Abstract
Secondary lymphoid organs (SLOs) provide a niche for the initiation and regulation of T-cell responses, but the mechanisms have been poorly understood. We investigated the influence of chemokines CCL19 and CCL21 constitutively expressed in SLOs on activation-induced cell death (AICD) of CD4+ T cells. When paucity of lymph node T cells (plt) mutant mice lacking expression of CCL19/CCL21 were primed with OVA/CFA, both expansion of OVA-responding CD4+ T cells in the draining lymph nodes and an in vitro recall response were prolonged as compared with responses in wild-type (WT) mice. The apoptotic cell frequency among OVA-responding CD4+ T cells was similarly low in plt/plt and WT mice during the clonal expansion phase. However, during the clonal contraction phase, the frequency never increased in plt/plt mice, whereas in WT mice it continuously increased to a peak 18 days after immunization. The presence of CCL19/CCL21 during the in vitro stimulation of CD4+ T cells with anti-CD3 plus anti-CD28 significantly enhanced in vitro AICD induction of the restimulated T cells, partially through enhancing expression of Fas ligand. Our results suggest that CCL19/CCL21 produced by stromal cells and antigen-presenting cells regulate CD4+ T-cell immune responses in SLOs by promoting AICD.
Introduction
Activation of naive T cells through engagement of the T-cell receptor (TCR) by antigen leads to proliferation and differentiation into helper and cytotoxic T cells. After expansion of antigen-specific T cells, the majority of the effector cells die to prevent undesirable immune responses such as autoimmunity,1,2 whereas a portion of them survive as memory T cells. One way homeostasis is maintained is the clearance of effector T cells through activation-induced cell death (AICD), in which activated T cells are triggered through various means to die by apoptosis.1-4 The importance of the lymphoid organ microenvironment in regulation of T-cell responses has been suggested by a previous report, in which promotion of T-cell retention in lymphoid organs by a sphingosine-derived drug, FTY720, suppresses in vivo T-cell responses.5 Secondary lymphoid organ T-cell zones contain antigen-presenting cells and stromal cells secreting various cytokines and chemokines with potential to regulate T-cell responses, but the roles of these cells in promoting T-cell AICD are mostly unknown.
The chemokines CCL19 and CCL21 are expressed by stromal cells in the T-cell zone of secondary lymphoid tissues.6,7 Additionally, a subset of dendritic cells (DCs) in the T-cell zone has also been shown to produce CCL19.8 There are 2 types of CCL21 in mouse; CCL21-Ser, which is expressed on high endothelial venules and in the T-cell zone of secondary lymphoid organs, and CCL21-Leu, which is expressed only in the lymphatic endothelium.6,7,9,10 CCL19 and CCL21 attract cells expressing their common receptor CCR7.11,12 These 2 chemokines mobilize naive or central memory T (TCM) cells and mature DCs to the T-cell zone,13-16 and thus can potentially affect T-cell responses.
We previously reported that plt/plt mice lacking CCL19 and CCL21 expression in secondary lymphoid tissues due to the lack of CCL19 and CCL21-Ser genes exhibit delayed but ultimately enhanced T-cell responses.10,17,18 Further, the T-cell responses in plt/plt mice persist for a long time, a phenomenon never observed in wild-type (WT) mice, suggesting a possible failure to appropriately clear antigen-specific T cells after their activation. One possibility might be that antigen-activated T cells may stay in the draining lymph nodes (LNs) in plt/plt mice much longer than in control mice because of little competition for a survival niche. Another possible explanation is that CCL19 and CCL21 may participate in the priming and preparing of CD4+ T cells for regulation of their response to the subsequent stimulation. Whether CCL19 and CCL21 regulate T-cell responses other than migration, such as AICD of activated T cells, is unknown. In this study, we analyzed AICD induction in CD4+ T cells in plt/plt mouse in vivo and in vitro. We found that the frequency of apoptotic antigen-specific CD4+ T cells was decreased in the draining LNs of plt/plt mice, when mice were subcutaneously immunized with a protein antigen in complete Freund adjuvant (CFA). Additionally, AICD of CD4+ T cells induced by repeated in vitro stimulation via TCR is increased in a dose-dependent manner by additional stimulation with CCL19 or CCL21 in vitro. Our results suggest that CCL19 and CCL21 promote AICD of antigen-specific CD4+ T cells, demonstrating that these chemokines regulate not only T-cell mobilization but also their fate after activation.
Materials and methods
Mice and immunization
This study was approved by Institutional Review Board of the Toho University School of Medicine (Tokyo, Japan). BALB/c, C57BL/6, C57BL/6-lpr/lpr, and C57BL/6-gld/gld mice were purchased from Charles River (Atsugi, Japan) or Shizuoka Laboratory Corporation (Hamamatsu, Japan). BALB/c-plt/plt mice were produced by back-crossing DDD/1-plt/plt mice to the BALB/c background for 10 generations.13 Where indicated, CCR7-deficient C57BL/6 mice also were used.19 OVA323-339/I-Ad-specific TCR transgenic DO11.10 mice were kindly provided by Dr Gilboa (Duke University, Durham, NC).20 Mice were bred and kept in the animal facilities of Toho University School of Medicine according to institutional guidelines. Experiments were performed on 8- to 12-week-old female mice. Mice were subcutaneously immunized in the hind footpads with 100 μg chicken OVA (Sigma-Aldrich, St Louis, MO) emulsified in CFA (Difco, Detroit, MI; OVA/CFA).
Adoptive transfer of antigen-specific naive CD4+ T cells
Naive CD4+ T cells were purified from DO11.10 mice.21 Briefly, CD4+ T cells were isolated from pooled LNs and spleen on a magnetic-activated cell sorting (MACS) column (Miltenyi Biotec, Auburn, CA) using anti-CD8 (53-6-7.2), -CD11b (M1/70), -CD11c (HL3), -CD49e (DX5), -B220 (RA6-3A1), –MHC class II (39-10-8), and -F4/80 monoclonal antibodies (mAbs) and rat anti–mouse IgM (Vector Laboratories, Burlingame, CA). Then high-density naive cells were collected by Percoll (Amersham Bioscience, Piscataway, NJ) gradient centrifugation. Over 95% of the purified cells were CD4+CD45RB+ cells. The frequency of transgenic TCR+ cells was determined with KJ1-26 mAb by flow cytometry. Purified DO11.10 CD4+ T cells were labeled with 5-(and 6-)carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene OR) according to the manufacturer's instructions; then 2.5 × 106 cells were intravenously transferred to recipients. The recipients were immunized with OVA/CFA 1 day after the adoptive transfer.
T-cell proliferation assay
Single-cell suspensions were prepared from popliteal LNs at indicated days after immunization. LN cells (5 × 105) were cultured for 4 days with OVA at indicated concentrations in 200 μL complete culture medium, RPMI 1640 supplemented with 10% FCS, 50 μM 2-ME, 10 mM HEPES, 50 U/mL penicillin, and 50 μg/mL streptomycin (Gibco, Gaithersburg, MD) in 96-well plates. The cells were pulsed with 1.25 μCi (0.046 MBq) [3H]-thymidine (Amersham Bioscience) during the final 10 hours of culture, then analyzed on a Matrix96 direct β counter (Packard Instrument, Meriden, CT).
AICD induction in vitro
LN cells were treated with 5 μg/mL concanavalin A (Amersham Bioscience) for 48 hours in complete culture medium, and then incubated for another 10 minutes in the presence of 10 μg/mL α-methyl-α-d-mannoside (Sigma-Aldrich). Viable T cells were purified by density gradient centrifugation over Lympholyte-M (Cedarlane Labs, Hornby, ON, Canada). Then, 5 × 104 viable T cells were recultured with 50 U/mL IL-2 for 48 hours in a 96-well plate coated with 1 μg/mL anti-CD3 (145-2C11) for 2 days (second culture). Then, apoptotic cells were analyzed as described in “Apoptotic cell analysis.”
For the chemokine-effect tests, 5 × 105 naive CD4+ T cells prepared from LNs and spleens were cultured in 2 mL complete RPMI 1640 with or without recombinant mouse CCL19, CCL21, CXCL9, CXCL10, CXCL12, CCL3, or CCL4 in the presence or absence of goat anti–mouse CCL19 neutralizing antibodies (R&D Systems, Minneapolis, MN) in 24-well plate coated with 1 μg/mL anti-CD3 and 1 μg/mL anti-CD28 (eBioscience, San Diego, CA) for 4 days (first culture). The treated and viable CD4+ T cells were incubated with anti-CD3 mAb plus IL-2 as just described (second culture) and assayed for apoptotic cells. Where indicated, the pancaspase inhibitor (Z-VAD-fmk), caspase-8–specific inhibitor (Z-IETD-fmk), or caspase-9–specific inhibitor (Z-LEHD-fmk; ICN Pharmaceuticals, Costa Mesa, CA) was added at 100 μM to the second culture.
Flow cytometric analysis
Popliteal LN cells were preincubated with anti-FcRγII (2.4G2) and 5% normal mouse and rat sera to avoid nonspecific staining and treated with the appropriate mAbs. mAbs against TCR Cβ (H57-597), Vβ6 (44-22-1), Vβ8.1 and 8.2 (KJ16), Vβ13 (MR12-3), Vβ14 (14.2), and DO11.10 clonotype TCR (KJ1-26) were purified and conjugated with FITC or biotin as described previously.22 FITC-, PE-, or cychrome-conjugated anti-CD4 (RM4-5) and biotinylated anti-Fas/CD95 (Jo2) were purchased from BD PharMingen (San Diego, CA). PE-conjugated anti-FasL/CD178 (MFL3) and rat IgG were purchased from eBioscience and Jackson ImmunoResearch (West Grove, PA), respectively. Treated cells were followed by staining with PE-conjugated (Dako, Carpinteria, CA) or allophycocyanin-conjugated (APC; Caltag, Burlingame, CA) streptavidin, and analyzed on a FACSCalibur (BD Bioscience, Mountain View, CA).
Apoptotic cell analysis
Cells were stained with the appropriate mAbs. After washing with cold PBS, the cells were further stained with FITC- or PE-conjugated annexin V (Trevigen, Gaithersburg, MD, and BD PharMingen, respectively) and propidium iodide (PI; Trevigen) according to the manufacturer's instructions, and were analyzed by flow cytometry.
Real-time reverse transcription-PCR analysis
Total cellular mRNA was isolated from CD4+ T cells using an EASYPrep RNA (Takara Bio, Otsu, Japan) following the manufacturer's instructions. RNA (500 ng/100 μL reaction) was reverse transcribed using the high-capacity cDNA archive kit (PE Applied Biosystems, Foster City, CA). For the quantitative analysis of gene expression, real-time polymerase chain reaction (PCR) was conducted using TaqMan gene expression assay kits (PE Applied Biosystems): Mm00433237_m1 for Fas and Mm00438864_1 for Fas ligand. Amplification reaction was performed on an ABI Prism 7000 sequence detector system (PE Applied Biosystems), under the following cycling protocol: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Subsequently the threshold cycle (Ct) was determined. As an endogenous reference GAPDH was used for normalization. Results were expressed as 2−ΔΔCt, where ΔCT = Ct of target gene − Ct of endogenous control gene (GAPDH), and ΔΔCT = ΔCT of CCL19/21-stimulated sample − ΔCT of unstimulated sample. Thus, all experimental samples are expressed as a n-fold difference to the control. Quantitative real-time PCR experiments were repeated twice in triplicate.
Analysis of transcription factors
Nuclear extract was prepared from CD4+ T cells using TransFactor extraction kit (BD Bioscience/Clontech), according to manufacturer's instructions. After centrifugation at 20 000g for 5 minutes at 4°C, supernatants (nuclear extracts) were assayed for NF-κB p65, NF-κB p50, or c-Jun content. The translocated transcription factors were detected using BD TransFactor kits (BD Bioscience/Clontech). Plates were read at 450 nm and expressed as OD values.
Results
Maintenance of antigen-reactive CD4+ T cells in plt/plt mice
To investigate the regulation of the T-cell response in vivo, draining popliteal LN cells were prepared from OVA/CFA-immunized mice at various time points, restimulated with OVA in vitro, and assessed for proliferation. In BALB/c mice, the recall response peaked by day 9 after immunization and was maintained until day 16 but declined rapidly thereafter (Figure 1). In plt/plt mice, the recall response remained maximal even 18 or 20 days after immunization. Surprisingly, strong proliferation was still observed 16 months after immunization (Figure 1).
In vitro proliferation of OVA-reactive T cells from immunized mice. BALB/c (○) and plt/plt (•) mice were immunized subcutaneously in the hind foot pads with OVA in CFA. Popliteal LN cells were harvested at indicated days or 16 months after immunization, stimulated in vitro with OVA for 4 days, and assessed for proliferation. Mean ± SD from triplicate tests is indicated.
In vitro proliferation of OVA-reactive T cells from immunized mice. BALB/c (○) and plt/plt (•) mice were immunized subcutaneously in the hind foot pads with OVA in CFA. Popliteal LN cells were harvested at indicated days or 16 months after immunization, stimulated in vitro with OVA for 4 days, and assessed for proliferation. Mean ± SD from triplicate tests is indicated.
We also counted the number of CD4+ T cells in the draining LNs (d-LNs). Consistent with a lymphocyte-homing deficiency, the total LN CD4+ T-cell number was much lower in plt/plt mice than in BALB/c mice throughout the observation period (Figure 2A). However, the number of CD4+ T cells in plt/plt mice increased at a quite similar rate to that in BALB/c mice after immunization (Figure 2B). The CD4+ T-cell number in the plt/plt mouse never declined during the observation period, whereas in BALB/c mice it peaked by day 16 and rapidly decreased thereafter (Figure 2A-B). Also in CCR7-deficient mice, CD4+ T-cell number never declined during the observation period (Figure 2C-D).
In vivo expansion of OVA-reactive T cells after immunization. (A,C) Absolute number of CD4+ T cells per LN and (B,D) relative increase in CD4+ T-cell number from preimmunization values were calculated from total cell number and percent CD4+ cells by flow cytometric analysis. For C57BL/6 and CCR7-deficient mice, draining popliteal LN cells were pooled from 2 mice and assessed, and the results were expressed as mean values. Baseline CD4+ T-cell number at day 0 was 4.38 × 105 ± 1.86 × 105 in BALB/c mice, 0.78 × 105 ± 0.04 × 105 in plt/plt mice, 6.4 × 105 in C57BL/6 mice, and 2.3 × 105 in CCR7-deficient mice. (E) Percentages of cells bearing Vβ6, Vβ8, or Vβ14 in CD4+ T cells were determined at the indicated days after immunization. Mean ± SD for 3 to 5 mice was indicated.
In vivo expansion of OVA-reactive T cells after immunization. (A,C) Absolute number of CD4+ T cells per LN and (B,D) relative increase in CD4+ T-cell number from preimmunization values were calculated from total cell number and percent CD4+ cells by flow cytometric analysis. For C57BL/6 and CCR7-deficient mice, draining popliteal LN cells were pooled from 2 mice and assessed, and the results were expressed as mean values. Baseline CD4+ T-cell number at day 0 was 4.38 × 105 ± 1.86 × 105 in BALB/c mice, 0.78 × 105 ± 0.04 × 105 in plt/plt mice, 6.4 × 105 in C57BL/6 mice, and 2.3 × 105 in CCR7-deficient mice. (E) Percentages of cells bearing Vβ6, Vβ8, or Vβ14 in CD4+ T cells were determined at the indicated days after immunization. Mean ± SD for 3 to 5 mice was indicated.
These results suggest that a larger population of OVA-reactive memory T cells was maintained for a longer period of time in plt/plt mice than in BALB/c mice. We kinetically quantified the OVA-reactive T cells in the d-LNs. The ratio of OVA-reactive Vβ8+ and Vβ14+CD4+ T cells increased in the LNs from OVA/CFA-immunized mice compared to that from PBS/CFA-immunized mice in both strains, but that of non-OVA–specific Vβ6+CD4+ T cells increased only minimally (Figure 2E), supporting the specific clonal expansion of Vβ8+CD4+ and Vβ14+CD4+ T cells. The percentage of Vβ8+CD4+ and Vβ14+CD4+ T cells peaked at day 16 then declined quickly by day 20 in BALB/c mice, whereas no such decrease was observed in plt/plt mice over the same period (Figure 2E).
Decreased apoptosis in antigen-specific CD4+ T cells in plt/plt mice in vivo
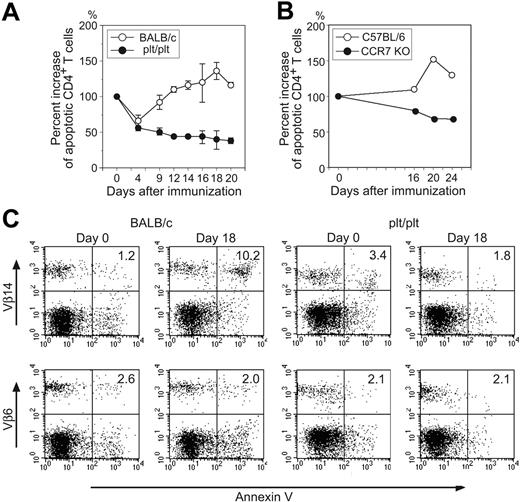
These findings suggest that absence of CCL19 or CCL21 signals results in longer life span of antigen-stimulated CD4+ T cells due to lowered sensitivity to AICD. Then, frequencies of apoptotic CD4+ T cells in the d-LNs were kinetically estimated. In BALB/c mice, annexin V+ apoptotic cells in the d-LNs dramatically increased from day 4 to day 18 then decreased thereafter. In plt/plt mice, however, the frequency slightly decreased after immunization and remained low until day 20 (Figure 3A). Similarly, the frequency decreased and remained low until day 24 in CCR7-deficient mice (Figure 3B). Among Vβ14+CD4+ T cells, representing OVA-reactive CD4+ T cells, the frequency of annexin V+ apoptotic cells increased in the LNs of BALB/c mice up to 8.5-fold at day 18 after immunization, when compared with unimmunized mice. However, the apoptotic cell frequency never increased in plt/plt mouse LNs at any time point (Figure 3C and data not shown). Among non-OVA–reactive Vβ6+CD4+ T cells, the apoptotic cell frequency was unchanged by immunization in either group of mice (Figure 3C).
Apoptosis of OVA-reactive CD4+ T cells. (A) Relative increase of annexin V+ PI− apoptotic CD4+ T cells in popliteal LNs of BALB/c (○) or plt/plt (•) mice was kinetically analyzed after immunization. At indicated days after immunization, popliteal LN cells were harvested and stained with anti-CD4 mAb, annexin V, and PI, then analyzed by flow cytometry. Percent increase of apoptotic CD4+ T cells was calculated according to the formula: % increase = (% annexin V+PI− apoptotic cells at indicated day in CD4+ T cells/% annexin V+PI− apoptotic cells at day 0 in CD4+ T cells) × 100. Mean ± SD from 3 to 5 mice is given. A representative result from 2 independent experiments is shown. (B) Relative increase of annexin V+PI− apoptotic CD4+ T cells in popliteal LNs of C57BL/6 (○) or CCR7-deficient (•) mice was kinetically analyzed after immunization. Percent increase was calculated as indicated in panel A. Draining popliteal LN cells were pooled from 2 mice and assessed. (C) Annexin V+ apoptotic cells in Vβ14+CD4+ or Vβ6+CD4+ T cells in BALB/c and plt/plt mouse LN were analyzed. We repeated these experiments at least 2 times and representative results at days 0 and 18 after immunization with OVA in CFA are shown.
Apoptosis of OVA-reactive CD4+ T cells. (A) Relative increase of annexin V+ PI− apoptotic CD4+ T cells in popliteal LNs of BALB/c (○) or plt/plt (•) mice was kinetically analyzed after immunization. At indicated days after immunization, popliteal LN cells were harvested and stained with anti-CD4 mAb, annexin V, and PI, then analyzed by flow cytometry. Percent increase of apoptotic CD4+ T cells was calculated according to the formula: % increase = (% annexin V+PI− apoptotic cells at indicated day in CD4+ T cells/% annexin V+PI− apoptotic cells at day 0 in CD4+ T cells) × 100. Mean ± SD from 3 to 5 mice is given. A representative result from 2 independent experiments is shown. (B) Relative increase of annexin V+PI− apoptotic CD4+ T cells in popliteal LNs of C57BL/6 (○) or CCR7-deficient (•) mice was kinetically analyzed after immunization. Percent increase was calculated as indicated in panel A. Draining popliteal LN cells were pooled from 2 mice and assessed. (C) Annexin V+ apoptotic cells in Vβ14+CD4+ or Vβ6+CD4+ T cells in BALB/c and plt/plt mouse LN were analyzed. We repeated these experiments at least 2 times and representative results at days 0 and 18 after immunization with OVA in CFA are shown.
To further clarify antigen specificity, mice were injected with DO11.10 CD4+ T cells specific for OVA323-339/I-Ad, immunized with OVA/CFA, and KJ1-26+ T-cell kinetics examined in the d-LNs. As shown in Figure 4A, the percentage of DO11.10 CD4+ T cells dramatically increased in both strains by day 7 and decreased rapidly thereafter in BALB/c recipient, whereas in plt/plt mice the percentage remained maximal until day 11 and then began to decrease gradually (Figure 4A). Thus, the proportion of DO11.10 cells in CD4+ T cells was significantly higher in plt/plt mice than in BALB/c mice at day 9 and thereafter.
Expansion and apoptosis of adoptively transferred CD4+ T cells. (A) Changes of DO11.10 cell frequency among CD4+ T cells in LN of BALB/c (○) or plt/plt (•) mice after immunization. (B) Annexin V+ cells in each division of transferred DO11.10 CD4+ T cells were detected after immunization, and results at day 11 are representatively depicted. The number of dividing time of DO11.10 CD4+ T cells is indicated in each column. (C,E) Quantitative comparison of annexin V+ DO11.10 CD4+ T cells in each division between BALB/c (□) and plt/plt (⊡) mice at day 5 (C) and day 11 (E). (D,F) The percentage of DO11.10 CD4+ T cells in each division in LN cells of BALB/c (□) and plt/plt (⊡) mice at day 5 (D) day 11 (F). Mean ± SD for 3 mice per group is given in panels C,E-F. Asterisk indicates significant difference by Student t test (P < .05) between BALB/c and plt/plt mice.
Expansion and apoptosis of adoptively transferred CD4+ T cells. (A) Changes of DO11.10 cell frequency among CD4+ T cells in LN of BALB/c (○) or plt/plt (•) mice after immunization. (B) Annexin V+ cells in each division of transferred DO11.10 CD4+ T cells were detected after immunization, and results at day 11 are representatively depicted. The number of dividing time of DO11.10 CD4+ T cells is indicated in each column. (C,E) Quantitative comparison of annexin V+ DO11.10 CD4+ T cells in each division between BALB/c (□) and plt/plt (⊡) mice at day 5 (C) and day 11 (E). (D,F) The percentage of DO11.10 CD4+ T cells in each division in LN cells of BALB/c (□) and plt/plt (⊡) mice at day 5 (D) day 11 (F). Mean ± SD for 3 mice per group is given in panels C,E-F. Asterisk indicates significant difference by Student t test (P < .05) between BALB/c and plt/plt mice.
To examine apoptosis in relationship to cell division, the annexin V positivity of CFSE-labeled DO11.10 cells was analyzed in the d-LNs after OVA-immunization. Figure 4B shows the results at day 11 when we detected the highest percentage of annexin V+ cells. The CFSE-dilution patterns were quite similar in BALB/c and plt/plt mice at any time point examined (Figure 4B,D,F), demonstrating that DO11.10 cells proliferated in response to OVA similarly in BALB/c and plt/plt mice. Most annexin V+ apoptotic cells were located in divisions 6 to 9, and the highest frequencies of apoptosis were at divisions 7 and 8 in both strains (Figure 4C,E). At day 11 the apoptotic cell frequency in divisions 7, 8, and 9 was significantly lower in plt/plt mice than in BALB/c mice (Figure 4B,E), suggesting that the efficiency of apoptosis induction in antigen-responding CD4+ T cells was lower in plt/plt mice than in BALB/c mice. A decreased frequency of apoptotic cells in the plt/plt mice was detected at day 9 and later but not before day 7 (Figure 4C,E and data not shown). Thus, during clonal expansion phase, the rate of apoptosis was quite similar between BALB/c and plt/plt mice, but the rate rapidly increased only in BALB/c mice during the clonal contraction phase.
CCL19 and CCL21 enhance sensitivity of T cells to AICD
The failure to appropriately regulate AICD of OVA-specific CD4+ T cells in the plt/plt mouse suggests the role of chemokines CCL19 or CCL21 (or both) in this regulation, or a defect intrinsic to the T cells. To evaluate the latter possibility, we assessed apoptosis in vitro, in cultures stimulated with concanavalin A followed by restimulation with anti-CD3 and IL-2. The frequencies of apoptotic CD4+ T cells were comparable between LN T cells from BALB/c and plt/plt mice, indicating that CD4+ T cells from plt/plt mice do not have any intrinsic defect in AICD (data not shown).
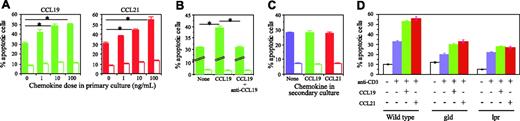
Next, we examined whether CCL19 or CCL21 (or both) provides signals for cell death. To test the effect of these chemokines on AICD, naive CD4+ T cells from BALB/c mice were activated with anti-CD3 and anti-CD28 mAbs in the presence or absence of CCL19 or CCL21 for 4 days in vitro, and restimulated for 48 hours with anti-CD3 mAb and IL-2. In the absence of CCL19 or CCL21 in the first culture, approximately 30% of CD4+ T cells underwent apoptosis after restimulation, whereas the presence of CCL19 or CCL21 in the first culture increased the apoptotic cell frequency in a dose-dependent manner (Figure 5A). Over 10 ng/mL, both chemokines elicited a significant enhancement of apoptosis. At 100 ng/mL, CCL19 and CCL21 increased the frequency of apoptotic cells 1.67 and 1.83 times, respectively. CCL19-enhanced apoptosis was completely abolished by neutralizing antibodies against CCL19, indicating that the enhanced apoptosis is mediated by CCL19 (Figure 5B). However, the presence of CCL19 or CCL21 in the second culture, but not in the first culture, did not alter the frequencies of apoptotic CD4+ T cells, demonstrating that neither CCL19 nor CCL21 influences apoptosis of previously activated CD4+ T cells (Figure 5C). We tried to confirm the chemokine effect on CD4+ T-cell AICD by inhibition with pertussis toxin. However, CD4+ T cells were mostly dead during the 4-day culture with pertussis toxin at appropriate concentration for inhibition of chemokine effect (data not shown).
Effects of CCL19 and CCL21 on CD4+ T-cell AICD. Naive CD4+ T cells were stimulated for 4 days with anti-CD3 and anti-CD28 mAbs with or without CCL19 (green bar) or CCL21 (red bar) in primary culture and were restimulated with anti-CD3 mAb and IL-2 (▪) or IL-2 alone (□) in secondary culture. After 2 days in the secondary culture, annexin V+PI− apoptotic cells were detected on flow cytometer. (A-B) In primary culture, T cells were stimulated in the presence of various doses of chemokines (A) or with or without 10 ng/mL CCL19 in the presence or absence of 10 μg/mL anti-CCL19 mAb (B). (C) T cells stimulated in the primary culture without chemokine were restimulated with anti-CD3 mAb and IL-2 in the absence (blue bar) or presence of 100 ng/mL CCL19 (green bar) or CCL21 (red bar). The results are shown as mean ± SD in triplicate tests. Asterisks indicate significant differences by Student t test (P < .05). A representative result from 2 (A-B) or 3 (C) independent experiments is indicated. (D) Naive CD4+ T cells were prepared from gld FasL-deficient, lpr Fas-deficient, and WT mice, and treated as shown in panel A with 100 ng/mL CCL19 or CCL21.
Effects of CCL19 and CCL21 on CD4+ T-cell AICD. Naive CD4+ T cells were stimulated for 4 days with anti-CD3 and anti-CD28 mAbs with or without CCL19 (green bar) or CCL21 (red bar) in primary culture and were restimulated with anti-CD3 mAb and IL-2 (▪) or IL-2 alone (□) in secondary culture. After 2 days in the secondary culture, annexin V+PI− apoptotic cells were detected on flow cytometer. (A-B) In primary culture, T cells were stimulated in the presence of various doses of chemokines (A) or with or without 10 ng/mL CCL19 in the presence or absence of 10 μg/mL anti-CCL19 mAb (B). (C) T cells stimulated in the primary culture without chemokine were restimulated with anti-CD3 mAb and IL-2 in the absence (blue bar) or presence of 100 ng/mL CCL19 (green bar) or CCL21 (red bar). The results are shown as mean ± SD in triplicate tests. Asterisks indicate significant differences by Student t test (P < .05). A representative result from 2 (A-B) or 3 (C) independent experiments is indicated. (D) Naive CD4+ T cells were prepared from gld FasL-deficient, lpr Fas-deficient, and WT mice, and treated as shown in panel A with 100 ng/mL CCL19 or CCL21.
We also examined the effect of CXCL9, CXCL10, CXCL12, CCL3, or CCL4 on AICD. The inclusion of one of these chemokines in the first culture did not enhance AICD of naive CD4+ T cells after restimulation with anti-CD3 mAb and IL-2 (data not shown).
Enhancement of FasL expression by CCL19 and CCL21
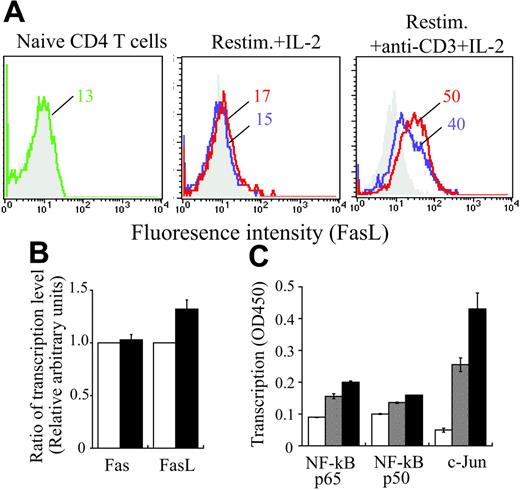
Because Fas and FasL are responsible for triggering induction of AICD of CD4+ T cells in many experimental systems, the ability of CCL19 and CCL21 to up-regulate Fas and FasL expression on CD4+ T cells was examined. FasL expression was low or negligible on naive CD4+ T cells and those once activated by anti-CD3 and anti-CD28 mAbs (Figure 6A and data not shown). However, FasL expression was clearly increased by repeated activation with anti-CD3 mAb plus IL-2, and further enhanced by addition of CCL19 and CCL21 to the first culture (Figure 6A), suggesting that CCL19 and CCL21 are able to play some role in enhancing FasL expression on CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs. This enhancement of FasL expression was confirmed at mRNA level (Figure 6B). Consistently, DNA binding of the transcription factors, c-Jun and NF-κB, was increased in CD4+ T cells by addition of CCL19 and CCL21 to the first culture (Figure 6C). This increase has been reported to enhance FasL transcription.23-26
Induction of Fas or FasL expressions on CD4+ T cells. (A) Naive or cultured CD4+ T cells prepared from BALB/c mice were stained with anti-Fas or -FasL mAb (green, blue, or red line) or isotype-matched control antibody (gray). Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs for 4 days in the absence (blue line) or presence of 100 ng/mL CCL19 and CCL21 (red line) in primary culture, and then restimulated with IL-2 alone or anti-CD3 mAb and IL-2 in secondary culture. Two days after, the cells were stained for Fas and FasL. Mean fluorescence intensity (MFI) is indicated in each panel. Similar results were obtained in another independent experiment. (B-C) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs with or without CCL19 and CCL21 (100 ng/mL each) for 4 days, washed, and restimulated with anti-CD3 mAb and IL-2 for 6 hours. Then, CD4+ T cells were negatively purified using the mouse CD4+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA; purity: 90%-95%). (B) Total cellular RNA was prepared from the T cells treated in the absence (▪) or presence (□) of CCL19 and CCL21 as described in “Materials and methods,” under “Real-time reverse transcription–PCR analysis.” Quantitative real-time PCR was carried out, and the results were expressed as 2−ΔΔCt, which corresponded to the fold change between the T cells treated in the absence of CCL19 and CCL21 and those in the presence of these chemokines. (C) For determining the translocation of transcription factors indicated, nuclear extracts were prepared from CD4+ T cells untreated (□), treated in the absence of (⊡), or presence (▪) of CCL19 and CCL21 in the primary culture, and were assayed for the contents of these factors using BD TransFactor kits (BD Bioscience/Clontech). Results were expressed as OD values at 450 nm ± SD of triplicate tests.
Induction of Fas or FasL expressions on CD4+ T cells. (A) Naive or cultured CD4+ T cells prepared from BALB/c mice were stained with anti-Fas or -FasL mAb (green, blue, or red line) or isotype-matched control antibody (gray). Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs for 4 days in the absence (blue line) or presence of 100 ng/mL CCL19 and CCL21 (red line) in primary culture, and then restimulated with IL-2 alone or anti-CD3 mAb and IL-2 in secondary culture. Two days after, the cells were stained for Fas and FasL. Mean fluorescence intensity (MFI) is indicated in each panel. Similar results were obtained in another independent experiment. (B-C) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs with or without CCL19 and CCL21 (100 ng/mL each) for 4 days, washed, and restimulated with anti-CD3 mAb and IL-2 for 6 hours. Then, CD4+ T cells were negatively purified using the mouse CD4+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA; purity: 90%-95%). (B) Total cellular RNA was prepared from the T cells treated in the absence (▪) or presence (□) of CCL19 and CCL21 as described in “Materials and methods,” under “Real-time reverse transcription–PCR analysis.” Quantitative real-time PCR was carried out, and the results were expressed as 2−ΔΔCt, which corresponded to the fold change between the T cells treated in the absence of CCL19 and CCL21 and those in the presence of these chemokines. (C) For determining the translocation of transcription factors indicated, nuclear extracts were prepared from CD4+ T cells untreated (□), treated in the absence of (⊡), or presence (▪) of CCL19 and CCL21 in the primary culture, and were assayed for the contents of these factors using BD TransFactor kits (BD Bioscience/Clontech). Results were expressed as OD values at 450 nm ± SD of triplicate tests.
Fas expression on naive CD4+ T cells was slightly enhanced by repeated stimulation with IL-2 or with anti-CD3 mAb plus IL-2 (data not shown). Addition of CCL19 or CCL21 to the first culture further enhanced marginally the Fas expression (data not shown). However, this enhancement was not noticeable at mRNA level (Figure 6B).
To examine the involvement of Fas and Fas-L in AICD, CCL19 or CCL21 was assessed for the ability to enhance sensitivity of Fas- or FasL-deficient CD4+ T cells to AICD. As shown in Figure 5D, anti-CD3 stimulation in the absence of CCL19 or CCL21 induced apoptosis in about 20% of CD4+ T cells from FasL-deficient gld or Fas-deficient lpr mice, compared with about 30% of those from WT mice. Stimulation in the presence of CCL19 or CCL21 in the first culture enhanced AICD from 20% to about 30% in CD4+ T cells from gld or lpr mice, but the enhancement was lower than in WT mice, where apoptosis was increased from 30% to about 50% (Figure 5D). These results strongly supported the possibility that CCL19 or CCL21 regulates AICD in CD4+ T cells at least partly through Fas- and FasL-dependent pathway.
Involvement of caspase-8 and -9 in the enhancement of sensitivity to AICD by CCL21
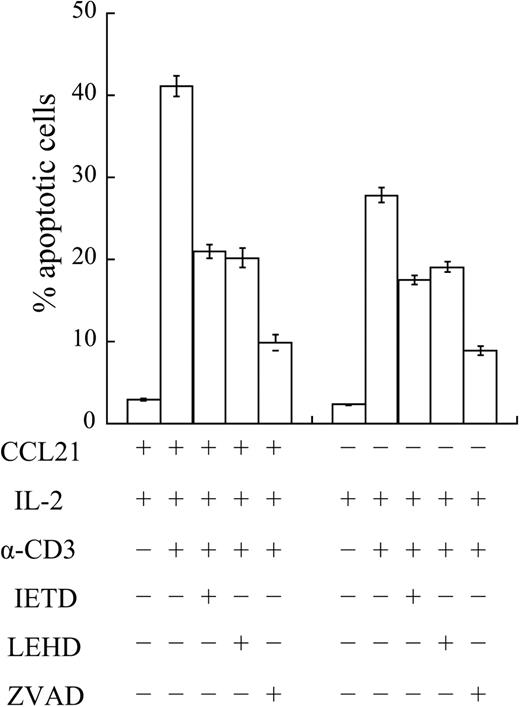
To determine the involvement of caspase-8 and -9 in the chemokine-enhanced sensitivity to AICD, BALB/c T cells primed in the presence or absence of CCL21 were restimulated with anti-CD3 mAb and IL-2 in the absence or presence of the caspase-8–specific inhibiter (Z-IETD-fmk), caspase-9–specific inhibitor (Z-LEHD-fmk), or pancaspase inhibitor (Z-VAD-fmk). As shown in Figure 7, when CCL21 was included in the first culture, the ratio of annexin V+ apoptotic cells was decreased from about 40% to 20% by the addition of the inhibitor for caspase-8 or caspase-9 to the restimulation culture. The ratio was further decreased to about 10%, when the pancaspase inhibitor was present in the restimulation. Similarly, the apoptotic cell ratio in the T cells primed in the absence of CCL21 was decreased from about 30% to 20% in the presence of the inhibitor for caspase-8 or caspase-9, and to 10% in the presence of the pancaspase inhibitor (Figure 7). These results suggested that the major part of CCL21-enhanced sensitivity to AICD involves caspase-8–mediated and caspase-9–mediated apoptotic pathway.
Involvement of caspase-8 and caspase-9 in the CCL21-induced enhancement of AICD. Naive CD4+ T cells from BALB/c mice were stimulated with anti-CD3 and anti-CD28 mAbs for 4 days in the absence (−CCL21) or presence (+CCL21) of 100 ng/mL CCL21 in primary culture, and then restimulated with IL-2 alone or anti-CD3 mAb and IL-2 in secondary culture in the presence or absence of the pancaspase inhibitor (zVAD), caspse-8–specific inhibitor (IETD), or caspase-9–specific inhibitor (LEHD). Two days after, annexin V+PI− apoptotic cells were detected on flow cytometer. Caspase inhibitors were dissolved in DMSO and used at 100 μM. Where any inhibitors were not added, DMSO was used as a solvent control. Results are shown as mean ± SD of triplicate tests.
Involvement of caspase-8 and caspase-9 in the CCL21-induced enhancement of AICD. Naive CD4+ T cells from BALB/c mice were stimulated with anti-CD3 and anti-CD28 mAbs for 4 days in the absence (−CCL21) or presence (+CCL21) of 100 ng/mL CCL21 in primary culture, and then restimulated with IL-2 alone or anti-CD3 mAb and IL-2 in secondary culture in the presence or absence of the pancaspase inhibitor (zVAD), caspse-8–specific inhibitor (IETD), or caspase-9–specific inhibitor (LEHD). Two days after, annexin V+PI− apoptotic cells were detected on flow cytometer. Caspase inhibitors were dissolved in DMSO and used at 100 μM. Where any inhibitors were not added, DMSO was used as a solvent control. Results are shown as mean ± SD of triplicate tests.
Discussion
Expression of CCL19 and CCL21 in the T-cell zone of secondary lymphoid organs where naive T cells are primed and activated raises the possibility that these chemokines are involved in regulation of T-cell responses.6-8 The possibility that CCL19 and CCL21 are involved in AICD is consistent with our previous finding that T-cell immune responses in plt/plt mice are dysregulated.18 Indeed, analysis of Vβ14+CD4+ T cells or adoptively transferred DO11.10 CD4+ T cells revealed a decrease in the frequency of apoptotic OVA-specific CD4+ T cells in the d-LNs of plt/plt mice as compared with WT BALB/c mice during the clonal contraction phase (Figures 3A and 4). The involvement of CCL19/CCL21 in AICD is further supported by the finding that AICD of CD4+ T cells was also decreased in CCR7-deficient mice (Figure 3B). Adoptive transfer experiments indicate that diminished AICD of CD4+ T cells in the plt/plt mouse is unlikely due to an intrinsic defect of CD4+ T cells (Figure 4A). Supporting our interpretation, CD4+ T cells from plt/plt and BALB/c mice undergo apoptosis equally well in vitro (data not shown).
The results shown in Figure 5 demonstrate that both CCL19 and CCL21 promote in vitro AICD of CD4+ T cells when naive CD4+ T cells are activated by TCR agonists. However, these chemokines alone were unable to promote AICD and did not affect AICD of previously activated CD4+ T cells, demonstrating that TCR and CCL19/CCL21 signals have to be given simultaneously or in close succession. In vivo, OVA-specific CD4+ T cells are probably primed in the d-LNs by OVA-bearing DCs in the presence of CCL19/CCL21, suggesting that OVA-peptide/MHC complex and CCL19/CCL21 simultaneously stimulate OCA-reactive T cells. Increased AICD rate in vivo was observed day 9 or later (Figure 4). Thus, simultaneous stimulation of naive CD4+T cells through TCR and CCR7 might increase AICD frequency at later contraction phase.
It is well known that repeated stimulation of T cells induces expression of FasL, which then binds to its receptor Fas on activated T cells, thus activated T cells are eliminated.27-29 CCL19/CCL21-mediated stimulation of anti-CD3–activated CD4+ T cells enhanced Fas-L mRNA accumulation and FasL-expression (Figure 6A-B). Consistently, an increase in nuclear translocation of transcription factors, c-Jun and NF-κB, was observed (Figure 6C). These transcription factors have been shown to up-regulate FasL expression of TCR-stimulated lymphocytes.23-25 In general AP-1 is involved in the Fas-FasL–mediated pathway in lymphoid cell AICD.30 However, we cannot formally exclude a possibility that these transcription factors are anticipated in the mitochondria-mediated apoptotic pathway, because they were also involved in the mitochondria-mediated pathway.31,32 Enhancement of FasL expression seems to be at least one of the major mechanisms for CCL19/CCL21-induced promotion of AICD in CD4+ T cells. Supporting our interpretation, an active caspase-8 fragment was similarly detected in anti-CD3 mAb-stimulated CD4+ T cells from plt/plt and WT mice (data not shown), and the caspase-8–specific inhibitor decreased in vitro induction of AICD (Figure 7). Furthermore, much less enhancement of AICD by CCL19/CCL21 was observed in Fas- or FasL-deficient CD4+ T cells (Figure 5D). However, reduced enhancement of AICD by stimulation in the presence of CCL19/CCL21 was still detected in these Fas- or FasL-deficient CD4+ T cells, indicating that other pathways are also involved. In accordance, the caspase-9–specific inhibitor decreased in vitro AICD in CD4+ T cells to the similar level obtained with the caspase-8–specific inhibitor (Figure 7A). Other molecules reportedly involved in AICD and mitochondria pathway are now under investigation.33-36
Enhanced FasL expression suggests that the CD4+ T cells are highly activated when stimulated by antigens in the presence of CCL19/CCL21 and that these chemokines may promote T-cell maturation or memory induction or both. Activated T cells differentiate into memory T cells that are classified into TCM and TEM cells.15 TCM cells are CCR7+CD62Lhigh, whereas TEM cells are CCR7−CD62Llow.15,37 The phenotypic analysis of OVA-reactive Vβ14+CD4+ T cells in the d-LNs revealed that at the peak frequency of annexin V+ apoptotic cells, day 18 or 20 after immunization, most apoptotic cells were CD44high and CD62Llow, typical for TEM cells (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). At these time points, the frequency of CD44highCD62Llow cells in annexin V− Vβ14+CD4+ T cells decreased in the LNs from BALB/c mice (Figure S1), suggesting that TEM cells are eliminated at least partly in the LNs and diluted by naive T cells. Also in plt/plt mice, the frequency of CD62Llow cells decreased at day 18 or 20 in annexin V− Vβ14+CD4+ T cells, but that of CD44high cells remained high, suggesting that CD62Llow cells are partially eliminated and diluted by CD44highCD62Lhigh T cells migrating from the immunization site. The latter phenotype is typical for TCM cells, which are relatively resistant to AICD.37 Thus, TCM cells possibly accumulate in the d-LNs in plt/plt mice, consistent with our previous results.18 The phenotypic analysis also supported our interpretation that the low frequency of AICD in the d-LNs of plt/plt mice is not due to an activation failure.
CCL19/CCL21 alone neither triggered CD4+ T-cell apoptosis nor affected AICD frequency of previously activated CD4+ T cells (Figure 5C). Although the precise mechanism is presently unknown, CCL19/CCL21 might enhance susceptibility of CD4+ T cells to AICD through modification of TCR-mediated signals for priming, or provide additional signals independent of those via TCR. In support of the former possibility, recent reports have demonstrated that TCR and chemokine receptors, CXCR4 and CCR5, share some signal transduction molecules including PI3K and JAK/STAT, in addition to MEK and ERK pathways that are involved in T-cell activation and maturation.38,39 TCR ligation and ligation of CXCR4 or of CCR5 also activates ERK1/2 and p38 phosphorylation via MEK1/2, and CXCL12 up-regulates FasL expression in T-cell lines.38-43 TCR, CXCR4, or CCR5 ligation also activates PKC, which up-regulates Fas expression.42,44-46 In addition, signals via CCR7 and CXCR4 share transduction molecules.38,47,48 These findings support the possibility that signals derived from CCR7 augment the TCR-mediated signals for T-cell maturation and for Fas and FasL expression.
Thus, chemokine receptors including CXCR4, CCR5, and CCR7, seems to share, at least partly, the intracellular signaling pathway. However, CXCR3, CXCR4, or CCR5 ligand did not affect AICD frequency of CD4+ T cells when added during priming with anti-CD3 and anti-CD28 mAbs, which might be due to low or negligible expression of these chemokine receptors on naive and unstimulated CD4+ T cells.49-52 It is also possible that the signaling pathway of CCR7 could be distinct from that of CXCR4. The exact CCR7-signaling pathway remains to be determined and will lead to an understanding of the mechanisms for CCL19/CCL21-enhanced apoptosis.
Our in vitro finding that CCL19/CCL21 enhances T-cell apoptosis independently of cell migration strongly suggests that these chemokines directly affect T cells and increase their susceptibility to AICD. Similarly, it has been reported that these chemokines are responsible not only for cellular migration but also for terminal activation of DCs to prime T-cell responses.53 However, it is also possible that these chemokines indirectly affect T-cell AICD, especially in vivo. These chemokines regulate trafficking of T cells and DCs,16 and efficient interaction between CD4+ T cells and antigen-presenting cells such as DCs is proposed to be necessary for induction of full activation of CD4+ T cells and ultimately for cell death.54 Our findings do not exclude the possibility that inefficient interaction between T cells and antigen-presenting cells causes reduced AICD of CD4+ T cells in the plt/plt mouse. Defective migration of those cells in plt/plt mouse could alter the strength of interaction or reduce the probability of repeated stimulation. Defective migration of a particular DC subset could also potentially alter T-cell activation in plt/plt mice.2,17,55 The possibility also remains to be elucidated that antigen-activated T cells stay longer in the d-LNs in plt/plt mouse than in BALB/c mice because of a small number of LN T cells and little competition for a survival niche, which results in longer survival of antigen-activated T cells.
In contrast to the dysregulated T-cell responses in plt/plt mice, increased T-cell responsiveness to chemokines induced by a sphingosine-derived immunosuppressive drug, FTY720, has been shown to extensively enhance T-cell homing into and retention in secondary lymphoid organs and to suppress in vivo T-cell responses,5,56 further supporting an immunosuppressive role of secondary lymphoid organs. CCL19/CCL21-induced promotion of T-cell AICD may be a key mechanism linked to immunosuppression in secondary lymphoid organs.
Recent reports suggested that CCL19/21 protects CD8+ T cells and DCs derived from human peripheral blood nuclear cells and kidney mesangial cells from apoptosis.57-59 These studies were carried out using cells at steady state, not on AICD induction. This difference might explain different observations.
This is the first report demonstrating that homeostatic chemokines are involved in AICD of antigen-specific CD4+ T cells in vivo. CCL19/CCL21 regulates immune responses not only by attracting leukocytes but also by providing signals for modifying T-cell fate, seeming to promote the differentiation of naive CD4+ T cells into effector cells after antigen stimulation. Functional inhibition in vivo of CCR7 or CCL19/CCL21 may prevent efficient maturation of CD4+ T cells into effector cells, resulting in the accumulation of antigen-specific CD4+ TCM cells that are relatively resistant to AICD. Accumulated TCM cells will permit stronger responses to secondary antigenic challenge. Indeed, T-cell recall responses, as assessed by proliferation or production of IL-2, were strikingly enhanced and prolonged in plt/plt mice (Figure 1 and Mori et al18 ). Alterations of chemokine function or T-cell trafficking may be useful targets for developing new strategies for vaccination or successful transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
T.Y. and T. Kuwabara contributed equally to this work.
We thank Drs M. D. Gunn and E. Gilboa (Duke University, Durham, NC) for providing DO11.10 mice and Drs A. Matsuzawa (University of Tokyo, Japan), H. Yoshida (RIKEN), F. Ishikawa, Y. Okada, and Y. Tanaka (Toho University) for their cooperation. We also thank to Dr T. Hasegawa (Ohno Chuo Hospital, Ichikawa, Japan) for his financial support.
This work was supported in part by Project Research of Toho University School of Medicine (T.Y., T. Kuwabara, and H.N.), grants from the Uehara Memorial Foundation and Kao Foundation for Arts and Sciences (H.N.), a grant from Research on Health Sciences Focusing on Drug Innovation of the Japan Health Sciences Foundation (KH51052; T. Kakiuchi), the Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (12670621, 15590911; T. Kakiuchi), and the Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology (14021121; T. Kakiuchi).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal