Abstract
By generating IgM and IgA switch variants of the 34-3C IgG2a anti–red blood cell (RBC) autoantibody, we evaluated the pathogenic activity of these 2 isotypes in view of the Fc-associated effector functions (ie, complement activation and polyvalency-dependent agglutination). We found that polymeric forms of 34-3C IgM and IgA anti-RBC autoantibody were as pathogenic as IgG2a, which was the most pathogenic among 4 different IgG subclasses, whereas their monomeric variants completely lacked pathogenic effects. Histological examination showed that 34-3C IgM and IgA autoantibodies caused anemia as a result of multivalency-dependent hemaggultination and subsequent sequestration of RBC in the spleen, in contrast to Fc receptor– and complement receptor–mediated erythrophagocytosis by Kupffer cells with IgG isotypes. In addition, the development of anemia induced by IgM and IgA isotypes of 34-3C antibody and by 2 additional IgM anti-RBC monoclonal autoantibodies was not inhibited at all in C3-deficient mice, indicating the lack of involvement of complement activation in the pathogenesis of IgM- and IgA-induced anemia. Our data demonstrate a remarkably high pathogenic potential of polymeric forms of IgM and IgA anti-RBC autoantibodies due to their ability to induce hemagglutination but completely independent of complement activation.
Introduction
NZB mice spontaneously develop autoimmune hemolytic anemia as a result of the production of IgG anti–red blood cell (RBC) autoantibodies and have been widely used to define the immunological mechanisms underlying this disease.1,2 Although the molecular nature of the RBC autoantigens responsible for the induction of this autoimmune response has not been well characterized, it is clear that IgG anti-RBC autoantibodies specific for exposed surface determinants of intact RBC are of primary importance in the development of autoimmune hemolytic anemia.3
The analysis of different monoclonal autoantibodies derived from NZB mice has shown that the IgG Fc region plays a critical role in the pathogenicity of anti-RBC autoantibodies by activating IgG Fc receptor (FcγR)-bearing effector cells as well as the complement cascade.4–8 Indeed, the analysis of IgG class-switch variants of anti-RBC monoclonal autoantibodies has demonstrated the remarkably different pathogenic potential of 4 IgG subclasses as a function of their respective capacities to interact with FcγR and to activate complement in vivo.6–8 Histological analysis of mice deficient in FcγR or C3 revealed that FcγR- and/or complement receptor (CR)-mediated erythrophagocytosis is the mechanism responsible for the development of anemia induced by IgG anti-RBC monoclonal antibodies (mAb). In contrast to IgG anti-RBC autoantibodies, the IgM class of anti-RBC autoantibodies induced anemia in association with massive agglutination of RBC in the spleen and liver but was unable to trigger FcR-mediated erythrophagocytosis.6,9 Although the IgM isotype is known to be highly efficient in activating complement, the possible role of CR-mediated erythrophagocytosis and complement-mediated intravascular hemolysis in the development of anemia induced by IgM anti-RBC mAb has not yet been well defined.
Among the different isotypes of immunoglobulin, IgA represents the second most abundant isotype present in sera. Although secretory IgA, the major contributor to mucosal immunity, is secreted as dimers, serum distribution of polymeric and monomeric forms of IgA varies from species to species. For reasons that remain unclear, approximately 90% of serum IgA is monomeric in human but dimeric in mice.10 It has been shown, however, that the majority of pathogen-specific serum IgA induced after vaccination or natural infection is present as the polymeric form in humans.11 It should also be stressed that IgA and IgM antibodies play a role in host defense distinct from that of IgG. Indeed, polymeric IgA and IgM can be secreted through polymeric Ig receptors at mucosal surfaces,12 and macrophages express a receptor specific for both IgA and IgM Fc fragments (Fcα/μR), which is involved in endocytosis of IgA- and IgM-opsonized microorganisms.13 However, it is not clear whether this receptor is able to trigger phagocytosis.
Spontaneous production of IgA anti-RBC autoantibodies has also been described in NZB mice.14 Because the avidity of antigen binding of polymeric IgA is expected to be higher than that of monomeric IgA, and because it is still controversial whether the IgA isotype is able to efficiently activate complement in vivo, it is of interest to determine the pathogenic potential of polymeric and monomeric IgA and their capacity to activate complement in vivo. To better define the pathogenic activity of IgM and IgA autoantibodies in relation to their Fc-associated effector functions in the development of autoimmune hemolytic anemia, we have generated IgM and IgA class-switch variants of the 34-3C IgG2a anti-RBC mAb derived from NZB mice.9 Their pathogenic effect was then assessed in mice deficient in C3 or the common FcR γ-chains (FcRγ). We show in the present study that: 1) polymeric forms of both IgM and IgA isotypes are highly pathogenic and induce anemia as a result of sequestration of agglutinated RBC in the spleen, whereas their monomeric forms induce neither hemagglutination nor anemia; 2) polymeric IgM anti-RBC mAb efficiently activate complement in vivo, but this activation hardly plays a role in the development of IgM anti-RBC-induced anemia; and 3) both polymeric and monomeric IgA anti-RBC mAb fail to activate complement in vivo.
Materials and methods
Mice
BALB/c and C57BL/6 (B6) mice were purchased from Gl. Bomholtgard Ltd, Ry, Denmark. C3-deficient mice, provided by Dr M. Carroll, Harvard Medical School, Boston, MA,15 were backcrossed for 6 generations with B6 mice. Mice deficient in FcRγ (ie, lacking functional expression of activating FcγRI, FcγRIII, and FcγRIV)16 with a pure B6 background were provided by Dr T. Saito, RIKEN Research Center for Allergy and Immunology, Yokohama, Japan.17
DNA constructions
The VDJ34-3C-Cμ and -Cα plasmids containing the complete 34-3C heavy-chain gene of IgM or IgA class were constructed using the following DNA fragments: the rearranged variable-diversity-joining (VDJ) region isolated from cDNA encoding the variable region of the heavy chain of the 34-3C mAb, the promoter region isolated from pSV-Vμ1,18 the heavy-chain enhancer region isolated from pSVE2-neo,19 and the Cμ or Cα region derived from the respective genomic clones, pMO-μ4C8 or pIgα-8.20,21 The VDJ34-3C-Cμ(S414/575) plasmid containing the mutations at position 414 and 575 (cysteine to serine) was previously described.22 The VDJ34-3C-Cα(S471) plasmid containing a mutation at position 471 (cysteine to serine) was generated by oligonucleotide-directed mutagenesis, as described in Kuroki et al.23
Monoclonal antibodies
The hybridoma secreting 34-3C IgG2a, 4C8 IgM, or 1E10 IgM anti-RBC monoclonal autoantibodies was derived from unmanipulated NZB mice.9,24 The polymeric forms of 34-3C IgM and IgA class-switch variants were obtained by transfecting 34-3C heavy-chain-loss mutant cells with VDJ34-3C-Cμ or VDJ34-3C-Cα plasmid together with a pSVE2-neo plasmid containing the neomycin-resistant gene, as previously described.6 Their monomeric variants were obtained with the use of VDJ34-3C-Cμ(S414/575) or VDJ34-3C-Cα(S471) plasmid, because both cysteines at positions 414 and 575 are necessary for efficient assembly of polymeric forms of IgM but not for monomers,22 and because cysteine at position 471 is critical for IgA dimerization.25 IgM and IgA mAb in culture supernatants were concentrated by ammonium sulfate precipitation and used without further purification in the present study. Concentrations of polymeric IgM and IgA were determined by IgM and IgA-specific enzyme-linked immunosorbent assay using a standard curve established with affinity-purified mouse IgM or IgA (eBioscience, San Diego, CA). Concentrations of monomeric IgM and IgA variants were estimated by assessing their RBC-binding activities in vitro in comparison with that of IgG2a isotype, purified by protein A column chromatography, by flow cytometric analysis using a biotinylated rat anti-mouse k-chain mAb (H139.52.1.5) followed by phycoerythrin (PE)-conjugated streptavidin as previously described.6,9
Gel filtration
The size distribution of 34-3C IgM and IgA mAb was analyzed by using a Sephadex G200 gel filtration column (Pharmacia, Uppsala, Sweden) equilibrated with 50 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, and elution was performed using the same buffer at a flow rate of 0.2 mL/min at 4°C. The column was calibrated with blue dextran (void volume), IgG (150 kD), and bovine serum albumin (67 kD). Concentrations of IgM and IgA in each fraction were estimated by IgM and IgA-specific enzyme-linked immunosorbent assay using standard curves established with polymeric and monomeric forms of 34-3C IgM and IgA.
Experimental autoimmune hemolytic anemia
Autoimmune hemolytic anemia was induced by a single intraperitoneal or intravenous injection of anti-RBC mAb into 2- to 3-month-old mice. The injection of mAb was controlled 24 h later by assessing the level of antibody opsonization of circulating RBC by a flow cytometric analysis using biotinylated rat anti-mouse k-chain mAb or goat anti-mouse C3 (Cappel Laboratories, Durham, NC) followed by PE-conjugated streptavidin as previously described.6 Blood samples were collected into heparinized microhematocrit tubes every 2 days after the injection and hematocrits (Ht) were directly determined after centrifugation as previously described.9 Livers and kidneys were obtained 2 or 8 days after injection of mAb, processed for histological examination, and stained with hematoxylin and eosin. The extent of in vivo RBC destruction by Kupffer cell–mediated phagocytosis was determined by Perls iron staining. Light microscopy images were visualized with an Optiphot microscope (Nikon Corp., Tokyo, Japan) equipped with a 10× eyepiece, a Plan 4×/0.13objective lens, and a Plan 20×/0.50 objective lens. Images were captured with a Nikon COOLPIX 4500 camera (Nikon Corp., Tokyo, Japan) and were processed using Adobe Photoshop software (Adobe Systems, San Jose, CA). In some experiments, 107 transfectoma cells were injected intraperitoneally into pristane-treated mice. Plasma levels of C3 were determined by enzyme-linked immunosorbent assay as described.8
Complement-mediated lysis assay
Fifty microliters of 2% mouse RBC suspension were incubated with 50 μL of serial dilutions of mAb, starting at a concentration of 10 μg/mL, at room temperature for 60 minutes in round-bottomed microtiter plates as previously described.6,9 After washing with Hanks medium, 50 μL of 10% rabbit serum was added as a source of complement, and the suspensions were incubated at 37°C. After 30 minutes, hemolysis was determined macroscopically.
Measurement of hemoglobin in plasma and urine
To determine plasma levels of hemoglobin as an indicator of intravascular hemolysis,26 freshly prepared plasma samples collected into EDTA-containing tubes were diluted 1:10 in 0.942 M Na2CO3 and absorbance was measured at 380 nm, 415 nm, and 450 nm in a spectrophotometer. The hemoglobin concentration (g/L) was calculated as 1.65 × A415 − 0.93 × A380 − 0.73 × A450, according to the method of Harboe.27 Urine samples collected from urinary bladder at autopsy were diluted 1:10, and the absorbance at 415 nm was measured as an index of hemoglobin content as described by Holt et al.26
Hemagglutination assay
A one-step hemagglutination assay was performed as follows: Fifty μL of serial dilutions of culture supernatants, starting at a concentration of 1 μg/mL, were mixed with 50 μL of 0.1% mouse RBC suspension in 1% bovine serum albumin–phosphate buffered saline (BSA-PBS) in round-bottomed microtiter plates and incubated at room temperature for 1 hour.
Statistical analysis
Statistical analysis was performed with the Wilcoxon 2-sample test. Probability values less than 5% were considered significant.
Results
High pathogenic activity of the 34-3C IgM and IgA class-switch variants
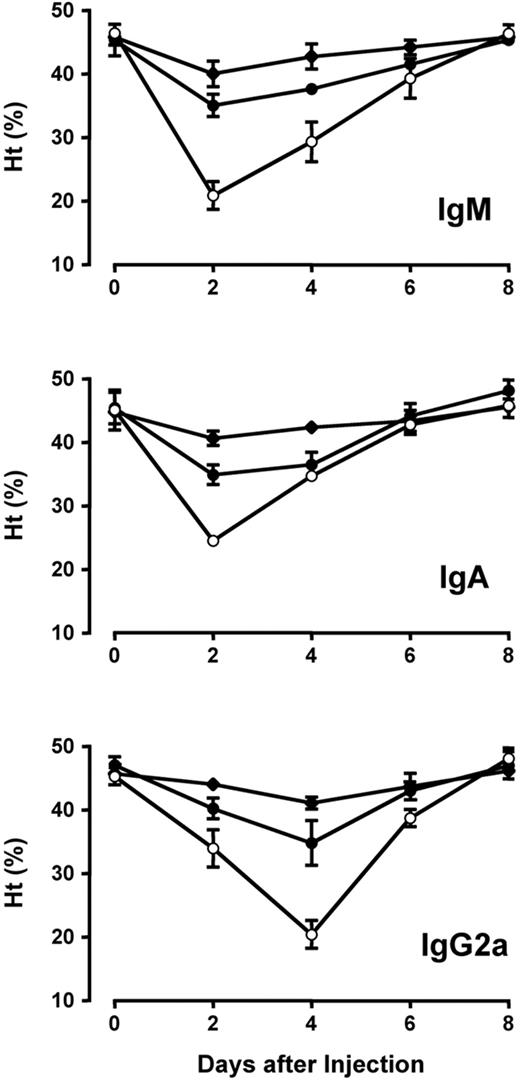
The pathogenic activity of 34-3C IgM and IgA anti-RBC mAb was analyzed by a single injection of 100 μg of polymeric forms of these mAb into BALB/c mice. Both IgM and IgA isotypes of the 34-3C mAb induced a severe form of anemia as documented by a marked decrease in mean hematocrit (Ht) values peaking at day 2 (Figure 1). The evolution in Ht values in mice receiving IgM and IgA anti-RBC mAb was different from that in mice injected with IgG anti-RBC mAb, which induced maximal drops of Ht values peaking at day 4.
Development of anemia induced by the 34-3C IgM, IgA, and IgG2a variants in BALB/c mice. Mice were injected intraperitoneally with 10 (◆), 25 (•), or 100 (○) μg of 34-3C variants on day 0. Results are expressed as mean Ht values (±SD) of 3 mice. Decreases in Ht values 2 and 4 days after injection of 34-3C IgM, IgA, or IgG2a, as compared with Ht values at day 0, were significant for all 3 doses tested (P < 0.05).
Development of anemia induced by the 34-3C IgM, IgA, and IgG2a variants in BALB/c mice. Mice were injected intraperitoneally with 10 (◆), 25 (•), or 100 (○) μg of 34-3C variants on day 0. Results are expressed as mean Ht values (±SD) of 3 mice. Decreases in Ht values 2 and 4 days after injection of 34-3C IgM, IgA, or IgG2a, as compared with Ht values at day 0, were significant for all 3 doses tested (P < 0.05).
To compare more quantitatively the pathogenic activity of IgM and IgA isotypes of the 34-3C mAb, we estimated minimal quantities of polymeric forms of respective mAb required to induce anemia (defined as Ht values <40%). Anemia was provoked by the injection of 25 μg of the 34-3C IgM and IgA mAb, the dose of which was comparable to that of 34-3C IgG2a mAb (Figure 1).
Activation of complement by polymeric IgM, but not IgA, class-switch variants of the 34-3C anti-red blood cell monoclonal antibodies
To assess the ability of IgM and IgA variants of the 34-3C anti-RBC mAb to activate complement in vivo, we analyzed by flow cytometry the extent of C3 deposition on circulating RBC 24 h after a single intraperitoneal injection into BALB/c mice of 100 μg of polymeric forms of IgM or IgA variant in comparison with the IgG2a isotype. Substantial C3 deposition was observed in mice injected with the IgM isotype at levels similar to that obtained with the IgG2a isotype (Figure 2). In contrast, no significant C3 deposition was detectable in mice receiving polymeric IgA anti-RBC mAb. The lack of complement activation by the IgA isotype was further confirmed by the analysis of mice implanted with IgA anti-RBC-secreting transfectoma cells, in which excessive amounts of IgA anti-RBC mAb were secreted as documented by the presence of free IgA anti-RBC antibodies in sera (data not shown).
Flow cytometric analysis of complement activation in vivo by the 34-3C IgM, IgA, and IgG2a variants. Mouse RBC were obtained 24 h after an intraperitoneal injection of 100 μg of 34-3C anti-RBC Ig variants into BALB/c mice and stained with biotinylated goat anti-mouse C3 antibodies followed by PE-conjugated streptavidin. Shaded areas indicate the background staining obtained with untreated BALB/c mice.
Flow cytometric analysis of complement activation in vivo by the 34-3C IgM, IgA, and IgG2a variants. Mouse RBC were obtained 24 h after an intraperitoneal injection of 100 μg of 34-3C anti-RBC Ig variants into BALB/c mice and stained with biotinylated goat anti-mouse C3 antibodies followed by PE-conjugated streptavidin. Shaded areas indicate the background staining obtained with untreated BALB/c mice.
No role of complement and Fc receptors in autoimmune hemolytic anemia induced by the 34-3C IgM and IgA class-switch variants
Because the 34-3C IgM mAb efficiently activated complement in vivo, we evaluated the contribution of complement to the anemia induced by 2 different doses (25 and 100 μg) of the 34-3C IgM mAb in C3-deficient B6 mice. The development of anemia occurring in wild-type (WT) B6 mice after injection with the 34-3C IgM mAb was unchanged in C3−/− B6 mice (Table 1). The lack of differences in 34-3C IgM-induced anemia between WT and C3−/− mice could be attributable to a rapid consumption of complement early after injection, thereby masking the effect of genetic C3 deficiency on the development of anemia after 2 days. To address this question, 100 μg of 34-3C IgM mAb was intravenously injected, and plasma levels of C3 and hemoglobin were measured as an indicator of complement depletion and complement-mediated intravascular hemolysis, respectively, at different time points after injection (10 minutes, 1 hour, 6 hours, 24 hours, and 48 hours). Neither a significant reduction of C3 nor a measurable increase in hemoglobin was observed at any time points tested (data not shown). The absence of significant intravascular hemolysis after the injection of IgM anti-RBC mAb was further confirmed by the absence of hemoglobinuria tested at 48 h (data not shown). Notably, the lack of protective effect of C3 deficiency on the development of IgM-induced anemia was also confirmed by the analysis of 2 other IgM anti-RBC mAb, 4C8 and 1E10, derived from NZB mice.9,24 Although highly improbable in view of the absence of C3 opsonization observed before, we wanted to formally exclude a possible role of complement activation in IgA-induced anemia by assessing the development of anemia between C3-deficient and C3-sufficient B6 mice. As expected, no significant inhibition of the anemia development after injection of 100 μg of polymeric 34-3C IgA mAb was observed in C3−/−mice as compared with WT mice (Table 1). In contrast, the development of anemia occurring in WT mice after injection of 100 μg of 34-3C IgG2a mAb was significantly inhibited in C3−/−mice (P < .05). It should be noted that, when mouse RBC opsonized with 34-3C IgM or IgA were subjected to complement-mediated lysis in vitro in the presence of rabbit serum, no macroscopically visible hemolysis was observed, as is the case for 4C8 and 1E10 IgM mAb.9 Collectively, these data indicated a lack of contribution of complement to the development of autoimmune hemolytic anemia induced by IgM and IgA isotypes of anti-RBC autoantibodies.
Development of anemia in WT, C3−/−, and FcRγ−/− B6 mice injected with IgM, IgA, and IgG2a anti-RBC mAb
| mAb . | Dose . | WT* . | C3−/−* . | FcRγ−/−* . |
|---|---|---|---|---|
| 34-3C IgM | 25 μg | 30.1 ± 2.8 | 32.6 ± 1.5 | ND |
| 100 μg | 21.1 ± 5.8 | 22.4 ± 3.6 | ND | |
| 4C8 IgM | 100 μg | 24.9 ± 2.8 | 24.9 ± 3.1 | ND |
| 1E10 IgM | 100 μg | 19.8 ± 2.0 | 20.5 ± 0.5 | ND |
| 34-3C IgA | 100 μg | 25.4 ± 1.4 | 25.9 ± 3.7 | 24.7 ± 1.2 |
| 34-3C IgG2a | 100 μg | 20.4 ± 2.2† | 29.0 ± 3.2† | 37.1 ± 0.5† |
| mAb . | Dose . | WT* . | C3−/−* . | FcRγ−/−* . |
|---|---|---|---|---|
| 34-3C IgM | 25 μg | 30.1 ± 2.8 | 32.6 ± 1.5 | ND |
| 100 μg | 21.1 ± 5.8 | 22.4 ± 3.6 | ND | |
| 4C8 IgM | 100 μg | 24.9 ± 2.8 | 24.9 ± 3.1 | ND |
| 1E10 IgM | 100 μg | 19.8 ± 2.0 | 20.5 ± 0.5 | ND |
| 34-3C IgA | 100 μg | 25.4 ± 1.4 | 25.9 ± 3.7 | 24.7 ± 1.2 |
| 34-3C IgG2a | 100 μg | 20.4 ± 2.2† | 29.0 ± 3.2† | 37.1 ± 0.5† |
Ht values (means of 3 to 5 mice ± SD) 2 days after an intraperitoneal injection of IgM or IgA anti-RBC mAb or 4 days after injection of IgG2a anti-RBC mAb in WT, C3−/− or FcRγ−/− B6 mice. Ht values before injection of anti-RBC mAb in WT, C3−/− and FCRγ−/− mice were 44% to 48%.
Differences in Ht values among WT, C3−/−, and FcRγ−/− mice injected with 100 μg of 34-3C IgG2a mAb were significant (P < .05). ND indicates not determined.
In addition, we tested the development of IgA-induced anemia in B6 mice deficient in FcRγ, which is required for the cell surface expression and activation of IgA-specific FcR (FcαRI)28,29 if this receptor is expressed on murine phagocytes. Again, the development of anemia after injection of 100 μg of polymeric IgA anti-RBC mAb was unaffected in FcRγ−/− B6 mice, which contrasted with a marked inhibition of IgG2a anti-RBC-induced anemia in FcRγ−/− mice (P < .05) (Table 1). Thus, there was no role for a putative FcRγ-associated FcαRI in the development of IgA-mediated autoimmune hemolytic anemia in mice.
Role of hemagglutination in autoimmune hemolytic anemia induced by the 34-3C IgM and IgA class-switch variants
Histological examinations showed an enormous accumulation of agglutinated RBC in the spleen, rendering its architecture hardly recognizable, 2 days after injection of 100 μg of 34-3C IgM or IgA mAb (Figure 3A-B). In addition, mice recovering from anemia 8 days after injection of IgM mAb displayed focal necrotic lesions of hepatic parenchymal cells, which can be a consequence of microaggregates of RBC in liver (Figure 3E-F), although these lesions were not observed in IgA-injected mice. No signs of erythrophagocytosis were detectable as judged by the absence of iron deposits in Kupffer cells (Figure 3G-H), which contrasted with the marked erythrophagocytosis associated with anemia induced by IgG isotypes of 34-3C mAb (Figure 3I).8 The formation of a massive hemagglutination in mice receiving a high dose of 34-3C IgM mAb could interfere with CR-mediated erythrophagocytosis. However, mice injected with lower doses (25 or 50 μg) of IgM mAb also failed to display detectable levels of iron deposits in Kupffer cells (data not shown), thus excluding any implication of CR-dependent erythrophagocytosis in the pathogenesis of IgM anti-RBC-induced anemia. Furthermore, it should be mentioned that no histological alterations in kidneys associated with intravascular hemolysis such as tubular necrosis and pigmented cast formation were observed in mice injected with 100 μg of IgM or IgA mAb (data not shown).
Representative histological appearance of spleen and liver in BALB/c mice injected with 100 μg of 34-3C IgM, IgA, and IgG2a anti-RBC mAb. Note an enormous accumulation of agglutinated RBC in red pulp of spleen 2 days after injection of polymeric forms of IgM or IgA anti-RBC mAb (A, IgM; B, IgA; C, IgG2a; hematoxylin & eosin [H&E], original magnification ×40), focal necrotic lesions of hepatic parenchymal cells in liver 8 days after injection of IgM anti-RBC mAb (E,F; H&E, original magnification ×40 and ×200), and extensive iron deposits by Kupffer cells in liver 8 days after injection of IgG2a anti-RBC mAb (G, IgM; H, IgA; I, IgG2a; Perls iron staining, original magnification ×200). As a control, normal histological appearances of spleen and liver from mice injected with 500 μg of monomeric form of 34-3C IgM anti-RBC mAb are shown (D,J).
Representative histological appearance of spleen and liver in BALB/c mice injected with 100 μg of 34-3C IgM, IgA, and IgG2a anti-RBC mAb. Note an enormous accumulation of agglutinated RBC in red pulp of spleen 2 days after injection of polymeric forms of IgM or IgA anti-RBC mAb (A, IgM; B, IgA; C, IgG2a; hematoxylin & eosin [H&E], original magnification ×40), focal necrotic lesions of hepatic parenchymal cells in liver 8 days after injection of IgM anti-RBC mAb (E,F; H&E, original magnification ×40 and ×200), and extensive iron deposits by Kupffer cells in liver 8 days after injection of IgG2a anti-RBC mAb (G, IgM; H, IgA; I, IgG2a; Perls iron staining, original magnification ×200). As a control, normal histological appearances of spleen and liver from mice injected with 500 μg of monomeric form of 34-3C IgM anti-RBC mAb are shown (D,J).
Lack of pathogenicity of monomeric forms of the 34-3C IgM and IgA class-switch variants
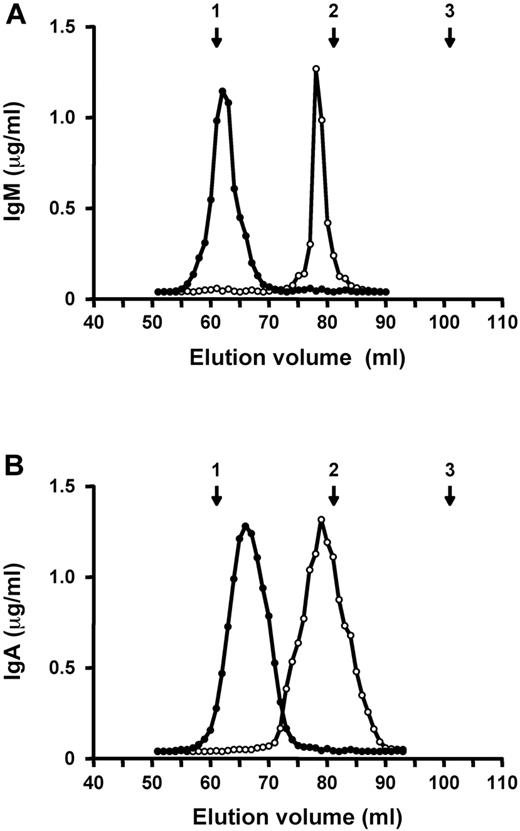
Histological analysis in mice developing anemia after injection of 34-3C IgM or IgA mAb revealed that sequestration of agglutinated RBC in the spleen was the main mechanism responsible for the development of autoimmune hemolytic anemia by these 2 isotypes. To test whether the polymeric form of these 2 mAb is crucial in the induction of anemia, because of polyvalency-dependent hemagglutination, we generated monomeric forms of 34-3C IgM and IgA by substituting cysteine by serine at positions 414 and 575 in the IgM constant region (IgM-S414/575) and at position 471 in the IgA constant region (IgA-S471). Gel filtration chromatography showed that IgM-S414/575 and IgA-S471 mutants eluted at the volumes characteristic of monomers, almost comparable to that of 34-3C IgG mAb, whereas IgM and IgA WT mAb eluted as polymers (Figure 4). Notably, monomeric IgM-S414/575 and IgA-S471 mutants failed to exhibit hemagglutinating activity in vitro.
Elution profiles of polymeric and monomeric forms of 34-3C IgM or IgA mAb on Sephadex G200 gel filtration column. Polymeric (•) and monomeric (○) forms of 34-3C IgM (A) and IgA (B) mAb were individually subjected to a Sephadex G200 column equilibrated with 50 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, and elution was performed using the same buffer at a flow rate of 0.2 mL/min at 4°C. The column was calibrated with blue dextran (void volume), IgG (150 kD), and BSA (67 kD), and their positions are indicated by arrows: 1, void volume; 2, IgG; 3, BSA.
Elution profiles of polymeric and monomeric forms of 34-3C IgM or IgA mAb on Sephadex G200 gel filtration column. Polymeric (•) and monomeric (○) forms of 34-3C IgM (A) and IgA (B) mAb were individually subjected to a Sephadex G200 column equilibrated with 50 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, and elution was performed using the same buffer at a flow rate of 0.2 mL/min at 4°C. The column was calibrated with blue dextran (void volume), IgG (150 kD), and BSA (67 kD), and their positions are indicated by arrows: 1, void volume; 2, IgG; 3, BSA.
To assess the pathogenic activity of monomeric forms of 34-3C IgM and IgA mAb, the development of anemia was analyzed by a single injection of various amounts of IgM-S414/575 or IgA-S471 mutant into BALB/c mice. In contrast to the remarkable pathogenic effects of 100 μg of polymeric IgM and IgA mAb, up to 500 μg of monomeric IgM-S414/575 and IgA-S471 mutants had no detectable pathogenic effects: neither decreases in Ht values (Figure 5A) nor histological alterations in the spleen and liver (Figure 3D,J). It should be also stressed that these mAb were unable to activate complement in vivo as judged by the absence of C3 deposition on circulating RBC despite extensive opsonization with each monomeric form of mAb (Figure 5B).
Development of anemia and complement activation in BALB/c mice after the injection of polymeric and monomeric forms of 34-3C IgM or IgA mAb. (A) Ht values of individual mice measured 2 days after intraperitoneal injection of 100 μg of polymeric IgM or IgA (○) or 500 μg of monomeric IgM or IgA (•) mAb are shown. Note significant decreases in Ht values in mice injected with polymeric IgM (P < 0.01) or IgA (P < 0.05) compared with mice receiving monomeric IgM or IgA. Ht values 4 days after injection of monomeric IgM or IgA remained in the normal range. The normal range of Ht values (mean ± 3 SD) of 2- to 3-month-old BALB/c mice is represented as shaded areas. (B) Opsonization of circulating RBC 24 h after intraperitoneal injection of 100 μg of polymeric IgM (pIgM), 500 μg of monomeric IgM (mIgM), 100 μg of polymeric IgA (pIgA), or 500 μg of monomeric IgA (mIgA) mAb is shown. RBC were stained with biotinylated goat anti-mouse C3 or rat anti-mouse k-chain antibody followed by PE-conjugated streptavidin. Shaded areas indicate the background staining obtained with untreated BALB/c mice.
Development of anemia and complement activation in BALB/c mice after the injection of polymeric and monomeric forms of 34-3C IgM or IgA mAb. (A) Ht values of individual mice measured 2 days after intraperitoneal injection of 100 μg of polymeric IgM or IgA (○) or 500 μg of monomeric IgM or IgA (•) mAb are shown. Note significant decreases in Ht values in mice injected with polymeric IgM (P < 0.01) or IgA (P < 0.05) compared with mice receiving monomeric IgM or IgA. Ht values 4 days after injection of monomeric IgM or IgA remained in the normal range. The normal range of Ht values (mean ± 3 SD) of 2- to 3-month-old BALB/c mice is represented as shaded areas. (B) Opsonization of circulating RBC 24 h after intraperitoneal injection of 100 μg of polymeric IgM (pIgM), 500 μg of monomeric IgM (mIgM), 100 μg of polymeric IgA (pIgA), or 500 μg of monomeric IgA (mIgA) mAb is shown. RBC were stained with biotinylated goat anti-mouse C3 or rat anti-mouse k-chain antibody followed by PE-conjugated streptavidin. Shaded areas indicate the background staining obtained with untreated BALB/c mice.
Discussion
We have generated IgM and IgA class-switch variants bearing identical VH and Vk regions as the 34-3C IgG2a anti-RBC autoantibody derived from autoimmune-prone NZB mice and determined the pathogenic potential of these 2 isotypes in relation to the Fc-associated effector functions (complement activation and polyvalency-dependent agglutination). Polymeric forms of 34-3C IgM and IgA mAb were as pathogenic as the IgG2a isotype, which was previously shown to be the most pathogenic IgG among 4 different IgG subclasses.7,8 The pathogenesis of autoimmune hemolytic anemia induced by IgM and IgA anti-RBC autoantibodies, however, was distinct from that of IgG anti-RBC autoantibodies. IgM and IgA anti-RBC autoantibodies provoked anemia attributable to polyvalency-mediated hemagglutination in the spleen and occasionally in the liver, whereas the mechanism of IgG anti-RBC autoantibodies was FcγR- and CR-mediated erythrophagocytosis by Kupffer cells. Our results thus indicate that Fcα/μR, a receptor specific for both IgA and IgM expressed on macrophages,13 does not participate in the process of erythrophagocytosis. Furthermore, the lack of any protection in C3-deficient mice revealed that the pathogenicity of IgM and IgA anti-RBC autoantibodies was totally independent of the activation of complement. Likewise, our results demonstrated that IgA antibodies were unable to efficiently activate complement in vivo.
Our analysis of the IgM switch variant of 34-3C anti-RBC mAb revealed that this isotype was highly pathogenic, because the minimal amounts of antibody required to induce anemia were comparable to those of 34-3C IgG2a mAb (Table 2). It has been previously shown that a low-affinity 4C8 IgM anti-RBC mAb was also as pathogenic as 34-3C IgG2a mAb.6 Because the affinity of the 4C8 mAb is more than 1,000 times weaker than that of the 34-3C mAb, this suggested that the high pathogenic potential of 34-3C IgM variant was not exclusively attributable to the high-affinity feature of the 34-3C mAb. Rather, it appeared that the affinity of IgM anti-RBC autoantibodies is of limited importance for their pathogenic activity, because their polymeric form dramatically enhances the binding avidity for autoantigens, which are likely to be expressed abundantly on the surface of RBC.6
Pathogenicity and effector functions of 34-3C Ig class-switch variants*
| Class . | Pathogenicity* . | C′† . | FcR‡ . | Pathogenesis . |
|---|---|---|---|---|
| pIgM§ | 25 μg | ++ | − | Hemagglutination |
| mIgM§ | — | − | − | — |
| pIgA§ | 25 μg | − | − | Hemagglutination |
| mIgA§ | — | − | − | — |
| IgG1 | 500 μg | − | + | Erythrophagocytosis |
| IgG2a | 25 μg | ++ | ++ | Erythrophagocytosis |
| IgG2b | 25 μg | ++ | ++ | Erythrophagocytosis |
| IgG3 | 100 μg | + | − | Erythrophagocytosis |
| Class . | Pathogenicity* . | C′† . | FcR‡ . | Pathogenesis . |
|---|---|---|---|---|
| pIgM§ | 25 μg | ++ | − | Hemagglutination |
| mIgM§ | — | − | − | — |
| pIgA§ | 25 μg | − | − | Hemagglutination |
| mIgA§ | — | − | − | — |
| IgG1 | 500 μg | − | + | Erythrophagocytosis |
| IgG2a | 25 μg | ++ | ++ | Erythrophagocytosis |
| IgG2b | 25 μg | ++ | ++ | Erythrophagocytosis |
| IgG3 | 100 μg | + | − | Erythrophagocytosis |
The quantity of mAb required for inducing anemia (defined as Ht values <40%) in BALB/c mice based on the results obtained in previous8 and the present studies.
The capacity of each Ig isotype to activate complement was judged by the extent of C3 deposition on circulating RBC in vivo based on the results obtained in previous8 and the present studies.
FcR involved in phagocytosis. Three different activating FcγR (FcγRI, FcγRIII, and FcγRIV) expressed on murine phagocytes display different specificities to IgG subclasses: FcγRI for IgG2a, FcγRIII for IgG1, IgG2a and IgG2b, and FcγRIV for IgG2a and IgG2b.16
pIgM and mIgM: polymeric and monomeric IgM. pIgA and mIgA: polymeric and monomeric IgA.
It should be stressed that the major pathogenic mechanism of anemia induced by 3 different NZB-derived, IgM anti-RBC mAb was massive hemagglutination and subsequent sequestration of RBC in the spleen and occasionally in the liver, as shown previously6,9 and in the present study (Table 2). This process apparently occurs more rapidly than the process of IgG anti-RBC-induced erythrophagocytosis through recognition by FcγR and CR on Kupffer cells in the liver. This was illustrated by a more rapid onset of anemia with IgM (peaking at day 2) than with IgG (peaking at day 4). The polymeric form of IgM is critical for its pathogenic activity, because it is able to efficiently induce hemagglutination. In contrast, the monomeric mutant form of 34-3C IgM mAb completely failed to induce anemia despite its high-affinity binding to circulating RBC in vivo. This lack of pathogenicity is explained by the inability to induce hemagglutination and by its poor activation of complement.
It is somehow unexpected that, despite strong activation of complement as documented by C3 deposition of RBC in mice injected with IgM anti-RBC mAb, no signs of CR-mediated erythrophagocytosis by Kupffer cells were detectable. In contrast, CR-dependent erythrophagocytosis significantly contributed to the development of a severe form of anemia induced by a high dose (>100 μg) of complement-activating IgG classes of 34-3C anti-RBC mAb.8 The lack of CR-dependent erythrophagocytosis in IgM anti-RBC-injected mice can be in part attributable to massive hemagglutination, which likely interferes with the process of recognition and subsequent phagocytosis by CR. In addition, studies with 34-3C IgG2a mAb revealed that CR-mediated erythophagocytosis requires an extensive opsonization of RBC with C3 fragments, whereas a limited opsonization with IgG2a antibodies is sufficient to trigger FcγR-dependent erythrophagocytosis.8 Thus, lack of contribution of complement to the development of anemia induced by low doses (<50 μg) of the 34-3C IgG2a mAb could also explain the absence of CR-mediated erythrophagocytosis at these doses of IgM anti-RBC mAb.
In addition, the lack of protective effect of C3 deficiency on the development of anemia induced by 3 different IgM anti-RBC mAb also argued against the involvement of complement-mediated intravascular hemolysis. This is in agreement with the observation that C5-deficient B6 mice developed anemia, an equally severe anemia as WT B6 mice, after injection of 34-3C IgG2a anti-RBC mAb (unpublished observations). The absence of complement-mediated intravascular hemolysis in mice injected with highly pathogenic IgM and IgG anti-RBC mAb is likely to be attributable to the activity of a number of complement inhibitory proteins present on RBC membranes. This idea was supported by the observations that 34-3C IgG2a-opsonized RBC deficient in the membrane-bound complement regulatory proteins, decay accelerating factor or CR1-related gene/protein y, were eliminated more rapidly,30 and that decay accelerating factor-deficient RBC were highly sensitive to antibody-induced complement-dependent hemolysis in vitro.31 In addition, no induction of complement-mediated hemolysis by our IgM anti-RBC mAb could also be related to the specificity of the target RBC antigen. Because ABO-incompatible transfusion results in typical acute intravascular hemolysis, it is likely that certain antigenic sites on RBC are more vulnerable for complement-mediated immune hemolysis. This notion was supported by the finding that some lymphocyte surface antigens are better targets for complement-mediated lysis than others, independent of their densities.32 Thus, we cannot completely exclude the possibility that some IgM anti-RBC autoantibodies bearing a particular specificity could induce anemia as a result of complement-mediated intravascular hemolysis.
Our demonstration that all 3 IgM anti-RBC mAb tested were highly pathogenic, inducing severe anemia as a result of massive hemagglutination, is consistent with clinical findings in several reported human cases. Indeed, the presence of warm-reactive, agglutinating IgM autoantibodies was associated with a lethal form of anemia attributable to hemagglutination, rather than intravascular hemolysis, and RBC aggregates likely played a role in the development of multiple systemic infarctions reported in these patients.33–39 Notably, hepatic failure associated with intrasinusoidal hemagglutination and necrosis of hepatic parenchymal cells observed in several cases33,35,36 was reminiscent of a lethal form of anemia induced by a high dose of murine IgM anti-RBC mAb.6,9 It should be stressed that autoimmune hemolytic anemia caused by warm-reactive, agglutinating IgM autoantibodies, without the presence of IgG autoantibodies, is rare in humans.40 This is also the case in autoimmune-prone NZB mice, in which the major Ig isotype of anti-RBC autoantibodies is IgG41 and the most consistent histological change in liver is the accumulation of hemosiderin in Kupffer cells.1 However, NZB mice with severe anemia occasionally display focal areas of hepatic necrosis, which were considered to be a consequence of the accumulation of agglutinated RBC in the hepatic sinusoids.1 Thus, when warm-reactive, agglutinating IgM autoantibodies become dominant as a result of mono- or oligoclonal expansion of autoreactive B lymphocytes under certain clinical conditions, they could be responsible for the development of an aggressive, severe autoimmune hemolytic anemia as observed in mice expressing a transgenic 4C8 IgM antibody.42
The analysis of polymeric and monomeric IgA 34-3C anti-RBC variants revealed that only polymeric IgA was able to induce anemia, and its pathogenic potency was comparable to that of IgM and IgG2a variants (Table 2). Polymeric IgA anti-RBC mAb apparently induced anemia by a mechanism identical to that of IgM anti-RBC mAb, because it was associated with a marked accumulation of agglutinated RBC in the spleen, and because the development of anemia was as rapid as with IgM. The absence of any sign of erythrophagocytosis in mice injected with IgA anti-RBC mAb confirmed the lack of expression of phagocytic IgA receptors in mice consistent with the fact that despite exhaustive efforts, attempts to isolate the mouse Fcar gene encoding for FcαRI have been unsuccessful.43 This would explain why monomeric IgA failed to induce anemia, together with the lack of activation of complement by IgA, as discussed subsequently. Remarkable differences in the pathogenic potentials between polymeric and monomeric forms of IgA anti-RBC mAb are in agreement with the finding that polymeric IgA antibodies against H1 hemagglutinin of influenza virus were more effective in neutralization than monomeric IgA antibodies in vitro.44 However, in human expressing FcRγ-associated FcαRI, which is able to trigger phagocytosis,45 we cannot exclude the possibility that RBC-bound monomeric IgA autoantibodies may become pathogenic through interaction with FcαRI.
It has been claimed that IgA is able to activate complement by the alternative and lectin pathways in vitro,46–48 but this has never been confirmed in vivo. Our results with IgA anti-RBC mAb demonstrate that IgA is unable to efficiently activate complement in vivo (Table 2). This was shown by the absence of C3 deposition on RBC of mice injected with 34-3C IgA anti-RBC mAb or implanted with 34-3C IgA-secreting transfectoma cells and by the lack of protection from IgA-induced anemia through C3 deficiency. The failure of IgA antibodies to activate complement may not be totally surprising, because the major role of IgA is the inhibition of adherence and agglutination of pathogens and the neutralization of viruses and toxins at mucosal sites, where levels of complement are much more limited.
Our present and previous studies6–8 have defined the pathogenic potency of individual Ig isotypes, including IgG subclasses, in the development of autoimmune hemolytic anemia in relation to Ig Fc-dependent effector functions. It is striking to see that polymeric forms of IgM and IgA autoantibodies are as potent as the most pathogenic IgG2a subclass of autoantibodies, because of their multivalency-dependent agglutinating potential, independently of their antigen-binding affinity. Thus, our results support a significant role for IgM and IgA antibodies, particularly if their specificities are directed against antigens abundantly expressed on the surface of pathogens and host cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr T. Moll for his critical reading of the manuscript and Mr G. Celetta and Mr G. Brighouse for their excellent technical assistance.
This work was supported by a grant from the Swiss National Foundation for Scientific Research. L.F.-J. is a recipient of a fellowship from the Arthritis Research Campaign, United Kingdom.
Authorship
L.B. performed research (in vivo analysis of the development of anemia, generation of monomeric forms of IgM and IgA); L.F.-J. contributed to the generation of IgM switch variant and performed the analysis of complement activation; C.C. and E.M.-S. contributed to the generation of IgA class-switch variant and its in vivo analysis; M.S. contributed to the generation of monomeric form of IgM and participated in writing the manuscript; S.I. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shozo Izui, Department of Pathology and Immunology, C.M.U., 1211 Geneva 4, Switzerland; e-mail: Shozo.Izui@medecine.unige.ch.


![Figure 3. Representative histological appearance of spleen and liver in BALB/c mice injected with 100 μg of 34-3C IgM, IgA, and IgG2a anti-RBC mAb. Note an enormous accumulation of agglutinated RBC in red pulp of spleen 2 days after injection of polymeric forms of IgM or IgA anti-RBC mAb (A, IgM; B, IgA; C, IgG2a; hematoxylin & eosin [H&E], original magnification ×40), focal necrotic lesions of hepatic parenchymal cells in liver 8 days after injection of IgM anti-RBC mAb (E,F; H&E, original magnification ×40 and ×200), and extensive iron deposits by Kupffer cells in liver 8 days after injection of IgG2a anti-RBC mAb (G, IgM; H, IgA; I, IgG2a; Perls iron staining, original magnification ×200). As a control, normal histological appearances of spleen and liver from mice injected with 500 μg of monomeric form of 34-3C IgM anti-RBC mAb are shown (D,J).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-11-059899/4/m_zh80120701330003.jpeg?Expires=1767718034&Signature=KIBzORZa3vE6kfSw8M9pTRsrKBqKaMWLnHiFBDoKRUb7OGBSelNIczYkQIwTxsy02f-9OgtveQSxJbYKKvFt8g5nvqSj2H7sH8VXOP0wvoPfRCqEn2TuA3H0tqQ2uAsETAnLViCpVw6hW4MV7pl-1xdlNAk0k8fI7321-rJu2IxpKXO5vbff37klpWdArzYNZo74R-vqiIgd74-ynI4pvG0MoUmlyTnlR2shiy3B-u2aoNXcNnzBQEljBs1kcXkrsJR7Q50SD-kzPF8rk7wNqMMTUx-2dQe40O-gDlOXDSR20gDFvkPczUe6X4mQqlV~4W1oGmB2Ra-Ld3nsiTcAAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal