Abstract
The primary inhibitor of plasmin, α2-antiplasmin (α2AP), is secreted by the liver into plasma with Met as the amino-terminus. During circulation, Met-α2AP is cleaved by antiplasmin-cleaving enzyme (APCE), yielding Asn-α2AP, which is crosslinked into fibrin approximately 13 times faster than Met-α2AP. The Met-α2AP gene codes for either Arg or Trp as the sixth amino acid, with both polymorphic forms found in human plasma samples. We determined the Arg6Trp genotype frequency in a healthy population and its effects on Met-α2AP cleavage and fibrinolysis. Genotype frequencies were RR 62.5%, RW 34.0%, and WW 3.5%. The polymorphism related to the percentage of Met-α2AP in plasma was WW (56.4%), RW (40.6%), and RR (23.6%). WW plasma tended to have shorter lysis times than RR and RW plasmas. APCE cleaved purified Met-α2AP(Arg6) approximately 8-fold faster than Met-α2AP(Trp6), which is reflected in Asn-α2AP/Met-α2AP ratios with time in RR, RW, and WW plasmas. Removal of APCE from plasma abrogated cleavage of Met-α2AP. We conclude that the Arg6Trp polymorphism is functionally significant, as it clearly affects conversion of Met-α2AP to Asn-α2AP, and thereby, the rate of α2AP incorporation into fibrin. Therefore, the Arg6Trp polymorphism may play a significant role in governing the long-term deposition/removal of intravascular fibrin.
Introduction
The serine proteinase inhibitor, α2-antiplasmin (α2AP), is a member of the serpin family, with plasmin as its primary target. Plasmin, generated from the zymogen plasminogen, plays a critical role in fibrin proteolysis and tissue remodeling.1 To prevent excessive proteolysis, regulation of plasminogen activators and plasmin inhibitors must occur. α2AP has been shown to be the most important inhibitor of plasmin, forming an irreversible inactive complex in what has been described as among the fastest proteinase-inhibitor reactions in biology.2–4 α2AP is secreted into plasma as an approximately 70-kDa single polypeptide chain of 464 amino acids with Met as the amino-terminus.5 During circulation in plasma, α2AP undergoes proteolytic cleavage between Pro12-Asn13 to yield a slightly shortened version, with Asn as the amino-terminus.6 We have shown in vitro that the amino-terminally shortened Asn-α2AP is crosslinked into fibrin approximately 13 times faster than its precursive form and that plasma clot lysis time is increased inversely to the Met-α2AP/Asn-α2AP ratio.7 The enzyme responsible for this cleavage was unknown until isolated and characterized in our laboratory, ultimately being termed antiplasmin cleaving enzyme (APCE) by us.7 We have since shown that APCE is essentially a soluble form of fibroblast activation protein (FAP), a type II integral membrane protein of the prolyl oligopeptidase family.8
When the Met-form of α2AP was found in plasma and its gene sequenced, there initially appeared to be a discrepancy in one of the nucleotides encoding the sixth amino acid. Two groups found a cytidine (C), resulting in Arg as the sixth amino acid, and one group found thymidine (T), resulting in Trp at that position.9–11 It was suggested that the difference was due to one group having used liver carcinoma cells as a source of DNA, while the other 2 groups used normal cells. It now has been determined that both Arg6 and Trp6 forms of Met-α2AP exist in healthy human plasma samples. An investigation of a mutant α2AP in a family with bleeding tendencies identified the mutation responsible for the ineffective α2AP along with 3 polymorphisms in the α2AP gene, including this C/T single nucleotide polymorphism (SNP); this study examined 30 healthy blood donors and reported an allelic frequency of 0.81/0.19 for the C/T SNP.12 No larger studies of a healthy population have been done to examine the frequency of homozygotes and heterozygotes, or whether genotype might affect ratios of Met- to Asn-α2AP in plasma. The Arg6Trp SNP apparently was assumed to be a silent polymorphism, but biochemical examination of the 2 polymorphic forms of Met-α2AP on yielding the derivative form, Asn-α2AP, its incorporation into fibrin and the impact on fibrinolysis have never been assessed. In this study, we first determined the prevalence of the polymorphism in a much larger healthy population and then assessed whether it relates to the inhibitory function of α2AP. We now report (1) genotype frequencies of the Arg6Trp SNP in Met-α2AP; (2) how each form affects cleavage by APCE; (3) the percent of Met-α2AP in plasma for each of the 2 polymorphisms; (4) plasma clot lysis times in relation to genotype; and (5) that removal of circulating APCE prevents conversion of Met- to Asn-α2AP.
Materials and methods
Materials
Fresh frozen human plasma for the purification of proteins was purchased from the Sylvan Goldman Blood Institute (Oklahoma City, OK). Hybridoma cells secreting the F19 antibody were purchased from American Type Culture Collection (ATCC) (Manassas, VA) and grown in serum-free media; the F19 antibody was purified from culture media using MEP-Hypercel chromatography (Pall, East Hills, NY). Institutional review board (IRB) approval was obtained from University of Oklahoma Health Sciences Center for these studies (IRB #10142 and 12189).
Isolation of α2AP
Mixtures of Met-α2AP and Asn-α2AP were isolated by a modification7 of a published purification procedure using plasminogen kringles 1-3 attached to sepharose 4B as an affinity matrix.13 Met-α2AP and Asn-α2AP were separated by immunoaffinity chromatography as previously described.7 The ratio of Met-α2AP to Asn-α2AP in plasma samples was determined by comparison of picomole recovery of Met versus Asn in cycle one during automated protein sequencing by Edman degradation (Applied Biosystems Procise model 492, Foster City, CA).
Isolation of APCE
APCE was purified from human plasma as previously described.8 Briefly, a combination of ammonium sulfate precipitation, hydrophobic interaction, and immunoaffinity chromatography were used for purification. Before storing at −80°C, glycerol was added to the pure APCE to give a final concentration of 20%.
Determination of α2AP genotype
Two hundred randomly responding healthy volunteers, self-reported as healthy and free of acute illness, were recruited to donate blood for determination of genotype frequency and plasma clot lysis time (PCLT; see next section) in a normal population. DNA was isolated from whole blood of each donor using the AquaPure Genomic DNA Blood Kit (Bio-Rad, Hercules, CA). The portion of DNA encompassing the Arg6Trp SNP was amplified by polymerase chain reaction using oligonucleotide primers (5′-GACCTCCTATCCTCATCCCTTT and 5′-CTGGTTCGGCCCGCTAGTTAG), dNTPs (Takara Mirus Bio, Madison, WI), and Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). Following amplification, the polymerase chain reaction (PCR) product was purified, using the MinElute PCR Purification Kit (Qiagen Operon, Alameda, CA) and sequenced, using an ABI3730 automated DNA sequencer.
Measurement of plasma clot lysis
To measure plasma clot lysis time, a mixture of 1 U/mL thrombin, 16 mM CaCl2, and 45 IU/mL urokinase (uPA) (Abbott, Chicago, IL) was added to each volunteer's plasma to catalyze essentially instant fibrin clot formation and to initiate fibrinolysis; the rate of plasma clot lysis was determined by a turbidimetric microtiter plate method.14–16
Determination of cleavage rates
Reaction mixtures containing equal amounts (40 μg) of pure Met-α2AP(Arg6) and pure Met-α2AP(Trp6) were digested by APCE. At selected times the digestion was stopped by decreasing the pH from 7.5 to 4.0 with trifluoroacetic acid. Proteins were removed from the mixture by Microcon (Millipore, Bedford, MA) centrifugal ultrafiltration using a 30-kDa cutoff membrane. The peptides were isolated from the ultrafiltered digestion mixture by binding to POROS-50 reversed-phase media (Applied Biosystems, Foster City, CA) packed into a glass purification capillary (Proxeon, Odense, Denmark). The peptides were then eluted from the POROS-50 directly into a metal coated glass nanospray capillary with 2.0 μL of 0.5% acetic acid in 1:1 methanol/water. The nanospray capillary was mounted on the nanospray ionization source of a QSTAR ESI-Quad-TOF mass spectrometer operated under Analyst QS software version 1.0 (Applied Biosystems) with an ionspray voltage of 1400 volts. Data were collected over a mass range of 300 to 1500 Da. The relative quantities of the 2 α2AP amino-terminal 12-amino acid peptides produced, MEPLGRQLTSGP, Mr = 1284.65 for Met-α2AP(Arg6) and MEPLGWQLTSGP, Mr = 1314.63 for Met-α2AP(Trp6), were determined by summing the areas of the 4 most abundant isotope peaks for the observed charge forms for each peptide. The data for each time point was normalized by a similar quantification of an added inert internal standard peptide that contained no proline.
Determination of APCE antigen level
An enzyme-linked immunosorbant assay (ELISA) was developed to determine antigen levels in human plasma. A goat antibody to the amino-terminal 15-amino acid sequence of APCE was prepared, using as the immunogen a multiple antigenic peptide (MAP) constructed in our laboratory to contain 8 copies of the amino-terminal peptide linked via their carboxyl-termini to a core peptide of 7 lysines.17 This goat MAP (amino-terminal 15 residue APCE peptide) antibody was bound to white high-binding polystyrene assay plates (Corning, Corning, NY) and used as the capture antibody. After incubation with dilutions of plasma, a monoclonal antibody purified from commercially available F19 hybridomas (ATCC) was applied, followed by peroxidase conjugated antimouse antibody (Sigma, St Louis, MO). A chemiluminescent substrate, SuperSignal ELISA Pico (Pierce, Rockford, IL), was added, and luminescence was monitored using a BIO-TEK FL600 plate reader (Winooski, VT). Antigen level was quantitated using purified human APCE as the standard.
Removal of APCE from plasma
The F19 mAb was linked to POROS EP 20 poly(styrenedivinylbenzene) perfusion chromatography beads (Applied Biosystems) and a nonspecific antibody, rabbit antigoat, was linked to the same type of media. Plasma from a single donor of the RR genotype was divided into 3 aliquots, diluted 1:1 with phosphate buffered saline (PBS) and incubated separately with each of the 2 bead-linked antibodies, nonspecific Ab or the F19 mAb, overnight at 4°C. The third aliquot received no treatment. The beads were removed from the plasma by filtration, and the plasma was then incubated at 29°C. Aliquots were removed at zero time, 24 hours, and 48 hours. α2AP was purified from each aliquot, and the Asn-α2AP/Met-α2AP ratio was determined as previously described. After removal from the plasma, the F19 mAb beads were washed with 25 mM Na PO4/0.5 M NaCl and then boiled with sodium dodecyl sulfate (SDS) loading buffer. The SDS buffer extract was then separated by electrophoresis on a 10% Bis-Tris gel (Invitrogen, Carlsbad, CA) and blotted to nitrocellulose. APCE was identified by Western blotting using the goat amino-terminal MAP antibody as described previously in this Methods section and visualized using SuperSignal West Femto Maximum Sensitivity Chemiluminescent Substrate (Pierce).
Results
α2AP genotype determination
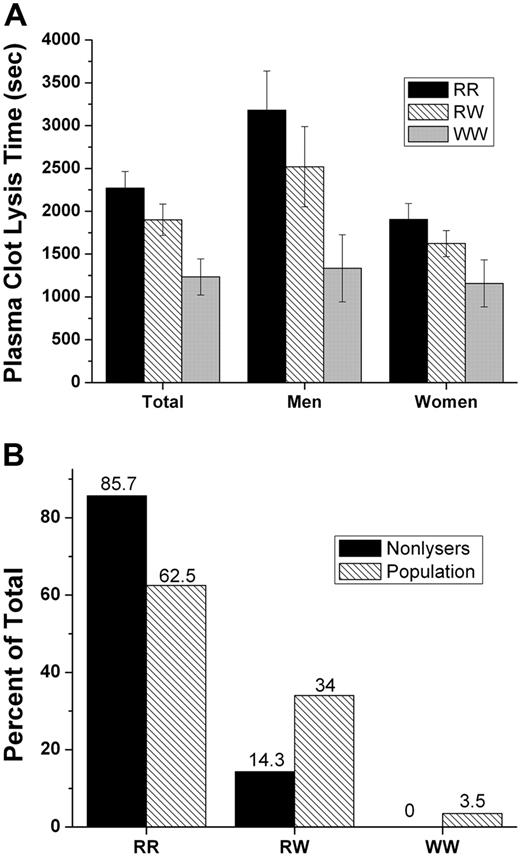
A group of 201 healthy volunteers, who were recruited in 2 sets of approximately 100 people each, separated by about 2 years, provided blood samples for determining the α2AP C/T SNP frequency. The total population consisted of 61 men and 139 women 21 to 69 years of age, with an ethnicity that closely matched the demographics for an Oklahoma population as listed for the year 2000 on the US Census web site (factfinder.census.gov). Genotype was determined for 200 of the subjects. Only one DNA sample did not amplify by polymerase chain reaction, possibly due to a mutation that prevented binding of one of the primers, although this has not been further explored. The genotype frequencies for the 2 normal populations were essentially the same, with less than 1% difference for any of the genotypes. After combining the 2 sets, the frequencies for the entire population were RR 62.5%, RW 34.0%, and WW 3.5%. The R allele had a frequency of 79.5%, and the W allele had a frequency of 20.5%. There was no difference in the genotype frequency between men and women. Because the population was 72% Caucasian, with the other 28% split between 6 ethnic categories, it was not possible to determine whether genotype varied among ethnic groups.
Met-α2AP and Asn-α2AP levels in plasma
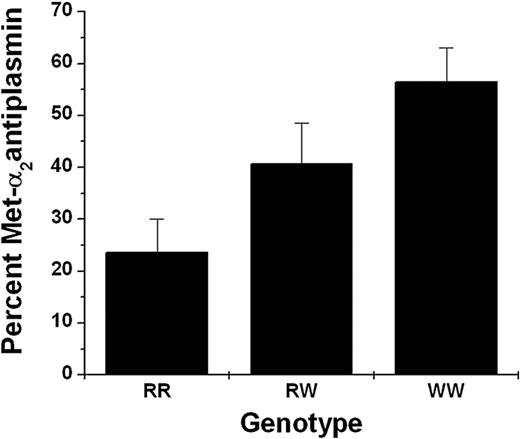
α2AP was purified from the plasmas of 15 persons of the RR genotype, 15 persons with RW, and 5 with WW genotype. The α2AP was sequenced by Edman degradation, and picomole recoveries of Met and Asn were determined for the first cycle to calculate the percent of Met-α2AP and Asn-α2AP. Figure 1 shows significantly different (P < .001) results that partitioned by genotype with WW having the highest percentage of Met-α2AP (56.4%); RR, the least (23.6%); and RW falling in between (40.6%).
Met-α2AP as percent of total α2AP and by genotype. α2AP was purified from each plasma of persons with RR (n = 15), RW (n = 15), and WW (n = 5) genotypes and amino-terminal sequences determined by Edman degradation. Percent Met-α2AP was calculated from picomole recoveries of Met and Asn in the first cycle.
Met-α2AP as percent of total α2AP and by genotype. α2AP was purified from each plasma of persons with RR (n = 15), RW (n = 15), and WW (n = 5) genotypes and amino-terminal sequences determined by Edman degradation. Percent Met-α2AP was calculated from picomole recoveries of Met and Asn in the first cycle.
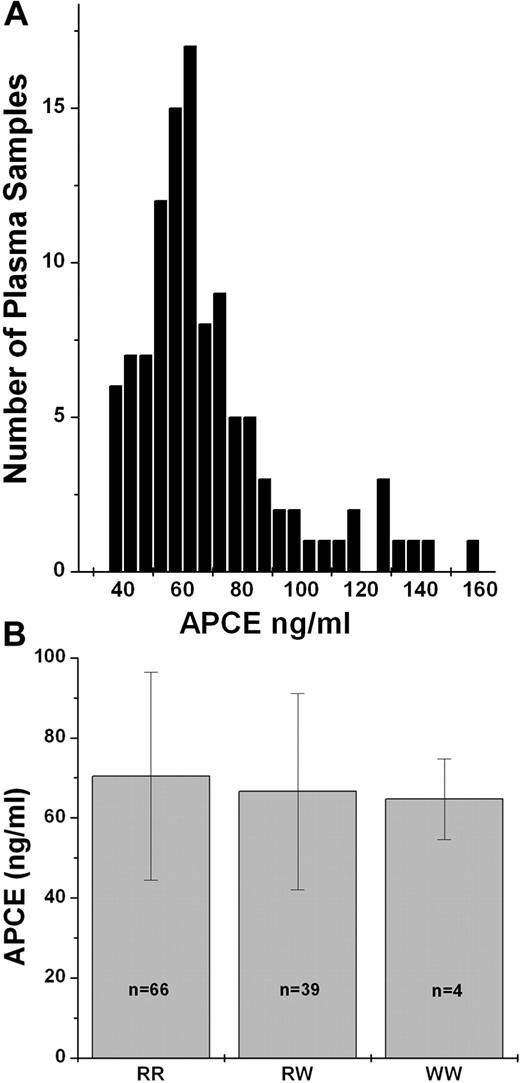
To understand the mechanism for the varying percentages of Met-α2AP in human plasma, we examined whether a variation in the level of enzyme that converts Met-α2AP to Asn-α2AP; that is, APCE, might explain the differences among the genotypes. To investigate this possibility, we developed an ELISA method for quantitating APCE antigen level in plasma and then determined APCE concentrations in plasma from 109 subjects in our normal population. As seen in Figure 2A, there is a distribution of APCE levels in normal human plasma, ranging from 38 to 159 ng/mL, that did not correlate with age or gender, and despite the suggestion of a possible association of APCE levels with genotype (RR, 70.4 ± 26; RW, 66.6 ± 24.5; WW, 64.7 ± 10.1), the differences were not statistically significant (Figure 2B). Therefore, APCE levels do not appear to account for the variation of Met-α2AP levels among genotypes.
Plasma APCE levels in a normal population and partitioned by genotype. APCE levels in plasma samples were determined by ELISA. (A) Histogram of APCE concentrations in a normal population (n = 109). (B) APCE levels by Met-α2AP genotype.
Plasma APCE levels in a normal population and partitioned by genotype. APCE levels in plasma samples were determined by ELISA. (A) Histogram of APCE concentrations in a normal population (n = 109). (B) APCE levels by Met-α2AP genotype.
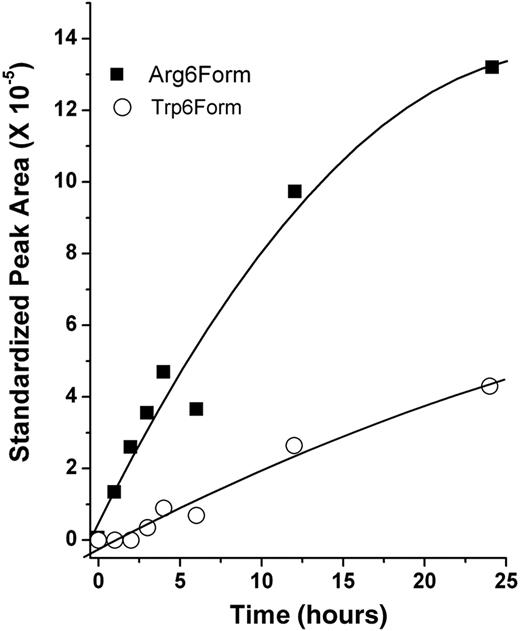
Another explanation for the different Met-α2AP percentages in the 3 genotypes might be that Met-α2AP(Arg6) is a better substrate for APCE than Met-α2AP(Trp6). To test this hypothesis, α2AP was purified from RR and WW plasma, and the cleavage rate of each polymorphic form, Met-α2AP(Arg6) and Met-α2AP(Trp6), was determined, using mass spectrometry to monitor the generation of the 12-residue amino-terminal peptide with time. As seen in Figure 3, comparisons of reaction rates were based on linear regression analysis of early time points and showed that APCE cleaves Met-α2AP(Arg6) approximately 8-fold faster than Met-α2AP(Trp6).
APCE cleavage of polymorphic forms of Met-α2AP. Equal amounts (40 μg) of purified Met-α2AP(Arg6) and Met-α2AP(Trp6) were digested by APCE. After stopping the reaction at selected times, samples were assessed by electrospray mass spectrometry for the quantity of the amino-terminal 12-amino acid peptide produced from each Met-α2AP form.
APCE cleavage of polymorphic forms of Met-α2AP. Equal amounts (40 μg) of purified Met-α2AP(Arg6) and Met-α2AP(Trp6) were digested by APCE. After stopping the reaction at selected times, samples were assessed by electrospray mass spectrometry for the quantity of the amino-terminal 12-amino acid peptide produced from each Met-α2AP form.
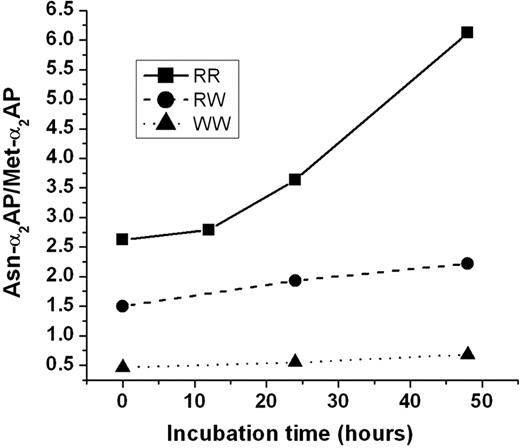
To examine whether the cleavage rate of Met-α2AP in whole plasma gave similar results based on the presence of R or W in position 6, fresh plasma samples from individuals whose genotype was determined to be RR, RW, and WW were obtained, incubated at 29°C, with each being analyzed at 0, 24, and 48 hours for Asn-α2AP/Met-α2AP ratios. α2AP was purified from each sample, and ratio of the 2 polymorphic forms was determined by picomole recovery of the amino-terminal residue in the first cycle of Edman degradation. As seen in Figure 4, Asn-α2AP increased at a much greater rate in plasma from a healthy volunteer of the RR genotype than in either the RW or WW genotype patients. These results obviously concur with those from reaction mixtures of pure α2AP and APCE that showed APCE cleaves Met-α2AP(Arg6) approximately 8-fold faster than Met-α2AP(Trp6).
Effect of Met-α2AP genotype on generation of Asn-α2AP in plasma with time. A plasma sample from a person of the RR, RW, or WW genotype was incubated at 29°C; at selected times α2AP was purified from each sample and subjected to amino-terminal sequence analysis. The ratio of Asn-α2AP/Met-α2AP was calculated from picomole recoveries of Met and Asn in the first cycle.
Effect of Met-α2AP genotype on generation of Asn-α2AP in plasma with time. A plasma sample from a person of the RR, RW, or WW genotype was incubated at 29°C; at selected times α2AP was purified from each sample and subjected to amino-terminal sequence analysis. The ratio of Asn-α2AP/Met-α2AP was calculated from picomole recoveries of Met and Asn in the first cycle.
Plasma clot lysis
Plasma clot lysis times (PCLTs) were determined on blood samples from the 200 women and men comprising the normal population. We did not attempt to control for variations of clot lysis that are known to occur due to activator release, activator-inhibitor interactions, fibrinogen level, or to effects of lipids on enzyme-substrate mechanisms; hence, gender, age, adrenergic status, smoking, obesity, alcohol consumption, serum lipid and fibrinogen measurements, medication, diurnal activities, etc, were disregarded as qualifiers for volunteer participation in the study. Instead, “all-comers” were entered so long as they reported themselves to be in good health, believing that study participants' lysis times should more or less reflect the “average” of all physiologic/pharmacologic effects within each genotype at any point in time and that if indeed the polymorphism detectably affected plasma clot lysis under our conditions, then its potential importance would be underscored. Important to emphasize is that mean PCLTs for men were significantly prolonged compared to values for women (P < .001). As depicted in Figure 5, mean PCLTs for the RR, RW, and WW genotype groups exhibited an impressive linear decrease, but the differences between mean PCLTs among the 3 genotypes were not statistically significant. As shown in panel A of Figure 5, after separating genotypes by gender, the differences for the mean PCLT among genotypes showed an obvious trend toward shorter lysis times for the WW genotype in both men and women. Since the distribution of PCLTs was skewed, nonparametric statistical methods were used to analyze the data. Medians for the RR and RW were similar and the distributions overlapped, suggesting that the R allele is dominant. When RR and RW groups were merged and compared to WW, after accounting for variation due to gender, the differences approached significance (P = .061). As noted in Figure 5B, 12 (10%) persons of the RR genotype and 2 (3%) of the RW genotype had plasma clots that remained totally intact during the entire one-hour assay period; no person of WW genotype had a lysis time more than 2100 seconds.
Plasma clot lysis times (PCLT) by Met-α2AP genotype. PCLTs were determined on plasma samples from RR, RW, and WW persons. (A) PCLT values were divided by genotype and plotted as mean ± SEM for the total population, men only and women only. (B) Percentage of plasmas that did not lyse (n = 14) compared to percentage of total population (n = 200) within each genotype.
Plasma clot lysis times (PCLT) by Met-α2AP genotype. PCLTs were determined on plasma samples from RR, RW, and WW persons. (A) PCLT values were divided by genotype and plotted as mean ± SEM for the total population, men only and women only. (B) Percentage of plasmas that did not lyse (n = 14) compared to percentage of total population (n = 200) within each genotype.
Removal of APCE from plasma
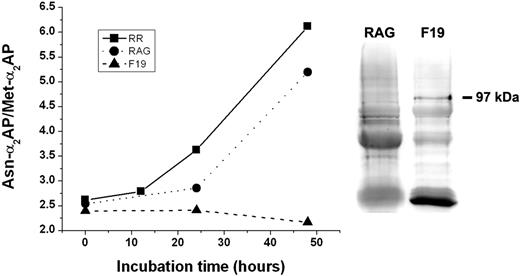
In an effort to definitively establish that APCE is the enzyme responsible for the conversion of Met-α2AP to Asn-α2AP, we removed APCE from plasma by incubating the plasma with the FAP-specific monoclonal antibody, F19, covalently attached to POROS chromatography beads. After removal of the beads, incubation of the plasma was continued for selected times at 29°C to allow conversion of Met-α2AP to Asn-α2AP. The graph in Figure 6 shows that the Asn-α2AP/Met-α2AP ratio in the control plasma, not incubated with the POROS-bound antibody, increased from 2.62 to 6.12 after 48 hours of incubation. In a second control, namely, plasma incubated with a nonspecific antibody bound to POROS chromatography beads, a significant increase in the Asn-α2AP/Met-α2AP ratio from 2.54 to 5.2 occurred after 48 hours. However, plasma incubated with the F19 antibody showed no increase in the Asn-α2AP/Met-α2AP ratio, 2.4 to 2.17, over the same incubation period, thereby indicating removal of APCE by the F19 antibody. These results clearly demonstrate that removal of APCE from plasma abrogates conversion of Met-α2AP to the shorter, faster fibrin-crosslinking form, Asn-α2AP. As further support for this conclusion, we also demonstrated by Western blot analysis that it was indeed APCE that was bound and removed by the bead-linked F19 mAb. Western blot analysis shown in Figure 6 shows an APCE monomeric 97-kDa band in the sample eluted from F19-linked beads; no such band was present on the rabbit antigoat (RAG) Ab-linked beads. Other bands visible were the result of nonspecific binding of the secondary Ab to immunoglobulin in the samples, which leaches off the beads during extraction by boiling in SDS.
Effect of APCE removal on conversion of Met-α2-AP to Asn-α2-AP with time. Plasma was drawn from a person of RR Met-α2AP genotype and divided into 3 aliquots. One aliquot was mixed with an APCE F19 mAb (F19) bound to chromatography beads and incubated at 4°C to remove APCE. The second aliquot was incubated at 4°C with a nonspecific rabbit α-goat Ab (RAG) bound to beads. The third aliquot (RR) received no treatment. After removal of beads, each sample was incubated at 29°C, and Asn-α2AP/Met-α2AP ratios determined at selected times. In addition, F19-bound beads and RAG-bound beads were boiled with SDS to remove antibody-bound protein. Samples were electrophoresed on 10% Bis-Tris SDS-PAGE gels and blotted to nitrocellulose. APCE (97 kDa) was identified by Western blotting using a goat Ab to its amino-terminal region and visualized with a chemiluminescent substrate.
Effect of APCE removal on conversion of Met-α2-AP to Asn-α2-AP with time. Plasma was drawn from a person of RR Met-α2AP genotype and divided into 3 aliquots. One aliquot was mixed with an APCE F19 mAb (F19) bound to chromatography beads and incubated at 4°C to remove APCE. The second aliquot was incubated at 4°C with a nonspecific rabbit α-goat Ab (RAG) bound to beads. The third aliquot (RR) received no treatment. After removal of beads, each sample was incubated at 29°C, and Asn-α2AP/Met-α2AP ratios determined at selected times. In addition, F19-bound beads and RAG-bound beads were boiled with SDS to remove antibody-bound protein. Samples were electrophoresed on 10% Bis-Tris SDS-PAGE gels and blotted to nitrocellulose. APCE (97 kDa) was identified by Western blotting using a goat Ab to its amino-terminal region and visualized with a chemiluminescent substrate.
Discussion
In the early nineteenth century, von Rokitansky and Virchow suggested that endothelial injury led to clotting activation and eventual platelet-fibrin accumulations at the site of damage.18,19 Since then, results of numerous studies support fibrin formation as integral in the developing atherogenic process.20–30 While the exact sequence of events that leads to plaque formation continues to be debated, there is more uniform agreement about fibrin deposition during the course of plaque growth, rupture, and acute thrombotic complications. Procoagulant processes that culminate in fibrin formation have been exhaustively studied, with elevations of selected clotting factor proteins or their activities considered as risk factors for atherogenesis or its acute complications.31–33 Similarly, diminished plasmin activity also has been implicated in plaque growth during the atherosclerotic process34–37 as well as in the persistence of occlusive thrombi when a plaque is disrupted.38,39
Rapid, effective plasmin inhibition over long periods of time would favor survival of intravascular platelet-fibrin deposits during the initiation of human atherogenesis and its progression. In contrast, enhanced plasmin activity would presumably digest and remove forming fibrin so that associated platelet deposits would become dispersed into the circulation. Genetic deficiencies of α2AP activity indicate that the homozygous state is associated with hemorrhage similar to that observed with factor XIII deficiencies; however, bleeding in heterozygotes appears to occur mainly after more serious injury or surgery, suggesting that enhanced fibrinolysis is clearly a feature of diminished α2AP function, but compatible with normal life.40 Conversely, functional α2AP levels have been purposefully raised to stabilize clot formation induced to occlude human patent ductus arteriosi,41 indicating that increased blood levels of α2AP make forming fibrin highly resistant to endogenous fibrinolysis. Finally, α2AP appears responsible for approximately 90% of plasmin inhibition in vivo,42–44 suggesting that functional levels of α2AP relate directly to thrombolytic rates. These observations indicate a significant regulatory role for α2AP in the thrombolytic process, perhaps even in the pathophysiology of atherosclerosis.
The 2 populations of healthy volunteers we analyzed in separate time frames were essentially identical, with the pooled genotype frequency being RR, 62.5%; RW, 34.0%; and WW, 3.5%. The allelic frequency values of 0.795/0.205 are in accord with the only other study of which we are aware, namely that of Lind and Thorsen,12 who reported values of 0.81/0.19 for the single nucleotide transition in 30 healthy blood donors. Because this polymorphism occurs in the 12-residue amino-terminal peptide that is removed from the longer, precursive form of α2AP,6 and given that a positively charged hydrophilic arginine (R) is substituted with a hydrophobic amino acid, tryptophan (W), we questioned whether such a difference might affect the rate of cleavage of the peptide by APCE and subsequent incorporation into forming fibrin. We found that pure APCE cleaved pure Met-α2AP of the WW genotype approximately 8 times slower when compared to pure Met-α2AP from plasma of RR individuals. Figure 4 clearly shows that the native precursive Met-α2AP/derivative Asn-α2AP ratios in plasma samples containing each of the 2 polymorphic forms of Met-α2AP change spontaneously with time when freshly drawn plasma is allowed to incubate at 29°C. This cleavage must be due to the naturally occurring plasma levels of APCE, since as shown in Figure 6, removal of APCE with a specific monoclonal antibody totally aborted generation of derivative Asn-α2AP during the same incubation time. Since this cleavage occurs spontaneously within circulating blood,6 ratios of precursive Met-α2AP/derivative Asn-α2AP should vary within the circulating plasma from the 3 genotypes, which in fact we demonstrated by quantitative amino-terminal analysis of the precursive/derivative α2AP forms for each of the 35 persons analyzed. As predicted, persons of the RR genotype had the least amount of circulating Met-α2AP (23.6%); with RW intermediate (40.6%), and WW the highest (56.4%). Our results cannot be explained by variation in APCE levels, since as depicted in Figure 2B, antigen levels were not significantly different among the 3 genotypes.
The relationship between RR, RW, and WW genotypes and corresponding Met-α2AP/Asn-α2AP ratios raises the question of whether the latter might impact individuals' fibrinolytic activities so that over the course of one's life, vulnerability of intravascularly generated fibrin to endogenous fibrinolysis, and consequently its survival, are differentially affected by Met-α2AP genotype. As a consequence, persons of the WW genotype would have Met-α2AP that is less susceptible to cleavage by APCE and therefore less effectively incorporated into forming fibrin,7 thereby making any generated fibrin more susceptible to digestion by plasmin. We attempted to demonstrate this using whole plasma to approximate native conditions as closely as possible. As indicated in the prior section, only minimal effort was made to standardize conditions under which all samples were drawn from healthy volunteers, thinking that if indeed the WW genotype group had shortened fibrinolysis times, then odds should favor this being the case for the majority of time. Most of the perturbants known to affect fibrinolytic times—either acutely or chronically—are in play over one's lifetime, and in spite of such influences, we posited that on the average, fibrinolytic status would segregate according to Met-α2AP genotype. Noteworthy is that in all our analyses, persons of RR genotype had the longest mean PCLT, with the RW group intermediate, and those in the WW genotype the shortest, suggesting that WW persons chronically have a more active fibrinolytic system than the RW group, and certainly greater activity than those with the RR genotype. The latter genotype contained a higher-than-expected percentage of persons whose fibrin never lysed, and if these were assigned PCLT values one second above the maximum measured value for any person in our study, then for men, the association of mean lysis times with RR and RW achieved significance at the P < .05 level. Our efforts were clearly compromised by the very significant difference in PCLTs we found for women compared to men, the relatively low prevalence of the WW polymorphism, and the characteristic mercurial responses of the human fibrinolytic system to a large array of physiologic and pharmacologic effectors. Despite these limitations, however, the apparent association of fibrin susceptibility to plasmin with the SNP described here prompts the interpretation that over one's lifetime, the W allele may serve as a “protection factor” (in contrast to the well-understood term, “risk factor”) by increasing the susceptibility of developing intravascular thrombi to removal by plasmin.
Evolutionarily the RR genotype may have posed an advantage for an individual's survival by maintaining structural integrity of blood clots so that life-threatening hemorrhage became fully staunched following wounding. While this would provide a breeding advantage during fertile years, over a full life span, the same genotype may become disadvantageous and indirectly participate in the pathogenesis of atherosclerotic disease by inhibiting fibrin digestion. Carefully designed genetic analyses of large-scale cardiovascular epidemiologic studies may determine if the prevalence of the WW genotype is significantly increased in those identified by multivariate cardiovascular risk analyses as having high risk for stroke, myocardial infarction, etc, but who otherwise remain free of events, or whether persons with late-onset, event-proven atherothrombotic cardiovascular disease have a disproportionate number of WW genotypes. Should the WW polymorphism be protective, the question of whether this can be addressed pharmacologically becomes apparent. It may be potentially useful to increase Met-α2AP/Asn-α2AP ratios to approximate those that accelerate fibrinolysis.7 If the function of α2AP—essentially the sole in vivo inhibitor of plasmin—could be decreased to levels that carry little risk of major bleeding, as exemplified in heterozygote deficiencies of α2AP function, and a chronic level of endogenous lytic activity sustained, then the survival and participation of intravascular fibrin-platelet thrombi in the atherosclerotic process should be reduced.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the University of Oklahoma Health Sciences Center General Clinical Research Center grant M01-RR14467, sponsored by National Center for Research Resources-National Institutes of Health; and by William K. Warren Medical Research Center and National Institutes of Health grant HL072995 (V.J.C., K.W.J., K.N.L., and P.A.M.)
We thank Ms D. Chissoe for excellent technical assistance.
National Institutes of Health
Authorship
Contribution: V.J.C. designed research, performed research, collected data, analyzed data, and wrote the paper. K.W.J. designed research, performed research, analyzed data, and assisted in writing of the paper. K.N.L. designed research and assisted in writing of the paper. P.A.M. directed and designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victoria J. Christiansen, William K. Warren Medical Research Center, PO Box 26901, BSEB 306, Oklahoma City, OK 73190; e-mail: victoria-christiansen@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal