Abstract
We have proposed that, unlike other HIV-vulnerable cell lineages, progenitor mast cells (prMCs), cultured in vitro from undifferentiated bone marrow–derived CD34+ pluripotent progenitors (PPPs), are susceptible to infection during a limited period of their ontogeny. As infected prMCs mature in culture, they lose expression of viral chemokine coreceptors necessary for viral entry and develop into long-lived, latently infected mature tissue mast cells (MCs), resistant to new infection. In vivo recruitment of prMCs to different tissue compartments occurs in response to tissue injury, growth, and remodeling or allergic inflammation, allowing populations of circulating and potentially HIV-susceptible prMCs to spread persistent infection to diverse tissue compartments. In this report, we provide in vivo evidence to confirm this model by demonstrating that HIV-infected women have both circulating prMCs and placental tissue MCs (PLMCs) that harbor inducible infectious HIV even after highly active antiretroviral therapy (HAART) during pregnancy. Furthermore, infectious virus, capable of infecting alloactivated fetal cord blood mononuclear cells (CBMCs), could be induced in isolated latently infected PLMCs after weeks in culture in vitro. These data provide the first in vivo evidence that tissue MCs, developed from infected circulating prMCs, comprise a long-lived inducible reservoir of persistent HIV in infected persons during HAART.
Introduction
Since the advent of highly active antiretroviral therapy (HAART), it is now accepted that persistent HIV infection is maintained by certain populations of latently infected, long-lived memory T cells and other non–T-cell lineages.1,2 Understanding which cell lineages are involved and how cellular reservoirs of persistent HIV infection are established has thus become an important priority in the development of effective new antiretroviral therapies. One of the most important and least studied cell lineages involved in persistent HIV-1 infection is the mast cell (MC). MCs are unique among HIV-vulnerable cell lineages in that they may only be exposed to infection for a brief period during their ontogeny. A limited number of studies using MCs developed in vitro from fetal cord blood–derived pluripotent progenitors (PPPs) have indicated that progenitor mast cells (prMCs), which transiently express viral chemokine coreceptors, are susceptible to R5-tropic HIV. Eventually, these prMCs become resistant to infection as they differentiate into mature MCs and lose expression of select chemokine coreceptors necessary for viral entry.3,4 These pioneering studies have laid the groundwork of a model for a MC reservoir of persistent HIV infection. The central concept of such a model is that susceptible circulating prMCs become infected with HIV and eventually become latently infected, long-lived mature MCs as they extravasate into various tissue compartments. The central concept of such a model is that productive HIV infection initially occurs only in susceptible, circulating prMCs. However, as infected prMCs extravasate into various tissue compartments, they can develop into “latently infected,” long-lived mature MCs. HIV-susceptible cells with a progenitor mast cell/basophil phenotype in the circulation of patients with AIDS with allergic disorders has been described.5 However, to our knowledge there has been no reported evidence for HIV-infected mature tissue MCs in vivo.
Recruitment of new MCs to different tissue compartments involves mechanisms by which bone marrow–derived, adult CD34+ PPPs differentiate into circulating (HIV-susceptible) prMCs that home to specific extravascular sites where they mature into adult tissue MCs with a potentially HIV infection–resistant phenotype. In the absence of allergy, such recruitment mechanisms may involve responses to injury, growth, or remodeling in damaged or in newly developing tissues. In this report we examined the placenta, a large complex organ that has been described as a rich source of tissue MCs.6 Evidence is presented that shows placental tissue MCs (PLMCs), which evolve from circulating HIV-susceptible prMCs and which can be isolated from uninfected pregnant women at term, are HIV-infection resistant. PLMCs isolated from HIV-infected women, however, were long lived (weeks to months) and harbored inducible virus capable of infecting alloactivated CD4+ fetal cord blood mononuclear cells (CBMCs). These data represent the first evidence that a MC reservoir of persistent virus infection does exist in HIV-infected persons and suggest that this reservoir can be established even during HAART, potentially within multiple tissue compartments and in the absence of allergy. These studies thus reinforce a model in which mature tissue MCs, recruited and developed in vivo from prMCs, comprise a long-lived reservoir of persistent HIV infection.
Materials and methods
In vitro culture and infection of MCs
Human MCs were cultured from adult CD34+ PPPs isolated from circulating peripheral blood mononuclear cells (PBMCs) under Emory University IRB approved protocols as described.7 PPPs were cultured in serum-free growth medium consisting of Stem Pro-34 medium (GIBCO/InVitrogen, Grand Island, NY) supplemented with rhu-IL3, rhu-IL6 and rhu-SCF for 1 week, then subsequently in the same growth medium without rhu-IL3. HIV-susceptible prMCs (in vitro–derived 4-6 weeks in culture) and HIV infection-resistant mature MCs (9-13 week in culture) were routinely used for infection studies. The human MC line LAD-2,8 which is susceptible to both CXCR4 (X4)–tropic and CCR5 (R5)–tropic HIV, was included in infection experiments for purposes of control. Virus titers of R5-tropic HIVBAL and X4-tropic HIVTybe strains, obtained from the NIH AIDS Reference and Reagent Program, were determined in TZMB1 cells9 by the Spearman-Karber method (from the Virology Manual for HIV Laboratories, NIAID). All virus infection experiments were conducted at a multiplicity of infection (MOI) of approximately 0.01. The following toll-like receptor (TLR) agonists were used in reactivation experiments with latently infected MCs: Escherichia coli lipopolysaccharide (LPS) 0111:B4 strain TLR4 ligand, CgG oligonucleotide (ODN) type A-Human TLR9 ligand, and Staphylococcus aureus peptidoglycan (SA-PGN) human TLR2 ligand (InvivoGen, San Diego, CA).
Collection and isolation of PLMCs and circulating prMCs
Samples of venous blood, cord blood, and placental tissues were obtained from patients who received prenatal care at a specialty obstetrics clinic established by Emory University's Department of Gynecology and Obstetrics for HIV+ women at Grady Memorial Hospital (GMH) in Atlanta, Georgia, and from HIV− women who delivered at GMH. All HIV+ pregnant patients were managed according to current guidelines from the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention. At the beginning of their second trimester (∼ 14 weeks' gestation) patients were started on triple drug antiretroviral therapy appropriate for their viral loads. Plasma viral loads were determined throughout pregnancy to assess responses to medication. Viral loads determined at late third trimester time points for enrolled patients on HAART (n = 12) ranged from less than 400 to 58 000 copies/mL plasma. All placental and fetal cord blood tissues were collected at term with informed consent under Emory University IRB-approved protocols. Placental tissue isolated from the basal plate (maternal side) was treated with type 2 collagenase (Worthington Biochemical, Lakewood, NJ), at 250 U/mL in serum-free HBSS for 45 to 60 minutes at room temperature on a rocker. Dissociated placental cells were purified by filtration through sterile nylon mesh, washed twice, resuspended in HBSS containing 5% FBS and antibiotics, then subjected to density gradient centrifugation on lymphocyte separation medium (LSM) to isolate placental cells of hematopoietic origin. Peripheral blood mononuclear cells containing circulating prMCs were isolated from venous blood specimens in a similar fashion. The populations of PBMCs or placental hematopoietic cells were then suspended in RPMI medium supplemented with 10% FBS, 2 mM glutamine, 100 μg gentamicin/mL, 100 ng rhu SCF/mL, and 1 μg biotinylated IgE myeloma protein/mL and then incubated overnight in a humidified environment at 37°C with 7% CO2. IgE-biotin pulsed PBMCs or placental cells were then washed, and IgE-positive cells were isolated using antibiotin imunomagnetic-positive selection (Easy-Sep; Stem Cell Technologies, Vancouver, BC) and cultured and maintained in serum-free medium (Stem Pro 34; Invitrogen) supplemented with rhu-SCF (100 ng/mL) in a humidified environment at 37°C with 7% CO2. Under these culture conditions, which uniquely support MC growth, further selection for prMCs or PLMCs ( ≥ 83% IgE/CD117 dual-positive cells) was achieved (described in further detail in “Results”).
Activation and infection of fetal CBMCs
Fetal cord blood was collected at term and mixed in HBSS containing 50 U heparin/mL to prevent coagulation. CBMCs were isolated on LSM, then placed in culture at 106 cells/mL in RPMI medium with 10% fetal bovine serum supplemented with 2 mM glutamine and antibiotics with an equal number of γ-irradiated (33 Gy) PBMCs collected from nonrelated adult donors. The mononuclear cells were next incubated in a humidified environment at 37°C for 96 to 120 hours. Alloactivation was confirmed by measuring incorporation of [3H]-thymidine (16-hour pulse). Activated CBMCs along with γ-irradiated stimulator PBMCs (control) were then incubated for 24 to 48 hours with virus released from infected PLMCs then washed and incubated in a similar fashion. Levels of HIV-p24 in culture supernatant fluids were measured 7 days after infection by enzyme-linked immunoabsorbent assay (ELISA) as described in “ELISA assay for HIV-p24 core antigen.”
Immunophenotyping by flow microfluorometry (FMF) and laser scanning confocal microscopy
MCs suspended in PBS were incubated with 1 μg/106 cells of phycoerythrin (PE)–conjugated anti-Hu CD117, PE–anti-Hu CCR3, PE–anti-Hu CXCR4, PE–anti-Hu CCR5, or -Ig isotype (PE-IgG2a,κ) control (Becton Dickinson PharMingen, Pleasantville, CA) in media for 30 minutes at 4°C then washed twice with PBS pH 7.2. To determine IgE receptor-positive cells, MCs pulse-labeled with an IgE-biotin myeloma protein conjugate were incubated in a similar fashion with an allophycocyanin streptavidin (SA) conjugate (1 μg/106 cells; BD-Pharmingen). The cells were then fixed with 1% (vol/vol) paraformaldehyde (pH 7.2) and analyzed by FMF with a Becton Dickinson FACSCaliber using CellQuest software. PLMCs were stained for confocal microscopy as previously described,4 using a Zeiss LSM 510 Meta Confocal microscope system, equipped with Argon and HeNe/3PMT lasers, using a Plan-APO 63× 1.4 oil immersion objective (630× final magnification) and Zeiss image analysis software v4.2.
Image analysis of isolated PLMCs by transmission electron microscopy (TEM)
Approximately 106 IgE-selected PLMCs were pelleted, then treated as a cell block: fixed initially in 2.5% buffered glutaraldehyde, postfixed in 1% osmium tetroxide in the same buffer, and dehydrated through graded ethanol. The fixed cells were infiltrated with propylene oxide and embedded in Embed-812 (Electron Microscopy Sciences, Fort Washington, PA). Initial coarse examinations were performed on 0.5-μm sections stained with toluidine blue to identify areas of interest. Subsequently, ultrathin sections (60-70 nM), prepared from representative areas of interest, were cut and stained with aqueous uranyl acetate and lead citrate, then examined using an H-7500 transmission electron microscope (Hitachi, Pleasanton, CA). Detection of tryptase-positive cytoplasmic granules was performed by indirect immunoperoxidase staining following cell fixation with 4% buffered paraformaldehyde plus 0.1% glutaraldehyde. Fixed cells were blocked with PBS containing 5% NGS, 5% BSA, and 0.1% gelatin for 30 minutes at 4°C. The cells were then incubated with mouse monoclonal anti-MC tryptase primary antibody for 1 hour, washed, and incubated with biotinylated goat anti–mouse antibody (1:200; clone mLR11; Vector Labs, Burlingame, CA). The cells were washed again, incubated in avidin-biotin complex (Vector ABC Elite; Vector Labs) for 2 hours at 4°C, then incubated with 0.05% in diaminobenzidine (Sigma, St Louis, MO) and 0.003% hydrogen peroxide in 0.1 M phosphate buffer for 5 to 15 minutes at room temperature. The cells were postfixed with 2.5% glutaraldehyde and prepared for TEM as described in this section.
Analysis of HIV− infection of MCs by real-time PCR
Determining viral entry into MCs by detection of viral strong stop (SS) cDNA.
Susceptibility of MCs to infection with HIV at the level of viral entry was determined by measuring levels of SS cDNA by real-time polymerase chain reaction (PCR). After an overnight incubation with either HIVBAL or HIVTybe, DNA was prepared from 1 to 2 × 106 MCs using the QIAamp DNA Mini Kit protocol (QIAGEN, Valencia, CA). Viral SS cDNA levels were determined by quantitative reverse transcription (qRT)–PCR in a SYBR Green assay system using a BioRad iCycler (Hercules, CA) programmed for 95°C for 3 minutes, then 50 cycles at 93°C for 1 minute, 50°C for 1 minute,; 72°C for 1 minute. SS (sense: M667, 5′-GGCTAACTAGGGAACCCACTG-3′; and antisense: AA55, 5′-CTGCTAGAGATTTTCCACACTGAC-3′) primers were used at a final concentration of 200 nM to amplify the products of interest from 400 ng of sample DNA.
Determining host-cell nuclear integration of HIV by detection of integrated proviral DNA.
Susceptibility of MCs to infection with HIV at the level of proviral nuclear integration into the host MC genome was determined by measuring the mean number of copies of integrated proviral DNA per 107 MCs. DNA was prepared as described in “Determining viral entry into MCs by detection of viral strong stop (SS) cDNA,” then a preamplification was performed as described.2 Briefly, 100 nM each of Alu 1 (5′-TCCCAGCTACTGGGGAGGCTGAGG-3′), Alu 2 (5′-GCCTCCCAAAGTGCTGGGATTACAG-3′), and a compound primer LM667 (5′-ATGCCACGTAAGCGAAACTCTGGCTAACTAGGGAACCCACTG-3′) were incubated with 200 ng of sample DNA or known concentrations of HIV cDNA (OM-10 cells) standards (gel purified; A260/A280 and ethidium bromide quantified). PCR was performed (Apollo ATC 401 thermocycler; 95°C for 3 minutes, then 23 cycles at 94°C for 1 minute, 55°C for 1 minute, 72°C for 1 minute). Integrated provirus or HIV cDNA standard concentrations were determined by a modified absolute concentration Nested Real-Time qPCR assay.2 Preamplified samples and standards (2 uL/well) were subjected to qPCR with 200 nM each of a quasi-nested primer pair (Lambda-T, 5′-ATGCCACGTAAGCGAAACT-3′; AA55M, 5′-GCTAGAGATTTTCCACACTGACTAA-3′) with SYBR Green on a BioRad iCycler (95°C for 3 minutes, then 50 cycles at 93°C for 1 minute, 55°C for 1 minute, 72°C for 1 minutes). Efficiencies of the qPCR assays were calculated from the slopes of the standard curves, and inverse natural logarithms of the Cts (adjusted for efficiency) were used to calculate starting copy numbers of integrated HIV provirus.
Determining proviral transcription of full-length (FL) and multiply spliced (MS) HIV mRNA.
RNA was prepared from 1 to 2 × 106 MCs using the QIAamp RNAeasy Mini Kit protocol (QIAGEN). FL and MS HIV mRNA transcript levels were determined by a modification of a protocol described by Brussels et al.10 Briefly, qRT-PCR was performed in a SYBR Green assay system using a BioRad iCycler programmed for 95°C 3 minutes, (94°C for 1minute, 56°C for 1 minute, 72°C for 1 minutes) × 40 cycles, 4°C for 10 minutes. Primers for FL (sense,: 5′-CTGAAGCGCGCACGGCAA-3′, and antisense, 5′-GACGCTCTCGCACCCATCTC-3′) and for MS sense (GACTCATCAAGTTTCTCTATCAAA) and MS antisense (GTCTCTCAAGCGGTGGT) were used at a final concentration of 200 nM to amplify the products of interest from 400 ng of sample RNA.
ELISA assay for HIV-p24 core antigen
An HIV-1 p24 antigen assay (Beckman Coulter, Miami, FL) was used as previously described4 according to the manufacturer's instructions to determine levels of HIV-1 p24 in cultures of infected PLMCs or CBMCs.
Results
PrMCs developed from circulating adult CD34+ PPPs are susceptible to R5-tropic HIV but are only marginally susceptible to X4-tropic virus
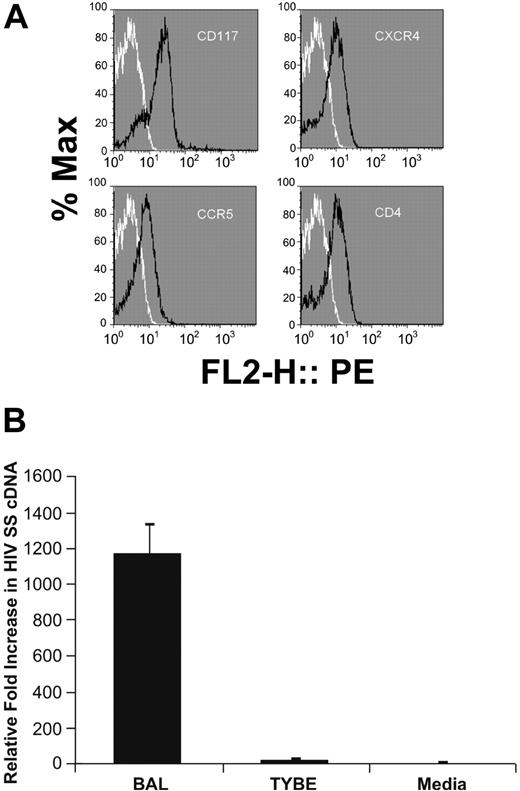
Evidence for MCs as a susceptible reservoir of persistent HIV infection is based on previous studies using MCs developed in vitro from human cord blood–derived PPPs.4 One limitation of this model is that in HIV-infected persons, the MC reservoir would naturally develop from circulating adult CD34+ PPPs and not from fetal progenitor cells. Furthermore, it has recently been shown that MCs derived from fetal cord blood are phenotypically different from adult circulating CD34+ PPPs in both function and gene expression profiles.11 Therefore, to provide stronger evidence that the MC reservoir can be created in vivo, prMCs were developed from adult human CD34+ PPPs isolated from peripheral blood and characterized for their susceptibility to infection with X4- and R5-tropic HIV. FcϵRIα+, CD117+, CXCR4+, CCR5+, CD4+ prMCs were present by 2 to 4 weeks in serum-free culture conditions and represented at least 92% of total viable cells at 5 weeks in culture (Figure 1A). When challenged overnight with virus, measurements of viral SS cDNA and integrated proviral DNA by real-time PCR indicated that these prMCs could become significantly infected with R5 tropic HIVBAL and marginally infected with X4-tropic HIVTybe (n ≥ 3 experiments), unlike what has been described with fetal cord blood–derived prMCs3,4 (Figure 1B).
PrMCs derived in vitro from CD34+ adult hematopoietic PPPs are susceptible to R5-tropic HIV. (A) FMF analysis of prMCs was performed by gating on populations of CD117/IgE dual-positive cells derived from adult CD34+ hematopoietic PPPs in serum-free mast cell medium. The y-axis (% Max) represents the percentage of total gated cells at a given fluorescent intensity (represented on the x-axis). At 5 weeks in culture prMCs (≥ 95% total cells) expressed CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors. Black lines note cell populations treated with antigen-specific antibody; white lines note cell populations treated with isotype-matched control. (B) PCR SS cDNA evidence that 5-week-old prMCs are strongly susceptible to R5-tropic (HIVBAL) but only marginally susceptible to X4-tropic (HIVTYBE) by PCR; LAD-2 control cells were susceptible to both HIVBAL and HIVTYBE. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
PrMCs derived in vitro from CD34+ adult hematopoietic PPPs are susceptible to R5-tropic HIV. (A) FMF analysis of prMCs was performed by gating on populations of CD117/IgE dual-positive cells derived from adult CD34+ hematopoietic PPPs in serum-free mast cell medium. The y-axis (% Max) represents the percentage of total gated cells at a given fluorescent intensity (represented on the x-axis). At 5 weeks in culture prMCs (≥ 95% total cells) expressed CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors. Black lines note cell populations treated with antigen-specific antibody; white lines note cell populations treated with isotype-matched control. (B) PCR SS cDNA evidence that 5-week-old prMCs are strongly susceptible to R5-tropic (HIVBAL) but only marginally susceptible to X4-tropic (HIVTYBE) by PCR; LAD-2 control cells were susceptible to both HIVBAL and HIVTYBE. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
Mature MCs developed from circulating CD34+ PPPs are not susceptible to infection with HIV
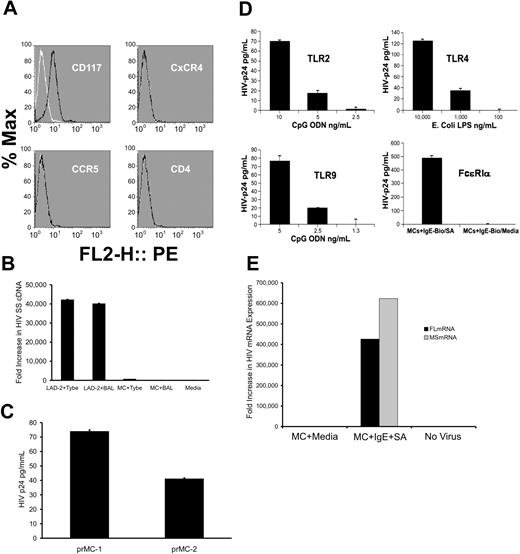
Circulating granulocytes with a progenitor mast cell/basophil phenotype have been detected in the circulation of HIV-infected persons with allergic disorders and have also been shown to be susceptible to infection with R5-tropic HIV-1.5 Basophils, unlike prMCs which ultimately leave the circulation and complete their maturation within extravascular tissues, require IL-3 for their propagation and development. Thus, prMCs were cultured using serum-free conditions in the presence of the essential MC growth factor, SCF, and in the absence of IL-3. By 8 to 9 weeks at least 95% of viable prMCs had routinely developed into homogenous phenotypically stable populations of FcϵRIα+, CD117+, CCR5−, CXCR4−, and CD4− mature MCs (Figure 2A).7 Results from real-time PCR measurements of viral SS cDNA indicated that these in vitro–derived mature MCs were highly resistant to infection with either HIVBAL (R5-tropic) or HIVTybe (X4-tropic) virus, unlike LAD-2 cells that were susceptible to both (Figure 2B). However, prMCs infected with HIVBAL at week 5 in culture developed into mature, productively infected MCs by 9 weeks in culture (Figure 2C). Thus, already infected prMCs were able to continue to develop into a stage of maturity with a phenotype that lacked HIV coreceptor expression and susceptibility to infection by virus de novo. By 12 weeks in culture, the productive HIV infection in mature MCs became latent, with undetectable levels of HIV-p24 by ELISA or MS or FL mRNA transcripts by PCR. Reactivation of latently infected mature MCs, however, demonstrated a dose-dependent response of viral replication and production of infectious virus after agonist stimulation by SA PGN (TLR2), E coli LPS (TLR4), CpG ODN (TLR9), or IgE cross-linking (Figure 2D-E). These in vitro–derived data thus support a mechanism whereby a MC reservoir of persistent HIV infection can be established in vivo.
Mature MCs derived in vitro from CD34+ adult hematopoietic progenitors are resistant to infection with HIV. (A) FMF analysis of (CD117+) mature MCs derived from adult CD34+ hematopoietic progenitors in serum-free mast cell medium at 9 weeks in culture in vitro showed a lack of surface expression of CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors. Black lines indicate cell populations treated with antigen-specific antibody; white lines indicate cell populations treated with isotype-matched control. (B) No detectable evidence by PCR of viral entry (SS cDNA) in mature MCs after challenge with either R5-tropic (HIVBAL) or with X4-tropic (HIVTYBE); LAD-2 control cells were susceptible to both HIVBAL and HIVTYBE. (C) Two separate cultures of productively infected HIV-infection resistant 9-week-old MCs express detectable levels of HIV p24 by ELISA. (D) Replication of infectious virus (HIV-p24+) could be reactivated in latently infected (HIV-p24 below detectable limits by ELISA) mature MCs. Activation of 13-week-old, latently infected mature MCs resulted in a dose-dependent elevation of HIV-p24 after signaling through TLR2, TLR4, TLR9, or after FcϵRIα coaggregation. (E) Latently infected 13-week-old mature MCs showed undetectable levels of HIV FL or MS mRNA that was significantly elevated (4-6 × 105-fold) after activation by IgE cross-linking. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
Mature MCs derived in vitro from CD34+ adult hematopoietic progenitors are resistant to infection with HIV. (A) FMF analysis of (CD117+) mature MCs derived from adult CD34+ hematopoietic progenitors in serum-free mast cell medium at 9 weeks in culture in vitro showed a lack of surface expression of CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors. Black lines indicate cell populations treated with antigen-specific antibody; white lines indicate cell populations treated with isotype-matched control. (B) No detectable evidence by PCR of viral entry (SS cDNA) in mature MCs after challenge with either R5-tropic (HIVBAL) or with X4-tropic (HIVTYBE); LAD-2 control cells were susceptible to both HIVBAL and HIVTYBE. (C) Two separate cultures of productively infected HIV-infection resistant 9-week-old MCs express detectable levels of HIV p24 by ELISA. (D) Replication of infectious virus (HIV-p24+) could be reactivated in latently infected (HIV-p24 below detectable limits by ELISA) mature MCs. Activation of 13-week-old, latently infected mature MCs resulted in a dose-dependent elevation of HIV-p24 after signaling through TLR2, TLR4, TLR9, or after FcϵRIα coaggregation. (E) Latently infected 13-week-old mature MCs showed undetectable levels of HIV FL or MS mRNA that was significantly elevated (4-6 × 105-fold) after activation by IgE cross-linking. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
MCs isolated from placental tissues are long-lived and are HIV-infection resistant
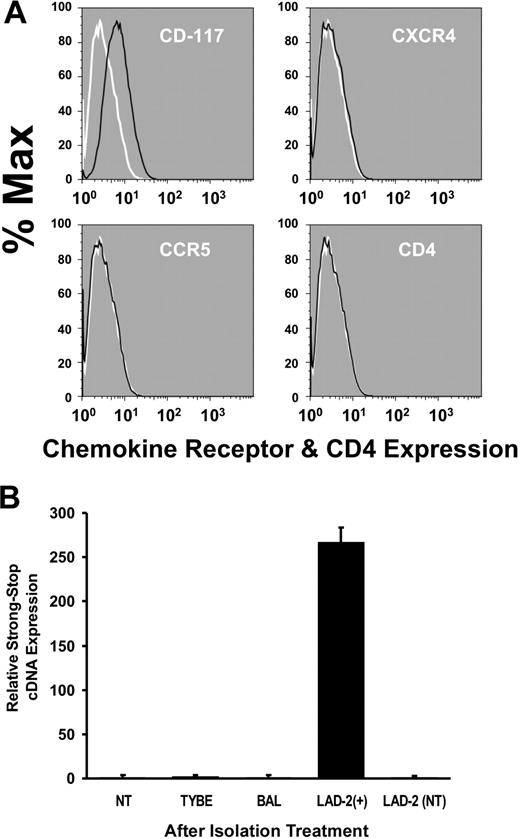
In vivo, HIV-susceptible prMCs receive diverse maturation signals after they leave the circulation and extravasate into different tissue compartments where they may differentiate into HIV infection–resistant mature MCs. Therefore, to confirm our in vitro findings, mature tissue MCs were isolated from fresh placental tissues (n = 6) collected from HIV− donors using IgE-biotin–positive selection and phenotyped within 24 hours (n = 3) or cultured in serum-free MC medium for an additional 7 to 14 days for further enrichment before phenotyping (n = 3). TEM and laser scanning confocal microscopic imaging (≥ 3 experiments) revealed that isolated and in vitro–cultured PLMCs were typically IgE/CD117 dual positive (≥ 83% total cells), exhibited an ovoid nucleus, multiple tryptase-positive cytoplasmic granules, an irregular outer cellular membrane characterized by microvilli, and surface expression of CD117 (Figure 3A-C). IgE-biotin–pulsed PLMCs subjected to FMF immediately following isolation were also IgE/CD117 dual positive and did not express CXCR4, CCR5, or CD4 (Figure 4A). Absence of chemokine receptor expression was confirmed by PCR measurements of mRNA (data not shown). Furthermore, results from real-time PCR measurements of viral SS cDNA indicated that PLMCs, isolated within 24 hours from placental tissues harvested at term (n = 3) and then experimentally challenged with HIV, were also resistant to infection by both HIVBAL (R5-tropic) and HIVTybe (X4-tropic) virus (Figure 4B). These results indicated that not only can living mature MCs be isolated from placental tissues, cultured, and phenotyped but also confirmed that MCs from this tissue compartment appear similar to PPP-derived in vitro–cultured mature MCs in their resistance to infection with HIV.
Mature PLMCs can be isolated from human placental tissues. TEM imaging revealed that isolated placental MCs typically exhibited (A) an ovoid nucleus and an irregular outer cellular membrane characterized by microvilli, and (B) TEM image showing multiple immunoperoxidase-positive cytoplasmic granules indirectly stained using a mast cell tryptase-specific monoclonal antibody. (C) Laser scanning confocal microscopic imaging confirmed that isolated PLMCs cultured in the presence of SCF are positive for cell surface–expressed CD117 (PE in red) and mast cell tryptase (FITC in green).
Mature PLMCs can be isolated from human placental tissues. TEM imaging revealed that isolated placental MCs typically exhibited (A) an ovoid nucleus and an irregular outer cellular membrane characterized by microvilli, and (B) TEM image showing multiple immunoperoxidase-positive cytoplasmic granules indirectly stained using a mast cell tryptase-specific monoclonal antibody. (C) Laser scanning confocal microscopic imaging confirmed that isolated PLMCs cultured in the presence of SCF are positive for cell surface–expressed CD117 (PE in red) and mast cell tryptase (FITC in green).
PLMCs are HIV infection resistant. (A) FMF analysis of IgE-selected and purified (CD117+ in green). PLMCs revealed a lack of cell surface–expressed CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors relative to controls (in red). Black lines demarcate cell populations treated with antigen-specific antibody; white lines demarcate cell populations treated with isotype-matched control. (B) No detectable evidence by PCR of viral entry (SS cDNA) in PLMCs after challenge with either R5-tropic (HIVBAL) or with X4-tropic (HIVTYBE); HIVBAL-infected LAD-2 + cells or non (virus) treated (NT) were included as controls. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
PLMCs are HIV infection resistant. (A) FMF analysis of IgE-selected and purified (CD117+ in green). PLMCs revealed a lack of cell surface–expressed CD4, CCR5, and CXCR4 (HIV) viral receptors and coreceptors relative to controls (in red). Black lines demarcate cell populations treated with antigen-specific antibody; white lines demarcate cell populations treated with isotype-matched control. (B) No detectable evidence by PCR of viral entry (SS cDNA) in PLMCs after challenge with either R5-tropic (HIVBAL) or with X4-tropic (HIVTYBE); HIVBAL-infected LAD-2 + cells or non (virus) treated (NT) were included as controls. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
PLMCs isolated from HIV-infected women harbor infectious HIV
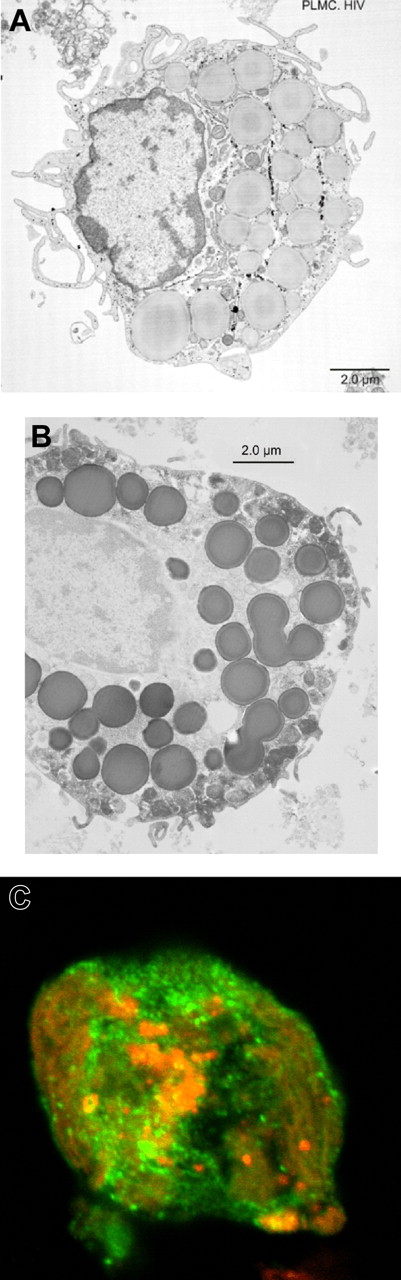
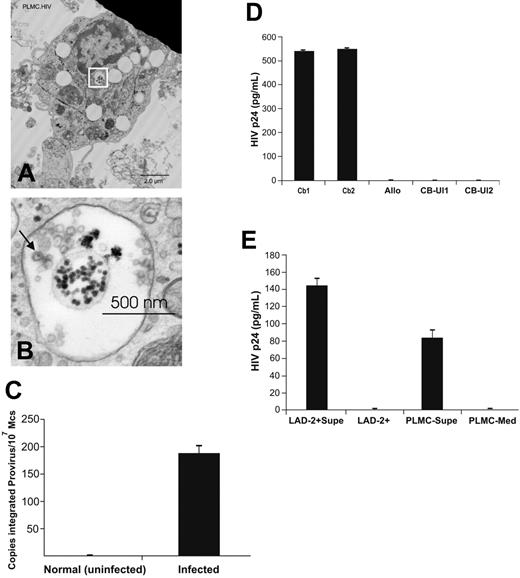
Having shown that, in our system, PLMCs lack HIV chemokine coreceptors and are infection resistant, the question of whether HIV-infected mature tissue MCs could be isolated from placentas donated by HIV-infected pregnant women was addressed. PLMCs were isolated from placentas collected at term (n = 3) from HIV-infected pregnant women on HAART. Representative electron photomicrographs of isolated PLMCs revealed the presence of 90- to 100-nm diameter virions within apparent cytoplasmic multivesicular bodies (MVBs) (Figure 5A-B). In addition, real-time PCR analysis provided evidence that isolated PLMC genomic DNA contained integrated HIV proviral cDNA (Figure 5C).
PLMCs isolated from infected placentas harbor infectious HIV virions within MVBs. (A) TEM imaging of PLMCs isolated from HIV+ women at term showed cytoplasmic MVBs (white box) containing (B) apparent 90-nm diameter HIV virions with typical nucleocapsid core particles (indicated by arrow). (C) DNA from IgE-biotin–selected PLMCs isolated from HIV+ women at term showed detectable levels of integrated HIV proviral DNA by PCR. (D) IgE-biotin–selected PLMCs isolated from HIV+ women at term and cultured in the presence of SA (to induce IgE cross-linking–mediated PLMC activation) release infectious virus that is able to infect (allo-) activated cord blood (CB) mononuclear cells. CB1 and CB2 represent 2 independent cord blood samples collected from HIV− fetal donors; allo, γ-irradiated allogeneic PBMCs stimulator cells;CBUI1 and CBUI2, results from uninfected CB cells. (E) IgE-biotin–selected PLMCs isolated from HIV+ women at term and cultured in the presence of SA released significant levels of HIV-p24+ virus (by day 6 after activation) that was used to infect susceptible LAD-2 MCs. Elevated levels of HIV-p24 (∼ 145 pg/mL) were detected in culture supernatant fluids of infected LAD-2 at day 7 after infection. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
PLMCs isolated from infected placentas harbor infectious HIV virions within MVBs. (A) TEM imaging of PLMCs isolated from HIV+ women at term showed cytoplasmic MVBs (white box) containing (B) apparent 90-nm diameter HIV virions with typical nucleocapsid core particles (indicated by arrow). (C) DNA from IgE-biotin–selected PLMCs isolated from HIV+ women at term showed detectable levels of integrated HIV proviral DNA by PCR. (D) IgE-biotin–selected PLMCs isolated from HIV+ women at term and cultured in the presence of SA (to induce IgE cross-linking–mediated PLMC activation) release infectious virus that is able to infect (allo-) activated cord blood (CB) mononuclear cells. CB1 and CB2 represent 2 independent cord blood samples collected from HIV− fetal donors; allo, γ-irradiated allogeneic PBMCs stimulator cells;CBUI1 and CBUI2, results from uninfected CB cells. (E) IgE-biotin–selected PLMCs isolated from HIV+ women at term and cultured in the presence of SA released significant levels of HIV-p24+ virus (by day 6 after activation) that was used to infect susceptible LAD-2 MCs. Elevated levels of HIV-p24 (∼ 145 pg/mL) were detected in culture supernatant fluids of infected LAD-2 at day 7 after infection. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
To confirm that these tissue MCs harbored “infectious” virus however, immunomagnetically selected IgE-biotin–positive PLMCs were then cultured for 6 days in serum-free MC media in the presence or absence of SA (125 ng/mL) to potently induce IgE cross-linking and reactivation of HIV-replication in latently infected PLMCs, analogous to latently infected mature MCs derived in vitro from CD34+ PPPs (Figure 2D). Supernatant fluids collected from both SA-treated and -untreated PLMC cultures were used to infect LAD-2 cells and alloactivated CBMCs, isolated from uninfected donors as described in “Materials and methods” (≥ 3 experiments). Supernatant fluids from LAD-2 and CBMC cultures at day 7 after infection and from PLMCs (at day 12 in culture) were then collected and assayed for HIV-p24 by ELISA. Elevated levels of p24 were measured in supernatant fluids from infected CBMC cultures (∼ 550 pg/mL), LAD-2 cells (∼ 145 pg/mL), and SA-treated PLMC cultures (∼ 84 pg/mL) but not SA-untreated PLMCs, indicating that PLMCs isolated from HIV-infected placentas were latently infected with replication competent, infectious HIV provirus (Figure 5D-E, representative of 3 separate experiments). Thus, HIV infection–resistant PLMCs can harbor inducible HIV capable of infecting not only susceptible CD4+ MCs (LAD-2) but also fetal CBMCs, suggesting their potential role in mother-to-child-transmission (MTCT) of HIV infection. Furthermore, latently infected PLMCs were isolated from some placenta donors on HAART with plasma viral loads fewer than 400 copies of HIV-1 RNA/mL (described in the “Materials and methods”). Thus, the MC reservoir of persistent HIV infection is maintained even during effective HAART.
Circulating prMCs isolated from blood of uninfected third-trimester pregnant women are susceptible to R5 and marginally susceptible to XR-tropic virus
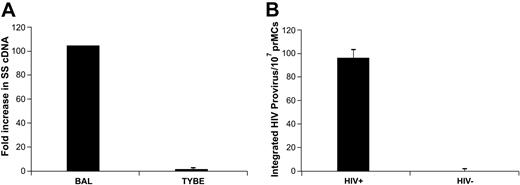
The rationale for choosing the placenta as a MC-rich tissue compartment was based on the hypothesis that increased numbers of MCs are recruited to populate this newly developing organ. On the basis of this reasoning and our findings of well-characterized mature PLMCs, we next looked for circulating prMCs in pregnant women. Mononuclear cells isolated from whole blood collected from late third-trimester pregnant women were primed with IgE-biotin, then subjected to immunomagnetic-positive selection. Isolated prMCs were cultured for 4 to 6 days in serum-free MC medium before immunophenotyping. IgE-selected CD117+ cultured prMCs were positive for cell surface–expressed CD4, CXCR4, and CCR5 (data not shown). Furthermore, cultured prMCs experimentally challenged with HIV were susceptible to HIVBAL (R5-tropic) and marginally susceptible to HIVTybe (X4-tropic) virus (Figure 6A). These results, consistent with those from prMCs cultured in vitro from adult CD34+ PPPs, demonstrate that prMCs present in the circulation are susceptible to infection with HIV and are available to extravasate and mature within developing placental tissues during pregnancy.
HIV-susceptible prMCs can be isolated from the circulation of pregnant women. (A) PCR evidence of viral entry (SS cDNA) shows that circulating prMCs (≥ 83% total cells), isolated from late third-trimester HIV-negative pregnant women, are strongly susceptible to R5-tropic HIVBAL but only marginally susceptible to X4-tropic HIVTybe in (B) PCR evidence that circulating prMCs isolated from HIV+ pregnant women at term harbor integrated (HIV) proviral cDNA. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
HIV-susceptible prMCs can be isolated from the circulation of pregnant women. (A) PCR evidence of viral entry (SS cDNA) shows that circulating prMCs (≥ 83% total cells), isolated from late third-trimester HIV-negative pregnant women, are strongly susceptible to R5-tropic HIVBAL but only marginally susceptible to X4-tropic HIVTybe in (B) PCR evidence that circulating prMCs isolated from HIV+ pregnant women at term harbor integrated (HIV) proviral cDNA. All experiments were performed a minimum of 3 times. All error bars represent standard deviation of the mean of replicate PCR reactions at each data point.
Circulating prMCs isolated from blood of HIV-infected third-trimester women are infected with HIV
Circulating prMCs were isolated and cultured in a similar manner from HIV-infected late third-trimester pregnant women (n ≥ 3). After 6 days in culture in serum-free MC medium, cellular DNA was isolated from prMCs, and real-time PCR analysis was used to confirm the presence of integrated proviral DNA (Figure 6B). These data confirm not only that HIV-susceptible, circulating prMCs are present in women during late stages of their pregnancy but also that HIV-infected prMC can be isolated from the circulation of infected pregnant women. These results provide evidence that the tissue MC reservoir of persistent HIV infection is likely created by susceptible circulating prMCs that become infected before extravasation and maturation in different tissue compartments.
Discussion
Clinical strategies to confront the problem of HIV persistence during therapy fundamentally involve knowledge of the cell lineages that can become persistently infected. A variety of susceptible non–T-cell lineages, including macrophages, natural killer cells, astrocytes, microglia, B cells, and dendritic cells have been identified, and each have been considered as potential sanctuaries of persistent infection.12–16 To date, however, macrophages and memory T cells are considered the most significant stable reservoirs of persistent HIV, based mainly on their longevity, wide tissue distribution, and ability to harbor replication-competent virus.17 The evidence presented in this report supports MCs as an important new member of this special class of HIV cellular reservoirs and also describes a unique model of how the MC reservoir of HIV infection is established in vivo. Two key features of this proposed model are that (1) circulating prMCs but not mature tissue MCs are readily susceptible to infection with HIV and (2) prMCs become infected as they encounter plasma-borne virus. Thus, creation of the MC reservoir would appear to be dependent on the active recruitment of circulating susceptible prMCs during infection. However, our findings that PLMCs phenotypically resemble mature HIV infection–resistant in vitro–cultured MCs may not universally hold for MCs found in all tissue compartments. Indeed, it has recently been reported that mouse intestinal MCs display a progenitor phenotype.18,19 In this context our data suggest that perhaps in some tissue compartments MCs may be susceptible to infection and yet still have the potential to develop HIV-infection resistance. If in vivo this is the case, our model would predict a larger more extensive MC reservoir of persistent HIV infection. However, the expression of functional chemokine coreceptors by PLMCs and prMCs in vivo may vary between different persons, thus potentially limiting the size and distribution of the MC reservoir in certain infected persons.
Increased levels of circulating prMCs, basophils, and eosinophils have been associated with active allergy and IgE-dependent responses to parasitic infections.20 There have been many reports of persons infected with HIV who are also coinfected with Schistosoma mansoni.21 HIV infection has been reported to be associated with allergic responses to drugs,22 and HIV-susceptible circulating cells with a progenitor mast cell/basophil phenotype have been identified in HIV-infected patients with allergic disorders.5 Thus, IgE-mediated allergy in HIV-infected persons provides a favorable environment for the creation of a MC reservoir of persistent infection. However, in this investigation we undertook the effort to make a stronger case for MCs as an HIV reservoir by studying HIV-infected persons without clinical evidence of allergy. The ability of the MC reservoir to become established in the absence of active allergy is clinically significant because it underscores the fact that prMC recruitment is an ongoing physiologic process that occurs both in the presence and absence of allergic disease, thus increasing the likelihood of the presence of this cellular reservoir of persistent viral infection in multiple tissue compartments. In this regard, it is important to note that the gut-associated lymphoid tissues (GALT) are the major targets of acute HIV-1 infection, and it is becoming increasingly clear that initial events of acute HIV infection dictate the disease course.23 MC progenitors are abundant in murine intestinal tissues19 ; however, the role of prMCs during acute HIV infection and subsequent GI dysregulation in humans has been the least studied. It is also evident that even aggressive HAART does not completely eliminate virus and does not fully reconstitute GALT.24,25
However, isolation and characterization of tissue MCs in vitro has been difficult. To meet this challenge, placental tissues were selected as a potential repository for mature tissue MCs for the following reasons: (1) MC recruitment to the newly developing placenta would attract migrating HIV-infected prMCs; (2) placentas are large temporary organs that are relatively easy to collect at term and are a source of large numbers of tissue MCs6 ; (3) according to our model, placentas from HIV-infected pregnant women would likely harbor HIV-infected mature tissue MCs. The fact that the production of infectious virus could be induced from HIV infection–resistant PLMCs that could be cultured for weeks to months in vitro offers compelling support for this model and has important implications for HIV persistence. Although the physiologic role of PLMCs has not been clearly defined, their newly described functional relationship with regulatory T cells26 may suggest their role in maintaining tolerance of the fetal allograft. It is also conceivable that this potential ability of MCs to collaborate with regulatory T cells in suppressing alloresponses may also indirectly contribute to their protection from HIV-specific antiviral cellular immune responses. Furthermore, the fact that virus from reactivated latently infected PLMCs was able to infect alloactivated fetal CBMCs suggests a potential role for MCs in MTCT.
Tissue MCs, like tissue macrophages, originate from circulating progenitors and complete their maturation after extravasation into different tissue compartments. However, mature tissue MCs, unlike macrophages, no longer express significant levels of viral chemokine coreceptors and have the potential to become resistant to infection with HIV (Figures 2 and 4A-B). Thus, HIV-infected tissue MCs may harbor and hence are potentially able to produce an archival clone of virus with which they were originally infected during their relatively brief tenure as susceptible circulating progenitors. This is because (1) virus is able to integrate into unactivated prMCs which mature into latently infected tissue MCs, thus minimizing levels of unintegrated virus and (2) there is no new RT, thus minimizing mutations because of RT errors and recombination between genomic viral RNA. Our data show that infected 9- to 10-week-old MCs derived in vitro from adult PPPs are HIV infection resistant (Figure 2A-B), supporting the observation that viral RT activity is absent in productively infected mature MCs. Furthermore, because (1) infected mature MCs are long lived within tissues with limited exposure to ART, (2) viral assembly appears to occur within cytoplasmic MVB (Figure 5A-B) (similar to macrophages, thus limiting their exposure to antiviral immunity), and (3) viral replication does not cause apparent cytopathic effects in MCs,4 they are able to serve as natural sources of residual viremia in the absence of viral evolution. In this context, viral fitness would be determined by the ability of the virus to infect susceptible circulating prMCs during recruitment, thus establishing a new stable reservoir of HIV-infected tissue MCs. Viral tropism and competition of different quasi-species for infection of prMCs may thus be influenced by such factors as different prMC recruitment environments, inflammatory cytokines, and IgE-mediated allergy. Note that prMCs appear significantly more susceptible to R5-tropic than X4-tropic HIV, despite their expression of CXCR4. It could be that exposure to such environmental factors in vivo, particularly IgE, may enhance the susceptibility of prMCs to X4-tropoic and dual-tropic virus.
Another significant feature of this model is that latent infection, which is readily established in HIV infection–resistant tissue MCs, can be reinitiated via agonist stimulation mediated through multiple TLR as well as FcϵRIα-mediated signal transduction pathways and perhaps many more yet undescribed physiologically relevant pathways. Thus, both latently infected and productively infected (HIV infection–resistant) mature MCs responding to environmental signals and serving as natural effectors of innate immunity or allergy, are a potential source of clonally conserved infectious virus. This unique characteristic of the MC reservoir may offer support and insight to a recent report of residual viremia from invariant clones not found in circulating T cells in some patients on ART.27 Bailey et al27 postulate that the source of these persistent invariant clones may be a monocyte-macrophage lineage progenitor capable of producing virus that can infect CD4+ T cells. We have shown that PLMCs isolated from HIV-infected women produce virus capable of infecting CD4+ LAD-2 and CD4+ CBMCs (Figure 5D-E). Furthermore, it is likely that in vivo, infected HIV infection–resistant MCs are capable of limited proliferation and expansion, thus multiplying HIV archival proviral clones in the reservoir. These observations thus have important clinical implications not only for the role of MCs in MTCT of HIV and in mucosal HIV infection but also for clinical strategies to eliminate this stable reservoir of persistent HIV infection. For instance, in the context of long-lived, latently HIV-infected, and -resistant tissue MCs, we must think beyond current chemotherapeutic strategies that target reverse transcription and perhaps explore new approaches to “prevent” rather than induce reactivation of certain populations of latently infected cells. To this end, MCs may serve as an important and useful model for understanding how latency is established at the cellular level and the relation between ontogeny and susceptibility to productive and latent infections.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the patients and the clinical staff from Emory University's Department of Gynecology and Obstetrics and from the Grady Memorial Hospital Specialty Obstetrics Clinic for HIV+ women in Atlanta, GA, who were involved in this study.
This work was supported in part by a grant from the National Institutes of Health (AI062383) (J.B.S.).
National Institutes of Health
Authorship
Contribution: H.Y. and A.C.C. performed research and collected data; M.K.L. supervised clinical studies; A.S.K. and D.D.M. analyzed and interpreted data, contributed vital new reagents, and provided critical reading of this manuscript; G.A.H. performed research, analyzed and interpreted data, and provided critical reading of this manuscript; J.E.E. performed research, analyzed and interpreted data, and supervised clinical studies; A.A.A. provided mentorship, analyzed and interpreted data, and provided critical reading of this manuscript; J.B.S. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Bruce Sundstrom, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, WMB Rm 2335, 101 Woodruff Cir, Atlanta, GA 30322; e-mail: jsundst@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal