Abstract
Intravascular large B-cell lymphoma (IVLBCL) is pathologically distinct with a broad clinical spectrum and immunophenotypic heterogeneity. A series of 96 patients with IVLBCL (median age, 67 years; range, 41-85 years; 50 men) was reviewed. Anemia/thrombocytopenia (84%), hepatosplenomegaly (77%), B symptoms (76%), bone marrow involvement (75%), and hemophagocytosis (61%) were frequently observed. The International Prognostic Index score was high or high-intermediate in 92%. For 62 patients receiving anthracycline-based chemotherapies, median survival was 13 months. CD5, CD10, Bcl-6, MUM1, and Bcl-2 were positive in 38%, 13%, 26%, 95%, and 91% of tumors, respectively. All 59 CD10− IVLBCL cases examined were nongerminal center B-cell type because they lacked the Bcl-6+MUM1− immunophenotype. CD5 positivity was associated with a higher prevalence of marrow/blood involvement and thrombocytopenia and a lower frequency of neurologic abnormalities among patients with CD10−IVLBCL. Compared with 97 cases of de novo CD5+CD10−diffuse LBCL, 31 cases of CD5+CD10−IVLBCL exhibited higher frequencies of poor prognostic parameters, except age. Multivariate analysis in IVLBCL revealed that a lack of anthracycline-based chemotherapies (P < .001, hazard ratio [HR]: 9.256), age older than 60 years (P = .012, HR: 2.459), and thrombocytopenia less than 100 × 109/L (P = .012, HR: 2.427) were independently unfavorable prognostic factors; CD5 positivity was not. Beyond immunophenotypic diversity, IVLBCL constitutes a unique group with aggressive behavior.
Introduction
Intravascular large B-cell lymphoma (IVLBCL) is an aggressive and systemic disease characterized by massive proliferation of large tumor cells within the lumina of small to medium-sized vessels.1 IVLBCL has been recently listed as a rare subtype of diffuse large B-cell lymphoma (DLBCL) in the new World Health Organization (WHO) classification.1 Since Pfleger and Tappeiner2 described the first case in 1959, more than 300 cases have been reported to date, mostly small series and case reports. These reports revealed the highly variable symptoms resulting from occlusion of small vessels by neoplastic cells in a variety of organs. The neurologic and dermatologic signs were originally described as specific to IVLBCL in Western countries. In addition, a considerable number of patients with IVLBCL present with nonspecific clinical manifestations including fever, malaise, and anemia, probably because of the dysregulation of inflammatory cytokines.3 We recently documented that most Japanese cases, which lack these specific signs, are associated with hemophagocytic syndrome and might be considered an Asian variant of IVLBCL because of their relatively high prevalence in Asian countries.4
DLBCL is the largest category, accounting for 30% to 40% of malignant lymphomas, and represents a heterogeneous group in terms of immunophenotype, cytogenetics, histology, and clinical features.5 Using cDNA microarray-generated gene-expression profiles, Alizadeh et al6 recently identified 2 main subgroups of DLBCL, the germinal center B cell-like (GCB) and the activated B cell-like types, with different prognoses. Since that identification, an increasing number of reports have addressed the prognostic relevance of CD10, Bcl-6, and MUM1/IRF4 in DLBCL based on the supposition that CD10 is a hallmark of GCB-cell differentiation; however, previous attempts to link CD10 expression with different clinical outcomes have produced controversial results.6-8 Nevertheless, Yamaguchi and colleagues demonstrated that de novo CD5+ DLBCL may constitute a distinct subtype with an aggressive clinical course.9 Subsequent analyses of gene expression and genome profiles have supported the idea of a distinct activated B cell–like type for this lymphoma, which accounts for approximately 10% of DLBCLs.10
The recent studies on IVLBCL simultaneously shed light on the heterogeneity of clinical presentation and immunophenotype among IVLBCL cases. Indeed, in the literature, the reported frequency of CD5 positivity in IVLBCL varies considerably (22%-75%), also with a subset of IVLBCL reported to express CD10, suggesting that this disease may represent an immunophenotypically heterogeneous group.4,11-13 Obviously, then, the immunophenotypic lineage of the tumor cells is the starting point in subclassifying most lymphoid neoplasms; however, to our knowledge, there is no documentation in the English-language literature of the clinicopathologic and prognostic significance of immunophenotypic heterogeneity in patients with IVLBCL. The relationship between CD5+ IVLBCL and de novo CD5+ DLBCL also remains to be elucidated because of the relative rarity of these diseases. To address these issues, we retrospectively analyzed clinically and immunophenotypically 96 patients with IVLBCL in Japan and compared their data with those of de novo CD5+CD10− DLBCL patients with and without intravascular or intrasinusoidal (intravascular/ sinusoidal) patterns.
Patients, materials, and methods
Patients and diagnostic studies
From February 1992 through October 2003, 96 patients with IVLBCL were retrospectively registered from 42 institutions affiliated with the refractory lymphoma study group in Japan, supported by the Ministry of Labor, Health and Welfare of Japan. Informed consent was obtained in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board from each institution that participated. The following information was collected for all patients: age, sex, diagnostic sites, number of extranodal sites involved, stage, performance status, serum lactate dehydrogenase (LDH) level, peripheral-blood (PB)–cell count with differentials including tumor cells, presence or absence of B symptoms, hepatomegaly, splenomegaly, neurologic abnormalities, skin lesion, and respiratory signs and symptoms. Initial therapies (99% of patients), results of bone marrow (BM) examination including tumor cells and hemophagocytosis (93%), and serum levels of C-reactive protein (CRP; 94%), albumin (90%), creatinine (95%), total bilirubin (90%), and soluble IL-2 receptor (69%) before therapy were obtained.
For the control group, we selected 97 cases of de novo CD5+CD10− DLBCL from the original 109 cases of Yamaguchi et al,9 excluding 7 true IVLBCL and 5 de novo CD5+CD10+ DLBCL cases.
Diagnoses of all patients, including those with de novo CD5+CD10− DLBCL as controls, were rendered by 4 expert hematopathologists (T.Y., N.M., J.T., and S.N.) according to the new WHO diagnostic criteria1 ; that is, our diagnosis of IVLBCL was restricted to cases characterized by the presence of neoplastic B cells only in the lumina of small vessels or sinuses (or both). Based on the presence or absence of intravascular/sinusoidal components in the specimens from the 97 patients with de novo CD5+CD10− DLBCL, these cases were assigned to 2 morphologic subgroups, one with and one without intravascular/sinusoidal patterns (DLBCL-IVL, n = 37, and DLBCL-NOS, n = 60, respectively).9 Divergent diagnoses were jointly discussed by the 4 hematopathologists until a final consensus diagnosis was established in each case.
Tissue samples were fixed in 10% formalin and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin. BM and PB smears were stained with Romanowsky (May-Grünwald-Giemsa or Wright-Giemsa) stain.
Immunohistochemistry was performed using the avidin-biotin complex (ABC) immunoperoxidase technique, as previously described.9 Paraffin sections were available for all cases and were analyzed after antigen retrieval was performed using a microwave oven heating treatment. Immunophenotypic analysis of BM or PB was performed by means of flow cytometric analysis in 41 patients; when fresh materials were available (5 patients: 2 from the liver, 1 from the spleen, and 2 at autopsy), immunohistochemistry in frozen sections was done. The tumor cells were confirmed to be of B-cell origin by identification of the expression of one or more pan–B-cell antigens. The following antibodies were used (manufacturers given in parentheses): a panel of monoclonal antibodies against human immunoglobulin light and heavy (anti-κ, anti-λ, anti-IgG, anti-IgA, anti-IgM, and anti-IgD) chains, CD3/F7.2.38, CD45RO/UCHL-1, CD20/L26, CD30/Ber-H2, CD79a/JCB117, Bcl-2/126, and Bcl-6/PG-B6p (Dako, Carpinteria, CA); CD5/4C7, CD10/270, and CD23/1B12 (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom); Bcl-1/cyclin D1 (IBL, Gunma, Japan); MUM1/IRF4 (Santa Cruz Biotechnology, Santa Cruz, CA); CD5/T1, CD10/J5, CD19/B4, and CD20/B1 (Beckman Coulter, Miami, FL); CD3/Leu4, CD5/Leu1, and CD10/HI10a (Becton Dickinson, Mountain View, CA); and CD23/H107 (Nichirei, Tokyo, Japan).4,9
The presence or absence of Epstein-Barr virus (EBV) small RNAs was assessed by means of in situ hybridization using EBV-encoded small nuclear early region (EBER) oligonucleotides and performed on formalin-fixed, paraffin-embedded sections.14 Briefly, a Dako hybridization kit was used with a cocktail of fluorescein isothiocyanate (FITC)–labeled EBER oligonucleotides (one oligonucleotide corresponding to EBER1 and one to EBER2, both 30 bases long; Dako A/S code Y 017). Hybridization products were detected with mouse monoclonal anti-FITC (Dako M878) and a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). RNase A or DNase I pretreatment was used for the negative controls and EBER+ Hodgkin lymphoma specimens for positive controls.
For the immunohistochemical evaluation, main markers of CD5, CD10, and CD20 were examined in all cases. The additional immunostains of CD23 (70 cases), Bcl-2 (57 cases), Bcl-6 (62 cases), MUM1 (61 cases), and cyclin D1 (51 cases), and in situ hybridization using EBER oligonucleotides (59 cases) were subsequently performed when paraffin sections were available. The paraffin blocks from the remaining cases did not contain tumor cells or had been discarded after long-term storage.
Statistics
Comparisons of frequency data between the 2 groups were performed using either χ2 or Fisher exact tests. For continuous data, intergroup comparisons were performed using the Mann-Whitney U test. Overall survival was calculated from the time of pathologic diagnosis to death from any cause or to the last follow-up. Patient survival data were analyzed using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression model, and variables were selected with the stepwise method. Data were analyzed with statistical software (STATA 8, StataCorp LP, College Station, TX).
Results
Clinical features
Our study consisted of 50 men and 46 women with a median age of 67 years (range, 41-85 years). Diagnosis was established in vivo in 81 (84%) patients and postmortem in 15 (16%). Diagnostic sites of disease are listed in Table 1 for the 81 patients with an in vivo diagnosis. Table 2 gives the clinical features of all 96 patients in the study, including prognostic components of the International Prognostic Index (IPI), IPI rating, and other related signs and symptoms.16 Eighty-eight patients (92%) were categorized in the high-risk or high intermediate-risk group for IPI. Hepatomegaly or splenomegaly and anemia (< 110 g/L hemoglobin or 3.50 × 1012/L red blood cell count) or thrombocytopenia (< 100 × 109/L) were seen in 74 (77%) and 81 (84%) patients, respectively. Hemophagocytosis had a statistically significant association with a constellation of symptoms and laboratory data, as follows: B symptoms (P = .003), splenomegaly (P = .044), higher levels of CRP (50 mg/L: P = .033) and soluble IL-2 receptor (≥ 5 × 103 U/mL, P = .003), lower albumin concentration (< 30 g/L; P = .002), and unexpectedly, absence of tumor cells in PB (P = .014) and lower creatinine level (< 15 mg/L; P =.048).
Diagnostic sites for 81 cases of IVLBCL with in vivo diagnosis
| Site . | No. of patients . |
|---|---|
| Bone marrow | 54* |
| Liver | 14 |
| Spleen | 13 |
| Skin | 6 |
| Lung | 5 |
| Lymph node | 3 |
| Adrenal gland | 2 |
| Brain | 2 |
| Kidney | 2 |
| Waldeyer ring | 1 |
| Psoas muscle | 1 |
| Uterus | 1 |
| Thyroid gland | 1 |
| Testis | 1 |
| Paranasal sinus | 1 |
| Ureter | 1 |
| Ileum | 1 |
| Site . | No. of patients . |
|---|---|
| Bone marrow | 54* |
| Liver | 14 |
| Spleen | 13 |
| Skin | 6 |
| Lung | 5 |
| Lymph node | 3 |
| Adrenal gland | 2 |
| Brain | 2 |
| Kidney | 2 |
| Waldeyer ring | 1 |
| Psoas muscle | 1 |
| Uterus | 1 |
| Thyroid gland | 1 |
| Testis | 1 |
| Paranasal sinus | 1 |
| Ureter | 1 |
| Ileum | 1 |
Including 23 cases with tumor cells in the peripheral blood.
Clinical characteristics of 96 patients with IVLBCL
| Phenotype . | Total . | CD5+ or CD5−CD10+* . | CD5−CD10− . | CD5+CD10− . | P† . | P‡ . |
|---|---|---|---|---|---|---|
| No. of patients | 96 | 12 | 53 | 31 | — | — |
| GCB | 12/71 (17) | 12/12 (100) | 0/35 (0) | 0/24 (0) | — | — |
| Age at diagnosis, y | ||||||
| Median | 67 | 68.5 | 67 | 68 | .455 | .667 |
| Range | 41-85 | 42-81 | 41-85 | 44-84 | — | — |
| Older than 60 | 66 (69) | 10 (83) | 35 (66) | 21 (68) | .873 | .489 |
| Sex, male | 50 (52) | 5 (42) | 27 (51) | 18 (58) | .528 | .714 |
| Performance status, above 1 | 79 (82) | 11 (92) | 42 (79) | 26 (84) | .602 | .444 |
| Serum LDH level: high§ | 89 (93) | 12 (100) | 48 (91) | 29 (94) | .633 | .581 |
| Stage: III or IV | 87 (91) | 11 (92) | 47 (89) | 29 (94) | .463 | > .999 |
| Extranodal involvement | 96 (100) | 12 (100) | 53 (100) | 31 (100) | — | — |
| Extranodal involvement, more than 1 site | 65 (68) | 7 (58) | 38 (72) | 20 (65) | .492 | .299 |
| IPI | ||||||
| Low | 2 (2) | 0 (0) | 2 (4) | 0 (0) | — | — |
| Low-intermediate | 6 (6) | 0 (0) | 3 (6) | 3 (10) | — | — |
| High-intermediate | 16 (17) | 2 (17) | 11 (21) | 3 (10) | > .999¶ | .581¶ |
| High | 72 (75) | 10 (83) | 37 (70) | 25 (81) | .276 | .722 |
| B symptoms present | 73 (76) | 10 (83) | 37 (70) | 26 (84) | .151 | .488 |
| Hepatomegaly | 53 (55) | 5 (42) | 31 (58) | 17 (55) | .744 | .366 |
| Splenomegaly | 64 (67) | 9 (75) | 31 (58) | 24 (77) | .078# | .520 |
| Respiratory signs and symptoms | 33 (34) | 2 (17) | 18 (34) | 13 (42) | .465 | .202 |
| Neurologic signs and symptoms | 26 (27) | 3 (25) | 19 (36) | 4 (13) | .023# | .742 |
| Skin lesions | 14 (15) | 3 (25) | 8 (15) | 3 (10) | .739 | .382 |
| Hemophagocytosis in BM | 54/89 (61) | 6/12 (50) | 20/47 (64) | 18/30 (60) | .735 | .717 |
| Tumor cells in BM | 67/89 (75) | 9/12 (75) | 31/47 (66) | 27/30 (90) | .017# | > .999 |
| Tumor cells in PB | 23 (24) | 5 (42) | 4 (8) | 14 (45) | < .001# | .113 |
| Anemia‖ | 63 (66) | 9 (75) | 38 (72) | 16 (52) | .064 | .739 |
| Thrombocytopenia, less than 100 × 109/L | 56 (58) | 10 (83) | 24 (45) | 22 (71) | .022 | .049 |
| Leukocytopenia, less than 4 × 109/L | 26 (27) | 5 (42) | 15 (28) | 6 (19) | .361 | .143 |
| Albumin level, less than 30 g/L | 40/86 (47) | 6/10 (60) | 23/49 (47) | 11/27 (41) | .603 | .735 |
| Bilirubin level, 15 mg/L or higher | 17/86 (20) | 1/11 (9) | 9/47 (19) | 7/28 (25) | .550 | .672 |
| Creatinine level, 15 mg/L or higher | 9/91 (10) | 0/11 (0) | 5/51 (10) | 4/29 (14) | .716 | .337 |
| CRP level, 50 mg/L or higher | 54/90 (60) | 3/12 (25) | 30/48 (63) | 21/30 (70) | .498 | .010 |
| sIL-2R level 5 × 103 U/mL or higher | 37/66 (56) | 6/10 (60) | 19/34 (56) | 12/22 (55) | .922 | .731 |
| Anthracycline-based chemotherapy | 62/94 (66) | 10/12 (83) | 31/51 (61) | 21/31 (68) | .642 | .116 |
| Phenotype . | Total . | CD5+ or CD5−CD10+* . | CD5−CD10− . | CD5+CD10− . | P† . | P‡ . |
|---|---|---|---|---|---|---|
| No. of patients | 96 | 12 | 53 | 31 | — | — |
| GCB | 12/71 (17) | 12/12 (100) | 0/35 (0) | 0/24 (0) | — | — |
| Age at diagnosis, y | ||||||
| Median | 67 | 68.5 | 67 | 68 | .455 | .667 |
| Range | 41-85 | 42-81 | 41-85 | 44-84 | — | — |
| Older than 60 | 66 (69) | 10 (83) | 35 (66) | 21 (68) | .873 | .489 |
| Sex, male | 50 (52) | 5 (42) | 27 (51) | 18 (58) | .528 | .714 |
| Performance status, above 1 | 79 (82) | 11 (92) | 42 (79) | 26 (84) | .602 | .444 |
| Serum LDH level: high§ | 89 (93) | 12 (100) | 48 (91) | 29 (94) | .633 | .581 |
| Stage: III or IV | 87 (91) | 11 (92) | 47 (89) | 29 (94) | .463 | > .999 |
| Extranodal involvement | 96 (100) | 12 (100) | 53 (100) | 31 (100) | — | — |
| Extranodal involvement, more than 1 site | 65 (68) | 7 (58) | 38 (72) | 20 (65) | .492 | .299 |
| IPI | ||||||
| Low | 2 (2) | 0 (0) | 2 (4) | 0 (0) | — | — |
| Low-intermediate | 6 (6) | 0 (0) | 3 (6) | 3 (10) | — | — |
| High-intermediate | 16 (17) | 2 (17) | 11 (21) | 3 (10) | > .999¶ | .581¶ |
| High | 72 (75) | 10 (83) | 37 (70) | 25 (81) | .276 | .722 |
| B symptoms present | 73 (76) | 10 (83) | 37 (70) | 26 (84) | .151 | .488 |
| Hepatomegaly | 53 (55) | 5 (42) | 31 (58) | 17 (55) | .744 | .366 |
| Splenomegaly | 64 (67) | 9 (75) | 31 (58) | 24 (77) | .078# | .520 |
| Respiratory signs and symptoms | 33 (34) | 2 (17) | 18 (34) | 13 (42) | .465 | .202 |
| Neurologic signs and symptoms | 26 (27) | 3 (25) | 19 (36) | 4 (13) | .023# | .742 |
| Skin lesions | 14 (15) | 3 (25) | 8 (15) | 3 (10) | .739 | .382 |
| Hemophagocytosis in BM | 54/89 (61) | 6/12 (50) | 20/47 (64) | 18/30 (60) | .735 | .717 |
| Tumor cells in BM | 67/89 (75) | 9/12 (75) | 31/47 (66) | 27/30 (90) | .017# | > .999 |
| Tumor cells in PB | 23 (24) | 5 (42) | 4 (8) | 14 (45) | < .001# | .113 |
| Anemia‖ | 63 (66) | 9 (75) | 38 (72) | 16 (52) | .064 | .739 |
| Thrombocytopenia, less than 100 × 109/L | 56 (58) | 10 (83) | 24 (45) | 22 (71) | .022 | .049 |
| Leukocytopenia, less than 4 × 109/L | 26 (27) | 5 (42) | 15 (28) | 6 (19) | .361 | .143 |
| Albumin level, less than 30 g/L | 40/86 (47) | 6/10 (60) | 23/49 (47) | 11/27 (41) | .603 | .735 |
| Bilirubin level, 15 mg/L or higher | 17/86 (20) | 1/11 (9) | 9/47 (19) | 7/28 (25) | .550 | .672 |
| Creatinine level, 15 mg/L or higher | 9/91 (10) | 0/11 (0) | 5/51 (10) | 4/29 (14) | .716 | .337 |
| CRP level, 50 mg/L or higher | 54/90 (60) | 3/12 (25) | 30/48 (63) | 21/30 (70) | .498 | .010 |
| sIL-2R level 5 × 103 U/mL or higher | 37/66 (56) | 6/10 (60) | 19/34 (56) | 12/22 (55) | .922 | .731 |
| Anthracycline-based chemotherapy | 62/94 (66) | 10/12 (83) | 31/51 (61) | 21/31 (68) | .642 | .116 |
Values before and after slash indicate numbers of positive and evaluable patients, respectively. In the absence of a slash, all patients are evaluable. Values in parentheses are percentages. P values less than .05 are italicized.
sIL-2R indicates soluble IL-2 receptor; and —, not applicable.
Including CD5−CD10+ (n = 7) and CD5+CD10+ types (n = 5).
CD5−CD10− IVLBCL (n = 53) versus CD5+CD10− IVLBCL (n = 31).
GCB type (n = 12: CD5−CD10+ [n = 7] and CD5+CD10+ [n = 5]) versus non-GCB type (n = 59: CD5−CD10− [n = 35] and CD5+CD10− [n = 24]) according to the decision tree proposed by Hans et al.15
Higher than upper limit of the standard range defined by each institution.
Defined as < 110 g/L hemoglobin or 3.50 × 1012/L red blood cell count.
High or high-intermediate versus low or low-intermediate.
Significant (P < .05) when CD5+ or CD5− CD10−IVLBCL was limited to the 59 cases of non-GCB type.
A small amount of monoclonal immunoglobulin was observed in 13 patients (14%) in the series, consisting of μ, γ, κ, or λ chain (7, 5, 5, and 1 cases, respectively). In 21 patients (22%), we identified some immunologic abnormalities, including antinuclear (n = 15) and anti-intrinsic factor (n = 1) antibodies and a positive reaction to the antiglobulin test (n = 5) and rheumatoid factor (n = 2).
Histopathologic features and immunologic studies
Prototypic histologic patterns of intravascular lymphoma and large lymphoid cells within vessel lumina or sinuses were observed in all 96 patients. These cells were large, with scant cytoplasm, vesicular nuclei, and one or more nucleoli. Immunophenotyping was never used as the sole source of diagnosis in any case.
CD5 was positive in 36 patients (38%). Table 3 shows the numbers and percentages of patients in the CD5− and CD5+ subgroups expressing CD20, CD79a, CD19, CD5, CD10, Bcl-2, Bcl-6, MUM1, CD23, cyclin D1, light-chain κ and λ, and EBER. There were no significant differences in the immunophenotypic features between the CD5− and CD5+ subgroups. Only 5 cases from the present series were simultaneously evaluated with paraffin and frozen sections, and they exhibited identical results: CD5 was positive in 2 of 5 cases examined, CD10 in 1 of 5, CD19 in 3 of 3, CD20 in 5 of 5, CD23 in 0 of 4, immunoglobulin light-chain κ in 2 of 3, and λ in 1 of 3. EBV has not been detected except for a single case with a small number of positive cells.
Immunophenotypic features and EBV status of 96 cases with IVLBCL
| . | Total series . | CD5− . | CD5+ . |
|---|---|---|---|
| No. of patients | 96 | 60 | 36 |
| CD10 | 12/96 (13) | 7/60 (12) | 5/36 (14) |
| CD19 | 35/41 (85) | 18/21 (86) | 17/20 (85) |
| CD20 | 92/96 (96) | 56/60 (93) | 36/36 (100) |
| CD23 | 3/70 (4) | 2/45 (4) | 1/25 (4) |
| CD79a | 49/49 (100) | 30/30 (100) | 19/19 (100) |
| Bcl-2 | 52/57 (91) | 33/37 (89) | 19/20 (95) |
| Bcl-6* | 16/62 (26) | 9/36 (25) | 7/26 (27) |
| MUM1/IRF4† | 58/61 (95) | 34/36 (94) | 24/25 (96) |
| Cyclin D1 | 0/51 (0) | 0/32 (0) | 0/19 (0) |
| κ chain | 20/28 (71) | 11/16 (69) | 9/12 (75) |
| λ chain | 5/28 (18) | 3/16 (19) | 2/12 (17) |
| EBERs-ISH | 0/59 (0) | 0/40 (0) | 0/19 (0) |
| . | Total series . | CD5− . | CD5+ . |
|---|---|---|---|
| No. of patients | 96 | 60 | 36 |
| CD10 | 12/96 (13) | 7/60 (12) | 5/36 (14) |
| CD19 | 35/41 (85) | 18/21 (86) | 17/20 (85) |
| CD20 | 92/96 (96) | 56/60 (93) | 36/36 (100) |
| CD23 | 3/70 (4) | 2/45 (4) | 1/25 (4) |
| CD79a | 49/49 (100) | 30/30 (100) | 19/19 (100) |
| Bcl-2 | 52/57 (91) | 33/37 (89) | 19/20 (95) |
| Bcl-6* | 16/62 (26) | 9/36 (25) | 7/26 (27) |
| MUM1/IRF4† | 58/61 (95) | 34/36 (94) | 24/25 (96) |
| Cyclin D1 | 0/51 (0) | 0/32 (0) | 0/19 (0) |
| κ chain | 20/28 (71) | 11/16 (69) | 9/12 (75) |
| λ chain | 5/28 (18) | 3/16 (19) | 2/12 (17) |
| EBERs-ISH | 0/59 (0) | 0/40 (0) | 0/19 (0) |
Values before and after slash indicate numbers of positive and evaluable patients, respectively. Values in parentheses are percentages.
EBERs-ISH indicates in situ hybridization using EBV-encoded small nuclear early region oligonucleotides.
All of the Bcl-6+ cases showed a partial and weak reaction in 10% to 50% of the tumor cells.
All 3 MUM1/IRF4− cases showed a negative reaction to CD10 and Bcl-6.
Following the decision tree proposed by Hans et al,15 the 96 patients with IVLBCL were assigned to 2 groups based on expression of CD10, Bcl-6, and MUM1 (Table 2). First, 12 IVLBCL cases with CD5+ or CD5−CD10+ type were classified into the GCB group. Among the remaining 84 patients with CD5+ or CD5−CD10− type, all 59 with available, tested paraffin sections were classified into the non-GCB group: CD10−Bcl-6+MUM1+ (n = 15), CD10−Bcl-6−MUM1+ (n = 41), and CD10−Bcl-6− MUM1− (n = 3); no case of someone in the GCB group with CD10−Bcl-6−MUM1− phenotype was found among the 84 patients with CD5+ or CD5−CD10− type.15 The 84 patients with CD5+ or CD5−CD10− type were also divided into 2 subgroups according to CD5 expression: CD5+CD10− and CD5−CD10− (n = 31 and 53, respectively).
Thus, the statistically comparative analyses of the clinical data were performed for the following 4 groups: GCB, non-GCB, CD5+CD10−, and CD5−CD10− (n = 12, 59, 31, and 53, respectively). There were no significant differences in clinical features or parameters between the GCB and non-GCB groups in the present series except for lower levels of CRP and a higher frequency of thrombocytopenia in the former (P = .010 and .049, respectively; Table 2). In comparisons between the 31 CD5+CD10− and 53 CD5−CD10− patients, only 4 parameters were significant in the former: higher prevalence of thrombocytopenia, tumor cells in BM and in PB, and fewer neurologic abnormalities (P = .022, .017, < .001, and .023, respectively; Table 2). When the analysis was limited to the 59 cases in the non-GCB group, the 24 non-GCB CD5+ cases were significantly associated with a higher prevalence of splenomegaly, tumor cells in BM and in PB, and fewer neurologic abnormalities (P = .007, .004, < .001, and .007, respectively) compared to the 35 non-GCB CD5− cases.
Comparison of the clinical features between CD5+CD10− IVLBCL and de novo CD5+CD10− DLBCL
In the current study, the lymphatic organs, such as Waldeyer ring and the spleen, were not counted as extranodal sites. Because these organs were counted as extranodal sites in the original report by Yamaguchi et al,9 for the 97 patients with de novo CD5+CD10− DLBCL in the current study, we reassessed the numbers of extranodal sites.
In a comparison of the 2 subgroups of de novo CD5+CD10− DLBCL with and without intravascular/sinusoidal patterns (ie, DLBCL-IVL and DLBCL-NOS), the CD5+CD10− IVLBCL group showed a significantly closer association with poor prognostic features or parameters in the IPI, as follows (comparisons with DLBCL-IVL and DLBCL-NOS, respectively): performance status < 1, P < .001 and < .001; serum LDH level more than normal, P = .046 and .001; clinical stage III or IV, P = .008 and < .001; number of extranodal sites involved more than 1, P = .004 and < .001; and the presence of B symptoms (P = .001 and < .001; Table 4) The exception was age: for age, P = .579 in the comparison of CD5+CD10− IVLBCL and DLBCL-IVL, and P = .880 in the comparison of CD5+CD10− IVLBCL and DLBCL-NOS. As a result, patients with CD5+CD10− IVLBCL were more frequently categorized as high risk for IPI than those with de novo CD5+CD10− DLBCL-IVL or DLBCL-NOS. The possibility of being categorized in the high-risk or high intermediate-risk group for IPI was also significantly higher in the CD5+CD10− IVLBCL group compared with either the de novo CD5+CD10− DLBCL-IVL or DLBCL-NOS groups.
Comparison of clinical features among de novo CD5+CD10− DLBCL with and without intravascular/sinusoidal patterning and CD5+CD10− IVLBCL
| Diagnosis . | De novo CD5+CD10− DLBCL . | CD5+CD10− IVLBCL . | P* . | P† . | P‡ . | |
|---|---|---|---|---|---|---|
| No IVL pattern . | IVL pattern . | IVL pattern, primarily . | ||||
| Grouping | DLBCL-NOS | DLBCL-IVL | IVLBCL | — | — | — |
| No. of patients | 60 | 37 | 31 | — | — | — |
| Age at diagnosis, y | ||||||
| Median | 66 | 63 | 68 | .588 | .880 | .579 |
| Range | 22-91 | 36-85 | 44-84 | — | — | — |
| Older than 60 | 41 (68) | 25 (68) | 21 (68) | .937 | .954 | .988 |
| Sex, male/female | 26/34 | 17/20 | 18/13 | .801 | .183 | .319 |
| Performance status, greater than 1 | 17 (28) | 14 (38) | 26 (84) | .330 | < .001 | < .001 |
| Serum LDH level, high§ | 37 (62) | 28 (76) | 29 (94) | .154 | .001 | .046 |
| Stage III/IV | 32 (53) | 25 (68) | 29 (94) | .167 | < .001 | .008 |
| Extranodal involvement‖ | 34 (57) | 25 (68) | 31 (100) | .285 | < .001 | < .001 |
| Extranodal involvement, more than 1 site | 9 (15) | 11 (30) | 20 (65) | .082 | < .001 | .004 |
| IPI | ||||||
| Low | 22 (37) | 7 (19) | 0 (0) | — | — | — |
| Low-intermediate | 14 (23) | 10 (27) | 3 (10) | — | — | — |
| High-intermediate | 7 (12) | 6 (16) | 3 (10) | .177§ | < .001¶ | .001¶ |
| High | 17 (28) | 14 (38) | 25 (81) | .330 | < .001 | < .001 |
| B symptoms present | 17 (28) | 17 (46) | 26 (84) | .077 | < .001 | .001 |
| Tumor cell in BM/PB | 10 (17) | 10 (27) | 27 (87) | .221 | < .001 | < .001 |
| Hepatomegaly | 6 (10) | 10 (27) | 17 (55) | .028 | < .001 | .020 |
| Splenomegaly | 7 (12) | 14 (38) | 24 (77) | .002 | < .001 | .001 |
| Lymphadenopathy | 45 (75) | 26 (70) | 4 (13) | .609 | < .001 | < .001 |
| Diagnosis . | De novo CD5+CD10− DLBCL . | CD5+CD10− IVLBCL . | P* . | P† . | P‡ . | |
|---|---|---|---|---|---|---|
| No IVL pattern . | IVL pattern . | IVL pattern, primarily . | ||||
| Grouping | DLBCL-NOS | DLBCL-IVL | IVLBCL | — | — | — |
| No. of patients | 60 | 37 | 31 | — | — | — |
| Age at diagnosis, y | ||||||
| Median | 66 | 63 | 68 | .588 | .880 | .579 |
| Range | 22-91 | 36-85 | 44-84 | — | — | — |
| Older than 60 | 41 (68) | 25 (68) | 21 (68) | .937 | .954 | .988 |
| Sex, male/female | 26/34 | 17/20 | 18/13 | .801 | .183 | .319 |
| Performance status, greater than 1 | 17 (28) | 14 (38) | 26 (84) | .330 | < .001 | < .001 |
| Serum LDH level, high§ | 37 (62) | 28 (76) | 29 (94) | .154 | .001 | .046 |
| Stage III/IV | 32 (53) | 25 (68) | 29 (94) | .167 | < .001 | .008 |
| Extranodal involvement‖ | 34 (57) | 25 (68) | 31 (100) | .285 | < .001 | < .001 |
| Extranodal involvement, more than 1 site | 9 (15) | 11 (30) | 20 (65) | .082 | < .001 | .004 |
| IPI | ||||||
| Low | 22 (37) | 7 (19) | 0 (0) | — | — | — |
| Low-intermediate | 14 (23) | 10 (27) | 3 (10) | — | — | — |
| High-intermediate | 7 (12) | 6 (16) | 3 (10) | .177§ | < .001¶ | .001¶ |
| High | 17 (28) | 14 (38) | 25 (81) | .330 | < .001 | < .001 |
| B symptoms present | 17 (28) | 17 (46) | 26 (84) | .077 | < .001 | .001 |
| Tumor cell in BM/PB | 10 (17) | 10 (27) | 27 (87) | .221 | < .001 | < .001 |
| Hepatomegaly | 6 (10) | 10 (27) | 17 (55) | .028 | < .001 | .020 |
| Splenomegaly | 7 (12) | 14 (38) | 24 (77) | .002 | < .001 | .001 |
| Lymphadenopathy | 45 (75) | 26 (70) | 4 (13) | .609 | < .001 | < .001 |
Values in parentheses expressed as percentage. P values less than .05 are italicized.
DLBCL-NOS indicates de novo CD5+CD10− DLBCL, not otherwise specified, without intravascular/sinusoidal pattern; DLBCL-IVL, de novo CD5+CD10− DLBCL with intravascular/sinusoidal pattern; IVL pattern, intravascular/sinusoidal pattern; and —, not applicable.
DLBCL-NOS versus DLBCL-IVL.
DLBCL-NOS versus CD5+CD10− IVLBCL.
DLBCL-IVL versus CD5+CD10− IVLBCL.
Higher than the upper limit of the standard range defined by each institution.
Waldeyer ring or spleen was not counted as an extranodal site.
High or high-intermediate versus low or low-intermediate.
Patients in the CD5+CD10− IVLBCL group showed significantly higher frequencies of BM/PB involvement, hepatomegaly, and splenomegaly than either group of de novo CD5+CD10− DLBCL. On the other hand, the frequency of lymph node swelling was significantly higher in either group of de novo CD5+CD10− DLBCL compared with CD5+CD10− IVLBCL. Interestingly, the de novo CD5+CD10− DLBCL-IVL group showed significantly higher frequencies of both hepatomegaly and splenomegaly compared with the de novo CD5+CD10− DLBCL-NOS group.
Survival analysis
Among 81 IVLBCL patients with the in vivo diagnosis, survival data were available for 79; 2 were lost to follow-up soon after the diagnosis. Median survival was 5 months (range, 0.5-47.0 months) for 48 patients with lethal clinical course, whereas the median follow-up duration was 14.5 months (range, 1-95.5 months) for the 31 survivors. In total, the estimated 3-year survival rate was 27.0% (±6.5%, with a median follow-up of 9.5 months).
In the present series, 62 patients were treated with anthracycline-based chemotherapy, with 32 of 58 cases (55%) evaluated achieving complete remission and 7 (12%) achieving partial remission; median survival overall for the 62 cases was 13 months. Of the 62 patients, 57 were treated with cyclophosphamide, hydroxydoxorubicin, Oncovin (vincristine), prednisolone (CHOP) or a CHOP-like regimen; 2 of the 57 received involved-field radiotherapy subsequent to a complete remission.
This series included 7 patients who received high-dose chemotherapy supported by autologous stem cell transplantation as a consolidation therapy; 5 patients are alive and relapse-free having had follow-up at 10.5, 18.5, 29, 39, and 95.5 months from diagnosis, and 2 died of the disease at 35 and 39 months from diagnosis, respectively. An additional 2 patients who received anthracycline-based chemotherapy combined with rituximab achieved complete remission but eventually died of the disease at 8.5 and 24.5 months, respectively, from diagnosis. Of the 79 cases included for evaluation of survival, 17 did not receive anthracycline-based chemotherapy; these 17 had a median survival of 1.5 months, 10 received supportive care with corticosteroids, 3 with a corticosteroid and single chemotherapeutic agent, and 4 with cyclophosphamide, a vinca alkaloid, and a corticosteroid.
In a comparison of patients receiving anthracycline-based chemotherapy (62 in all) with those not receiving it (17 in all), there were few statistically significant differences in prognostic factors such as higher levels of albumin, lower levels of bilirubin, lower performance status (PS), or the absence of B symptoms (data not shown); however, a higher frequency of 2 or more sites of extranodal involvement was significantly associated with those not receiving the treatment (P = .049).
For the 79 patients evaluated for survival, univariate Cox analysis identified an association with the following unfavorable prognostic factors: no use of anthracycline-based chemotherapy, older age, thrombocytopenia, elevated soluble IL-2 receptor level, presence of tumor cells in BM or PB (or both), and clinical stage higher than II; however, there was no association identified with expression of CD5 antigen (Table 5) Multivariate analysis using the Cox regression model revealed that older age, thrombocytopenia, and a lack of anthracycline-based chemotherapy were independent and significant unfavorable prognostic factors for overall survival (Table 5).
Prognostic factors affecting overall survival for 79 cases with IVLBCL diagnosed in vivo
| Variable . | Unfavorable factor . | Univariate . | Multivariate; final model . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| CD5 | Positive | 1.188 (0.661-2.134) | .567 | ND | ND |
| PS | 2-4 | 1.472 (0.685-3.163) | .321 | ND | ND |
| Extranodal | > 1 site | 1.535 (0.821-2.871) | .18 | ND | ND |
| Serum LDH level | High† | 3.923 (0.540-28.508) | .177 | ND | ND |
| Stage | III/IV | 4.247 (1.026-17.573) | .046 | — | — |
| TCs in BM/PB | Yes | 2.369 (1.046-5.365) | .039 | — | — |
| sIL-2R | ≥ 5 × 103 U/mL | 2.458 (1.206-5.010) | .013 | — | — |
| Platelet | < 100 × 109/L | 2.375 (1.216-4.639) | .011 | 2.427 (1.217-4.840) | .012 |
| Age | > 60 y | 2.471 (1.256-4.859) | .009 | 2.459 (1.219-4.960) | .012 |
| Chemotherapy* | No | 6.413 (2.818-14.594) | < .001 | 9.256 (3.711-23.089) | < .001 |
| Variable . | Unfavorable factor . | Univariate . | Multivariate; final model . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| CD5 | Positive | 1.188 (0.661-2.134) | .567 | ND | ND |
| PS | 2-4 | 1.472 (0.685-3.163) | .321 | ND | ND |
| Extranodal | > 1 site | 1.535 (0.821-2.871) | .18 | ND | ND |
| Serum LDH level | High† | 3.923 (0.540-28.508) | .177 | ND | ND |
| Stage | III/IV | 4.247 (1.026-17.573) | .046 | — | — |
| TCs in BM/PB | Yes | 2.369 (1.046-5.365) | .039 | — | — |
| sIL-2R | ≥ 5 × 103 U/mL | 2.458 (1.206-5.010) | .013 | — | — |
| Platelet | < 100 × 109/L | 2.375 (1.216-4.639) | .011 | 2.427 (1.217-4.840) | .012 |
| Age | > 60 y | 2.471 (1.256-4.859) | .009 | 2.459 (1.219-4.960) | .012 |
| Chemotherapy* | No | 6.413 (2.818-14.594) | < .001 | 9.256 (3.711-23.089) | < .001 |
TCs in BM/PB indicates tumor cells in the bone marrow or peripheral blood or both; ND, not done; —, not applicable.
Anthracycline-based chemotherapy.
Higher than the upper limit of the standard range defined by each institution.
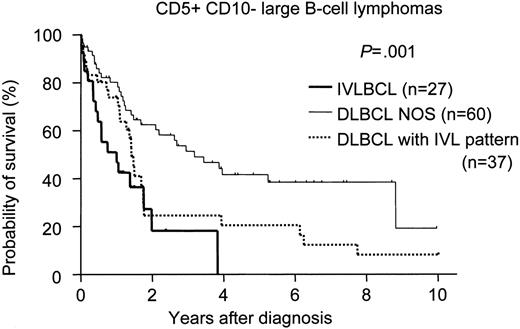
There was no significant difference in survival among the phenotypically delineated IVLBCL groups (Figure 1). We also compared the survival of CD5+CD10− IVLBCL patients with that of de novo CD5+CD10− DLBCL-NOS and DLBCL-IVL patients with the same phenotype. The survival curve of the patients with CD5+CD10− IVLBCL overlapped with that of the CD5+CD10− DLBCL-IVL patients but was significantly inferior to that of the patients with CD5+CD10− DLBCL-NOS (Figure 2).
Survival curves for intravascular large B-cell lymphoma (IVLBCL). No significant difference was found for survival among the 3 IVLBCL groups: CD5−CD10−, CD5+CD10−, and CD5+ or CD5−CD10+. The expression of CD5 or CD10 had no significant effect on survival for IVLBCL.
Survival curves for intravascular large B-cell lymphoma (IVLBCL). No significant difference was found for survival among the 3 IVLBCL groups: CD5−CD10−, CD5+CD10−, and CD5+ or CD5−CD10+. The expression of CD5 or CD10 had no significant effect on survival for IVLBCL.
Survival curves for CD5+CD10− large B-cell lymphomas. De novo CD5+CD10− DLBCL without an intravascular/sinusoidal pattern (DLBCL-NOS) showed significantly better survival than either de novo CD5+CD10− DLBCL with an intravascular/sinusoidal pattern (DLBCL with IVL pattern; log-rank P = .018) or CD5+CD10− IVLBCL (P < .001). No significant difference for survival was found between CD5+CD10− IVLBCL and DLBCL with an IVL pattern (P = .158).
Survival curves for CD5+CD10− large B-cell lymphomas. De novo CD5+CD10− DLBCL without an intravascular/sinusoidal pattern (DLBCL-NOS) showed significantly better survival than either de novo CD5+CD10− DLBCL with an intravascular/sinusoidal pattern (DLBCL with IVL pattern; log-rank P = .018) or CD5+CD10− IVLBCL (P < .001). No significant difference for survival was found between CD5+CD10− IVLBCL and DLBCL with an IVL pattern (P = .158).
Discussion
We report here on 96 patients from Japan with IVLBCL, to our knowledge the largest such study to date addressing clinical profiles, phenotypic features, outcome, and prognostic factors in this disease. In our group, 36 cases (38%) were positive for CD5. We also investigated the relationship of this disease with an additional 97 patients with de novo CD5+CD10− DLBCL with and without an intravascular/sinusoidal pattern. Our study provides additional evidence that IVLBCL constitutes a distinct aggressive group within DLBCL that is classifiable beyond the criteria of heterogeneity in clinical presentation and immunophenotype. More than half of the patients presented with hepatosplenomegaly (77%), anemia or thrombocytopenia (84%), hemophagocytosis (61%), and BM involvement of tumor cells (75%), but rarely with neurologic symptoms (27%) or cutaneous lesions (15%); these findings conform to the clinicopathologic picture we have proposed for an Asian variant of IVLBCL.3,4
These clinical features at presentation were largely attributable to the disseminated disease, including B symptoms (76%), hypoalbuminemia (47%), and respiratory symptoms (34%). Interestingly, in the current series, the prevalence (84%) of patients with an in vivo diagnosis and the overall response rate (67%) of their therapeutic management were generally in keeping with those (79% and 59%, respectively) of Western IVLBCL patients reported by Ferreri et al17 and the International Extranodal Lymphoma Study Group (IELSG); this consistency suggests an increasing recognition of this disease over the past 2 decades. Wick et al18 reported 15 cases with IVLBCL in 1986, all of which were diagnosed at autopsy; DiGiuseppe et al19 documented the frequency of in vivo diagnosis as 70% among 10 cases with IVLBCL in 1994; and in 2004, Ferreri et al20 described 38 IVLBCL cases, 79% of which were in vivo diagnoses. However, there appear to be some distinct differences in some of the clinical manifestations between Western and Japanese patients.
In the present series, BM was the most frequently involved organ, and its involvement was usually accompanied by hemophagocytosis (61%), symptomatically developing anemia (66%), thrombocytopenia (58%), and sometimes leukocytopenia (27%). We previously documented that most DLBCL patients with hemophagocytic syndrome, reported mainly from Asian countries, exhibited the pathologic features of IVLBCL but rarely with neurologic abnormalities or cutaneous lesions; we designated this distinct cluster of presentation as the Asian variant of IVLBCL.3,4 The present observations support our assertion that, based on clinicopathologic features, many Japanese patients with IVLBCL (54 of the 96 patients in this series) conform to this proposed Asian variant of the disease.21 In the Western series of the IELSG, BM involvement was observed in 32% of patients, the highest frequency from Western countries, and was significantly associated with hepatosplenic involvement and thrombocytopenia (Table 6)20 Although this figure was significantly lower than ours and their series had no documentation of hemophagocytosis, a fraction of those patients may be regarded as having the Asian variant of IVLBCL. This possibility requires further investigation.
Comparison of sites of disease and laboratory findings between the present series and those of Ferreri et al20
| . | Present series . | Ferreri et al20 . | P . |
|---|---|---|---|
| No. of patients | 96 | 38 | — |
| B symptoms, % | 76 | 55 | .018 |
| Sites of disease, % | |||
| Bone marrow | 75 | 32 | < .001 |
| Spleen | 67 | 26 | < .001 |
| Liver | 55 | 26 | .003 |
| Peripheral blood | 24 | 5 | .012 |
| Central nervous system | 27 | 39 | .171 |
| Skin | 15 | 39 | .002 |
| Lymph nodes | 11 | 11 | > .999 |
| Laboratory findings, % | |||
| Anemia, less than 120 g/L hemoglobin* | 78 | 63 | .075 |
| Thrombocytopenia, less than 150 × 109/L | 76 | 29 | < .001 |
| Serum LDH level, high† | 93 | 86 | .278 |
| Hypoalbuminemia: less than 36 g/L | 84 | 18 | < .001 |
| . | Present series . | Ferreri et al20 . | P . |
|---|---|---|---|
| No. of patients | 96 | 38 | — |
| B symptoms, % | 76 | 55 | .018 |
| Sites of disease, % | |||
| Bone marrow | 75 | 32 | < .001 |
| Spleen | 67 | 26 | < .001 |
| Liver | 55 | 26 | .003 |
| Peripheral blood | 24 | 5 | .012 |
| Central nervous system | 27 | 39 | .171 |
| Skin | 15 | 39 | .002 |
| Lymph nodes | 11 | 11 | > .999 |
| Laboratory findings, % | |||
| Anemia, less than 120 g/L hemoglobin* | 78 | 63 | .075 |
| Thrombocytopenia, less than 150 × 109/L | 76 | 29 | < .001 |
| Serum LDH level, high† | 93 | 86 | .278 |
| Hypoalbuminemia: less than 36 g/L | 84 | 18 | < .001 |
— indicates not applicable.
Values for this term are based on the definition by Ferreri et al.20
Higher than the upper limit of the standard range defined by each institution.
Hemophagocytosis in T/natural-killer (NK)–cell lymphoma usually means an extremely poor prognosis22 ; however, it was not an unfavorable prognostic factor for IVLBCL in the present study. In fact, the presence of hemophagocytosis was associated with some parameters suggestive of disseminated disease, including B symptoms, higher serum levels of soluble IL-2 receptor, and lower albumin concentrations, but also with some suggestive of a favorable outcome, including the absence of PB involvement and lower levels of creatinine. Further study will be needed to confirm our observations.
In the Far East, nasal type NK/T-cell lymphoma with an association of EBV is more prevalent and frequently associated with hemophagocytosis.23 Of note, in the present IVLBCL series, EBV has so far not been detected except for a single case with a small number of positive cells. Thus, the exact mechanism of the hemophagocytosis is uncertain, although humoral factors, such as IL-6 and macrophage colony-stimulating factor, may contribute to this phenomenon in IVLBCL.4
Ferreri et al20 and the IELSG recently shed light on a unique group, the cutaneous variant, comprising 10 (26%) of 38 IVLBCL patients in Europe; this variant is characterized by the exclusive limitation of tumors to the skin at presentation with an invariably female predominance and normal platelet count, and it is regarded as an independent favorable prognostic factor.20 Indeed, cutaneous lesions are well known as one of the main clinical presentations in reports from Western countries, reaching 39% in the Ferreri et al series. However, in our series, cutaneous lesions or involvement were low, achieving a maximum of only 15%. All cases with cutaneous lesions in our series were associated with thrombocytopenia or lesions at other sites and thus did not meet the diagnostic criteria required to designate the cutaneous variant as a localized disease (Table 6). We also identified neurologic signs and symptoms in 26 (27%) of our patients, a percentage that is lower than that (39%) of the IELSG report, the lowest for neurologic abnormalities in Western countries; however, our percentage was not significantly lower.18,19 On the other hand, both hepatomegaly (54%) and splenomegaly (67%) were more frequently seen in the current series than in the IELSG study (each 26%).
The clinicopathologic significance of CD5 and CD10 expression among the IVLBCL patients remains to be elucidated because of the limited number of reported cases.12,13 Hans et al15 recently reported that classification as GCB or non-GCB, based on immunostains of CD10, Bcl-6, and MUM1, could be considered an independent predictor analogous to the results of gene-expression profiles in the DLBCL category. In our series, according to their definition, all 12 CD10+ IVLBCL patients were classified as GCB. Of note, of the 59 CD10− IVLBCL patients tested in our series, Bcl-6 and MUM1 immunostains classified all of them as non-GCB. Thus, most of the 84 patients with CD10− IVLBCL in the present series may correspond with the non-GCB type. Based on the presence of somatic mutation in variable regions of immunoglobulin heavy-chain genes, Kanda et al24 suggested that most IVLBCL cases might originate from the post-GC cells. The present series may support their assertion that many or most IVLBCL cases are grouped into a non-GCB type. However, this immunophenotypic delineation of GCB and non-GCB types resulted in no significant differences in the current study except for the lower levels of CRP and thrombocytopenia in the GCB group.
The frequency of CD5 expression in IVLBCL cases has varied considerably from 22% to 75% in previous reports4,11-13,25 and was 38% in the present study. Having CD5+CD10− tumors was significantly associated with higher frequencies of thrombocytopenia and BM/PB involvement and lower frequencies of neurologic abnormalities as compared with the CD5−CD10− type; however, there were no significant differences for any other clinical features or parameters, including survival. When limited to the 59 cases in our study with a non-GCB type, the difference between CD5+ and CD5− cases was almost the same. The expression of CD5, as well as the presence of hemophagocytosis, might not be a prognostic factor in IVLBCL.
However, the clinicopathologic significance of the immunophenotypes (CD5 and CD10 and the GCB and non-GCB types) in IVLBCL still remains to be elucidated because of the limited number of patients in the series.12,24,26 Further studies should address these issues in a larger series of patients with IVLBCL.
Yamaguchi et al9 previously reported that de novo CD5+ DLBCL is associated with more aggressive clinical features and a more unfavorable prognosis than CD5− DLBCL. It is still unknown if CD5+CD10− IVLBCL might constitute a distinct subgroup of de novo CD5+CD10− DLBCL. To investigate the relationship between CD5+CD10− IVLBCL (n = 31) and de novo CD5+CD10− DLBCL (n = 97) with and without intravascular/sinusoidal patterning, we performed a comparative analysis. There was no significant difference in the age distribution between the patient groups with these diseases. In addition to a greater association with all the unfavorable factors listed in the IPI, except older age, CD5+CD10− IVLBCL also was more strongly associated than CD5+CD10− DLBCL with the following features and parameters: B symptoms, BM involvement, splenomegaly, hepatomegaly, and the absence of nodal presentation. Of interest, there was no significant difference in survival between patients with CD5+CD10− IVLBCL and de novo CD5+CD10− DLBCL partly featuring intravascular/sinusoidal patterning; both manifested a more aggressive clinical course than de novo CD5+CD10− DLBCL lacking these morphologic features. These results suggest that the histopathologic assessment of the intravascular/sinusoidal location of neoplastic cells in DLBCL might be a reliable prognostic parameter, although further examination, especially for cases with CD5− DLBCL, is required to confirm this assertion.
Identification of clinicopathologic features is of relevance for categorizing IVLBCL patients into different risk groups. Here we have shown that in the case of in vivo diagnosed IVLBCL, age over 60 years, thrombocytopenia, and a lack of anthracycline-based chemotherapy are factors independently predictive of a poor prognosis.
In conclusion, IVLBCL appears to constitute a distinct group within DLBCL, with features that extend beyond its broad clinical manifestations and the phenotypic heterogeneity of CD5 and CD10. There was no significant difference in prognosis between CD5+ and CD5− IVLBCL patients, most of whom seemed to be classified as non-GCB. Further investigation is required to examine the possibility that CD5+ IVLBCL and de novo CD5+ DLBCL may lie in a continuous spectrum. The clinical features in our Japanese series were distinct from those identified in Western series; the Japanese series is associated with hemophagocytic syndrome, frequent BM involvement, and the lack of a cutaneous variant. The validity of these differences will be resolved only with future studies involving larger series.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the contributing members of the Refractory Lymphoma Study Group appears as a data supplement to the online version of this article (Document S1, available on the Blood website; click on the Supplemental Document link at the top of the online article).
Contribution: T.M., M.Y., R.S., and S.N. participated in designing and performing the research; S.N., J.-i.T., N.M., and T.Y. centralized the pathologic review; R.S., M.Y., M.O., Y.S., J.-i.T., M.K., I.M., N.M., T.Y., and T.M. controlled and analyzed data; T.M., M.Y., R.S., and S.N. wrote the paper; and all authors checked the final version of the manuscript.
This work was supported by Grants-in-Aid from the Ministry of Health, Labor, and Welfare of Japan, and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal