assessed on cells preincubated in IMDM + 10% fetal bovine serum (FBS) at 37°C, 5% CO2 with ToxB (100 ng/mL) for 18 hours or with PTX (1 μg/mL) for 90 minutes, respectively, prior to transwell migration assays. Rho kinase inhibitor Y27632 (Calbiochem, Nottingham, United Kingdom) was included in the top well for the duration of the migration assay.22
The neutralizing effect of anti-CXCR4 monoclonal antibody (mAb) (clone 12G5; R&D Systems, Wiesbaden, Germany) on HSC migration was assessed by preincubating cells with anti-CXCR4 mAb (50 μg/mL) or normal mouse IgG for 30 minutes at room temperature, prior to chemotaxis experiments.23
Hematopoietic colony assay
The colony-forming efficiency of migrated cells was evaluated by standard semisolid cultures. Briefly, CD34+ migrated cells were cultured at 37°C, 5% CO2 in methylcellulose medium (MethoCult H4434; Stem Cell Technologies, Vancouver, BC, Canada), as previously described.18 Granulocyte-macrophage colony-forming units (GM-CFUs), erythroid burst-forming units (BFU-Es), and multilineage colonies (CFU-Mixs) (together referred to as colony-forming unit cells, CFU-Cs), were scored after 14 days of incubation at 37°C in a fully humidified 5% CO2 atmosphere.
Flow cytometry
Migrated and nonmigrated CD34+ cells were incubated for 20 minutes in the dark at room temperature with the following fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mAbs: CXCR4-PE (R&D Systems, Minneapolis, MN), CD49d-FITC, and CD49e-PE (Becton Dickinson, Heidelberg, Germany). Isotype-identical antibodies were used as controls.
For intracellular CXCR4 staining, cell-surface expression was blocked by incubation with 10 μg/mL nonconjugated anti–human CXCR4 mAb for 1 hour at 4°C. The cells were than washed, fixed, and permeabilized with FIX & PERM Reagents (CALTAG Laboratories, Burlingame, CA) before staining intracellular CXCR4 with anti–human CXCR4-PE mAb. The cells were analyzed with a fluorescence-activated cell sorting (FACS) Calibur flow cytometer using CellQuest analysis software (Becton Dickinson). The mean fluorescence intensity (MFI) was calculated from the fluorescence histogram and expressed in arbitrary units.
Adhesion assay
Fibronectin (FN; Sigma-Aldrich) was adsorbed to 24-well plates at 20 μg/cm2 in phosphate-buffered saline solution (PBS); the coating step was performed overnight at 4°C. Control plates were coated with 1% bovine serum albumin (BSA; Sigma-Aldrich).24 The coating solution was removed by aspiration and plates were incubated with RPMI 1640 (Cambrex Bio Science) containing 1% fraction V BSA at 37°C for 30 minutes to block nonspecific binding sites. After 2 washes in RPMI 1640 containing 25 mM HEPES (BioWhittaker Europe, Verviers, Belgium), 1 × 105 to 2 × 105 CD34+ cells were plated in RPMI 1640 containing 0.1% BSA at 37°C in the presence of UTP, ATP, CXCL12, or control medium. After 1-hour incubation, nonadherent cells were harvested by 2 standardized washes using warm PBS. Adherent cells were recovered after a 10-minute incubation in the Accutase solution (Innovative Cell Technologies, La Jolla, CA) at 37°C by vigorous pipetting. Adherent and nonadherent cells were counted and percent adhesion was calculated as (number of adherent cells)/(number of adherent cells + number of nonadherent cells) × 100. Adhesion on BSA-coated control wells was less than 1%.
Competitive repopulation assay in NOD/SCID mice
BM homing capacity of human HSCs was assessed as recently described18 with few modifications. Highly purified CD34+ cells were divided into 2 aliquots and stained with a red (PKH26; Sigma) or a green (PKH67; Sigma-Aldrich) fluorescent dye. PKH67-stained CD34+ cells were then incubated in serum-free medium in the presence of 10 μM UTP at 37°C, 5% CO2 for 1, 6, or 24 hours, whereas PKH26-stained CD34+ cells were incubated with mock medium. In 2 different experiments, NOD/SCID mice (n = 6 per study group for each experiment) were sublethally irradiated (350 cGy) and subsequently coinjected with UTP-primed and control CD34+ cells (106 cells/mouse) stained with the green or red fluorescent dye, respectively. As internal control of the staining procedure, other NOD/SCID mice were injected with PKH26-UTP–treated CD34+ cells and PKH67-control cells.
The number of human CD34+ cells injected into NOD/SCID mice was chosen so as to give 1% to 5% of human cell homing in the BM the day after xenotransplantation.
Twenty-four hours after transplantation, NOD/SCID mice were killed and their BM and PB collected for the evaluation of human BM-homed cells by flow cytometry. All procedures involving animal models were performed in accordance with national and international laws and policies.
Measurement of human competitive repopulation in NOD/SCID mice by flow cytometry
BM and PB samples of NOD/SCID mice were evaluated by flow cytometry for quantification of allophycocyanin-labeled CD34+ cells and peridinin chlorophyll protein (PerCP)–labeled CD45-, PKH26-, and PKH67-positive cells.18
After red cell lysis, cell suspensions were evaluated by FACS Calibur using analysis gates designed to exclude dead cells, debris, and platelets. After acquisition of at least 100 000 cells/sample, analyses were considered as informative when adequate numbers of events (ie, > 100; typically 100-200) were collected in the human cell enumeration gates.
Percentages of stained cells were determined and compared with appropriate negative controls. The expression of human CD45 antigen in the BM and PB of mice that underwent transplantation was confirmed by reverse-transcription–polymerase chain reaction (RT-PCR) and Southern blotting as previously reported.25 For comparative homing assessment in the BM and PB samples, CD34+ CD45+ cells were further evaluated for their relative content in PKH67- and PKH26-positive cells (ie, cells treated with UTP or mock medium, respectively).
Microarray analysis
Highly purified CD34+ cells from 6 healthy donors were seeded at 106 cells/mL in serum-free medium (X-VIVO 15) for 24 hours with or without 10 μM UTP, 150 ng/mL CXCL12, or the combination of the 2 factors. This time point was chosen because preliminary experiments demonstrated the up-regulation of mRNA of many genes involved in cell motility after 1 hour of treatment, but the most significant transcriptional regulation occurred after 24 hours (data not shown). Total RNA was isolated from each cell population using RNeasy MinElute Cleanup kit (Qiagen, Valencia, CA) following the manufacturer's recommendations. Disposable RNA chips (Agilent RNA 6000 Nano LabChip kit; Agilent Technologies, Waldbrunn, Germany) were used to determine the concentration and purity/integrity of RNA samples using Agilent 2100 bioanalyzer. RNAs originating from the 6 donors were pooled in order to obtain at least 2 μg/sample in both untreated and treated cells.
Using Affymetrix standard protocols (Affymetrix, Santa Clara, CA),26 the biotin-labeled target synthesis reactions, as well as the Affymetrix HG-U133A GeneChip arrays hybridization, staining, and scanning, were performed, starting from 2 μg total cellular RNA.
The GeneChip Operating Software (GCOS) absolute analysis algorithm was used to determine the amount of a transcript mRNA (signal), while the GCOS comparison analysis algorithm was used in order to compare gene expression levels between samples. Differentially expressed genes were selected, as the probe sets showing a change called “I” (increasing) or “D” (decreasing) at least once in the pair-wise comparisons between treated samples (UTP, CXCL12, and UTP + CXCL12) and untreated cells. The gene list passing this filter was selected as “changing genes.” Genes showing a detection called “A” (absent) in all samples were excluded.25 The generated lists and, independently, the GCOS-generated absolute analysis data were uploaded onto GeneSpring software version 7.2 (Silicon Genetics, Redwood City, CA). To normalize data, each measurement was divided for the 50th percentile of all signals in that sample. The percentile was calculated with all normalized signals above 10. Each gene was divided by the median of its measurements in all samples.
Then, using the GeneSpring package filtering options, “poorly changed” genes (ie, those showing a normalized intensity in all conditions between 0.5 and 2) were filtered out.
DAVID TOOL 2.1 Beta (National Institute of Allergy and Infectious Diseases, http://david.abcc.ncifcrf.gov/) was used to examine selected lists of genes in order to identify overrepresentation of functional classes accordingly with gene-ontology classification.
Statistical analysis
The results are expressed as mean ± SEM of at least 3 different experiments. Results of in vitro studies were analyzed with the paired nonparametric Wilcoxon rank sum test. In transplantation experiments, statistical comparisons were performed using the t test and analysis of variance (ANOVA) when data were normally distributed and the nonparametric analysis of Spearman and Mann-Whitney when data were not normally distributed. All P values were considered statistically significant at less than .05.
Results
UTP enhances CXCL12-mediated migration of CD34+ HSCs
UTP alone, added to the cell suspension in the upper chamber of transwells, did not significantly enhance the percentage of spontaneously migrating HSCs. However, UTP significantly improved the migration of CD34+ cells toward a gradient of CXCL12 (2.1-fold increase; P < .05; Figure 1A). The activity of UTP on CXCL12-induced migration of HSCs was exerted within 1 hour (Figure 1A, UTP-primed cells; P < .05).
Chemotaxis and in vitro migration of CD34+ HSCs in response to extracellular nucleotides. The chemotactic effect of UTP on CD34+ HSCs was assessed in transwell migration assays, as described in “Materials and methods.” UTP alone (10 μM) slightly improved HSC migration but significantly enhanced CXCL12-induced chemotaxis (P < .05; A). The activity of UTP was exerted within 1 hour as shown in priming experiments (UTP/ATP-primed cells). UTP alone showed, per se, a slight chemotactic activity, but it significantly enhanced HSC migration rate when combined with CXCL12 in the lower chamber of transwell assays (P < .05; B). These results were confirmed by clonogenic assays (C-D). Conversely, ATP (1 nM) gave a borderline priming effect on CXCL12-induced chemotaxis (E,G) and no significant chemotactic activity (F,H). Results are expressed as mean ± SEM and were obtained from 6 independent experiments. *P values less than .05.
Chemotaxis and in vitro migration of CD34+ HSCs in response to extracellular nucleotides. The chemotactic effect of UTP on CD34+ HSCs was assessed in transwell migration assays, as described in “Materials and methods.” UTP alone (10 μM) slightly improved HSC migration but significantly enhanced CXCL12-induced chemotaxis (P < .05; A). The activity of UTP was exerted within 1 hour as shown in priming experiments (UTP/ATP-primed cells). UTP alone showed, per se, a slight chemotactic activity, but it significantly enhanced HSC migration rate when combined with CXCL12 in the lower chamber of transwell assays (P < .05; B). These results were confirmed by clonogenic assays (C-D). Conversely, ATP (1 nM) gave a borderline priming effect on CXCL12-induced chemotaxis (E,G) and no significant chemotactic activity (F,H). Results are expressed as mean ± SEM and were obtained from 6 independent experiments. *P values less than .05.
Similarly, UTP did not induce a significant chemotactic effect per se, as shown by the addition of the nucleotide to the lower chamber of the transwell system (Figure 1B). However, when combined with CXCL12, UTP synergistically improved HSC migration (P = .05).
The enhancement of the transwell migration rate of UTP-treated HSCs was further demonstrated by CFU-C clonogenic assays (3.4-fold increase of CFU-Cs in UTP-treated samples; P < .05) (Figure 1C-D). Extracellular ATP gave similar but less impressive results than UTP (P = .06 for the number of migrated cells and P = .04 for CFU-Cs; Figure 1E-H).
Thus extracellular nucleotides, particularly UTP, may improve CXCL12-dependent HSC migration by (1) enhancing the responsiveness of HSCs to the CXCL12 gradient; and (2) synergistically promoting CXCL12 chemoattractant effect. Furthermore, we showed that stable dinucleotide analogs of ATP/UTP enhanced the migration of HSCs in response to CXCL12 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, we demonstrated that (1) the biologic effects of UTP are unlikely to be exerted by metabolites; and (2) the activation of P2Y2R is crucial to induce HSC motility, as P2Y4R and P2Y6R, which are the only other 2 receptors activated by UTP and its analogs, are not expressed by CD34+ cells.18
Subsequently, we investigated whether the activity of UTP may be mediated by the enhanced expression of CXCR4 on the cell membrane. When a bulk population of CD34+ HSCs was incubated with UTP up to 24 hours, no significant effects on CXCR4 expression were detected (data not shown). However, when CXCR4 receptor was analyzed on migrating and nonmigrating HSCs collected from transwell assays, we observed a lower percentage of CXCR4+ cells migrating toward CXCL12 gradients compared with control samples (Figure 2A). Down-regulation of membrane CXCR4 was paralleled by the internalization of the molecule as a consequence of CXCL12 binding to the receptor (Figure 2B). Conversely, when UTP was added to the upper transwell chamber, the down-regulation of cell membrane CXCR4 on migrating cells was inhibited (Figure 2A), and this finding was associated with the lower expression of intracytoplasmic CXCR4 (Figure 2B). Taken together, these results suggest that UTP may improve HSC migration by preventing membrane CXCR4 down-regulation and its internalization. In contrast, UTP did not modify the expression of CD49d and CD49e on both migrating and nonmigrating HSCs (data not shown).
Membrane and intracytoplasmic CXCR4 expression in migrating and nonmigrating HSCs in response to CXCL12 and UTP. (A) Percentages of CXCR4+ cells were evaluated in migrating and nonmigrating CD34+ HSCs, collected from the lower and upper chamber of transwell plates, respectively. Among CD34+ cells migrating toward a CXCL12 gradient, we found the down-regulation of membrane CXCR4 compared with nonmigrating cells. However, when UTP was added to the upper chamber, no difference in the percentage of CXCR4+ cells was detected between migrating and nonmigrating HSCs. Intracytoplasmic staining of CXCR4 (B) demonstrated that the down-regulation of cell membrane CXCR4 is due to its internalization, which is inhibited by addition of UTP. Results were from 3 independent experiments and are shown as mean ± SEM. (C) In inhibition assays, human CD34+ HSCs were preincubated with PTX (1 μg/mL) as described in “Materials and methods.” PTX treatment almost completely abrogated spontaneous migration of UTP-treated cells and CXCL12-dependent chemotaxis. Furthermore, the CXCL12-induced/UTP-supported migration was completely blocked by treatment with PTX (93% inhibition; P < .05). Cells treated with anti-CXCR4 mAb (50 μM/mL) showed a decreased response to CXCL12 gradients, even when simultaneously cultured with UTP. Results were from 4 independent experiments and are shown as mean ± SEM. (D) Cytosolic Ca2+ concentration changes induced by UTP and CXCL12. Cells were loaded with fura-2/AM as reported in “Materials and methods.” UTP concentration was 10 μM, while CXCL12 concentration was 150 ng/mL. The arrow indicates addition of the stimuli. UTP (dashed line), CXCL12 (dotted line), and UTP + CXCL12 (continuous line). Results were from 3 independent experiments and are shown as mean ± SEM. *P values less than .05.
Membrane and intracytoplasmic CXCR4 expression in migrating and nonmigrating HSCs in response to CXCL12 and UTP. (A) Percentages of CXCR4+ cells were evaluated in migrating and nonmigrating CD34+ HSCs, collected from the lower and upper chamber of transwell plates, respectively. Among CD34+ cells migrating toward a CXCL12 gradient, we found the down-regulation of membrane CXCR4 compared with nonmigrating cells. However, when UTP was added to the upper chamber, no difference in the percentage of CXCR4+ cells was detected between migrating and nonmigrating HSCs. Intracytoplasmic staining of CXCR4 (B) demonstrated that the down-regulation of cell membrane CXCR4 is due to its internalization, which is inhibited by addition of UTP. Results were from 3 independent experiments and are shown as mean ± SEM. (C) In inhibition assays, human CD34+ HSCs were preincubated with PTX (1 μg/mL) as described in “Materials and methods.” PTX treatment almost completely abrogated spontaneous migration of UTP-treated cells and CXCL12-dependent chemotaxis. Furthermore, the CXCL12-induced/UTP-supported migration was completely blocked by treatment with PTX (93% inhibition; P < .05). Cells treated with anti-CXCR4 mAb (50 μM/mL) showed a decreased response to CXCL12 gradients, even when simultaneously cultured with UTP. Results were from 4 independent experiments and are shown as mean ± SEM. (D) Cytosolic Ca2+ concentration changes induced by UTP and CXCL12. Cells were loaded with fura-2/AM as reported in “Materials and methods.” UTP concentration was 10 μM, while CXCL12 concentration was 150 ng/mL. The arrow indicates addition of the stimuli. UTP (dashed line), CXCL12 (dotted line), and UTP + CXCL12 (continuous line). Results were from 3 independent experiments and are shown as mean ± SEM. *P values less than .05.
To further investigate the role of CXCL12 cognate receptor in UTP-promoted migration, we performed CXCR4-blocking experiments. Both CXCR4 and P2YRs are 7-spanning receptors coupled to G-proteins. PTX from Bordetella pertussis is an inhibitor of signal transduction pathway mediated by inhibitory G-protein alpha-subunits (Gαi) and almost completely abrogated CXCL12-dependent in vitro migration of human CD34+ HSCs (87% inhibition; P = .03) (Figure 2C). A 48% inhibition rate was observed in spontaneously migrating (ie, in the absence of chemoattractants), UTP-stimulated cells (P = .06). Furthermore, CXCL12-induced/UTP-supported migration was completely blocked by PTX (93% inhibition; P < .05) (Figure 2C). These findings suggest that UTP-mediated motility depends upon Gαi protein–coupled P2YRs, and that a Gαi protein pathway is shared by UTP and CXCL12.
In addition, CXCL12-induced/UTP-supported migration was partially blocked by the treatment with anti-CXCR4 mAb prior to migration assays (57% inhibition; P < .05), thus confirming that inhibition of CXCR4 down-regulation in migrating cells may be one of the mechanisms activated by extracellular nucleotides (Figure 2C).
Intracellular Ca2+ increase and actin polymerization, which are initial steps in cellular motility, were also investigated. Figure 2D shows that UTP induced a small and long-lasting Ca2+ increase, while CXCL12-induced Ca2+ spike was much higher and transient. Application of both stimuli increased the amplitude of the Ca2+ spike.
Actin polymerization was analyzed by cytofluorimetric analysis of the FITC-phalloidin marker. Preincubation of CD34+ cells with UTP did not significantly increase intracellular actin polymerization mediated by CXCL12 (data not shown).
UTP improves adhesion of HSCs to FN
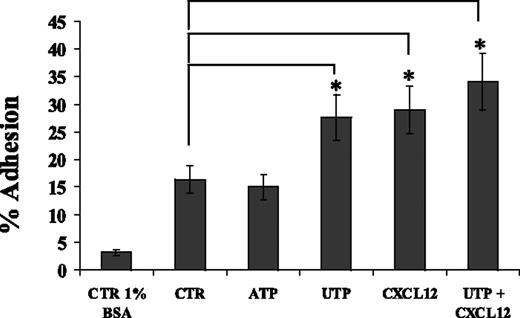
By using FN-coated culture wells, we demonstrated that whereas ATP did not improve the percentage of adherent CD34+ cells, UTP improved the adhesive capacity of HSCs (27.6% ± t4.9% vs 16.4% ± t2.5%; P < .05) similarly to CXCL12 (Figure 3). The combination of UTP and CXCL12 did not induce any further significant improvement in HSC adhesion. UTP did not affect the expression of integrins CD49d and CD49e (data not shown). Therefore, these data suggest that UTP might improve HSC homing to BM and engraftment by enhancing CD34+ adhesion to FN and interaction with extracellular matrix components, without affecting CD49d and CD49e expression levels.
UTP enhances CD34+ HSC adhesion onto FN-coated culture wells. Adhesion assays were performed using culture wells coated overnight with FN (20 μg/cm2). CD34+ HSCs were treated with control medium, UTP (10 μM), ATP (1 nM), and CXCL12 (150 ng/mL) during adhesion assays (60 minutes at 37°C, 5% CO2). Similarly to CXCL12, extracellular UTP, but not ATP, significantly enhanced the percentage of CD34+ HSCs adhering to FN (P < .05). The combination of UTP and CXCL12 did not improve the adhesion of HSCs over each single factor. Cell adhesion was calculated as described in “Materials and methods.” Results are expressed as mean ± SEM and were obtained from 4 independent experiments. *P values less than .05.
UTP enhances CD34+ HSC adhesion onto FN-coated culture wells. Adhesion assays were performed using culture wells coated overnight with FN (20 μg/cm2). CD34+ HSCs were treated with control medium, UTP (10 μM), ATP (1 nM), and CXCL12 (150 ng/mL) during adhesion assays (60 minutes at 37°C, 5% CO2). Similarly to CXCL12, extracellular UTP, but not ATP, significantly enhanced the percentage of CD34+ HSCs adhering to FN (P < .05). The combination of UTP and CXCL12 did not improve the adhesion of HSCs over each single factor. Cell adhesion was calculated as described in “Materials and methods.” Results are expressed as mean ± SEM and were obtained from 4 independent experiments. *P values less than .05.
Extracellular UTP enhances BM homing of human CD34+ HSCs in NOD/SCID mice
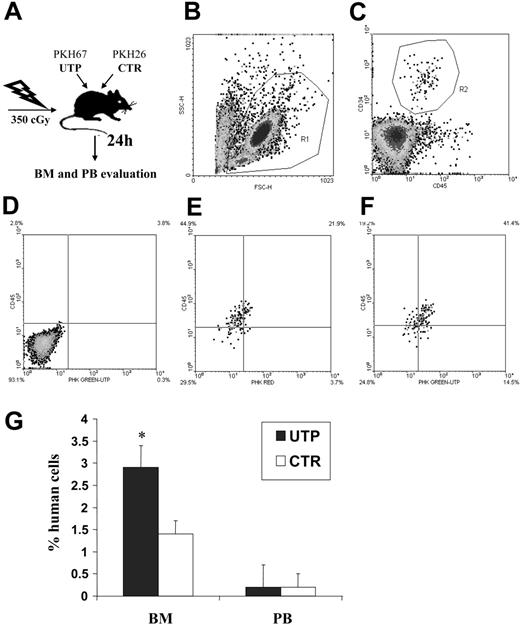
To assess the activity of UTP on HSC migration in vivo, NOD/SCID mice were sublethally irradiated and intravenously coinjected with human CD34+ HSCs incubated, for 1 hour, with and without UTP. Treated and untreated cells had been previously stained with green and red PKH dye, respectively, to trace their distribution after transplantation (Figure 4A-C). The frequency of human CD34+ HSCs, expressing red or green fluorescence, was evaluated by flow cytometry 24 hours after transplantation in the BM and PB (Figure 4D-F).
Competitive homing assay in sublethally irradiated NOD/SCID mice coinjected with UTP- and control medium–incubated CD34+ HSCs. Sublethally irradiated NOD/SCID mice (2 different experiments with 6 animals for each group in each experiment) were injected with UTP- and control medium–treated CD34+ HSCs that had been previously stained with red and green PKH dyes (“Materials and methods”). Twenty-four hours after transplantation, BM and PB samples were evaluated by flow cytometry to assess the frequency of human CD34+ HSCs bearing red or green fluorescence. Panels B and C show the gate used to exclude platelets, dead cells, and debris and the gate used to select human CD45+CD34+ HSCs, respectively. (D) Negative control obtained from a mouse that did not undergo transplantation. (E) Representative evaluation of PKH fluorescence in control BM samples. (F) Representative evaluation of PKH fluorescence in UTP-treated BM samples. In PB, no significant difference in the number of circulating UTP- or control medium–primed CD34+ cells was detected. Conversely, in the BM, the percentage of human CD34+ cells was significantly increased by 1-hour preincubation with UTP compared with untreated cells (P < .001; G). Longer times of preincubation with the extracellular nucleotide (ie, 6 and 24 hours) did not give different results. These data indicate that short-term incubation with UTP favors the BM homing of CD34+ cells. Results are expressed as mean ± SEM. *P values less than .05.
Competitive homing assay in sublethally irradiated NOD/SCID mice coinjected with UTP- and control medium–incubated CD34+ HSCs. Sublethally irradiated NOD/SCID mice (2 different experiments with 6 animals for each group in each experiment) were injected with UTP- and control medium–treated CD34+ HSCs that had been previously stained with red and green PKH dyes (“Materials and methods”). Twenty-four hours after transplantation, BM and PB samples were evaluated by flow cytometry to assess the frequency of human CD34+ HSCs bearing red or green fluorescence. Panels B and C show the gate used to exclude platelets, dead cells, and debris and the gate used to select human CD45+CD34+ HSCs, respectively. (D) Negative control obtained from a mouse that did not undergo transplantation. (E) Representative evaluation of PKH fluorescence in control BM samples. (F) Representative evaluation of PKH fluorescence in UTP-treated BM samples. In PB, no significant difference in the number of circulating UTP- or control medium–primed CD34+ cells was detected. Conversely, in the BM, the percentage of human CD34+ cells was significantly increased by 1-hour preincubation with UTP compared with untreated cells (P < .001; G). Longer times of preincubation with the extracellular nucleotide (ie, 6 and 24 hours) did not give different results. These data indicate that short-term incubation with UTP favors the BM homing of CD34+ cells. Results are expressed as mean ± SEM. *P values less than .05.
In PB samples, no significant difference in the number of circulating UTP- or control medium–primed CD34+ cells was detected (Figure 4G). Conversely, the percentage of BM CD34+ cells was significantly increased after UTP treatment (2.9% ± t0.5% vs 1.4% ± t0.3%, P < .001) (Figure 4G). The homing capacity of human HSCs to murine BM was not further enhanced by longer times of incubation with UTP (ie, 6 and 24 hours) before transplantation (data not shown). Thus, data from competitive homing assays indicated that UTP-primed CD34+ cells gain a significant in vivo homing advantage over untreated cells.
UTP affects CD34+ HSC migration via the activation of the axis of RhoGTPase
We then assessed the transcriptome profile of UTP-, CXCL12-, and UTP + CXCL12–treated cells using Affymetrix HG-U133A GeneChip array. All the data have been deposited in the Gene Expression Omnibus MIAME compliant public database27 (Table S1). Using the filtering procedure described in “Materials and methods,” we identified a number of genes significantly modulated by different treatments (Table S2).
Microarray data showed that mRNA complexity seems to be unmodified by all treatments. In fact, the numbers of sequences called “present” by Affymetrix GCOS absolute analysis algorithm in treated and untreated cells were almost the same (data not shown).
Using DAVID TOOL 2.1 Beta, we assessed which GO categories were significantly modulated. As detailed in Table 1, many genes belonging to GO categories such as “Cell Motility,” “Cytoskeleton Organization and Biogenesis,” and “Cell Adhesion” were significantly up-regulated by UTP and/or CXCL12 treatment. In particular, GO categories “Small GTPase-Mediated Signal Transduction” and “Rho Protein Signal Transduction” were significantly up-regulated by UTP alone or combined with CXCL12 (Table 1).
GO biologic process categories
| Category . | P . |
|---|---|
| Increased UTP versus CTR | |
| Protein modification | .003 |
| Cellular protein metabolism | .005 |
| Small GTPase-mediated signal transduction | .005 |
| Protein amino acid phosphorylation | .006 |
| Cellular macromolecule metabolism | .009 |
| Macromolecule metabolism | .017 |
| Cell motility | .017 |
| Cytoskeleton organization and biogenesis | .021 |
| Cell adhesion | .028 |
| Phosphate metabolism | .028 |
| Protein kinase cascade | .033 |
| M phase | .043 |
| RHO protein signal transduction | .043 |
| Intracellular signaling cascade | .061 |
| Increased CXCL12 versus CTR | |
| Cellular physiological process | .001 |
| Cell proliferation | .001 |
| M phase | .001 |
| Response to stress | .001 |
| Cellular metabolism | .001 |
| M phase of mitotic cell cycle | .001 |
| Cell organization and biogenesis | .001 |
| Mitotic cell cycle | .001 |
| Cellular process | .001 |
| Organelle organization and biogenesis | .001 |
| Protein polymerization | .010 |
| Cytoskeleton organization and biogenesis | .010 |
| Regulation of cell cycle | .017 |
| Cell adhesion | .017 |
| Cytokinesis | .025 |
| Cell motility | .027 |
| Regulation of physiological process | .030 |
| Protein amino acid phosphorylation | .031 |
| Chemotaxis | .032 |
| Taxis | .032 |
| Physiological process | .033 |
| Response to abiotic stimulus | .036 |
| Protein metabolism | .045 |
| Microtubule cytoskeleton organization and biogenesis | .045 |
| Microtubule polymerization or depolymerization | .045 |
| DNA replication checkpoint | .046 |
| Increased UTP + CXCL12 versus CTR | |
| Response to stress | .001 |
| Response to external biotic stimulus | .001 |
| Cell proliferation | .001 |
| Small GTPase-mediated signal transduction | .001 |
| Cellular metabolism | .001 |
| Cell cycle | .001 |
| Mitotic cell cycle | .002 |
| Cellular protein metabolism | .002 |
| Protein metabolism | .002 |
| Cytoskeleton organization and biogenesis | .002 |
| Cellular macromolecule metabolism | .002 |
| Protein amino acid phosphorylation | .003 |
| Intracellular signaling cascade | .004 |
| Macromolecule metabolism | .005 |
| Cell organization and biogenesis | .005 |
| Actin cytoskeleton organization and biogenesis | .007 |
| Response to wounding | .008 |
| Cytokinesis | .009 |
| Actin filament-based process | .010 |
| Response to external stimulus | .013 |
| M phase | .013 |
| Cell motility | .019 |
| Transcription initiation | .019 |
| Inflammatory response | .022 |
| RHO protein signal transduction | .023 |
| Protein modification | .024 |
| Cell adhesion | .027 |
| Phosphate metabolism | .027 |
| Response to pest, pathogen, or parasite | .032 |
| Protein complex assembly | .032 |
| Microtubule-based process | .041 |
| Chemotaxis | .048 |
| Taxis | .048 |
| Cell communication | .048 |
| Phosphorylation | .049 |
| Category . | P . |
|---|---|
| Increased UTP versus CTR | |
| Protein modification | .003 |
| Cellular protein metabolism | .005 |
| Small GTPase-mediated signal transduction | .005 |
| Protein amino acid phosphorylation | .006 |
| Cellular macromolecule metabolism | .009 |
| Macromolecule metabolism | .017 |
| Cell motility | .017 |
| Cytoskeleton organization and biogenesis | .021 |
| Cell adhesion | .028 |
| Phosphate metabolism | .028 |
| Protein kinase cascade | .033 |
| M phase | .043 |
| RHO protein signal transduction | .043 |
| Intracellular signaling cascade | .061 |
| Increased CXCL12 versus CTR | |
| Cellular physiological process | .001 |
| Cell proliferation | .001 |
| M phase | .001 |
| Response to stress | .001 |
| Cellular metabolism | .001 |
| M phase of mitotic cell cycle | .001 |
| Cell organization and biogenesis | .001 |
| Mitotic cell cycle | .001 |
| Cellular process | .001 |
| Organelle organization and biogenesis | .001 |
| Protein polymerization | .010 |
| Cytoskeleton organization and biogenesis | .010 |
| Regulation of cell cycle | .017 |
| Cell adhesion | .017 |
| Cytokinesis | .025 |
| Cell motility | .027 |
| Regulation of physiological process | .030 |
| Protein amino acid phosphorylation | .031 |
| Chemotaxis | .032 |
| Taxis | .032 |
| Physiological process | .033 |
| Response to abiotic stimulus | .036 |
| Protein metabolism | .045 |
| Microtubule cytoskeleton organization and biogenesis | .045 |
| Microtubule polymerization or depolymerization | .045 |
| DNA replication checkpoint | .046 |
| Increased UTP + CXCL12 versus CTR | |
| Response to stress | .001 |
| Response to external biotic stimulus | .001 |
| Cell proliferation | .001 |
| Small GTPase-mediated signal transduction | .001 |
| Cellular metabolism | .001 |
| Cell cycle | .001 |
| Mitotic cell cycle | .002 |
| Cellular protein metabolism | .002 |
| Protein metabolism | .002 |
| Cytoskeleton organization and biogenesis | .002 |
| Cellular macromolecule metabolism | .002 |
| Protein amino acid phosphorylation | .003 |
| Intracellular signaling cascade | .004 |
| Macromolecule metabolism | .005 |
| Cell organization and biogenesis | .005 |
| Actin cytoskeleton organization and biogenesis | .007 |
| Response to wounding | .008 |
| Cytokinesis | .009 |
| Actin filament-based process | .010 |
| Response to external stimulus | .013 |
| M phase | .013 |
| Cell motility | .019 |
| Transcription initiation | .019 |
| Inflammatory response | .022 |
| RHO protein signal transduction | .023 |
| Protein modification | .024 |
| Cell adhesion | .027 |
| Phosphate metabolism | .027 |
| Response to pest, pathogen, or parasite | .032 |
| Protein complex assembly | .032 |
| Microtubule-based process | .041 |
| Chemotaxis | .048 |
| Taxis | .048 |
| Cell communication | .048 |
| Phosphorylation | .049 |
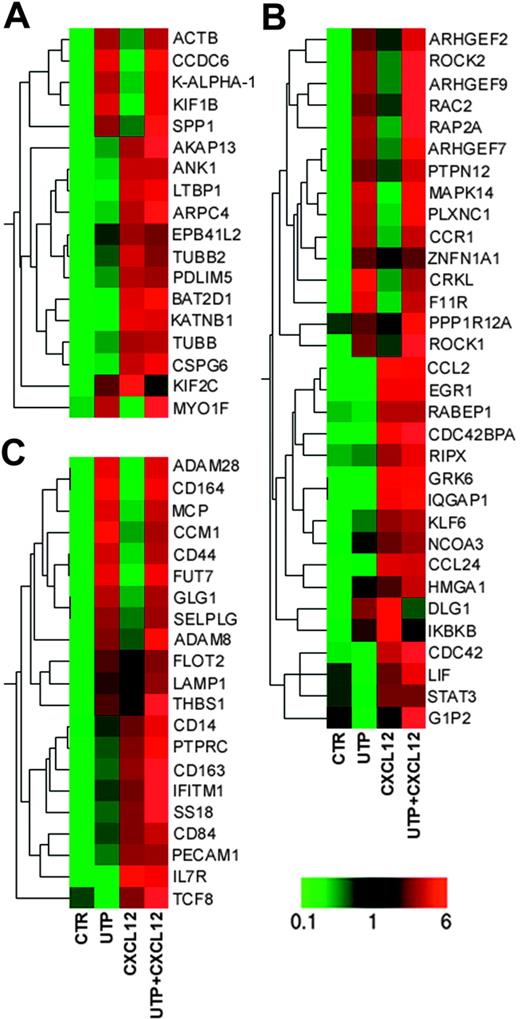
Among the sequences involved in cytoskeleton organization and biogenesis, ACTB, CCDC6, KALPHA1, KIF1B, and MYO1F (whose expression is required for the cytoskeleton reorganization before cell migration28 ) were increased following UTP treatment (Figure 5A; Table S2). Conversely, CXCL12 treatment preferentially induced other genes involved in cytoskeleton reorganization, such as ANK1, TUBB, ARPC4, and AKAP13.28
Microarray analysis. Eisen tree map computed using the GeneSpring “gene” tree and the Pearson correlation equation on the modulated probe sets belonging to GO categories of (A) “Cytoskeleton Organization and Biogenesis”; (B) “Cell Motility,” “Small GTPase Mediated Signal Transduction,” and “RHO Protein Signal Transduction”; and (C) “Cell Adhesion.” The signal-based coloring legend is shown at the bottom of the figure. Gene profiling of HSCs was performed after 24 hours of incubation with UTP and CXCL12.
Microarray analysis. Eisen tree map computed using the GeneSpring “gene” tree and the Pearson correlation equation on the modulated probe sets belonging to GO categories of (A) “Cytoskeleton Organization and Biogenesis”; (B) “Cell Motility,” “Small GTPase Mediated Signal Transduction,” and “RHO Protein Signal Transduction”; and (C) “Cell Adhesion.” The signal-based coloring legend is shown at the bottom of the figure. Gene profiling of HSCs was performed after 24 hours of incubation with UTP and CXCL12.
Furthermore, UTP treatment up-regulated a large number of genes involved in cell motility, such as CRKL,29 PTPN12,30 PLXNC1,31 F11R,32 MAPK14,33 and RAP2A34 and some members of Rho family proteins (ARHGEF2, ARHGEF7, ARHGEF9, ROCK1, ROCK2, and RAC2),35-39 while CXCL12 alone induced the preferential up-regulation of CDC42,40 CDC42BPA,41 GRK6,42 and IQGAP143 (Figure 5A; Table S2), which are strongly associated with cell migration.
We further assessed the transcriptional regulation of genes specifically involved in HSC homing. Remarkably, UTP- and UTP + CXCL12–treated cells showed increased expression of GLG1,44 RAC2,35 CD44,45 CD164,46 and SELPLG.47 Moreover, some genes governing leukocyte migration and extravasation, such as ADAM8, ADAM28,48 FUT7,49 and CCM1,50 were up-regulated in UTP- and UTP + CXCL12–treated cells (Figure 5C; Table S2). As expected, CXCL12 induced a set of genes that positively regulates homing and engraftment, such as IFITM1,51 IL7R,52 and SS18.53
Taken together, these data give the molecular support to the observed enhanced migration and homing/engraftment capacity of UTP-treated cells and indicate that the extracellular nucleotide and CXCL12 share some signal transduction pathways (for instance, they both activate members of the RhoGTPase family) and, more importantly, that they act synergistically by activating most of the molecular pathways leading to cell motility.
Because of the pivotal role played by RhoGTPases in cell locomotion35 and their significant up-regulation by UTP and UTP + CXCL12 treatment, as demonstrated by our transcriptome analysis, we then assessed, at the functional level, the extent to which RhoGTPases mediate UTP-promoted migration of CD34+ HSCs.
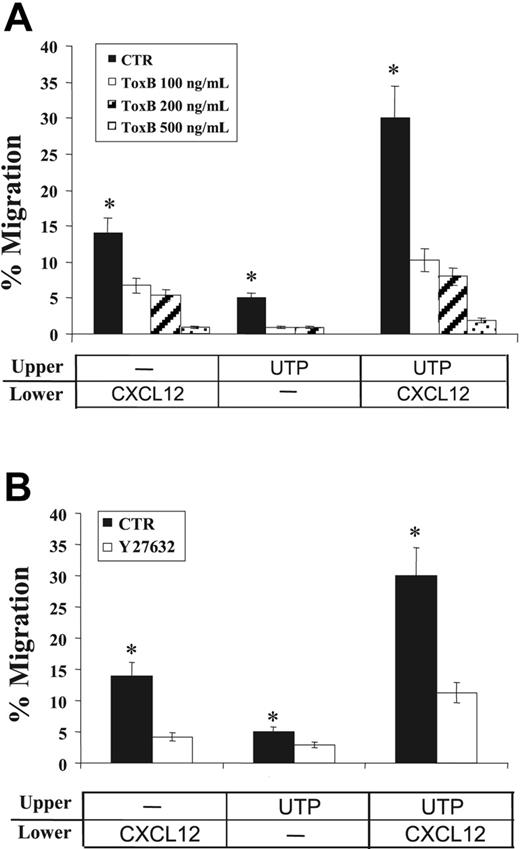
To this end, we treated human CD34+ HSCs with Clostridium difficile ToxB, a single-chained 270-kDa molecule that specifically inactivates the Rho proteins Rac/Rho/Cdc42 (Figure 6A). In transwell migration assays, CXCL12-dependent migration of CD34+ HSCs was significantly reduced by treatment with clostridial toxin in a dose-response manner (> 90% inhibition at 500 ng/mL). Similarly, the migration of cells treated with extracellular UTP alone (P = .05) or in cooperation with CXCL12 was almost completely abrogated by ToxB (> 90% inhibition; P = .02), suggesting that RhoGTPases play a pivotal role in both CXCL12- and UTP-dependent transduction pathways.
Effects of signal transduction inhibition on CD34+ HSC migration. The effects of clostridial ToxB and ROCK-inhibitor Y27632 on CD34+ HSC migration were assessed in transwell assays. Cells were incubated (37°C, 5% CO2) with increasing concentrations of ToxB (100, 200, and 500 ng/mL) for 18 hours prior to chemotaxis assays. Y27632 (10 μM) was included in the top chamber of transwells for the duration of the migration assay. (A) Pretreatment with ToxB markedly reduced the spontaneous migration of UTP-treated cells (P = .05) and toward CXCL12 gradients (P = .02). (B) Similarly, the migration of UTP-treated cells and their response to CXCL12 chemoattractant gradients were significantly inhibited by Y27632 treatment (P < .05). Results were obtained from 3 independent experiments and are shown as mean ± SEM. *P values less than .05.
Effects of signal transduction inhibition on CD34+ HSC migration. The effects of clostridial ToxB and ROCK-inhibitor Y27632 on CD34+ HSC migration were assessed in transwell assays. Cells were incubated (37°C, 5% CO2) with increasing concentrations of ToxB (100, 200, and 500 ng/mL) for 18 hours prior to chemotaxis assays. Y27632 (10 μM) was included in the top chamber of transwells for the duration of the migration assay. (A) Pretreatment with ToxB markedly reduced the spontaneous migration of UTP-treated cells (P = .05) and toward CXCL12 gradients (P = .02). (B) Similarly, the migration of UTP-treated cells and their response to CXCL12 chemoattractant gradients were significantly inhibited by Y27632 treatment (P < .05). Results were obtained from 3 independent experiments and are shown as mean ± SEM. *P values less than .05.
Furthermore, to investigate the potential role of a major downstream effector of RhoGTPases in HSC migration, the Rho GTPase-activated kinase (ROCK) inhibitor Y27632 was also used in chemotaxis assays. In the presence of Y27632, a 70% inhibition was achieved for CD34+ cells migrating toward CXCL12 (P < .05; Figure 6B). Similarly, the migration of UTP-treated cells and their responsiveness to CXCL12 gradients were significantly inhibited by Y27632 treatment (40% and 62% inhibition, respectively; P < .05; Figure 6B). Inhibition of HSC migration was not due to a putative toxicity of the compound, since cell viability evaluation by trypan blue exclusion demonstrated that more than 90% of cells remained viable at the end of the tests (not shown).
These results demonstrated the involvement of RhoGTPases and ROCK in the CXCL12-induced/UTP-supported migration of human CD34+ HSCs.
Discussion
We recently demonstrated that human CD34+ HSCs express P2XRs and P2YRs for extracellular nucleotides and that their ligation increases the proliferation of CD34+ HSCs, lineage-negative CD34− progenitors, and more primitive CD34+-derived long-term culture–initiating cells. In addition, xenotransplant studies showed that short-term preincubation with UTP expands the number of marrow-repopulating HSCs in NOD/SCID mice.18
In this investigation, we asked whether the significant improvement of human HSC engraftment in NOD/SCID mice could depend upon the capacity of extracellular nucleotides to improve HSC homing and lodgment to BM niches.
ATP and UTP have been demonstrated to play pivotal roles in the regulation of hematopoietic and immune cells, acting as chemotactic molecules for different cell types, including neutrophils, dendritic cells (DCs), and monocytes.54-56 Recent investigations indicate that extracellular nucleotides may also be involved in the regulation of HSC motility as well. The receptor subtype P2Y14 (GPR105) has indeed been identified as a marker of a quiescent BM HSC population and seems to mediate primitive HSC responses such as chemotaxis to specific BM niches.57
In the present study, migration assays clearly demonstrated the capacity of extracellular UTP to improve CXCR4-dependent HSC migration in vitro. In vivo, BM homing of human CD34+ HSCs in sublethally irradiated NOD/SCID mice was significantly increased by preincubation with UTP. Consistent with our previous studies,18 these experiments suggest that extracellular UTP exerts its effect on HSCs within 1 hour, by significantly improving their responsiveness to CXCL12 gradients, in vitro and in vivo. This finding may be important to understand the physiological activity of extracellular nucleotides whose half-life is very short. UTP treatment did not produce any significant change on CXCR4 expression on steady-state CD34+ HSCs. However, cytofluorimetric analysis performed separately on migrating and nonmigrating CD34+ cells suggests that UTP may affect CXCR4 dynamics by inhibiting the down-regulation of cell membrane CXCR4 and preventing its internalization after CXCL12 binding. Moreover, transwell migration assays performed in the presence of anti-CXCR4 mAb further indicated that UTP-dependent promotion of HSC migration requires a functional CXCR4 receptor, capable of binding CXCL12.
HSC homing to the BM microenvironment depends upon anchorage to specific adhesive niches in BM stroma.1 Likewise, our in vitro functional assays demonstrated that the adhesive properties of CD34+ HSCs are markedly enhanced by UTP, suggesting that the improved BM homing of UTP-treated HSCs in NOD/SCID mice might also depend upon UTP's capability to affect HSC adhesiveness, without changing CD49d and CD49e expression. In this view, recent findings described the capability of FTY720, an immunosuppressant molecule also involved in HSC migration, to synergize the CXCL12 chemotactic activity without affecting the expression of integrins.58 Of interest, microarray analysis demonstrated that UTP treatment up-regulates several adhesion-related genes acting as positive regulators of HSC homing such as GLG1, RAC2, CD44, CD164, and SELPLG.
Both CXCR4 and P2YRs belong to the family of G-protein–coupled 7-transmembrane receptors. This receptor structure is characteristic of a number of chemokine receptors that share similar signaling pathways coupled to trimeric G-proteins, resulting in chemotactic responses. Several Gαi-coupled 7-transmembrane receptors and their cognate ligands, already described in phlogosis phenomena, have recently emerged as key players in the modulation of HSC homing. Similarly, our data indicate that extracellular nucleotides, already reported to be involved in inflammation, might physiologically tune HSC trafficking as well. Signal transduction pathways leading to chemotaxis can be blocked by PTX from B pertussis. Our inhibition assays with PTX indicate that both CXCL12- and UTP-activated signaling cascades depend upon the functional recruitment of Gαi proteins. Therefore, HSCs appear to follow molecular pathways similar to those used by mature cells, such as phlogosis-activated leukocytes, to migrate from the PB to extravascular sites.59,60 Furthermore, PTX's capability to inhibit BM homing and short-term engraftment by blocking a wide spectrum of signaling pathways strongly suggests that several molecules cooperatively transduce homing signals, which converge in Gαi protein–dependent pathways. The results presented here thus suggest that Gαi proteins might also provide a converging signal for the cooperative modulation of HSC migration by CXCL12 and extracellular UTP. Earlier studies demonstrated that PTX induces the partial inhibition of UTP effects mediated by P2Y2R ligation, indicating that this receptor recruits both PTX-sensitive (Gi/o) and PTX-insensitive (Gq) G-proteins.61,62 Similarly, in the present work, PTX treatment induced only the partial inhibition of UTP-dependent spontaneous migration of CD34+ cells.
Downstream, UTP-treated cells showed a positive transcriptional regulation of different genes involved in cellular motility and morphology. Microarray analyses indicate RhoGTPases and other proteins related to their signal transduction pathway as major players in UTP-promoted HSC migration. RhoGTPases have recently emerged as privileged mediators of chemotaxis, capable of affecting cell motility, cell polarity, as well as actin polymerization. Small GTPases belonging to the Rho family have been identified as key players also in CXCL12-dependent signaling. Furthermore, sphingosine 1-phosphate receptor, a novel class of G-protein–associated receptors, has been recently demonstrated to synergize with CXCL12 via the recruitment of RhoGTPase family members. UTP treatment up-regulated genes coding for RhoGTPase members, such as Rac2, as well as upstream (ARHGEF2, ARHGEF7, and ARHGEF9) and downstream (ROCK1 and ROCK2) effectors, while CXCL12 preferentially increased other molecules of the same family, such as CDC42 and CDC42BPA, and their combination was synergistic. In vitro inhibition assays, performed in the presence of Rho-inhibitor ToxB form C difficile and anti-ROCK Y27632, further confirmed the crucial role of Rho-dependent signaling in UTP-promoted/CXCL12-dependent HSC migration. Thus, we demonstrate that both CXCR4- and P2YR-signaling pathways recruit RhoGTPases and related effectors, leading to migratory responses, suggesting that activation of identical signal transduction pathways, either by CXCL12 or extracellular nucleotides, might contribute to the enhancement of CXCL12-dependent effects.
Recent findings suggest that many nonpeptide molecules, such as leukotrienes and other lipid mediators,63 are involved in the modulation of both phlogosis and HSC homing to the BM. Of note, inflammation and homing phenomena simultaneously occur, in vivo, in the BM microenvironment after exposure to DNA-damaging agents.64 Ponomaryov et al65 reported that the capacity of human HSCs to home to the BM of NOD/SCID mice significantly increases when the cells are transplanted 24 to 48 hours after treatment with DNA-damaging agents, which is when the secretion of survival and migration factors, such as CXCL12, peaks. In turn, the increased secretion of CXCL12 is capable of enhancing the homing and survival potential of donor HSCs. Thus, increased production of CXCL12, during alarm situations, is part of host defense mechanisms capable of counteracting the effects of DNA-damaging agents leading to cell death and anemia.
Similarly, extracellular nucleotides released mainly by activated lymphocytes, macrophages, and platelets, as well as necrotic and apoptotic cells, are known to dramatically increase under inflammatory situations in vivo.66 Nucleotides, in turn, behave as danger molecules and actively participate in the inflammatory response. These findings, together with the demonstrated capacity of extracellular UTP to expand human HSCs18 and to improve their BM homing (present results), contribute to define the hypothesis that nucleotides released under alarm conditions may induce paracrine or autocrine signaling pathways, along with cascades initiated by other chemokines, such as CXCL12, to recruit and activate HSCs. Inflammatory molecules may also participate in stem cell trafficking to different organs and tissues. Indeed, CXCL12 is secreted not only in the BM microenvironment but also in several other organs (eg, heart, skeletal muscle, neural tissue, and kidney) to recruit HSCs participating in tissue regeneration.67
In summary, our results suggest that extracellular UTP may be a pivotal player in the cytokine milieu regulating paraphysiological trafficking and BM homing of human HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: L.R., M.F., and V.S. performed the in vitro migration assays, the inhibitory experiments, and flow cytometry analysis; R.M., R.Z., and S.S. performed the microarray studies and analyzed the data; F.B. performed the xenogenic transplant experiments; D.F., S.G., and E.A. performed the cytosolic calcium concentration measurements and contributed to data analysis; L.R. and R.M.L. designed the research and wrote the paper; and S.F., F.D.V., and M.B. supervised the research and contributed to writing the paper.
The authors thank Dr Diletta Di Mitri for technical support, Dr Joságe Boyer (Inspire Pharmaceutical) for providing dinucleotide analogs INS415 and INS45973, and David C. Weksberg (Baylor College of Medicine, Houston, TX) for reviewing.
This work was supported by the Italian Ministry of Education, University and Scientific Research (MIUR), the National Research Council (CNR) of Italy, the Italian Association for Cancer Research (AIRC), the Italian Association against Leukemia, section of Bologna (BolognAIL), the Italian Space Agency (ASI), the University of Ferrara and the University of Bologna (funds for selected topics), Istituto Superiore di Sanitága (ISS), and the sixth European Union (EU) Framework Programme (Integrated Project “Angiotargeting”; contract no. 504743) in the area of “Life Sciences, Genomics and Biotechnology for Health.”



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal