Abstract
Recent molecular analyses of leukemic blasts from pretreatment marrow or blood of patients with acute myeloid leukemia (AML) and a normal karyotype, the largest cytogenetic subset (ie, 40%-49%) of AML, have revealed a striking heterogeneity with regard to the presence of acquired gene mutations and changes in gene expression. Multiple submicroscopic genetic alterations with prognostic significance have been discovered, including internal tandem duplication of the FLT3 gene, mutations in the NPM1 gene, partial tandem duplication of the MLL gene, high expression of the BAALC gene, and mutations in the CEBPA gene. Application of gene-expression profiling has also identified a gene-expression signature that appears to separate cytogenetically normal AML patients into prognostic subgroups, although gene-expression signature-based classifiers predicting outcome for individual patients with greater accuracy are needed. These and similar future findings are likely to have a major impact on the clinical management of cytogenetically normal AML not only in prognostication but also in selection of appropriate treatment, since many of the identified genetic alterations already constitute or will potentially become targets for specific therapeutic intervention. In this report, we review prognostic genetic findings in karyotypically normal AML and discuss their clinical implications.
Introduction
The development of acute myeloid leukemia (AML) is associated with accumulation of acquired genetic alterations and epigenetic changes in hematopoietic progenitor cells that alter normal mechanisms of cell growth, proliferation, and differentiation. At diagnosis, most patients with AML harbor at least 1 chromosome aberration in their marrow blasts. Numerous recurrent structural and numeric cytogenetic aberrations have been identified and many of them not only are diagnostic markers for specific AML subtypes but also constitute independent prognostic factors for attainment of complete remission (CR), relapse risk, and overall survival (OS).1-5 However, in a sizable group of AML patients, 40% to 49% of adults and 25% of children, no microscopically detectable chromosome abnormality can be found on standard cytogenetic analysis.2-7
These cytogenetically normal (CN) patients have usually been classified in an intermediate-risk prognostic category because their CR rate, relapse risk, and survival are worse than those of adequately treated patients with such favorable aberrations as t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22), or t(15;17)(q22;q21) but better than those of patients with unfavorable cytogenetic findings [eg, −7, inv(3)(q21q26)/t(3;3)(q21;q26), balanced translocations involving 11q23 other than t(9;11)(p22;q23), or a complex karyotype].3-5 The outcome of CN patients has varied among studies, with 5-year survival rates between 24% and 42% reported.2,8 A recent Cancer and Leukemia Group B (CALGB) study found the relapse risk and disease-free survival (DFS) of these patients was improved by postremission treatment including 4 cycles of high-dose cytarabine (HDAC) or intermediate-dose cytarabine or 1 cycle of HDAC/etoposide followed by autologous stem-cell transplantation (ASCT) as opposed to regimens that include fewer cytarabine cycles or no ASCT.8 However, these therapeutic approaches do not improve outcome for all CN patients, likely because this cytogenetic subset is very heterogeneous at the molecular level. Indeed, during the last 12 years, several gene mutations9-12 and changes in gene expression13-16 have been discovered that strongly affect clinical outcome of CN AML patients.15-22 In this review, we will first discuss what constitutes a normal karyotype in AML and then those genetic alterations that are clinically relevant as both prognostic markers and potential targets for risk-adapted therapies in CN AML patients. We will begin with the internal tandem duplication (ITD) of the FLT3 gene, shown by many studies to be the most important prognostic factor, and follow with other genetic alterations discussed in order of the number of studies reporting their prognostic significance in CN AML.
The importance of ensuring that the patient's karyotype is truly normal
The proportion of adults with de novo CN AML has varied between 40% and 49% in the largest cytogenetic studies,3-7 although both lower and higher percentages have been reported.2 The differences among studies could relate to several factors including the number of older patients studied, since the proportion of CN cases increases with age,3,23 and differences in cytogenetic methodologies and the criteria used to consider a karyotype normal.
Leukemic blasts carrying AML-associated chromosome aberrations can constitute only a fraction of cells dividing in vitro. Occasionally, the cytogenetically abnormal clone is detectable in cells cultured in vitro for 24 to 48 hours but not in a direct preparation of the same sample (ie, when harvesting occurs immediately after the specimen is delivered to the cytogenetic laboratory). Moreover, a blood specimen can sometimes be cytogenetically normal when the marrow is abnormal. In the CALGB database, this was found in approximately 5% of AML patients whose marrow and blood specimens were studied simultaneously.24 Therefore, to reliably deem a patient as karyotypically normal it is recommended that full analysis of at least 20 metaphase cells originating from a marrow sample cultured in vitro for 24 to 48 hours is performed. Additionally, for multi-institutional research studies in which cytogenetic investigations are done in local laboratories, central karyotype review is important. In the CALGB experience, cases submitted as cytogenetically normal were found on central karyotype review to harbor such prognostically relevant chromosome aberrations as inv(3), t(9;11)(p22;q23), t(11;19)(q23;p13.1), and inv(16).25 Rarely, the opposite was true (ie, an institutionally reported abnormal karyotype was found to be normal on central review).
Although rare,26-28 there are patients who, despite having a normal karyotype on standard cytogenetic investigation, carry 1 of the fusion genes identical to those generated by recurrent translocations [eg, PML-RARA/t(15;17), RUNX1-RUNX1T1 (AML1-ETO)/t(8;21)] or inversions [CBFB-MYH11/inv(16)]. In most instances, these fusion genes are created by cryptic insertions of very small chromosome segments that do not alter the chromosome morphology.27,29,30 Patients with these cryptic rearrangements are usually categorized with and treated as patients with typical t(15;17), t(8;21), and inv(16). Both reverse transcriptase–polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) can be used to detect the presence of the aforementioned hidden rearrangements. Such testing is especially warranted in CN patients with FAB M3, M3v, and M4Eo marrow morphology but is otherwise not routinely recommended outside of a clinical trial.31 Studies employing spectral karyotyping,32,33 FISH with a comprehensive set of genomic DNA probes,34,35 and comparative genomic hybridization36 suggest that most patients with a normal karyotype on standard cytogenetic examination do not harbor other unrecognized chromosome aberrations. Instead, leukemic blasts of these patients carry acquired mutations and/or changes in gene expression that are unrelated to recurrent chromosome aberrations in AML but are important for predicting outcome and selecting treatment (Table 1)
Genes whose mutations or changes in expression occur recurrently in cytogenetically normal AML and have clinical significance
| Gene symbol (aliases and previous gene symbols) . | Gene name . | Chromosomal location . |
|---|---|---|
| FLT3 (STK1, FLK2, CD135) | Fms-related tyrosine kinase 3 | 13q12 |
| NPM1 (B23)* | Nucleophosmin (nucleolar phosphoprotein B23, numatrin) | 5q35 |
| MLL (ALL-1, HRX, HTRX1, CXXC7, TRX1, MLL1A) | Myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila) | 11q23 |
| BAALC | Brain and acute leukemia gene, cytoplasmic | 8q22.3 |
| CEBPA (CEBP) | CCAAT/enhancer binding protein (C/EBP), alpha | 19q13.1 |
| ERG | v-ets erythroblastosis virus E26 oncogene-like (avian) | 21q22.3 |
| Gene symbol (aliases and previous gene symbols) . | Gene name . | Chromosomal location . |
|---|---|---|
| FLT3 (STK1, FLK2, CD135) | Fms-related tyrosine kinase 3 | 13q12 |
| NPM1 (B23)* | Nucleophosmin (nucleolar phosphoprotein B23, numatrin) | 5q35 |
| MLL (ALL-1, HRX, HTRX1, CXXC7, TRX1, MLL1A) | Myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila) | 11q23 |
| BAALC | Brain and acute leukemia gene, cytoplasmic | 8q22.3 |
| CEBPA (CEBP) | CCAAT/enhancer binding protein (C/EBP), alpha | 19q13.1 |
| ERG | v-ets erythroblastosis virus E26 oncogene-like (avian) | 21q22.3 |
Mutations of the FMS-related tyrosine kinase 3 (FLT3) gene
The first report demonstrating that internal tandem duplications (ITDs) within the juxtamembrane domain (JMD) of the FLT3 gene are recurrent genetic alterations in AML was published in 1996.10 The FLT3 gene encodes a member of the class III receptor tyrosine kinase family that is normally expressed on the surface of bone marrow (BM) hematopoietic progenitor cells and plays an important role in the survival and/or differentiation of multipotent stem cells.41 Since 1996, FLT3-ITD has been shown to be among the most prevalent genetic abnormalities in AML, being especially frequent in t(15;17)–positive and CN patients.42 Among the latter, FLT3-ITD has been detected in 28% to 33% of patients.18,21,37,43-47 In addition, 2 types of point mutations in FLT3 have been reported: those localized within the activation loop of the tyrosine kinase domain (TKD), found in 5% to 14% of CN patients21,43,45,48 ; and those localized in the JMD, whose incidence is 2% among all AML patients but has not yet been established in CN AML.49,50
The FLT3-ITDs occur in exons 14 and 15 (previously exons 11 and 12) and vary in length of the duplicated JMD region from 3 to over 400 nucleotides.51 Despite such heterogeneity, and the fact that in some cases the FLT3-ITD is accompanied by insertions of additional nucleotides, the resultant transcripts are always in-frame. FLT3-ITD encodes an abnormal protein that undergoes ligand-independent receptor dimerization, autophosphorylation, and constitutive activation, which turns on downstream signaling pathways involved in cell proliferation, differentiation, and survival. These include the Janus kinase 2 (JAK2) signal transducer and activator of transcription 5 (STAT5) pathway and mitogen-activated protein kinase (MAPK) pathway.50,52,53 Similarly, both FLT3-TKD and JMD mutations bestow constitutive phosphorylation and activation of the FLT3 receptor, although the autophosphorylation of FLT3 and consequently transforming potential conferred by JMD mutations are weaker than those of FLT3-ITD and FLT3-TKD mutations.50 Furthermore, there is mounting evidence suggesting that the downstream cellular responses to FLT3-ITD and FLT3-TKD are not equivalent.52,53 In murine bone marrow transplantation (BMT) models, FLT3-ITD induces an oligoclonal myeloproliferative disease,54 and FLT3-TKD induces an oligoclonal lymphoid disorder with longer latency.52
Clinically, CN AML patients carrying FLT3-ITD differ from those without FLT3-ITD with regard to pretreatment characteristics and treatment outcome (Table 2) FLT3-ITD–positive patients present with increased white blood cell counts (WBCs)18,43,45,46 and percentages of blood and BM blasts43 and are more often diagnosed with de novo than secondary AML.43,45 Although the CR rates of FLT3-ITD–positive patients are often inferior, no study has observed a statistically significant difference between patients with and without FLT3-ITD (Table 2). However, an inferior prognosis for both remission duration and survival conferred by FLT3-ITD, initially reported by studies analyzing patients belonging to the intermediate-risk cytogenetic group that contained both CN patients and those with “intermediate-risk” chromosome abnormalities,42,56-58 has been firmly established in CN AML.18,37,43-46
Studies reporting clinical outcome of karyotypically normal AML patients according to the FLT3 status
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| FLT3-ITD+ versus FLT3-ITD− | |||||||
| CR rate: no significant difference (77% vs 86%) | — | De novo | 82 (23/59) | 20-59 (NR) | 1.7 y | Higher WBC | Whitman et al18 |
| OS: no significant difference (median, 11 vs 46 mo) | — | 82 (23/59) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (median, 8 vs 52 mo; P = .03) | ND | 68 (17/51) | |||||
| CR rate: no significant difference | — | De novo | 106 (33/73) | 17-65 (44) | NR | NR | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 37% vs 43%) | — | 106 (33/73) | |||||
| EFS: significantly shorter for FLT3-ITD+ pts (5-year EFS rates, 19% vs 34%; P = .05) | No | 106 (33/73) | |||||
| CR rate: no significant difference (84% vs 85%) | — | De novo (84%), secondary (16%) | 67 (19/48) | 18-71 (52/48)‡ | 38 mo | Higher WBC, higher LDH | Bienz et al46 |
| OS: significantly shorter for FLT3-ITD+ pts (median, 10 vs 15.5 mo; P = .015) | Yes | 67 (19/48) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (median, 8 vs 12.5 mo; P = .033) | Yes | 57 (16/41) | |||||
| CR rate: no significant difference (50% vs 76%) | — | De novo | 53 (16/37) | Adults (58)§ | NR | Higher WBC, higher LDH, lower lactoferrin expression | Kainz et al44 |
| OS: significantly shorter for FLT3-ITD+ pts (5-year OS rates, 6% vs 28%; P < .003) | Yes | 53 (16/37) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (5-year DFS rates, 13% vs 41%; P < .02) | NR | 36 (8/28) | |||||
| FLT3-ITD+ versus FLT3-WT† | |||||||
| OS: significantly shorter for FLT3-ITD+ pts (P < .001) | Yes‖ | De novo and secondary | 192 (67/125) | 16-60 (47) | 34 mo¶ | Higher WBC, higher LDH, higher percentage of PB and BM blasts | Fröhling et al 43 |
| CRD: significantly shorter for FLT3-ITD+ pts (P = .007) | Yes‖ | 142 (47/95) | |||||
| CR rate: no significant difference | — | De novo and secondary | 84 (33/51) | 18-60 (NR) | NR | NR | Beran et al45 |
| OS: significantly shorter for FLT3-ITD+ pts (median, 32 vs 108 wk; P < .001) | ND | 84 (33/51) | |||||
| CRD: significantly shorter for FLT3-ITD+ pts (median, 42 vs 75 wk; P = .04) | ND | 58 (17/41) | |||||
| CR rate: no significant difference | — | De novo and secondary | 84 (20/64) | 61-82 (NR) | NR | NR | Beran et al45 |
| OS: no significant difference (median, 14 vs 34 wk) | — | 84 (20/64) | |||||
| CRD: no significant difference (median, 20 vs 45 wk) | — | 39 (11/28) | |||||
| FLT3ITD/− versus FLT3-ITD− | |||||||
| CR rate: no significant difference (75% vs 86%) | — | De novo | 67 (8/59) | 20-59 (37/45)# | 1.7 y | Higher WBC | Whitman et al18 |
| OS: significantly shorter for FLT3ITD/− pts (median, 7 vs 46 mo; P = .001) | Yes | 67 (8/59) | |||||
| DFS: significantly shorter for FLT3ITD/− pts (median, 4 vs 52 mo; P = .001) | Yes | 57 (6/51) | |||||
| FLT3-ITD+ versus FLT3-Asp835+ versus FLT3-WT | |||||||
| CR rate: no significant difference (65% vs 82% vs 76%) | — | De novo and secondary | 220 (67/28/125)** | 16-60 (47) | 34 mo | Secondary AML less Common in FLT3-ITD+ and FLT3-Asp835+ pts | Fröhling et al43 |
| FLT3-ITD+ versus FLT3-Asp835+ | |||||||
| OS: no significant difference | — | De novo and secondary | 95 (67/28) | 16-60 (47) | 34 mo¶ | Higher LDH, higher percentage of PB blasts | Fröhling et al43 |
| CRD: no significant difference | — | 70 (47/23) | |||||
| FLT3-Asp835+ versus FLT3-WT | |||||||
| OS: no significant difference | — | De novo and secondary | 153 (28/125) | 16-60 (47) | 34 mo¶ | Higher percentage of BM blasts | Fröhling et al43 |
| CRD: no significant difference | — | 118 (23/95) |
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| FLT3-ITD+ versus FLT3-ITD− | |||||||
| CR rate: no significant difference (77% vs 86%) | — | De novo | 82 (23/59) | 20-59 (NR) | 1.7 y | Higher WBC | Whitman et al18 |
| OS: no significant difference (median, 11 vs 46 mo) | — | 82 (23/59) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (median, 8 vs 52 mo; P = .03) | ND | 68 (17/51) | |||||
| CR rate: no significant difference | — | De novo | 106 (33/73) | 17-65 (44) | NR | NR | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 37% vs 43%) | — | 106 (33/73) | |||||
| EFS: significantly shorter for FLT3-ITD+ pts (5-year EFS rates, 19% vs 34%; P = .05) | No | 106 (33/73) | |||||
| CR rate: no significant difference (84% vs 85%) | — | De novo (84%), secondary (16%) | 67 (19/48) | 18-71 (52/48)‡ | 38 mo | Higher WBC, higher LDH | Bienz et al46 |
| OS: significantly shorter for FLT3-ITD+ pts (median, 10 vs 15.5 mo; P = .015) | Yes | 67 (19/48) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (median, 8 vs 12.5 mo; P = .033) | Yes | 57 (16/41) | |||||
| CR rate: no significant difference (50% vs 76%) | — | De novo | 53 (16/37) | Adults (58)§ | NR | Higher WBC, higher LDH, lower lactoferrin expression | Kainz et al44 |
| OS: significantly shorter for FLT3-ITD+ pts (5-year OS rates, 6% vs 28%; P < .003) | Yes | 53 (16/37) | |||||
| DFS: significantly shorter for FLT3-ITD+ pts (5-year DFS rates, 13% vs 41%; P < .02) | NR | 36 (8/28) | |||||
| FLT3-ITD+ versus FLT3-WT† | |||||||
| OS: significantly shorter for FLT3-ITD+ pts (P < .001) | Yes‖ | De novo and secondary | 192 (67/125) | 16-60 (47) | 34 mo¶ | Higher WBC, higher LDH, higher percentage of PB and BM blasts | Fröhling et al 43 |
| CRD: significantly shorter for FLT3-ITD+ pts (P = .007) | Yes‖ | 142 (47/95) | |||||
| CR rate: no significant difference | — | De novo and secondary | 84 (33/51) | 18-60 (NR) | NR | NR | Beran et al45 |
| OS: significantly shorter for FLT3-ITD+ pts (median, 32 vs 108 wk; P < .001) | ND | 84 (33/51) | |||||
| CRD: significantly shorter for FLT3-ITD+ pts (median, 42 vs 75 wk; P = .04) | ND | 58 (17/41) | |||||
| CR rate: no significant difference | — | De novo and secondary | 84 (20/64) | 61-82 (NR) | NR | NR | Beran et al45 |
| OS: no significant difference (median, 14 vs 34 wk) | — | 84 (20/64) | |||||
| CRD: no significant difference (median, 20 vs 45 wk) | — | 39 (11/28) | |||||
| FLT3ITD/− versus FLT3-ITD− | |||||||
| CR rate: no significant difference (75% vs 86%) | — | De novo | 67 (8/59) | 20-59 (37/45)# | 1.7 y | Higher WBC | Whitman et al18 |
| OS: significantly shorter for FLT3ITD/− pts (median, 7 vs 46 mo; P = .001) | Yes | 67 (8/59) | |||||
| DFS: significantly shorter for FLT3ITD/− pts (median, 4 vs 52 mo; P = .001) | Yes | 57 (6/51) | |||||
| FLT3-ITD+ versus FLT3-Asp835+ versus FLT3-WT | |||||||
| CR rate: no significant difference (65% vs 82% vs 76%) | — | De novo and secondary | 220 (67/28/125)** | 16-60 (47) | 34 mo | Secondary AML less Common in FLT3-ITD+ and FLT3-Asp835+ pts | Fröhling et al43 |
| FLT3-ITD+ versus FLT3-Asp835+ | |||||||
| OS: no significant difference | — | De novo and secondary | 95 (67/28) | 16-60 (47) | 34 mo¶ | Higher LDH, higher percentage of PB blasts | Fröhling et al43 |
| CRD: no significant difference | — | 70 (47/23) | |||||
| FLT3-Asp835+ versus FLT3-WT | |||||||
| OS: no significant difference | — | De novo and secondary | 153 (28/125) | 16-60 (47) | 34 mo¶ | Higher percentage of BM blasts | Fröhling et al43 |
| CRD: no significant difference | — | 118 (23/95) |
Only studies comprising 50 or more patients are included. A smaller study on 34 patients receiving high-dose chemotherapy and ASCT in first CR was also published.55 pts indicates patients; MVA, multivariable analysis; FLT3-ITD+, patients with FLT3-ITD; FLT3-ITD−, patients without FLT3-ITD; CR, complete remission; OS, overall survival; DFS, disease-free survival; EFS, event-free survival; FLT3-WT, patients with FLT3 wild-type; CRD, CR duration; —, no significant difference in univariable analysis (MVA not performed); ND, not done; NR, not reported; WBC, white blood cell count; LDH, serum lactate dehydrogenase level; FLT3-Asp835−, patients without activating point mutations in codon 835 within the activation loop in the tyrosine kinase domain of the FLT3 gene; PB, blood; BM, bone marrow; FLT3ITD/−, patients with the FLT3-ITD lacking an FLT3-wild-type allele; FLT3-Asp835+, patients with FLT3-Asp835 mutations.
Numbers of patients for whom clinical data were available.
FLT3-WT (ie, FLT3-ITD− and FLT3-Asp835−).
Median age of patients with FLT3-ITD/patients without FLT3-ITD.
Mean age.
In this analysis, patients with FLT3-ITD, FLT3-Asp835 mutation, or both were combined into I group (FLT3 mutation) and compared with patients without FLT3-ITD and/or FLT3-Asp835 mutation.
Follow-up reported for all 220 patients studied.
Median age of patients with FLT3ITD/−/patients with FLT3WT/WT.
Number of patients with FLT3-ITD/patients with FLT3-Asp835/patients with FLT3-WT.
Several studies restricted to CN patients have demonstrated that patients harboring at least 1 allele with FLT3-ITD have a significantly worse complete remission duration (CRD), DFS, event-free survival (EFS), and OS than patients without FLT3-ITD (Table 2). Moreover, FLT3-ITD has been found to be an independent prognostic factor for OS,46,59 cumulative incidence of relapse (CIR),59 and CRD.19,46 Notably, in the first study limited to CN adult patients, FLT3-ITD–positive patients had a significantly shorter DFS than patients without FLT3-ITD.18 However, in one third of patients with FLT3-ITD, the FLT3 wild-type allele was not detectable. Their prognosis was particularly poor, with both DFS and OS significantly worse than those of patients without FLT3-ITD.18 In agreement with these results, Thiede et al48 reported that among AML patients with intermediate-risk cytogenetics, only cases with an FLT3-mutant/FLT3–wild-type allele ratio above the median value had a significantly shorter OS and DFS compared with patients without FLT3-ITD. Recent studies have shown that the absence of the FLT3 wild-type allele in this subset of patients with very poor prognosis likely results from acquired uniparental disomy, which could be either partial, due to somatic recombination between homologous chromosomes occurring after FLT3-ITD had been generated, or could involve a loss of the entire chromosome 13 with the FLT3 wild-type allele and subsequent duplication of the remaining homologous chromosome 13 with FLT3-ITD.60,61 The latter mechanism, however, seems less likely because in 2 patients studied by Whitman et al,18 the 2 homologous chromosomes 13 were heteromorphic for the chromosome 13 short arm (13p).
In contrast to FLT3-ITD, the FLT3-TKD has not been reported to have an adverse prognosis among either CN patients43 or those with intermediate-risk cytogenetics.48 Interestingly, a relatively small group of CN patients with FLT3-TKD who concurrently harbored a mutation in the NPM1 gene had significantly longer EFS than patients without these mutations.21 This finding requires corroboration, and further studies are necessary to determine the prognostic role of FLT3-TKD, especially in relation to other molecular prognostic factors in CN AML.
A recent study,62 not limited to CN AML, examined the prognostic impact of the length of the FLT3-ITD and found that patients with a duplicated segment consisting of at least 40 nucleotides had the worst relapse-free survival (RFS) compared with patients with smaller duplications and those without FLT3-ITD. Since length of the FLT3-ITD has not been correlated with level of aberrant autophosphorylation of the protein,63 it is unknown whether the different length of FLT3-ITDs can directly affect the protein's activity and response to treatment. It is also unknown whether the prognosis of CN patients is affected by FLT3-ITD length. Likewise, the adverse prognostic significance of overexpression of wild-type FLT3 alleles in FLT3-ITD– and FLT3-TKD–negative patients, suggested by a relatively small study of AML patients with diverse karyotypes,64 remains to be established in CN AML.65,66
In addition to being a prognostic marker, FLT3-ITD is a potential therapeutic target. Several small-molecule inhibitors of FLT3 tyrosine kinase activity are currently in clinical trials (Table 3)72 In patients with FLT3-ITD or FLT3-TKD mutations, clinical responses are usually manifested as a transient reduction in blood and, less frequently, BM blast counts, with few patients attaining a CR or a long-lasting partial response.67,68,70-72 Nevertheless, in vivo inhibition of FLT3 autophosphorylation by PKC41267 and CEP-70168 has been demonstrated in responding patients, supporting the further clinical development of these compounds. Notably, all these compounds are also capable of inhibiting multiple tyrosine kinases (Table 3), which may also explain clinical responses in AML patients without FLT3-ITD.72
FLT3 inhibitors currently in clinical trials for AML
| Compound . | Chemical class . | Manufacturer . | Targeted kinases . | FLT3 IC50,* nM . | Selected clinical trials . |
|---|---|---|---|---|---|
| PKC412 | Benzoyl-staurosporine | Novartis (Basel, Switzerland) | PKC, PDGFR, KDR, KIT, FLT3, ABL, FGFR1, FGFR3 | 528 | Stone et al67 : Phase 2 trial in advanced MDS or relapsed or refractory AML with FLT3-ITD or FLT3-TKD |
| CEP-701 (lestaurtinib) | Indolocarbazole | Cephalon (Frazer, PA) | FLT3, TRKA, KDR, PKC, PDGFR, EGFR | 2-3 | Smith et al68 ; Phase 1/2 trial in refractory, relapsed, or poor-risk AML with FLT3-ITD or FLT3-TKD mutations |
| MLN518 (CT53518, tandutinib) | Piperazinyl quinazoline | Millennium (San Francisco, CA) | KIT, PDGFR, FLT3, FMS | 170-220 | De Angelo et al69 : Phase 2 trial in relapsed, refractory, or untreated (not fit for induction therapy) AML with FLT3-ITD |
| SU11248 (sunitinib malate) | Indolinone | Pfizer (New York, NY; synthesized by Sugen, South San Francisco, CA) | FLT3, KDR, PDGFR, KIT, VEGFR | 10 | O'Farrell et al70 : Phase 1 trial in refractory, relapsed, or untreated AML; Fiedler et al71 : Phase 1 trial in relapsed or refractory AML with FLT3-ITD or FLT3-TKD |
| Compound . | Chemical class . | Manufacturer . | Targeted kinases . | FLT3 IC50,* nM . | Selected clinical trials . |
|---|---|---|---|---|---|
| PKC412 | Benzoyl-staurosporine | Novartis (Basel, Switzerland) | PKC, PDGFR, KDR, KIT, FLT3, ABL, FGFR1, FGFR3 | 528 | Stone et al67 : Phase 2 trial in advanced MDS or relapsed or refractory AML with FLT3-ITD or FLT3-TKD |
| CEP-701 (lestaurtinib) | Indolocarbazole | Cephalon (Frazer, PA) | FLT3, TRKA, KDR, PKC, PDGFR, EGFR | 2-3 | Smith et al68 ; Phase 1/2 trial in refractory, relapsed, or poor-risk AML with FLT3-ITD or FLT3-TKD mutations |
| MLN518 (CT53518, tandutinib) | Piperazinyl quinazoline | Millennium (San Francisco, CA) | KIT, PDGFR, FLT3, FMS | 170-220 | De Angelo et al69 : Phase 2 trial in relapsed, refractory, or untreated (not fit for induction therapy) AML with FLT3-ITD |
| SU11248 (sunitinib malate) | Indolinone | Pfizer (New York, NY; synthesized by Sugen, South San Francisco, CA) | FLT3, KDR, PDGFR, KIT, VEGFR | 10 | O'Farrell et al70 : Phase 1 trial in refractory, relapsed, or untreated AML; Fiedler et al71 : Phase 1 trial in relapsed or refractory AML with FLT3-ITD or FLT3-TKD |
IC50 indicates the half-maximal inhibitory concentration in vitro; FLT3-ITD, internal tandem duplication of the FLT3 gene; and FLT3-TKD, activating point mutations in codon 835 or 836 of the FLT3 gene.
FLT3 autophosphorylation in vitro.
Resistance to the tyrosine kinase inhibitors targeting FLT3-ITD has recently been observed in treated patients. Initial evidence suggests that such resistance is likely mediated by gain of additional point mutations in the FLT3-ITD gene that result in single–amino-acid substitutions within the ATP pocket of the protein, but confirmatory studies are required.76
Mutations of the nucleophosmin, member 1 (NPM1) gene
Mutations of NPM1, a partner in gene fusions generated by recurrent chromosome translocations [ie, t(2;5)(p23;q35) in anaplastic large-cell lymphoma, t(3;5)(q25;q35) in AML, and t(5;17)(q35;q21) in acute promyelocytic leukemia],38,39,78 are the most frequent gene mutations in CN AML patients, being reported in 46% to 62% of cases.12,21,37,47,79 The most common NPM1 mutation is a 4–base pair duplication, 956dupTCTG in exon 12 (called type A), that causes a shift in the reading frame leading to replacement of the last 7 amino acids by 11 different ones in the C-terminal portion of nucleophosmin. This and several other less-frequent mutations, which are always heterozygous, result in both loss of tryptophan residues 290 and 288 (or 290 only), critical for nucleolar protein localization, and acquisition of an additional nuclear export signal at the C-terminus of nucleophosmin, causing its aberrant cytoplasmic localization.40 Altered distribution in cell compartments likely interferes with the normal functions of nucleophosmin, which include preventing nucleolar protein aggregation, regulation of ribosomal protein assembly and the nucleocytoplasmic transport of these proteins, as well as the regulation of the ARF and p53 tumor-suppressor pathways.12
NPM1 mutations occur predominantly in CN patients12,47 and are associated with female sex21,79 and such pretreatment characteristics as higher WBCs21,37,47,79 and platelet counts,21,47,79 increased BM blast percentages,47,79 and low or absent CD34+ expression.12,21,47,79 Notably, patients with mutated NPM1 harbor FLT3-ITDs and FLT3-TKD mutations approximately twice as often as those with wild-type NPM1.12,21,47,79 Thiede et al47 provided evidence that NPM1 mutations usually precede the acquisition of FLT3-ITDs, suggesting that NPM1 mutations might constitute a primary event in leukemogenesis. CEBPA mutations occur with a similar incidence in patients with and without NPM1 mutations,21,79 whereas the partial tandem duplication (PTD) of MLL is extremely rare in patients with NPM1 mutations.21,47,79
The prognostic relevance of NPM1 mutations has been shown by most, but not all,37 recent studies that analyzed CN patients21,47,79 or those classified in the intermediate-risk cytogenetic category.80 Falini et al12 first reported that cytoplasmic localization of nucleophosmin, which is highly correlated with the presence of NPM1 mutation, was an independent favorable prognostic factor for achievement of CR. In subsequent studies, comparisons of the clinical outcome involving patients with and without NPM1 mutations regardless of the presence of other genetic rearrangements have yielded somewhat inconsistent results (Table 4) However, when FLT3-ITDs, which are detected in approximately 40% of patients with NPM1 mutations, have also been considered, all studies have found that NPM1 mutations are associated with a significantly improved outcome in the absence of FLT3-ITD. Patients with NPM1 mutations lacking FLT3-ITD had significantly better CR rates,47,79 EFS,21 RFS,79 DFS,47 and OS,21,47,79 whereas NPM1 mutations did not impact the poor outcome of patients harboring FLT3-ITD (Table 4). In multivariable analyses, the combined status of mutated NPM1 and the absence of FLT3-ITD constituted an independent favorable prognostic factor for the achievement of CR,79 DFS,47 RFS,79 EFS,21 and OS.47,79
Studies reporting clinical outcome of karyotypically normal AML patients according to the NPM1 mutational status
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| Cytoplasmic localization of NPM1 vs nucleolar localization of NPM1 | |||||||
| CR rate: no significant difference in univariable analysis (77% vs 62%): independent favorable prognostic factor for CR achievement (odds ratio 2.98, CI: 1.2-7.43; P = .019) | Yes | De novo | 126 (79/47) | 19-60 (52/42)† | NA | Older age | Falini et al12 |
| NPM1+ versus NPM1− | |||||||
| CR rate: significantly higher in NPM1+ pts (70.5% vs 54.7%; P = .003) | ND | De novo (93%), secondary (7%) | 401 (212/189) | 17-82 (56/58)† | 484 d | More often women; higher WBC; lower CD34, CD133, and MPO expression | Schnittger et al21 |
| OS: no significant difference (1012 vs 549 d) | — | 401 (212/189) | |||||
| EFS: significantly longer (median, 428 vs 336 d; P = .012). | Yes | 401 (212/189) | |||||
| RFS: no significant difference (median, 473 vs 386 d) | — | 226 (136/90) | |||||
| OS: no significant difference | — | De novo (86%), secondary (8%), unknown (6%) | 300 (145/155) | 16-60 (NR) | 46 mo | More often women, higher WBC, higher platelet counts, higher LDH, higher percentage of BM blasts, more FAB M4/M5, more extramedullary, involvement and lymphadenopathy, lower CD34 expression | Döhner et al79 |
| RFS: significantly longer for NPM1+ pts (P = .002) | NR | 217 (111/106) | |||||
| CR rate: no significant difference (86% vs 88%) | — | De novo | 106 (50/56) | 17-65 (43/46)† | NR | Higher WBC, more FAB M4/M5, less FAB M0, and less common CEBPA mutations | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 43% vs 37%) | No | 106 (50/56) | |||||
| EFS: no significant difference (6-year EFS rates, 32% vs 26%) | No | 106 (50/56) | |||||
| RFS: no significant difference (6-year RFS rates, 47% vs 34%) | — | 92 (43/49) | |||||
| OS: no significant difference | — | De novo and secondary | 658 (298/360) | 15-87 (NR) | 20.2 mo‡ | More often women, lower CD34 expression‡ | Thiede et al47 |
| DFS: significantly longer for NPM1+ pts (P = .043) | NR | 301 (166/135) | |||||
| CR rate: significantly higher in NPM1+ pts (86.4% vs 64.3%; P = .037) | Yes | De novo | 79 (37/42) | 15-85 (58/47)†§ | NR | Older age, higher WBC, higher percentage of PB blasts, less FAB M2§ | Suzuki et al81 |
| OS: no significant difference | No | 79 (37/42) | |||||
| RR: significantly higher in NPM1+ pts (P = .032) | Yes | 59 (32/27) | |||||
| NPM1+/FLT3-ITD− versus NPM1+/FLT3-ITD+ versus NPM1−/FLT3-ITD+ versus NPM1−/FLT3-ITD− | |||||||
| CR rates: a significant difference according to the NPM1 and FLT3-ITD mutational status (86% vs 63% vs 76% vs 68.5%; P < .001) | Yes‖ | De novo (86%), secondary (8%), unknown (6%) | 300 (86/59/38/117)¶ | 16-60 (49/47/43/49)¶ | 46 mo | WBC, LDH, percentage of PB and BM blasts highest in NPM1+/FLT3-ITD+ pts, WBC, LDH, percentage of PB and BM blasts lowest in NPM1−-/FLT3-ITD− pts | Döhner et al79 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with the 3 other groups (P = .001) | Yes‖ | 300 (86/59/38/117)¶ | |||||
| RFS: significantly longer for NPM1+/FLT3-ITD− pts compared with the 3 other groups (P < .001) | Yes‖ | 217 (74/37/28/78)¶ | |||||
| CR rate: a significant difference according to the NPM1 and FLT3-ITD mutational status (61% vs 52.8% vs 50% vs 42.1%; P < .001) | NR | De novo and secondary | 709 (182/142/76/309)¶ | 15-87 (NR) | 20.2 mo‡ | NR | Thiede et al47 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .001) NPM1−/FLT3-ITD+ (P = .032) and NPM1−/FLT3-ITD− (P = .03) pts | Yes‖ | 658 (169/129/70/290)¶ | |||||
| DFS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .04), NPM1−/FLT3-ITD+ (P < .001), and NPM1−/FLT3-ITD− (P = .04) pts | Yes‖ | 301 (103/63/31/104)¶ | |||||
| CR rate: no significant difference (68.8% vs 57.7% vs 68.9% vs 60.2%) | — | De novo | 352 | < 60 (NR) | 20.2 mo‡ | NR | Thiede et al47 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .003) and NPM1−/FLT3-ITD− (P = .02) pts; no difference between NPM1+/FLT3-ITD− and NPM1−/FLT3-ITD+ pts | Yes‖ | 359 (74/95/46/144)¶ | |||||
| DFS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .02), NPM1−/FLT3-ITD+ (P < .001) and NPM1−/FLT3-ITD− (P = .006) pts | Yes‖ | 204 (43/67/26/68)¶ | |||||
| CIR: significantly reduced for NPM1+/FLT3-ITD− pts compared with the 3 other groups (40-mo CIR 25% vs 57.2% vs 51.3% vs 32.7%; P = .004) | NR | 204 (43/67/26/68)¶ | |||||
| NPM1+/FLT3-ITD− versus NPM1−/FLT3-ITD− | |||||||
| OS: significantly longer for NPM1+/FLT3-ITD− pts (median, 1183 vs 601 d; P = .022) | NR | De novo and secondary | 264 (126/138) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD− pts (median, 773 vs 365 d; P = .001) | NR | 264 (126/138) | |||||
| NPM1+/FLT3-ITD− versus NPM1+/FLT3-ITD+ | |||||||
| OS: significantly longer for NPM1+/FLT3-ITD− pts (median, 1183 vs 321 d; P = .014) | NR | De novo and secondary | 212 (126/86) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD− pts (median, 773 vs 234 d; P = .001) | NR | 212 (126/86) | |||||
| NPM1−/FLT3-ITD+ versus NPM1+/FLT3-ITD+ | |||||||
| OS: no significant difference (median, 405 vs 321 d) | — | De novo and secondary | 131 (45/86) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: no significant difference (median, 279 vs 234 d) | — | 131 (45/86) | |||||
| NPM1+/FLT3-TKD+ versus NPM1−/FLT3-TKD− | |||||||
| OS: no significant difference (median, not reached vs 676 d) | — | De novo and secondary | 173 (20/153) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD+ (median, not reached vs 336 d; P = .014) | NR | 173 (20/153) |
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| Cytoplasmic localization of NPM1 vs nucleolar localization of NPM1 | |||||||
| CR rate: no significant difference in univariable analysis (77% vs 62%): independent favorable prognostic factor for CR achievement (odds ratio 2.98, CI: 1.2-7.43; P = .019) | Yes | De novo | 126 (79/47) | 19-60 (52/42)† | NA | Older age | Falini et al12 |
| NPM1+ versus NPM1− | |||||||
| CR rate: significantly higher in NPM1+ pts (70.5% vs 54.7%; P = .003) | ND | De novo (93%), secondary (7%) | 401 (212/189) | 17-82 (56/58)† | 484 d | More often women; higher WBC; lower CD34, CD133, and MPO expression | Schnittger et al21 |
| OS: no significant difference (1012 vs 549 d) | — | 401 (212/189) | |||||
| EFS: significantly longer (median, 428 vs 336 d; P = .012). | Yes | 401 (212/189) | |||||
| RFS: no significant difference (median, 473 vs 386 d) | — | 226 (136/90) | |||||
| OS: no significant difference | — | De novo (86%), secondary (8%), unknown (6%) | 300 (145/155) | 16-60 (NR) | 46 mo | More often women, higher WBC, higher platelet counts, higher LDH, higher percentage of BM blasts, more FAB M4/M5, more extramedullary, involvement and lymphadenopathy, lower CD34 expression | Döhner et al79 |
| RFS: significantly longer for NPM1+ pts (P = .002) | NR | 217 (111/106) | |||||
| CR rate: no significant difference (86% vs 88%) | — | De novo | 106 (50/56) | 17-65 (43/46)† | NR | Higher WBC, more FAB M4/M5, less FAB M0, and less common CEBPA mutations | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 43% vs 37%) | No | 106 (50/56) | |||||
| EFS: no significant difference (6-year EFS rates, 32% vs 26%) | No | 106 (50/56) | |||||
| RFS: no significant difference (6-year RFS rates, 47% vs 34%) | — | 92 (43/49) | |||||
| OS: no significant difference | — | De novo and secondary | 658 (298/360) | 15-87 (NR) | 20.2 mo‡ | More often women, lower CD34 expression‡ | Thiede et al47 |
| DFS: significantly longer for NPM1+ pts (P = .043) | NR | 301 (166/135) | |||||
| CR rate: significantly higher in NPM1+ pts (86.4% vs 64.3%; P = .037) | Yes | De novo | 79 (37/42) | 15-85 (58/47)†§ | NR | Older age, higher WBC, higher percentage of PB blasts, less FAB M2§ | Suzuki et al81 |
| OS: no significant difference | No | 79 (37/42) | |||||
| RR: significantly higher in NPM1+ pts (P = .032) | Yes | 59 (32/27) | |||||
| NPM1+/FLT3-ITD− versus NPM1+/FLT3-ITD+ versus NPM1−/FLT3-ITD+ versus NPM1−/FLT3-ITD− | |||||||
| CR rates: a significant difference according to the NPM1 and FLT3-ITD mutational status (86% vs 63% vs 76% vs 68.5%; P < .001) | Yes‖ | De novo (86%), secondary (8%), unknown (6%) | 300 (86/59/38/117)¶ | 16-60 (49/47/43/49)¶ | 46 mo | WBC, LDH, percentage of PB and BM blasts highest in NPM1+/FLT3-ITD+ pts, WBC, LDH, percentage of PB and BM blasts lowest in NPM1−-/FLT3-ITD− pts | Döhner et al79 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with the 3 other groups (P = .001) | Yes‖ | 300 (86/59/38/117)¶ | |||||
| RFS: significantly longer for NPM1+/FLT3-ITD− pts compared with the 3 other groups (P < .001) | Yes‖ | 217 (74/37/28/78)¶ | |||||
| CR rate: a significant difference according to the NPM1 and FLT3-ITD mutational status (61% vs 52.8% vs 50% vs 42.1%; P < .001) | NR | De novo and secondary | 709 (182/142/76/309)¶ | 15-87 (NR) | 20.2 mo‡ | NR | Thiede et al47 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .001) NPM1−/FLT3-ITD+ (P = .032) and NPM1−/FLT3-ITD− (P = .03) pts | Yes‖ | 658 (169/129/70/290)¶ | |||||
| DFS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .04), NPM1−/FLT3-ITD+ (P < .001), and NPM1−/FLT3-ITD− (P = .04) pts | Yes‖ | 301 (103/63/31/104)¶ | |||||
| CR rate: no significant difference (68.8% vs 57.7% vs 68.9% vs 60.2%) | — | De novo | 352 | < 60 (NR) | 20.2 mo‡ | NR | Thiede et al47 |
| OS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .003) and NPM1−/FLT3-ITD− (P = .02) pts; no difference between NPM1+/FLT3-ITD− and NPM1−/FLT3-ITD+ pts | Yes‖ | 359 (74/95/46/144)¶ | |||||
| DFS: significantly longer for NPM1+/FLT3-ITD− pts compared with NPM1+/FLT3-ITD+ (P = .02), NPM1−/FLT3-ITD+ (P < .001) and NPM1−/FLT3-ITD− (P = .006) pts | Yes‖ | 204 (43/67/26/68)¶ | |||||
| CIR: significantly reduced for NPM1+/FLT3-ITD− pts compared with the 3 other groups (40-mo CIR 25% vs 57.2% vs 51.3% vs 32.7%; P = .004) | NR | 204 (43/67/26/68)¶ | |||||
| NPM1+/FLT3-ITD− versus NPM1−/FLT3-ITD− | |||||||
| OS: significantly longer for NPM1+/FLT3-ITD− pts (median, 1183 vs 601 d; P = .022) | NR | De novo and secondary | 264 (126/138) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD− pts (median, 773 vs 365 d; P = .001) | NR | 264 (126/138) | |||||
| NPM1+/FLT3-ITD− versus NPM1+/FLT3-ITD+ | |||||||
| OS: significantly longer for NPM1+/FLT3-ITD− pts (median, 1183 vs 321 d; P = .014) | NR | De novo and secondary | 212 (126/86) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD− pts (median, 773 vs 234 d; P = .001) | NR | 212 (126/86) | |||||
| NPM1−/FLT3-ITD+ versus NPM1+/FLT3-ITD+ | |||||||
| OS: no significant difference (median, 405 vs 321 d) | — | De novo and secondary | 131 (45/86) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: no significant difference (median, 279 vs 234 d) | — | 131 (45/86) | |||||
| NPM1+/FLT3-TKD+ versus NPM1−/FLT3-TKD− | |||||||
| OS: no significant difference (median, not reached vs 676 d) | — | De novo and secondary | 173 (20/153) | 17-82# (NR) | 484 d# | NR | Schnittger et al21 |
| EFS: significantly longer for NPM1+/FLT3-ITD+ (median, not reached vs 336 d; P = .014) | NR | 173 (20/153) |
MVA indicates multivariable analysis; pts, patients; CR, complete remission; NPM1+, patients with mutations of the NPM1 gene; NPM1−, patients without mutations of the NPM1 gene; OS, overall survival; EFS, event-free survival; RFS, relapse-free survival; DFS, disease-free survival; RR, risk of relapse; FLT3-ITD−, patients without FLT3-ITD; FLT3-ITD+, patients with ITD of the FLT3 gene; CIR, cumulative incidence of relapse; FLT3-TKD+, patients with mutations in the tyrosine kinase domain (TKD) of the FLT3 gene; FLT3-TKD−, patients without FLT3-TKD mutations; NA, not applicable; —, no significant difference in univariable analysis (MVA not performed); ND, not done; WBC, white blood cell count; MPO, myeloperoxidase; NR, not reported; LDH, serum lactate dehydrogenase level; BM, bone marrow; FAB, French-American-British; and PB, blood.
Numbers of patients for whom clinical data were available.
Median age of patients with NPM1 mutations/patients without NPM1 mutations.
Number and median follow-up and differences in pretreatment features reported for all 1485 patients, including 709 with a normal, 686 with an abnormal, and 90 with an unknown karyotype.
Age range and median and differences in pretreatment features reported for all 190 patients, including 79 with a normal, 87 with an abnormal, and 24 with an unknown karyotype.
NPM1+/FLT3-ITD− is an independent prognostic factor.
Number and median age of patients with NPM1+/FLT3-ITD−/patients with NPM1+/FLT3-ITD+/patients with NPM1−/FLT3-ITD+/patients with NPM1−/FLT3-ITD−.
Age range and median follow-up reported for all 401 patients studied.
Partial tandem duplication (PTD) of the myeloid/lymphoid or mixed-lineage leukemia (MLL) gene
MLL-PTD was the first gene mutation shown to affect prognosis in CN AML patients. The discovery of MLL-PTD was made after Caligiuri et al,82 using Southern analysis, unexpectedly detected rearrangements of the MLL gene in patients who did not harbor structural chromosome aberrations involving band 11q23 and the MLL gene but had instead either an isolated trisomy of chromosome 11 or a normal karyotype.9,83 Among CN AML patients, MLL-PTD occurs in approximately 8% (range, 5%-11% among the largest studies17,84-86 ).
MLL-PTD most often involves a duplication of a genomic region spanning exons 5 through 11 and insertion of the duplicated region into intron 4 of the MLL gene; in a minority of cases the duplicated region encompasses exons 5 through 12. Both main types of MLL-PTD result in in-frame fusions of exons 11 or 12 upstream of exon 5 and a duplication of the N-terminal region of MLL, thereby producing an elongated protein. Unlike the MLL chimeric fusion proteins, the MLL-PTD retains all functional domains of the MLL wild-type protein, including the C-terminal SET domain that confers histone methyltransferase activity,87 along with duplication of the N-terminal AT hook DNA-binding motifs, a transcriptional repression domain, and a domain preferentially binding an unmethylated cytosine in cytidine phosphate guanosine (CpG) dinucleotides on DNA.88 The presence of MLL-PTD is concurrent with silencing of the MLL wild-type allele in AML blasts.88 The mechanism for the monoallelic gene silencing in MLL-PTD–positive AML appears to involve differential DNA methylation and histone modifications regulating either directly or indirectly the promoter of the MLL wild-type allele.
Pretreatment characteristics do not differ significantly between CN patients with and without MLL-PTD.17,85 Between 30% and 40% of MLL-PTD–positive patients also harbor FLT3-ITD,79,86 whereas coexistence of MLL-PTD with CEBPA19 or NPM121,79 mutations is rare. There is no significant difference in the probability of achieving a CR or OS (Table 5)17,85 However, in both the initial study on prognostic impact of the MLL-PTD17 and the largest series to date, comprising 221 CN patients,85 CRD was significantly shorter for MLL-PTD–positive patients. Likewise, RFS and EFS were also worse for CN patients with MLL-PTD in 2 smaller studies.84,89 In a multivariable analysis that did not include other molecular genetic markers, MLL-PTD status was the single prognostically significant factor for CRD.85
Studies reporting clinical outcome of karyotypically normal AML patients according to the MLL-PTD status: MLL-PTD+ versus MLL-WT
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up, mo . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| CR rate: no significant difference (70% vs 71%) | — | De novo | 98 (11/87) | 18-84 (41/53)† | No significant differences | Caligiuri et al17 | |
| OS: no significant difference (median, 13.8 vs 20.1 mo) | — | 98 (11/87) | 27 | ||||
| CRD: significantly shorter for MLL-PTD+ pts (median, 7.1 vs 23.2 mo; P = .01) | ND | 68 (7/61) | 22 | ||||
| Median survival: significantly shorter for MLL-PTD+ pts (10 with normal and 5 with abnormal karyotype) compared with that of age-matched karyotypically normal control group (median, 5 vs 12 mo; P = .006) | ND | De novo and secondary | 45 (15/30) | 50-73‡ (61) | NR | NR | Schnittger et al84 |
| Relapse-free interval: significantly shorter for MLL-PTD+ pts (5 with normal and 4 with abnormal karyotype) compared with that of age-matched karyotypically normal control group (median, 4 mo vs median not reached; P < .001) | ND | 26 (9/17) | |||||
| CR rate: no significant difference (89% vs 78%) | — | De novo and secondary | 221 (18/203) | 16-60 (49/45)† | 19 | No significant differences | Döhner et al85 |
| OS: no significant difference (13.4 vs 20.8 mo) | — | 221 (18/203) | |||||
| CRD: significantly shorter for MLL-PTD+ pts (median, 7.75 vs 19 mo; P = .02) | Yes | 174 (16/158) | |||||
| DFS: no significant difference (median, 3.2 vs 19.6 mo) | — | De novo and secondary | 169 (8/161) | 16-60 (NR) | NR | NR | Steudel et al86 |
| EFS: significantly shorter for MLL-PTD+ pts (2-year EFS rates, 20% vs 66%; P = .03) | ND | De novo | 30 (5/25) | 16-60 (NR) | NR | NR | Muñoz et al89 |
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up, mo . | Differences in pretreatment features . | Source . |
|---|---|---|---|---|---|---|---|
| CR rate: no significant difference (70% vs 71%) | — | De novo | 98 (11/87) | 18-84 (41/53)† | No significant differences | Caligiuri et al17 | |
| OS: no significant difference (median, 13.8 vs 20.1 mo) | — | 98 (11/87) | 27 | ||||
| CRD: significantly shorter for MLL-PTD+ pts (median, 7.1 vs 23.2 mo; P = .01) | ND | 68 (7/61) | 22 | ||||
| Median survival: significantly shorter for MLL-PTD+ pts (10 with normal and 5 with abnormal karyotype) compared with that of age-matched karyotypically normal control group (median, 5 vs 12 mo; P = .006) | ND | De novo and secondary | 45 (15/30) | 50-73‡ (61) | NR | NR | Schnittger et al84 |
| Relapse-free interval: significantly shorter for MLL-PTD+ pts (5 with normal and 4 with abnormal karyotype) compared with that of age-matched karyotypically normal control group (median, 4 mo vs median not reached; P < .001) | ND | 26 (9/17) | |||||
| CR rate: no significant difference (89% vs 78%) | — | De novo and secondary | 221 (18/203) | 16-60 (49/45)† | 19 | No significant differences | Döhner et al85 |
| OS: no significant difference (13.4 vs 20.8 mo) | — | 221 (18/203) | |||||
| CRD: significantly shorter for MLL-PTD+ pts (median, 7.75 vs 19 mo; P = .02) | Yes | 174 (16/158) | |||||
| DFS: no significant difference (median, 3.2 vs 19.6 mo) | — | De novo and secondary | 169 (8/161) | 16-60 (NR) | NR | NR | Steudel et al86 |
| EFS: significantly shorter for MLL-PTD+ pts (2-year EFS rates, 20% vs 66%; P = .03) | ND | De novo | 30 (5/25) | 16-60 (NR) | NR | NR | Muñoz et al89 |
MLL-PTD+ indicates patients with partial tandem duplication of the MLL gene; MLL-WT, patients with wild-type MLL alleles; MVA, multivariable analysis; pts, patients; CR, complete remission; OS, overall survival; CRD, CR duration; DFS, disease-free survival; EFS, event-free survival; —, no significant difference in univariable analysis (MVA not performed); ND, not done; and NR, not reported.
Numbers of patients for whom clinical data were available.
Median age for patients with/patients without MLL-PTD.
Age range for patients with MLL-PTD.
Direct molecular targeting of MLL-PTD by pharmacologic agents has not been investigated, likely due to the acknowledged inherent difficulties in targeting a protein originally thought to be a transcription factor without enzymatic activity. However, Whitman et al88 have recently shown that the combination of decitabine (5′-aza-2′-deoxycytidine), a DNA methyltransferase inhibitor, and depsipeptide, a histone deacetylase inhibitor, can reactivate the transcription of the MLL wild-type allele in MLL-PTD–positive cells. Importantly, the induction of MLL wild-type expression was associated with enhanced cell death of MLL-PTD–positive leukemic blasts.88 These data suggest that therapy that includes DNA methyltransferase and/or histone deacetylase inhibitors should be explored in patients with MLL-PTD. However, until molecularly targeted therapies, such as the aforementioned drug combination or agents targeting the histone methyltransferase activity of MLL-PTD, are shown to be clinically effective, allogeneic SCT appears to be the best therapeutic option for CN AML patients with MLL-PTD.85
Overexpression of the BAALC gene
The BAALC gene is expressed primarily in neuroectoderm-derived tissues and hematopoietic precursors but not in mature marrow or blood mononuclear cells and encodes a protein with no homology to any known proteins or functional domains.13 In the initial report, high BAALC expression was detected in a subset of patients with AML, acute lymphoblastic leukemia, and chronic myelogenous leukemia (CML) in blast crisis but not in those with chronic-phase CML or chronic lymphocytic leukemia.13 In CN adults younger than 60 years with de novo AML, high BAALC expression (defined as expression above the median BAALC expression value) in blood at diagnosis negatively affected EFS, DFS, and OS in patients with wild-type FLT3 or those who harbored FLT3-ITD and concurrently expressed the wild-type FLT3 allele (Table 6)20
Studies reporting clinical outcome of karyotypically normal AML patients according to the changes in gene expression
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | References . |
|---|---|---|---|---|---|---|---|
| BAALC high expression versus BAALC low expression | |||||||
| CR rate: no significant difference (77% vs 81%) | — | De novo | 86 (43/43) | 18-59 (51/56)† | 3.3 y | Lower WBC, less often FAB M5 | Baldus et al20 |
| OS: significantly shorter for high BAALC pts (median, 1.7 vs 5.8 y; P = .02) | Yes | 86 (43/43) | |||||
| EFS: significantly shorter for high BAALC pts (median, 0.8 vs 4.9 y; P = .03) | Yes | 86 (43/43) | |||||
| DFS: significantly shorter for high BAALC pts (median, 1.4 vs 7.3 y; P = .03) | Yes | 68 (33/35) | |||||
| CR rate: no significant difference (82% vs 91%) | — | De novo (84%), secondary (16%) | 67 (44/23) | 18-71 (46/53)† | 38 mo | Higher CD34; lower CD11b, CD15, and MPO‡ | Bienz et al46 |
| OS: significantly shorter for high BAALC pts (median, 10 vs 21 mo; P = .021) | Yes | 67 (44/23) | |||||
| DFS: significantly shorter for high BAALC (median, 8.5 vs 21 mo; P = .015) | Yes | 57 (36/21) | |||||
| CR rate: significantly lower for high BAALC pts (62% vs 73%; P = .038) | No | De novo (91%), secondary (9%) | 307 (153/154) | 17-60 (46/50)† | 29 mo | Higher percentage of PB blasts, more FAB M0/M1, less FAB M5b, less gingival hyperplasia | Baldus et al59 |
| Primary resistant disease rate: significantly higher for high BAALC pts (16% vs 6%; P = .006) | Yes | 307 (153/154) | |||||
| OS: significantly shorter for high BAALC pts (3-year OS rates, 36% vs 54%; P = .001) | Yes | 307 (153/154) | |||||
| RR: significantly higher for high BAALC pts (43% vs 29%; P = .042) | ND | 208 (95/113) | |||||
| CIR: significantly higher for high BAALC pts (3-year CIR rates, 50% vs 32%; P = .018) | Yes | 208 (95/113) | |||||
| BAALC high expression/FLT3 high risk versus BAALC high expression/FLT3 low risk versus BAALC low expression/FLT3 high risk versus BAALC low expression/FLT3 low risk | |||||||
| CR rate: the lowest for BAALC high/FLT3 high-risk pts (57% vs 58% vs 83% vs 73%; P = .048) | ND | De novo and secondary | 268 (21/110/12/125)§ | 17-60 | 29 mo | NR | Baldus et al59 |
| OS: the worst for BAALC high/FLT3 high-risk pts (3-year OS rates, 0% vs 38% vs 22% vs 58%; P < .001) | ND | 268 (21/110/12/125)§ | |||||
| CIR: the highest for BAALC high/FLT3 high-risk pts (3-year CIR rates, 100% vs 44% vs 60% vs 28%; P < .001) | ND | 177 (12/64/10/91)§ | |||||
| ERG high expression versus ERG low expression | |||||||
| CR rate: no significant difference (76% vs 83%) | — | De novo | 48 (21/63) | 18-59 (48/47)‖ | 5.7 y | Higher percentage of PB and BM blasts, more often high BAALC expression | Marcucci et al16 |
| OS: significantly shorter for high ERG pts (median, 1.2 y vs not reached; P = .011) | Yes | 84 (21/63) | |||||
| CIR: significantly higher for high ERG pts (median, 0.7 y vs not reached, P < .001) | Yes | 68 (16/52) | |||||
| Wild-type FLT3 high expression versus wild-type FLT3 low expression | |||||||
| OS: a trend towards worse OS for high FLT3 pts (P = .059) | ND | De novo and secondary | 49 (21/28) | 17-84 (61)¶ | NR | NR | Kuchenbauer et al66 |
| EFS: a trend towards worse EFS for high FLT3 pts (P = .087) | ND | 50 (21/29) | |||||
| MN1 high expression versus MN1 low expression | |||||||
| CR rate: no significant difference (80 vs 89%) | — | De novo (87%), secondary (13%) | 142 (71/71) | 18-60 (46/45)# | 30 mo | Less often mutated NPM1, less often NPM1 mutated/FLT3-ITD absent, more often high CD34 expression | Heuser et al15 |
| OS: significantly shorter for high MN1 pts (3-year OS rates, 38.1% vs 58.8%; P = .03) | Yes | 142 (71/71) | |||||
| RFS: significantly shorter for high MN1 pts (3-year RFS rates, 23.4% vs. 51.4%; P = .002) | Yes | 120 (57/63) | |||||
| RR: significantly higher for high MN1 pts (56.1% vs 36.5%; P = .03) | — | 120 (57/63) |
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | References . |
|---|---|---|---|---|---|---|---|
| BAALC high expression versus BAALC low expression | |||||||
| CR rate: no significant difference (77% vs 81%) | — | De novo | 86 (43/43) | 18-59 (51/56)† | 3.3 y | Lower WBC, less often FAB M5 | Baldus et al20 |
| OS: significantly shorter for high BAALC pts (median, 1.7 vs 5.8 y; P = .02) | Yes | 86 (43/43) | |||||
| EFS: significantly shorter for high BAALC pts (median, 0.8 vs 4.9 y; P = .03) | Yes | 86 (43/43) | |||||
| DFS: significantly shorter for high BAALC pts (median, 1.4 vs 7.3 y; P = .03) | Yes | 68 (33/35) | |||||
| CR rate: no significant difference (82% vs 91%) | — | De novo (84%), secondary (16%) | 67 (44/23) | 18-71 (46/53)† | 38 mo | Higher CD34; lower CD11b, CD15, and MPO‡ | Bienz et al46 |
| OS: significantly shorter for high BAALC pts (median, 10 vs 21 mo; P = .021) | Yes | 67 (44/23) | |||||
| DFS: significantly shorter for high BAALC (median, 8.5 vs 21 mo; P = .015) | Yes | 57 (36/21) | |||||
| CR rate: significantly lower for high BAALC pts (62% vs 73%; P = .038) | No | De novo (91%), secondary (9%) | 307 (153/154) | 17-60 (46/50)† | 29 mo | Higher percentage of PB blasts, more FAB M0/M1, less FAB M5b, less gingival hyperplasia | Baldus et al59 |
| Primary resistant disease rate: significantly higher for high BAALC pts (16% vs 6%; P = .006) | Yes | 307 (153/154) | |||||
| OS: significantly shorter for high BAALC pts (3-year OS rates, 36% vs 54%; P = .001) | Yes | 307 (153/154) | |||||
| RR: significantly higher for high BAALC pts (43% vs 29%; P = .042) | ND | 208 (95/113) | |||||
| CIR: significantly higher for high BAALC pts (3-year CIR rates, 50% vs 32%; P = .018) | Yes | 208 (95/113) | |||||
| BAALC high expression/FLT3 high risk versus BAALC high expression/FLT3 low risk versus BAALC low expression/FLT3 high risk versus BAALC low expression/FLT3 low risk | |||||||
| CR rate: the lowest for BAALC high/FLT3 high-risk pts (57% vs 58% vs 83% vs 73%; P = .048) | ND | De novo and secondary | 268 (21/110/12/125)§ | 17-60 | 29 mo | NR | Baldus et al59 |
| OS: the worst for BAALC high/FLT3 high-risk pts (3-year OS rates, 0% vs 38% vs 22% vs 58%; P < .001) | ND | 268 (21/110/12/125)§ | |||||
| CIR: the highest for BAALC high/FLT3 high-risk pts (3-year CIR rates, 100% vs 44% vs 60% vs 28%; P < .001) | ND | 177 (12/64/10/91)§ | |||||
| ERG high expression versus ERG low expression | |||||||
| CR rate: no significant difference (76% vs 83%) | — | De novo | 48 (21/63) | 18-59 (48/47)‖ | 5.7 y | Higher percentage of PB and BM blasts, more often high BAALC expression | Marcucci et al16 |
| OS: significantly shorter for high ERG pts (median, 1.2 y vs not reached; P = .011) | Yes | 84 (21/63) | |||||
| CIR: significantly higher for high ERG pts (median, 0.7 y vs not reached, P < .001) | Yes | 68 (16/52) | |||||
| Wild-type FLT3 high expression versus wild-type FLT3 low expression | |||||||
| OS: a trend towards worse OS for high FLT3 pts (P = .059) | ND | De novo and secondary | 49 (21/28) | 17-84 (61)¶ | NR | NR | Kuchenbauer et al66 |
| EFS: a trend towards worse EFS for high FLT3 pts (P = .087) | ND | 50 (21/29) | |||||
| MN1 high expression versus MN1 low expression | |||||||
| CR rate: no significant difference (80 vs 89%) | — | De novo (87%), secondary (13%) | 142 (71/71) | 18-60 (46/45)# | 30 mo | Less often mutated NPM1, less often NPM1 mutated/FLT3-ITD absent, more often high CD34 expression | Heuser et al15 |
| OS: significantly shorter for high MN1 pts (3-year OS rates, 38.1% vs 58.8%; P = .03) | Yes | 142 (71/71) | |||||
| RFS: significantly shorter for high MN1 pts (3-year RFS rates, 23.4% vs. 51.4%; P = .002) | Yes | 120 (57/63) | |||||
| RR: significantly higher for high MN1 pts (56.1% vs 36.5%; P = .03) | — | 120 (57/63) |
MVA indicates multivariable analysis; pts, patients; CR, complete remission; OS, overall survival; EFS, event-free survival; DFS, disease-free survival; RR, risk of relapse; CIR, cumulative incidence of relapse; FLT3 high risk, patients with a high (ie, more than 0.8) FLT3-ITD/FLT3-wild-type allele ratio; FLT3 low risk, patients with FLT3 wild-type alleles or a low (ie, equal to or less than 0.8) FLT3-ITD/FLT3-wild-type allele ratio; RFS, relapse-free survival; —, no significant difference in univariable analysis (MVA not performed); WBC, white blood cell count; FAB, French-American-British; MPO, myeloperoxidase; ND, not done; PB, blood; NR, not reported; and BM; bone marrow.
Numbers of patients for whom clinical data were available.
Median age for patients with high/patients with low BAALC expression.
Differences in pretreatment features significant for comparisons between patients with high (n = 23) and low (n = 15) BAALC expression when patients with FLT3-ITD and CEBPA mutations were excluded.
Number of patients with BAALC high expression/FLT3 high-risk/patients with BAALC high expression/FLT3 low-risk/patients with BAALC low expression/FLT3 high-risk/patients with BAALC low expression/FLT3 low-risk.
Median age for patients with high/patients with low ERG expression.
Age range and median reported for all patients, both with normal and abnormal karyotype.
Mean age for patients with high/patients with low MN1 expression.
In a second study of CN patients,46 who were also analyzed for the presence of FLT3-ITD and CEBPA mutations, high BAALC expression, measured in marrow in relation to the median value of BAALC expression in marrow from healthy volunteers, predicted for a shorter DFS and OS. A subgroup analysis, restricted to patients without FLT3-ITD or CEBPA mutations, also showed high BAALC expression to be associated with worse DFS and OS.46 Bienz et al46 concluded that BAALC expression appears to be particularly useful as a prognostic marker in CN AML patients lacking FLT3-ITD and CEBPA mutations.
In the largest study to date, comprising 307 CN adults aged 60 years or younger, high expression of BAALC in blood was an independent adverse prognostic factor for resistance to initial induction chemotherapy, CIR, and OS.59 On multivariable analysis, high BAALC expression and high FLT3-ITD/FLT3–wild-type ratio were the only factors predicting a high CIR and shorter OS. Notably, preliminary data from the same study also suggested that allogeneic SCT in first CR might overcome the higher relapse rate associated with high BAALC expression compared with ASCT or chemotherapy.59 Future studies confirming this are required.
Mutations of the CCAAT/enhancer-binding protein α (CEBPA) gene
Mutations of CEBPA, a gene encoding a protein member of the family of basic region leucine zipper (bZIP) transcription factors that plays an essential role in granulopoiesis, were discovered in AML in 2001 and associated with FAB M2 and M1 morphology and a normal karyotype at diagnosis.11 There are 2 main categories of CEBPA mutations: C-terminal mutations that occur in the bZIP domain, which are usually in-frame and predict mutant proteins lacking DNA binding and/or homodimerization activities; and N-terminal nonsense mutations that prevent expression of the full-length protein and result in truncated isoforms with dominant-negative activity. Some patients present with biallelic mutations at the C-terminus, whereas others are heterozygous for separate mutations or found to have a C-terminal mutation coexisting with a mutation in the N-terminus.19,90
The initial clinical studies revealed that CEBPA mutations were associated with a significantly better EFS,90,91 DFS,91 and OS90,91 among patients included in the cytogenetic intermediate-risk category. This favorable prognostic significance of CEBPA mutations was subsequently confirmed by investigations restricted to CN patients (Table 7), 15% to 19% of whom carry CEBPA mutations.19,37,46 At diagnosis, patients with CEBPA mutations had higher percentages of blood blasts, lower platelet counts, less lymphadenopathy and extramedullary involvement, less frequent FLT3-ITD and FLT3-TKD, and no MLL-PTD.19 CR rates did not differ between patients with and without CEBPA mutations,19,37,46 whereas CRD,19 DFS,46 EFS,37 and OS19,46 were significantly better for patients with CEBPA mutations. In multivariable analyses, CEBPA mutational status has added prognostic information to that provided by MLL-PTD and FLT3-ITD status, age, and resistant disease after the first course of induction therapy with regard to CRD and by FLT3-ITD status, age, and WBC for OS.19 Similar results were obtained in another study, where CEBPA mutations, BAALC, and FLT3-ITD status were prognostic for DFS, and CEBPA mutations, age, BAALC, and FLT3-ITD status were prognostic for OS.46
Studies reporting clinical outcome of karyotypically normal AML patients according to the CEBPA mutational status
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | References . |
|---|---|---|---|---|---|---|---|
| CEBPA+ versus CEBPA− | |||||||
| Rates of CR, resistant disease, and early or hypoplastic death: no significant differences | — | De novo and secondary | 236 (36/200) | 16-60 (47/47)† | 30 mo | Higher hemoglobin level, lower platelet counts, higher percentage of PB blasts, less lymphadenopathy, less extramedullary involvement | Fröhling et al19 |
| OS: significantly longer for CEBPA+ pts (median, not reached vs 19 mo; P = .05) | Yes | 236 (36/200) | |||||
| CRD: significantly longer for CEBPA+ pts (median, not reached vs 26 mo; P = .01) | Yes | 187 (31/156) | |||||
| CR rate: no significant difference (100% vs 82%) | — | De novo (84%), secondary (16%) | 67 (12/55) | 18-71 (46/49)† | 38 mo | Lower LDH, higher CD7 | Bienz et al46 |
| OS: significantly longer for CEBPA+ pts (median, 45.5 vs 12 mo; P < .001) | Yes | 67 (12/55) | |||||
| DFS: significantly longer for CEBPA+ pts (median, 33.5 vs 10 mo; P = .002) | Yes | 57 (12/45) | |||||
| CR rate: no significant difference | — | De novo | 106 (20/86) | 17-65 (44) | NR | NR | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 53% vs 38%) | — | 106 (20/86) | |||||
| EFS: significantly longer for CEBPA+ pts (P = .01) | No | 106 (20/86) | |||||
| CEBPA N-terminal nonsense mutations versus other CEBPA mutations versus CEBPA wild-type | |||||||
| CRD: the longest for pts with CEBPA N-terminal nonsense mutations, the shortest for pts with CEBPA-WT (median, not reached vs 52 mo vs 26 mo; P = .04) | ND | De novo (88%), secondary (12%) | 187 | 16-60 | 30 mo | FAB M1/M2 more common in pts with N-terminal mutations than in those with other mutations | Fröhling et al19 |
| Prognostic significance . | Independent prognostic factor on MVA . | AML type . | No. of pts (no. with/no. without alteration)* . | Age range, y (median) . | Median follow-up . | Differences in pretreatment features . | References . |
|---|---|---|---|---|---|---|---|
| CEBPA+ versus CEBPA− | |||||||
| Rates of CR, resistant disease, and early or hypoplastic death: no significant differences | — | De novo and secondary | 236 (36/200) | 16-60 (47/47)† | 30 mo | Higher hemoglobin level, lower platelet counts, higher percentage of PB blasts, less lymphadenopathy, less extramedullary involvement | Fröhling et al19 |
| OS: significantly longer for CEBPA+ pts (median, not reached vs 19 mo; P = .05) | Yes | 236 (36/200) | |||||
| CRD: significantly longer for CEBPA+ pts (median, not reached vs 26 mo; P = .01) | Yes | 187 (31/156) | |||||
| CR rate: no significant difference (100% vs 82%) | — | De novo (84%), secondary (16%) | 67 (12/55) | 18-71 (46/49)† | 38 mo | Lower LDH, higher CD7 | Bienz et al46 |
| OS: significantly longer for CEBPA+ pts (median, 45.5 vs 12 mo; P < .001) | Yes | 67 (12/55) | |||||
| DFS: significantly longer for CEBPA+ pts (median, 33.5 vs 10 mo; P = .002) | Yes | 57 (12/45) | |||||
| CR rate: no significant difference | — | De novo | 106 (20/86) | 17-65 (44) | NR | NR | Boissel et al37 |
| OS: no significant difference (6-year OS rates, 53% vs 38%) | — | 106 (20/86) | |||||
| EFS: significantly longer for CEBPA+ pts (P = .01) | No | 106 (20/86) | |||||
| CEBPA N-terminal nonsense mutations versus other CEBPA mutations versus CEBPA wild-type | |||||||
| CRD: the longest for pts with CEBPA N-terminal nonsense mutations, the shortest for pts with CEBPA-WT (median, not reached vs 52 mo vs 26 mo; P = .04) | ND | De novo (88%), secondary (12%) | 187 | 16-60 | 30 mo | FAB M1/M2 more common in pts with N-terminal mutations than in those with other mutations | Fröhling et al19 |
MVA indicates multivariable analysis; pts, patients; CEBPA+, patients with mutations of the CEBPA gene; CEBPA−, patients without mutations of the CEBPA gene; CR, complete remission; OS, overall survival; CRD, CR duration; DFS, disease-free survival; EFS, event-free survival; —, no significant difference in univariable analysis (MVA not performed); PB, blood; LDH, serum lactate dehydrogenase level; NR, not reported; ND, not done; and FAB, French-American-British.
Numbers of patients for whom clinical data were available.
Median age for patients with CEBPA mutations/patients without CEBPA mutations.
Fröhling et al19 also analyzed clinical outcome separately for patients with N-terminal nonsense CEBPA mutations, those with other CEBPA mutation types, and those with wild-type CEBPA and found that the CRD of patients with N-terminal mutations was the longest, followed by CRD of patients with other mutations and of those without CEBPA mutations. However, there were no significant differences in CRD between patients with N-terminal mutations and those with other mutations, or between patients with other mutations and patients with wild-type CEBPA in pairwise comparisons.19 A larger study is needed to assess the prognostic role of different types of CEBPA mutations.
Interestingly, 3 patients with familial AML and a deletion of a cytosine residue at nucleotide 212 of the CEBPA gene, 2 of whom had a normal karyotype, also had an excellent prognosis.92
Overexpression of the ETS-related gene (ERG)
Overexpression of the ERG gene, a member of the ETS family of transcription factors that are involved in regulation of cell proliferation, differentiation, and apoptosis, was discovered in AML patients with prognostically unfavorable complex karyotypes that contained cryptic amplification of chromosome 21, first uncovered by spectral karyotyping.93,94 ERG overexpression was not always associated with genomic amplification and was also detected in CN AML,94 suggesting that it might bestow an adverse prognosis in CN AML patients.
Indeed, adults younger than 60 years with de novo AML, treated on CALGB 9621, whose high ERG expression in pretreatment blood placed them in the uppermost quartile of ERG expression, had a significantly worse CIR and OS than patients in the 3 quartiles with lower ERG expression.16 In multivariable analyses, high ERG expression and FLT3-ITD independently predicted worse CIR and OS. Analysis restricted to the more favorable subset of patients lacking FLT3-ITD or expressing both FLT3-ITD and the FLT3 wild-type allele revealed that high ERG expression and MLL-PTD both impacted on remission duration. With respect to OS, there was an interaction between ERG and BAALC expression, with ERG overexpression predicting shorter OS only in low-BAALC expressers.16 These results await independent corroboration.
Other prognostic factors in karyotypically normal AML
Although the majority of CN patients studied to date harbored at least 1 of the aforementioned genetic alterations, in a study by Döhner et al79 almost a quarter of patients did not carry FLT3-ITD, FLT3-TKD, MLL-PTD, or mutations in the CEBPA or NPM1 genes (Figure 1). Thus, it is likely that novel gene mutations and/or abnormal gene expression with prognostic significance will be discovered in the future. Expression of the meningioma 1 (MN1) gene might become such a novel prognostic factor. Heuser et al15 have reported high expression of the MN1 gene to bestow inferior RFS and OS, and a higher risk of relapse, in CN adults aged 60 years or younger with de novo or secondary AML. This observation requires confirmation.
Pie chart based on 246 patients analyzed for the presence or absence of mutations in the NPM1 and CEBPA genes, FLT3-ITD, FLT3-TKD, and MLL-PTD indicating the coexistence of mutations in individual patients. WT indicates patients with only wild-type alleles of genes tested. Adapted by Kenneth X. Probst from Döhner et al79 with permission.
Pie chart based on 246 patients analyzed for the presence or absence of mutations in the NPM1 and CEBPA genes, FLT3-ITD, FLT3-TKD, and MLL-PTD indicating the coexistence of mutations in individual patients. WT indicates patients with only wild-type alleles of genes tested. Adapted by Kenneth X. Probst from Döhner et al79 with permission.
A recent study found a significantly shortened CRD of CN AML patients associated with overexpression of breast cancer resistance protein (BCRP) encoded by the ABCG2 gene.95 Interestingly, in another study that used gene-expression profiling to classify adult AML, overexpression of the ABCG2 gene was a consistent finding in a cluster of patients with the worst DFS and high induction failure rate, 42% of whom had a normal karyotype.96 Additionally, it will be of interest to determine if high expression of the EVI1 gene, hitherto reported to bestow adverse prognosis in patients classified in the intermediate-risk cytogenetic group,97 or overexpression of the WT1 gene, shown to impact unfavorably on clinical outcome of cytogenetically heterogeneous AML patients in some98 but not all99 studies, also confers adverse prognosis in CN AML. Likewise, although a recent study revealed no difference in outcome between CN patients with and without NRAS mutations,6 further studies of NRAS, KRAS, and KIT mutations seem warranted given the prospect of developing therapies with tyrosine kinase inhibitors targeting these mutations. Finally, future analyses should address potential prognostic relevance in CN AML of epigenetic changes that in some studies have already been correlated with clinical outcome in adult AML.100-103
Gene-expression profiling
Relatively little information on the clinical utility of gene-expression profiling in AML is currently available. However, this methodology, capable of determining expression levels of thousands of genes in 1 experiment, was recently used to ascertain patterns of gene activation and silencing (ie, gene-expression signatures) in CN AML with the goal of identifying patient subsets with diverse prognoses. Bullinger et al14 found that the majority of CN patients segregated into 2 gene-expression clusters with significantly different survival. Patients in cluster I, who had worse survival, more frequently harbored FLT3 aberrations and were more commonly diagnosed with AML FAB M1 and M2 and less frequently with AML FAB M4 and M5. Although these results suggested that gene-expression profiling might become useful as a tool in outcome prediction, they required confirmation, especially because in other studies CN patients segregated into several distinct clusters.96,104 A recent CALGB study22 has validated the prognostic significance of the gene-expression signature identified by Bullinger et al14 using a different microarray platform in a patient population treated on a different protocol and having longer follow-up (4.7 versus < 2 years). Moreover, this study increased the clinical applicability of the signature because Radmacher et al22 created a class-prediction algorithm that allows for the prediction of outcome for subsequent individual patients. This signature-based classifier identified groups with differences in DFS and OS.22 However, a strong association of the outcome classifier with the FLT3-ITD was observed that might explain the prognostic significance of the signature. Moreover, the prediction accuracy of the classifier for dichotomized outcome classes was modest, with OS and DFS of approximately 60% of the patients being correctly predicted. Of potential importance is the observation that the classifier showed some ability to identify a subset of patients with wild-type FLT3 who fare poorly.22 Future studies should refine this strategy and aim at identifying different classifiers that might predict outcome for individual CN AML patients with greater accuracy.
Conclusions and future directions
It is now clear that CN AML is very heterogeneous at the molecular level. We believe that upcoming modification to the World Health Organization (WHO) classification of AML should consider recurrent molecular genetic rearrangements discussed in this review. Moreover, since 2 or more genetic alterations are present simultaneously in many patients, it is important to devise a prioritized schema that stratifies patients to risk-adapted therapies using information on all known prognostic markers. While many questions still remain, we believe that the available data, discussed in this article, allow us to propose as a starting point for future clinical studies the molecular-risk classification, depicted in Figure 2. Based on the current evidence showing that CN patients with FLT3-ITD, and especially those with high FLT3-ITD/FLT3–wild-type ratios, have a particularly poor prognosis, which is only modestly influenced by the concomitant presence of other molecular markers tested so far, these patients should be considered as candidates for clinical trials such as those investigating compounds that directly inhibit FLT3 tyrosine kinase activity or that might facilitate ubiquitination and disposal of the mutant protein (ie, inhibitors of the chaperone activity of heat-shock protein such as 17 AAG) in combination with chemotherapy. Additionally, these patients might be considered as candidates for allogeneic SCT, although a definitive role for this treatment in first CR remains to be established.105 Likewise, aggressive treatment is recommended for patients with high BAALC expression and MLL-PTD; patients in the latter group could also potentially benefit from enrollment in clinical trials testing efficacy of histone deacetylase and/or DNA methyltransferase inhibitors.88 For patients without these molecular markers, the situation is less clear. FLT3-ITD–negative patients who harbor mutations in the NPM1 and/or CEBPA genes and have low BAALC and ERG expression appear to have a relatively favorable outcome and are likely to benefit from ASCT or HDAC-based therapy. On the other hand, no or little information is available on prognosis of patients harboring multiple mutations and/or gene overexpression with contradictory influence on clinical outcome (eg, concurrent presence of CEBPA and/or NPM1 mutation and high BAALC and/or ERG expression). Studies that would test all currently known molecular alterations concurrently to determine the relative impact of each of them are underway. In addition, while the recent results of gene-expression profiling are promising, it remains to be determined whether microarray technology will become more useful clinically and thus be able to replace testing for several individual genetic alterations with prognostic significance in CN AML patients.
Proposed schema assigning AML patients with a normal karyotype to risk-adapted postremission therapies using information on currently known prognostic markers as a basis for designing future clinical studies. SCT denotes stem-cell transplantation; ASCT, autologous SCT; and HDAC, high-dose cytarabine. Illustration drawn by Kenneth X. Probst.
Proposed schema assigning AML patients with a normal karyotype to risk-adapted postremission therapies using information on currently known prognostic markers as a basis for designing future clinical studies. SCT denotes stem-cell transplantation; ASCT, autologous SCT; and HDAC, high-dose cytarabine. Illustration drawn by Kenneth X. Probst.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: All authors participated in writing this article and approved its final version.
Acknowledgments
This work was supported in part by National Cancer Institute (Bethesda, MD) grants CA77658, CA101140, CA31946, CA102031, and CA16058 and The Coleman Leukemia Research Foundation.

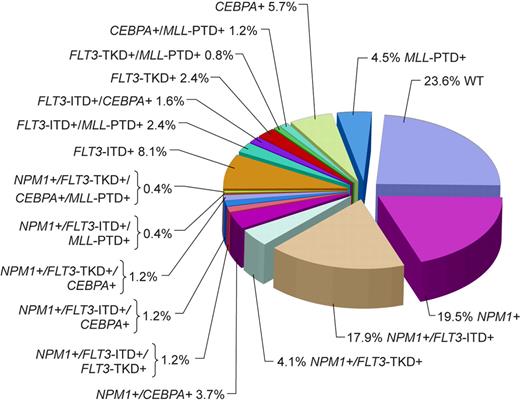
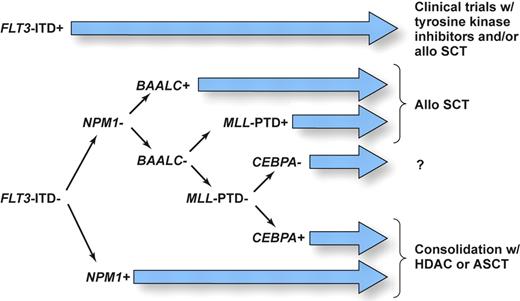
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal