Abstract
The impact of ligand density on integrin-mediated cell adhesion and outside-in signaling is not well understood. Using total internal reflection fluorescent microscopy, conformation-specific antibodies, and Ca2+ flux measurements, we found that the surface density of fibrinogen affects αIIbβ3-mediated platelet signaling, adhesion, and spreading. Adhesion to fibrinogen immobilized at low density leads to rapid increases in cytosolic Ca2+ and sequential formation of filopodia and lamellipodia. In contrast, adhesion to high-density fibrinogen results in transient or no increases in Ca2+ and simultaneous formation of filopodia and lamellipodia. αIIbβ3 receptors at the basal surface of platelets engage fibrinogen in a ringlike pattern at the cell edges under both conditions. This engagement is, however, more dynamic and easily reversed on high-density fibrinogen. Src and Rac activity and actin polymerization are important for adhesion to low-density fibrinogen, whereas PKC/PI3 kinases contribute to platelet spreading on high-density fibrinogen. We conclude that 2 fundamentally different signaling mechanisms can be initiated by a single integrin receptor interacting with the same ligand when it is immobilized at different densities.
Introduction
Integrins mediate interaction of cells with their environment, responding to activation and inhibition signals (inside-out signaling) and transmitting information initiated by ligand-receptor interaction into the cell (outside-in signaling). These interactions are vital to gene regulation, cell migration, cell proliferation, and cellular differentiation in many processes.1 The most abundant platelet integrin, αIIbβ3, and its signaling have been extensively studied using both platelets and other cell model systems.2 Structural data on this integrin and the related integrin αVβ3 have brought new insights into its function, especially with the regard to ligand binding.3,4 αIIbβ3-mediated platelet adhesion to fibrinogen has been implicated in a number of different physiologic and pathologic processes. After vascular injury, fibrinogen binds to the damaged surface and may act as one of the proteins to which platelets adhere.5 This is of particular significance, since αIIbβ3 can bind to immobilized fibrinogen without prior platelet activation.6,7 As fibrinogen is also present in atherosclerotic plaque,8,9 platelet adhesion to fibrinogen may contribute to thrombus formation on ruptured or eroded plaques, or even to the atherosclerotic process itself.10 Fibrinogen also preferentially deposits on artificial surfaces, including those used for vascular prostheses and biomaterials. Thus platelet adhesion to fibrinogen-coated surfaces is of importance in the biocompatibility of these surfaces.11
αIIbβ3 interactions with immobilized fibrinogen trigger outside-in signaling followed by filopodial extensions, development of lamellipodia, and subsequent attachment and spreading (reviewed in Shattil12 ). Platelet spreading on fibrinogen is associated with tyrosine phosphorylation of several platelet proteins including FAK,13 Src,13 and Syk.14 Kinases involved in these processes have been shown to include protein kinase C (PKC)15,16 ; phosphatidylinositol 3-kinase (PI3K)17,18 ; Csk, Src, and Syk kinases19 , and other molecules.20
In previous studies, αIIbβ3-mediated interactions with immobilized fibrinogen were often studied in cells not expressing αIIbβ3 endogenously and mostly at fibrinogen coating concentrations of 10 μg/mL or higher. However, we have shown that platelet spreading, activation of luminal αIIbβ3 receptors, and recruitment of additional platelets is dependent on the density of immobilized fibrinogen.21 The differences in platelet adhesive behavior follow a biphasic pattern, with decreased spreading and activation of luminal αIIbβ3 at fibrinogen coating concentrations higher than 10 μg/mL. In the present study, we analyze αIIbβ3-mediated adhesion to low-density and high-density fibrinogen and demonstrate that the density of fibrinogen affects platelet adhesion from the very beginning, resulting in differences in intracellular Ca2+ fluxes and the dynamics of αIIbβ3 interactions with fibrinogen. These differences are associated with differences in platelet morphology and the activation of the signaling pathways involved in platelet spreading.
Materials and methods
Reagents
Human fibrinogen (depleted of von Willebrand factor, plasminogen, and fibronectin) was from Enzyme Research Laboratories (South Bend, IN); prostaglandin E1, bovine serum albumin (grade V), and apyrase (grade VII) were from Sigma (St Louis, MO); bisindolylmaleimide, PP2, SU6656, PP3, H-1152, Y-27632, NSC23766, and cytochalasin D were from Calbiochem (La Jolla, CA); wortmannin was from Biomol Research Laboratories (Plymouth Meeting, PA); and Oregon Green BAPTA-1, AM, Fura Red, AM, Alexa-Fluor 594-phalloidin, latrunculin A, and Alexa-Fluor 488, 594, and 647 were from Molecular Probes (Eugene, OR). Type I collagen from the skin of lathyritic rats was prepared as previously described.22
All experiments were performed at 22°C.
Platelet preparation and adhesion assay
Gel-filtered platelets in HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid)-modified Tyrode buffer containing 0.35% bovine serum albumin (HBMT) were prepared from acid-citrate-dextrose (ACD) anticoagulated blood as described previously.23 In some experiments, platelets were incubated with vehicle (dimethyl sulfoxide, DMSO) or inhibitors of PKC (10 μM bisindolylmaleimide; 1-hour incubation), phosphoinositide 3-kinase (PI3K) (20 nM wortmannin; 10 minutes), Src family kinases (20 μM PP2 or SU6656; PP3 as a control; 30 minutes), actin polymerization (10 μM cytochalasin D or 10 μM latrunculin A; 10 minutes), Rho kinase (5 μM H-1152 or 10 μM Y-27632; 10 minutes), Rac1 (100 μM NSC23766; 10 minutes), or apyrase (3 U/mL; 5 minutes).
Fibrinogen was adsorbed at concentrations of 3 and 100 μg/mL for 1 hour. In some experiments, adhesion to collagen (33 μg/mL) was studied for comparison. After adsorption, washing, and blocking with HBMT, platelets in HBMT containing 2 mM Mg2+ were allowed to adhere for 1 hour, and further washed with HBMT containing 2 mM Mg2+. The number of adherent platelets was quantified by assessing the endogenous acid phosphatase activity using a pNpp buffer (0.1 M citrate, pH 5.4, 0.1% Triton X-100, 5 mM paranitrophenylphosphate).24
Time-lapse phase-contrast video microscopy
The kinetics of platelet adhesion was observed using previously described phase-contrast time-lapse videomicroscopy.23 In brief, fibrinogen was adsorbed to a polystyrene-coated microchamber and platelets were allowed to adhere for 2 hours. Images were obtained concurrently from 2 channels at 2.4-minute intervals using a Zeiss Axiovert microscope with Zeiss Plan-Neofluar 100 ×/1.3 NA oil immersion objective (Carl Zeiss, Mannheim, Germany), and acquired with an Oma4 camera (EG&G Instruments; Princeton Applied Research, Princeton, NJ) and Image-1 software (Universal Imaging, West Chester, PA).
The surface areas covered by an adherent platelet were calculated using the Image-1 software on 2 to 3 random fields in 6 separate experiments (average 68 platelets on each surface/experiment).
Total internal reflection fluorescence microscopy (TIR-FM)
The settings for TIR-FM illumination through the objective (Apo 60 ×/1.45 NA; Olympus, Melville, NY) were essentially the same as previously described.25 Alexa 488–conjugated proteins were excited with the 488-nm line of an Argon laser (Melles Griot, Carlsbad, CA) reflected off a dichroic mirror (498DCLP) and collected through emission band pass filters (HQ525/50M). For simultaneous dual-color imaging of Alexa 488/594, the emissions were collected through an emission splitter (Dual-view; Roper Biosciences, Tuscon, AZ) equipped with dichroic mirrors to split the emission (550DCLP) and emission band pass filters (Alexa 488, HQ525/50M; Alexa 594, HQ580LP). All filters were obtained from Chroma Technologies (Rockingham, VT). Images were acquired with a 12-bit cooled CCD ORCA-ER camera (Hamamatsu Photonics, Hamamatsu, Japan) with a resolution of 1280 × 1024 pixels (pixel size, 6.45 μm). For time-lapse experiments, platelets were allowed to adhere in the presence of Alexa 488– or Alexa 594–conjugated 7H2 Fab or IgG (20 and 50 ng/mL, respectively) and images were acquired at 1 frame/10 seconds. Preliminary experiments showed no difference when either IgG or Fab fragment was used for β3 labeling. In some experiments, platelets labeled with 7H2 were allowed to adhere for 1 hour and then AP5-Alexa 488 was added to image ligand-bound receptors. This imaging was done for 5 minutes only in order not to induce further spreading.
Immunofluorescence microscopy of adherent platelets
Platelets were adherent at low platelet count (30 × 109/L [30 000/μL]) in order to visualize single platelet morphology. Adherent platelets were stained simultaneously with Alexa 594-7H2 (anti-β3), FITC-PAC-1 (antibody to activated αIIbβ326 ; BD PharMingen, San Diego, CA), and Alexa 488-AP5 (anti-β327 ) for 5 minutes and then fixed. In some experiments, platelets were fixed, permeabilized, and after washing stained for F-actin with Alexa 594-phalloidin. The specimens were imaged using either a TIR-FM system as described in section Total internal reflexion fluoresence microscopy or a Zeiss LSM-510 confocal system with Axiovert 200 microscope (Carl Zeiss) using Plan-Apochromat 100 ×/1.4 NA oil DIC objective. Scanning with the 488- and 543-nm lasers was performed sequentially to avoid crosstalk between the fluorescent probes. In other experiments, platelets were incubated with mepacrine (10 μM; Sigma) for 30 minutes at 37°C to label dense bodies28 ; gel-filtered to remove excess mepacrine; and then allowed to adhere to fibrinogen for 1 hour. After washing, the number of dense bodies per platelet was determined and recorded as 0, 1, 2, 3, 4, or more.
Cytosolic calcium concentration in adherent platelets
Changes in cytosolic calcium concentration in platelets adhering to fibrinogen were monitored and analyzed by a dual ratiometric method using confocal microscopy (same equipment as in section Immunofluorescence microscopy of adherent platelets) as per Nesbitt et al29 and Yap et al.30 Platelets were loaded with Oregon Green BAPTA-1, AM (1 μM) and Fura Red, AM (1.25 μM) and added to the wells. Cytosolic calcium was monitored by acquiring images simultaneously in 2 fluorescence channels over a period of 140 seconds at 2-second intervals during the first 30 minutes of adhesion. Differential interference contrast (DIC) images were acquired at the same time to assure focus of imaging. Fluorescence intensities were determined using LSM-510 software (Carl Zeiss) and converted to intracellular calcium concentration, Δ[Ca2+], as previously described.30 Platelets were separated into categories based on the extent of adhesion (contact, filopodia, filopodia/lamellipodia, spread, other) to compare Ca2+ in platelets with similar morphologies. The calcium response was defined as transient when only 1 or 2 peaks of intracellular Ca2+ were observed during the 140-second observation period, and as sustained when 3 or more peaks were observed (Figure 3B). Platelets from 3 independent experiments (n ≥ 25/surface/experiment) were analyzed.
Protein tyrosine phosphorylation in adherent platelets
Adherent platelets were incubated for 1 minute with HBMT buffer containing 1 mM Na3VO4, 10 mM EDTA, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF, 0.1 mM leupeptin, 20 μg/mL trypsin inhibitor, and 6 mM N-ethylmaleimide, followed by addition of 90°C sodium dodecylsulfate (SDS) sample buffer. Lysates were then removed, heated to 100°C for 5 minutes, and subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotting with a mixture of 2 antiphosphotyrosine monoclonal antibodies, PY20 and PY99 (Santa Cruz Biotechnology, Santa Cruz, CA). The posttransfer gel was stained with Coomassie blue to assure that equal amounts of protein were loaded in each lane.
For immunoprecipitations, adherent platelets were washed with HBMT buffer containing 2 mM Mg2+ and 1 mM Na3VO4 and subsequently lysed in 1 mL lysis buffer13 for 30 minutes on ice. After centrifugation (15 000g, 15 minutes, 4°C), lysates containing equal amounts of protein were immunoprecipitated with antibody to Syk or FAK (rabbit polyclonal antibodies to Syk(C-20) and FAK(C-903); Santa Cruz Biotechnology) and protein G-Sepharose beads. Immunoprecipitates were then analyzed by immunoblotting for phosphotyrosine content with antibodies PY20 and PY99. The membranes were then stripped and reacted with an antibody to FAK (Upstate Biotechnology, Lake Placid, NY) or Syk (N-19; Santa Cruz Biotechnology). Densitometry was performed to quantitatively analyze the extent of protein phosphorylation (FluorChem; AlphaInnotech, San Leandro, CA).
ATP release from adherent platelets
Platelets were allowed to adhere to fibrinogen-coated microtiter wells for 1 hour. Adenosine triphosphate (ATP) substrate solution (50 μL, ATPlite Luminescence Assay System; Perkin Elmer, Waltham, MA) was then added and luminescence immediately measured with a Flexstation II 384 Plate Reader (Molecular Devices, Sunnyvale, CA). After this, platelet adhesion was quantified using pNpp substrate as in section Platelet preparation and adhesion assay and measured luminescence normalized per adherent platelets in each well (luminescence/OD405).
Statistical analysis
Data that were not normally distributed are presented as median and interquartile range (IQR); Mann-Whitney rank-sum test was used for statistical comparison (Sigma Stat; SPSS Science, Chicago, IL). For normally distributed data, means of 2 sets of data were compared using Student t test.
Results
The density of immobilized fibrinogen affects the kinetics of platelet-ligand interactions and the morphology of adherent platelets
A fibrinogen solution of 3 μg/mL was used to prepare surfaces with low-density fibrinogen, resulting in a surface density of approximately 80 ng/cm2 or 1.8 × 1011 molecules/cm2 (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). High-density fibrinogen was prepared using a solution of 100 μg/mL, resulting in a density of approximately 500 ng/cm2 or 8.9 × 1011 molecules/cm2. As observed by time-lapse phase-contrast video microscopy, platelet adhesion and spreading on low-density fibrinogen began approximately 10 minutes after the addition of the platelets to the chambers and was followed by recruitment of new platelets to the adherent ones and continuous platelet spreading for up to 2 hours (Figure S1B; Video S1). In contrast, platelet adhesion to high-density fibrinogen began earlier (within 5 minutes of platelet addition), and there was relatively little change in spreading or morphology after 40 minutes. Moreover, there was virtually no recruitment of additional layers of platelets. Also, platelets adherent to high-density fibrinogen covered significantly smaller areas than platelets adherent to low-density fibrinogen (32 ± 6 μm2 vs 50 ± 9 μm2, respectively [mean ± SD]; P = .003, n = 6). In control experiments, platelets did not adhere to the wells treated with HBMT alone, and monoclonal antibody (mAb) 10E5,31 which is specific for αIIbβ3, blocked platelet adhesion to both low- and high-density fibrinogen (not shown).
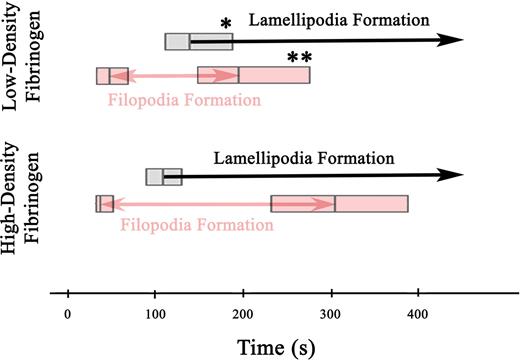
To study the effect of fibrinogen densities on the spatial and temporal distribution of integrin αIIbβ3 on the basal membrane of adhering platelets, αIIβ3 was labeled with a β3-specific, fluorescently conjugated mAb 7H232 and imaged using TIR-FM. Due to a high density of αIIβ3 on the platelet surface, TIR-FM imaging of labeled αIIβ3 allowed us to follow morphologic changes of platelets in contact with the surface during the adhesion. This revealed significant differences in the kinetics of filopodia and lamellipodia formation on high- versus low-density fibrinogen after the platelets first appeared in the evanescent field (< 200 nm from the fibrinogen coated surface) (Video S2). Filopodia started to form after approximately 40 to 50 seconds on both low- and high-density fibrinogen. However, new filopodia stopped forming earlier on low-density (median = 195 seconds, n = 79) than on high-density (median = 305 seconds, n = 62; P = .03) fibrinogen (Figure 1). Lamellipodia formation began later on platelets adhering to low- than to high-density fibrinogen (140 seconds vs 110 seconds, P = .04). Once lamellipodia started to form on platelets adhering to low-density fibrinogen, formation of new filopodia ceased within 55 seconds. In contrast, platelets adhering to high-density fibrinogen continued to form new filopodia for 195 seconds after the onset of lamellipodia formation. Thus, the period during which the adhering platelets were actively extending both filopodia and lamellipodia was shorter on low-density fibrinogen (P < .001).
Filopodia and lamellipodia formation in platelets adhering to low- and high-density fibrinogen. Time-lapse TIR-FM imaging of platelets adhering to low- or high-density fibrinogen in the presence of Alexa 488-7H2Fab was recorded for 30 minutes after the addition of platelets into the fibrinogen-coated wells (Video S2). Individual platelets were analyzed for the appearance of new filopodia and onset of lamellipodia formation, counting the time they first appeared in the evanescent field (ie, within 200 nm of the substrate) as t = 0 seconds. The boxes in the figure represent the median and the 25th and 75th percentiles of the time to the onset and end of filopodia formation and the onset of lamellipodia formation. A total of 79 platelets were analyzed on low-density and 62 platelets on high-density fibrinogen from 2 independent experiments; *P = .03, **P = .04 low-density versus high-density fibrinogen.
Filopodia and lamellipodia formation in platelets adhering to low- and high-density fibrinogen. Time-lapse TIR-FM imaging of platelets adhering to low- or high-density fibrinogen in the presence of Alexa 488-7H2Fab was recorded for 30 minutes after the addition of platelets into the fibrinogen-coated wells (Video S2). Individual platelets were analyzed for the appearance of new filopodia and onset of lamellipodia formation, counting the time they first appeared in the evanescent field (ie, within 200 nm of the substrate) as t = 0 seconds. The boxes in the figure represent the median and the 25th and 75th percentiles of the time to the onset and end of filopodia formation and the onset of lamellipodia formation. A total of 79 platelets were analyzed on low-density and 62 platelets on high-density fibrinogen from 2 independent experiments; *P = .03, **P = .04 low-density versus high-density fibrinogen.
Patterns of total and activated αIIbβ3 receptors on platelets adherent to low- and high-density fibrinogen
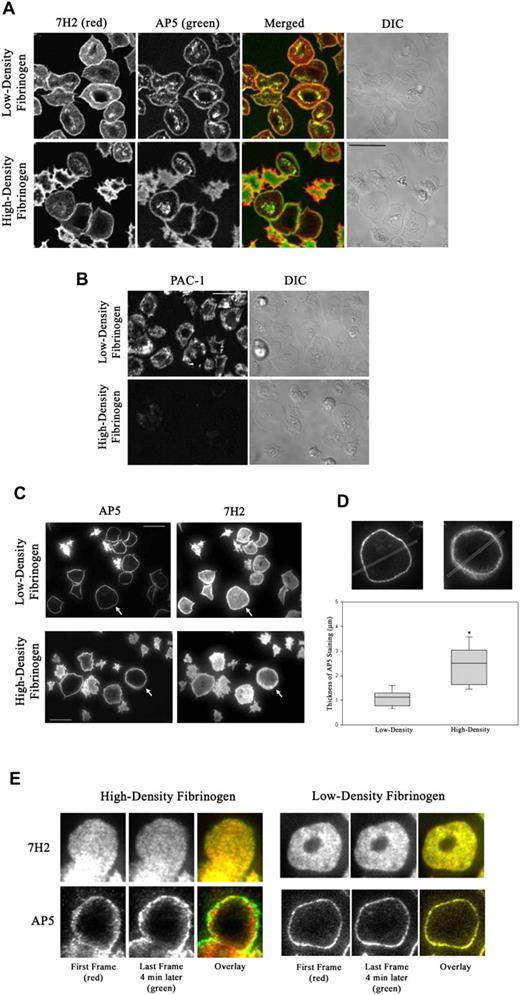
Conformation-specific mAbs were used to monitor the localization and activation state of αIIbβ3 60 minutes after the initiation of adhesion. 7H2 bound to the entire platelet surface, but the staining was most intense in a thin rim on the edge of spread platelets on both low- and high-density fibrinogen (Figure 2A). AP5, a mAb specific for a ligand-induced epitope in the PSI domain of β3,27 also stained spread platelets in a ring pattern, but the ring was thicker in platelets on high-density than on low-density fibrinogen. Unlike on low-density fibrinogen, not all spread platelets on high-density fibrinogen showed granulomere AP5-positive staining. Most importantly, PAC-1, a mAb that selectively recognizes activated αIIbβ3,26 stained spread platelets on low-density fibrinogen 4 times more intensely than on high-density fibrinogen (P < .001, n = 3; Figure 2B). The intensity of PAC-1 staining of platelets on high-density fibrinogen could be increased by adding mAb D3,33 which directly activates αIIbβ3 (Figure S2). From this we conclude that it is more likely that the αIIbβ3 receptors on platelets adherent to high-density fibrinogen are in an inactive conformation rather than all redistributed to the basal surface and engaged with ligand.
Patterns of total and activated αIIbβ3 in platelets adherent to low- and high-density fibrinogen. Platelets were added to wells coated with fibrinogen and incubated for 1 hour. Wells were then washed and adherent platelets incubated with fluorescently labeled antibodies as described in “Materials and methods.” (A) By confocal microscopy, 7H2 and AP5 staining produced similar patterns on platelets adherent to both low- and high-density fibrinogen, with AP5 staining being more pronounced in the granulomere region in platelets on low-density fibrinogen. (B) PAC-1, mAb specific for activated αIIbβ3, intensely stained the surface of spread platelets on low-density fibrinogen but, under the same conditions, stained only weakly the surface of platelets spread on high-density fibrinogen. Differential interference contrast images (DIC) are shown on the right side for comparison of the platelet morphology. (C) TIR-FM of platelets double stained with AP5 and 7H2 revealed that AP5 staining on the basal surface of platelets spread on low-density fibrinogen appears in a very thin rim at the edge, whereas AP5 staining of spread platelets on high-density fibrinogen is much thicker and diffuse. Cells marked with arrows are magnified in panel D. Bars represent 10 μm. Images shown are representative of at least 2 independent experiments. (D) TIR-FM images were analyzed for the width of AP5 staining by line scan analysis. The box plot shows the median and the 25th and 75th percentiles for 21 and 16 platelets on low- and high-density fibrinogen, respectively, from 3 independent experiments, *P < .001. (E) 7H2 staining of receptors on a platelet spread on high-density fibrinogen is static, whereas AP5 staining shows radial movement. Alexa 546-7H2–labeled platelets were allowed to adhere for 1 hour and then Alexa 488-AP5 was added and both antibodies were imaged using TIR-FM for 5 minutes. The image of a platelet spread on high-density fibrinogen (Video S3) was taken at the beginning of the acquisition period (red) and then 4 minutes later (green). On both low- and high-density fibrinogen, 7H2 staining did not change between the first and last frame as demonstrated by overlay (yellow). AP5 staining of platelet on high-density fibrinogen showed radial extension as judged by the appearance of strong green ring outside the red/yellow staining on the overlaid image. AP5 staining on low-density fibrinogen was without a change between the first and last frame (yellow overlay).
Patterns of total and activated αIIbβ3 in platelets adherent to low- and high-density fibrinogen. Platelets were added to wells coated with fibrinogen and incubated for 1 hour. Wells were then washed and adherent platelets incubated with fluorescently labeled antibodies as described in “Materials and methods.” (A) By confocal microscopy, 7H2 and AP5 staining produced similar patterns on platelets adherent to both low- and high-density fibrinogen, with AP5 staining being more pronounced in the granulomere region in platelets on low-density fibrinogen. (B) PAC-1, mAb specific for activated αIIbβ3, intensely stained the surface of spread platelets on low-density fibrinogen but, under the same conditions, stained only weakly the surface of platelets spread on high-density fibrinogen. Differential interference contrast images (DIC) are shown on the right side for comparison of the platelet morphology. (C) TIR-FM of platelets double stained with AP5 and 7H2 revealed that AP5 staining on the basal surface of platelets spread on low-density fibrinogen appears in a very thin rim at the edge, whereas AP5 staining of spread platelets on high-density fibrinogen is much thicker and diffuse. Cells marked with arrows are magnified in panel D. Bars represent 10 μm. Images shown are representative of at least 2 independent experiments. (D) TIR-FM images were analyzed for the width of AP5 staining by line scan analysis. The box plot shows the median and the 25th and 75th percentiles for 21 and 16 platelets on low- and high-density fibrinogen, respectively, from 3 independent experiments, *P < .001. (E) 7H2 staining of receptors on a platelet spread on high-density fibrinogen is static, whereas AP5 staining shows radial movement. Alexa 546-7H2–labeled platelets were allowed to adhere for 1 hour and then Alexa 488-AP5 was added and both antibodies were imaged using TIR-FM for 5 minutes. The image of a platelet spread on high-density fibrinogen (Video S3) was taken at the beginning of the acquisition period (red) and then 4 minutes later (green). On both low- and high-density fibrinogen, 7H2 staining did not change between the first and last frame as demonstrated by overlay (yellow). AP5 staining of platelet on high-density fibrinogen showed radial extension as judged by the appearance of strong green ring outside the red/yellow staining on the overlaid image. AP5 staining on low-density fibrinogen was without a change between the first and last frame (yellow overlay).
TIR-FM imaging was used to selectively determine the conformational state of the αIIbβ3 receptors on the basal membrane of adherent platelets. Platelets labeled with subsaturating concentrations of fluorescent 7H2-Fab were allowed to adhere for 60 minutes and then additionally labeled with AP5 (Figure 2C). 7H2 labeled β3 at the basal surface of platelets in a punctate pattern (Figure S3), suggesting that the receptors were organized into small clusters. The pattern was not affected by ligand density and did not change over time. AP5 stained nearly the entire basal surface of partially spread platelets, with accentuation at the platelet edge. On fully spread platelets, AP5 produced a pure ringlike pattern, and the ring was narrower and sharper in platelets spread on low-density fibrinogen (Figure 2C-D). Time-lapse TIR-FM imaging of platelets double labeled with 7H2 and AP5 confirmed the progression of AP5 staining from diffuse staining of the surface to more ringlike pattern as platelets underwent spreading. On fully spread platelets, the AP5-stained ring in platelets on low-density fibrinogen remained nearly unchanged over a period of 5 minutes (Video S3; Figure 2E). In contrast, on high-density fibrinogen, the AP5-labeling pattern was more dynamic, with movement both within the thick, mobile ring around the edges of spread platelets, and extending radially outward beyond the initial positions (Video S3). The corresponding 7H2 staining, also dynamic, did not demonstrate radial movement (Figure 2E).
These observations suggested that αIIbβ3 interactions with high-density fibrinogen are more dynamic than with low-density fibrinogen. To test this hypothesis, platelets adherent for 60 minutes were treated with αIIbβ3 antagonists (the mAb c7E3 Fab34 or the nonpeptide RGD mimetic tirofiban) or EDTA and reversal of adhesion was measured. In fact, twice as many platelets adherent to high-density fibrinogen were detached by either the αIIbβ3 antagonists or EDTA (Table 1)
Reversibility of platelet adhesion by αIIbβ3 antagonists or EDTA
| Inhibitor . | Concentration . | Residual platelet adhesion, % of control . | n . | |
|---|---|---|---|---|
| Low-density fibrinogen . | High-density fibrinogen . | |||
| c7E3 Fab | 10 μg/mL | 75 ± 3 | 34 ± 13* | 3 |
| EDTA | 10 mM | 66 ± 14 | 43 ± 21* | 7 |
| Tirofiban | 100 μM | 69 ± 21 | 37 ± 25* | 4 |
| Inhibitor . | Concentration . | Residual platelet adhesion, % of control . | n . | |
|---|---|---|---|---|
| Low-density fibrinogen . | High-density fibrinogen . | |||
| c7E3 Fab | 10 μg/mL | 75 ± 3 | 34 ± 13* | 3 |
| EDTA | 10 mM | 66 ± 14 | 43 ± 21* | 7 |
| Tirofiban | 100 μM | 69 ± 21 | 37 ± 25* | 4 |
Platelets were allowed to adhere to fibrinogen-coated microtiter wells for 1 hour, unbound platelets were removed by washing, and wells were further filled with buffer (control) or one of the inhibitors for 1 hour. After washing, the residual platelet adhesion was assessed by measuring endogenous acid phosphatase activity and expressed as a percentage of platelets adherent in the control wells.
Results are expressed as mean ± SD.
P ≤ .01 compared to low-density fibrinogen.
To assess whether any other integrin receptors contribute to the differences in platelet adhesion, we used mAbs LM609 (anti-αVβ3),35 BIIG2 (anti-α5),36 and AIIB2 (anti-β1),37 which selectively block ligand binding to their respective targets. At concentrations known to inhibit ligand binding (5-20 μg/mL), none of these antibodies affected either the number or spreading of adherent platelets (data not shown).
Platelet adhesion to low-density, but not to high-density, fibrinogen elicits rapid and sustained intracellular calcium responses
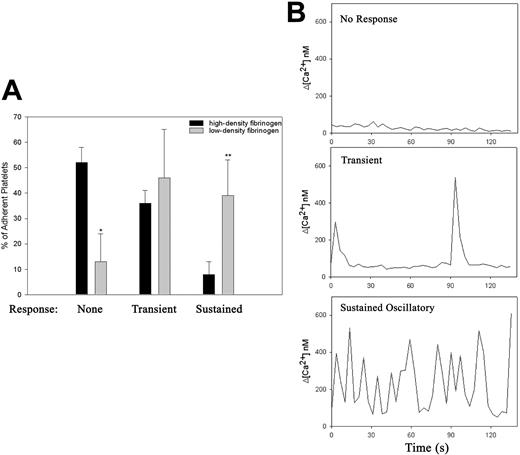
Intracellular calcium flux during the first 30 minutes of platelet adhesion was measured using a dual ratiometric method.29,30 As previously reported, adhesion to fibrinogen triggered changes in intracellular calcium concentration38 ; however, the frequency of occurrence and character of the intracellular Ca2+ response varied according to the fibrinogen density (Figure 3; Video S4). Within 30 minutes of the initiation of adhesion to low-density fibrinogen, 90% of adherent platelets demonstrated some increase in intracellular calcium and in 40% of platelets the Ca2+ oscillations were sustained (Figure 3A). In contrast, only 50% of platelets adhering to high-density fibrinogen demonstrated any increase in cytoplasmic Ca2+, and in the vast majority of responding platelets the increase was only transient (Figure 3A). The amplitude of the individual Ca2+ signals in platelets was, however, independent of the density of fibrinogen (Figure 3B). The time to onset of sustained Ca2+ oscillation also differed in platelets adhering to low- versus high-density fibrinogen. Thus, on low-density fibrinogen, 8% of adhering platelets developed sustained Ca2+ oscillations immediately after the initial interaction with the surface, whereas on high-density fibrinogen, sustained Ca2+ oscillations developed only after platelets extended both filopodia and lamellipodia. Thus, platelet interaction with low-density fibrinogen triggers intracellular Ca2+ response more rapidly, more often, and in a more sustained manner than platelet interaction with high-density fibrinogen.
Platelet adhesion to low-density fibrinogen leads to more rapid and sustained calcium response. Platelets loaded with calcium-sensitive dyes were added to wells coated with low- or high-density fibrinogen, and the cytosolic calcium fluxes during the first 30 minutes of the adhesion process were recorded using confocal microscopy as described in “Materials and methods” (Video S3). The adherent platelets were then analyzed according to their morphology and the characteristics of their calcium responses. (A) Population analysis demonstrated that most platelets adherent to high-density fibrinogen did not show any elevation in intracellular Ca2+ (*P < .001 high vs low-density fibrinogen, n = 3), whereas on low-density fibrinogen, approximately 40% of adherent platelets showed sustained Ca2+ oscillation (**P = .01). (B) Selected single platelet recordings of intracellular Ca2+ fluxes typical of nonresponsive platelet on high-density fibrinogen, a transient Ca2+ elevation in a platelet on high-density fibrinogen, and sustained oscillatory Ca2+ response in a platelet on low-density fibrinogen. Results are presented as mean ± SD.
Platelet adhesion to low-density fibrinogen leads to more rapid and sustained calcium response. Platelets loaded with calcium-sensitive dyes were added to wells coated with low- or high-density fibrinogen, and the cytosolic calcium fluxes during the first 30 minutes of the adhesion process were recorded using confocal microscopy as described in “Materials and methods” (Video S3). The adherent platelets were then analyzed according to their morphology and the characteristics of their calcium responses. (A) Population analysis demonstrated that most platelets adherent to high-density fibrinogen did not show any elevation in intracellular Ca2+ (*P < .001 high vs low-density fibrinogen, n = 3), whereas on low-density fibrinogen, approximately 40% of adherent platelets showed sustained Ca2+ oscillation (**P = .01). (B) Selected single platelet recordings of intracellular Ca2+ fluxes typical of nonresponsive platelet on high-density fibrinogen, a transient Ca2+ elevation in a platelet on high-density fibrinogen, and sustained oscillatory Ca2+ response in a platelet on low-density fibrinogen. Results are presented as mean ± SD.
Platelet adhesion to low-density fibrinogen leads to more intense protein tyrosine phosphorylation
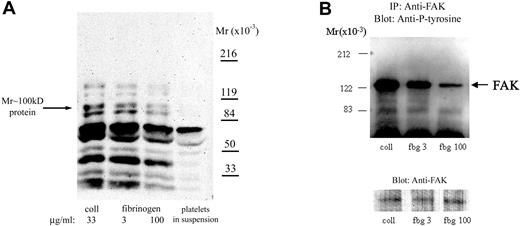
To further understand how fibrinogen surface density affects intracellular signaling, we studied its effect on tyrosine phosphorylation. The intensity of antiphosphotyrosine staining of proteins in adherent platelets varied with the fibrinogen density, with less intensity in platelets adherent to high-density fibrinogen (Figure 4A). Densitometric analysis demonstrated that the intensity of staining of a tyrosine-phosphorylated protein of Mr approximately 100 kDa, which was previously identified in platelets adherent to fibrinogen,13 was approximately 50% more in platelets adherent to low- than to high-density fibrinogen (Figure 4A). The intensity of staining of tyrosine-phosphorylated proteins in platelets adherent to low-density fibrinogen was similar to that in the platelets adherent to collagen, a substrate recognized as among the most active in initiating platelet signaling.13
Platelet adhesion to low-density fibrinogen induces greater protein tyrosine phosphorylation than adhesion to high-density fibrinogen. Platelets were allowed to adhere to fibrinogen (fbg)–coated or collagen (coll)–coated wells for 1 hour. After washing, adherent platelets were lysed in a buffer containing phosphatase inhibitors as described in “Materials and methods.” (A) Equal amounts of protein were subjected to electrophoresis and immunoblotting with mAbs specific for phosphotyrosine. Phosphotyrosine staining of proteins was less intense in platelets adherent to high-density compared with low-density fibrinogen; a protein of Mr approximately 100 kDa demonstrated approximately 50% less intense staining in platelets adherent to high-density than to low-density fibrinogen. For comparison, phosphotyrosine staining of proteins from platelets in suspension prior to adhesion is shown. (B) Equal amounts of protein lysates were used to immunoprecipitate FAK. Immunoprecipitated proteins were analyzed by immunoblotting for phosphotyrosine. Thereafter, the membranes were stripped and reanalyzed with antibody to FAK, to verify that the amounts of immunoprecipitated proteins were equal in all lanes. Results shown are representative of 3 independent experiments.
Platelet adhesion to low-density fibrinogen induces greater protein tyrosine phosphorylation than adhesion to high-density fibrinogen. Platelets were allowed to adhere to fibrinogen (fbg)–coated or collagen (coll)–coated wells for 1 hour. After washing, adherent platelets were lysed in a buffer containing phosphatase inhibitors as described in “Materials and methods.” (A) Equal amounts of protein were subjected to electrophoresis and immunoblotting with mAbs specific for phosphotyrosine. Phosphotyrosine staining of proteins was less intense in platelets adherent to high-density compared with low-density fibrinogen; a protein of Mr approximately 100 kDa demonstrated approximately 50% less intense staining in platelets adherent to high-density than to low-density fibrinogen. For comparison, phosphotyrosine staining of proteins from platelets in suspension prior to adhesion is shown. (B) Equal amounts of protein lysates were used to immunoprecipitate FAK. Immunoprecipitated proteins were analyzed by immunoblotting for phosphotyrosine. Thereafter, the membranes were stripped and reanalyzed with antibody to FAK, to verify that the amounts of immunoprecipitated proteins were equal in all lanes. Results shown are representative of 3 independent experiments.
Because both Syk and FAK tyrosine kinases have been implicated in signaling downstream of αIIbβ13,14 and have been reported to be tyrosine phosphorylated after platelet adhesion to fibrinogen, lysates of platelets adherent to collagen, or either low- or high-density fibrinogen, were immunoprecipitated with antibodies specific for Syk and FAK. Indeed, proteins from platelets adherent to collagen showed the most intense antiphosphotyrosine staining of both FAK (Figure 4B) and Syk (not shown). Densitometric analysis showed that the intensities of antiphosphotyrosine staining of Fak (n = 4) and Syk (n = 5) in platelets adherent to high-density fibrinogen were 30% ± 19% and 36% ± 18% lower, respectively, than the intensities in platelets adherent to low-density fibrinogen (P = .03 and P = .01, respectively). These observations suggest that there may be different signaling mechanisms after platelet adhesion to low- versus high-density fibrinogen.
Effect of apyrase and inhibitors of signal transduction and actin polymerization on morphology of adherent platelets and FAK tyrosine phosphorylation
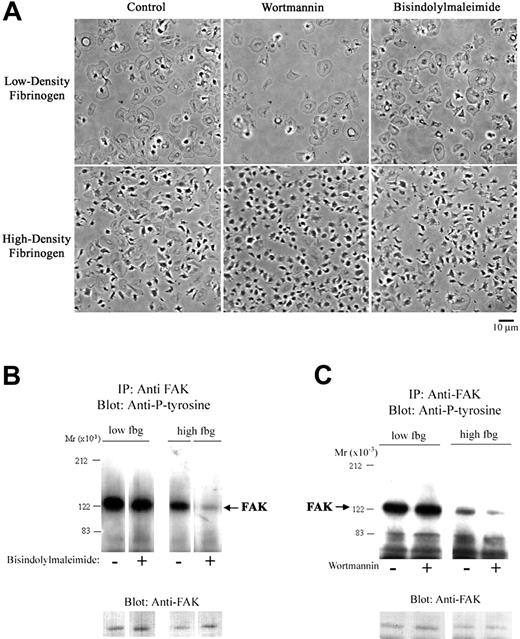
Since both PKC and PI3K have been implicated in signaling downstream from αIIbβ3 after interaction with fibrinogen, we studied the effect of their inhibition on adhesion to low- and high-density fibrinogen. Both bisindolylmaleimide, an inhibitor of PKC, and wortmannin, an inhibitor of PI3K, decreased adhesion to low-density fibrinogen by approximately 50% (Table 2), but had little effect on the morphology of adherent platelets or FAK tyrosine phosphorylation (Figure 5). In contrast, these 2 inhibitors reduced adhesion to high-density fibrinogen by only approximately 15%, but they markedly decreased platelet spreading and FAK tyrosine phosphorylation (Figure 5).
The effect of inhibitors on platelet adhesion to fibrinogen
| Inhibitor . | Dose . | Target . | Platelet adhesion, % of control . | |
|---|---|---|---|---|
| Low-density fibrinogen . | High-density fibrinogen . | |||
| Bisindolylmaleimide | 10 μM | PKC | 49 ± 15* | 85 ± 2* |
| Wortmannin | 20 nM | PI3K | 51 ± 15* | 84 ± 14 |
| PP2 | 20 μM | Src kinase | 43 ± 9* | 99 ± 10 |
| Cytochalasin D | 10 μM | Actin polymerization | 20 ± 14* | 104 ± 5 |
| Apyrase | 2 U/mL | ADP | 57 ± 12* | 86 ± 7* |
| H-1152 | 5 μM | Rho kinase | 103 ± 19 | 100 ± 9 |
| NSC23766 | 100 μM | Rac1 | 82 ± 14 | 103 ± 4 |
| Inhibitor . | Dose . | Target . | Platelet adhesion, % of control . | |
|---|---|---|---|---|
| Low-density fibrinogen . | High-density fibrinogen . | |||
| Bisindolylmaleimide | 10 μM | PKC | 49 ± 15* | 85 ± 2* |
| Wortmannin | 20 nM | PI3K | 51 ± 15* | 84 ± 14 |
| PP2 | 20 μM | Src kinase | 43 ± 9* | 99 ± 10 |
| Cytochalasin D | 10 μM | Actin polymerization | 20 ± 14* | 104 ± 5 |
| Apyrase | 2 U/mL | ADP | 57 ± 12* | 86 ± 7* |
| H-1152 | 5 μM | Rho kinase | 103 ± 19 | 100 ± 9 |
| NSC23766 | 100 μM | Rac1 | 82 ± 14 | 103 ± 4 |
Platelets were allowed to adhere to fibrinogen coated microtiter wells for 1 hour. After washing, platelet adhesion was assessed by endogenous acid phosphatase activity. Adhesion is expressed as a percentage of control adhesion and results are presented as mean ± SD (n = 3).
P < .05 compared to control.
PKC and PI3K inhibition decreases FAK tyrosine phosphorylation and platelet spreading on high-density fibrinogen. Platelets were incubated with bisindolylmaleimide (10 μM; A-B), wortmannin (20 nM; A,C), or vehicle (control) and then allowed to adhere to wells precoated with fibrinogen. (A) Morphology of platelets adherent to low-density fibrinogen did not change with treatment with bisindolylmaleimide or wortmannin. Platelets adherent to high-density fibrinogen in the presence of these inhibitors showed less spreading than the control platelets. (B-C) Presence of PKC or PI3K inhibitors led to a decrease in FAK tyrosine phosphorylation in platelets adherent to high-density fibrinogen only. Noncontiguous lanes from a single blot are shown in both panels B and C. Results shown are representative of 3 independent experiments.
PKC and PI3K inhibition decreases FAK tyrosine phosphorylation and platelet spreading on high-density fibrinogen. Platelets were incubated with bisindolylmaleimide (10 μM; A-B), wortmannin (20 nM; A,C), or vehicle (control) and then allowed to adhere to wells precoated with fibrinogen. (A) Morphology of platelets adherent to low-density fibrinogen did not change with treatment with bisindolylmaleimide or wortmannin. Platelets adherent to high-density fibrinogen in the presence of these inhibitors showed less spreading than the control platelets. (B-C) Presence of PKC or PI3K inhibitors led to a decrease in FAK tyrosine phosphorylation in platelets adherent to high-density fibrinogen only. Noncontiguous lanes from a single blot are shown in both panels B and C. Results shown are representative of 3 independent experiments.
Src family kinases have been shown to play a major role in αIIbβ3-mediated outside-in signaling on high-density fibrinogen,19 however, their role in αIIbβ3 outside-in signaling on low-density fibrinogen is unknown. The Src family kinase inhibitor PP2 significantly reduced platelet adhesion to low-density fibrinogen but not to high-density fibrinogen (Table 2). Platelet spreading was impaired on both low- and high-density fibrinogen in the presence of PP2 (Figure 6A). Similar results were obtained with SU6656, another Src family kinase inhibitor (not shown).
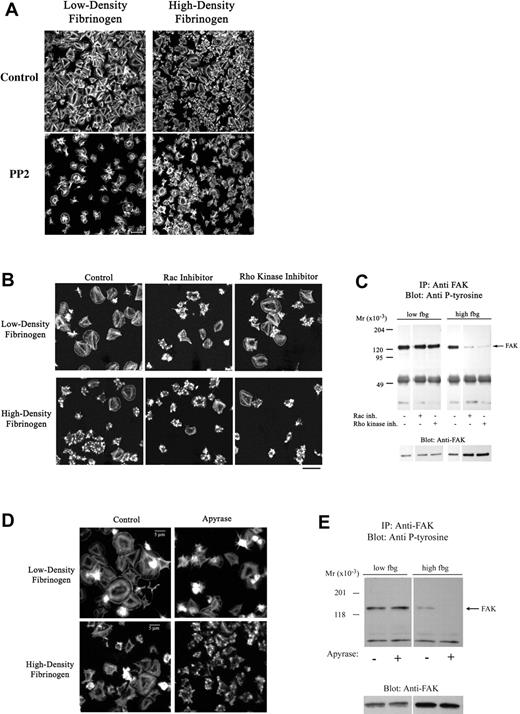
Apyrase and inhibitors of Src, Rac-1, or Rho kinase decrease platelet spreading on fibrinogen. Platelets were allowed to adhere to fibrinogen in the presence of a Src-kinase inhibitor (20 μM PP2), a Rac-1 inhibitor (100 μM NSC23766), or a Rho kinase inhibitor (5 μM H1152) for 1 hour. (A-B) Adherent platelets were fixed, permeabilized, and stained with fluorescently labeled phalloidin for F-actin. The Src (A) and Rac-1 (B) inhibitor and to a lesser extent the Rho kinase inhibitor (B) led to impaired spreading of platelets adhering to both low- and high-density fibrinogen. (C) Analysis of FAK immunoprecipitated from platelets adherent in the presence of Rac-1 and Rho kinase inhibitors revealed decreased phosphorylation of FAK in platelets adherent to high- but not to low-density fibrinogen. (D-E) Presence of apyrase led to less extensive spreading in platelets on both high- and low-density fibrinogen, but to decrease in FAK phosphorylation in platelets on high-density fibrinogen only. Noncontiguous lanes from a single blot are shown. Results shown are representative of 3 independent experiments.
Apyrase and inhibitors of Src, Rac-1, or Rho kinase decrease platelet spreading on fibrinogen. Platelets were allowed to adhere to fibrinogen in the presence of a Src-kinase inhibitor (20 μM PP2), a Rac-1 inhibitor (100 μM NSC23766), or a Rho kinase inhibitor (5 μM H1152) for 1 hour. (A-B) Adherent platelets were fixed, permeabilized, and stained with fluorescently labeled phalloidin for F-actin. The Src (A) and Rac-1 (B) inhibitor and to a lesser extent the Rho kinase inhibitor (B) led to impaired spreading of platelets adhering to both low- and high-density fibrinogen. (C) Analysis of FAK immunoprecipitated from platelets adherent in the presence of Rac-1 and Rho kinase inhibitors revealed decreased phosphorylation of FAK in platelets adherent to high- but not to low-density fibrinogen. (D-E) Presence of apyrase led to less extensive spreading in platelets on both high- and low-density fibrinogen, but to decrease in FAK phosphorylation in platelets on high-density fibrinogen only. Noncontiguous lanes from a single blot are shown. Results shown are representative of 3 independent experiments.
The small GTPases Rac and Rho have been implicated in filopodia and lamellipodia formation in platelets39,40 and other cells.41 Neither the Rac-1 inhibitor (NSC2376642 ) nor the Rho kinase inhibitor (H-1152) had an affect on the number of adherent platelets (Table 2); however, both Rac-1 and Rho kinase inhibitors decreased platelet spreading irrespective of the fibrinogen density (Figure 6B). Surprisingly, the presence of Rac-1 or Rho kinase inhibitors did not decrease the intensity of FAK phosphorylation in platelets adherent to low-density fibrinogen, whereas FAK phosphorylation in platelets adherent to high-density fibrinogen was almost eliminated (Figure 6C).
Since actin polymerization participates in platelet spreading, we studied the effect of an actin polymerization inhibitor. Cytochalasin D reduced the number of platelets adherent to low-density fibrinogen by approximately 80%, but did not inhibit the number of platelets adherent to high-density fibrinogen (Table 2). It did, however, inhibit platelet spreading on high-density fibrinogen, and this effect correlated with inhibition of actin polymerization as judged by phalloidin staining (not shown). The morphology of the few platelets on low-density fibrinogen in the presence of cytochalasin D could not be determined because the adhesion was weak and the remaining platelets were lost during the staining procedure. Latrunculin A, another inhibitor of actin polymerization, produced similar effects (not shown).
To assess the contribution of released endogenous ADP, previously implicated in platelet adhesion to, and spreading on, fibrinogen,43 studies were performed in the presence of apyrase. Apyrase led to an approximately 40% decrease in adhesion to low-density fibrinogen and only an approximately 15% decrease in adhesion to high-density fibrinogen (Table 2). Platelet spreading, however, was reduced in platelets adherent to both low- and high-density fibrinogen (Figure 6D). The presence of apyrase did not affect tyrosine phosphorylation of FAK in platelets adherent to low-density fibrinogen, but it nearly eliminated the small amount of FAK phosphorylation in platelets adherent to high-density fibrinogen (Figure 6E).
Platelet adhesion to low-density fibrinogen results in more platelet degranulation than to high-density fibrinogen, but release is not extensive on either surface
Adhesion to high-density fibrinogen has previously been shown to initiate only minimal release reaction.44 To assess whether low-density fibrinogen is a more active surface, we measured ATP concentration in the supernatant 60 minutes after platelet adhesion. In each of 5 separate experiments, the ATP release from the platelets adherent to low-density fibrinogen exceeded the ATP release from platelets adherent to high-density fibrinogen. Although absolute values for luminescence varied considerably from day to day and donor to donor (mean ± SD for low-density fibrinogen: 3000 ± 2800, and for high-density fibrinogen: 940 ± 940), the ratios of luminescence values on low- versus high-density fibrinogen for each experiment ranged only from 2 to 5 (3.2 ± 1.5, P = .008). To assess the release reaction in individual platelets, we studied degranulation by imaging platelets labeled with the fluorescent amine, mepacrine, which concentrates in platelet-dense bodies.28 After adhesion to low-density fibrinogen for 60 minutes, a higher percentage of platelets had only 0 or 1 dense body (46% vs 31%; medians; n = 4, P = .05 period t-test) and a lower percentage of platelets had more than 4 dense bodies (15% vs 26%, P = .03) compared to platelets adherent to high-density fibrinogen. These data support the results of the ATP experiments in indicating greater dense granule release on low-density fibrinogen. Even after 60 minutes of platelet adhesion to low-density fibrinogen, however, the majority of adherent platelets retained some dense bodies. The addition of a thrombin receptor activating peptide (TRAP) to the adherent platelets resulted in nearly immediate release of most of the dense bodies, as judged by the loss of the mepacrine staining (Figure S4). In addition, the morphology of the platelets on high-density fibrinogen dramatically changed after addition of TRAP, with many platelets immediately extending lamellipodia and spreading further. In contrast, there was little change in morphology of platelets adherent to low-density fibrinogen.
Discussion
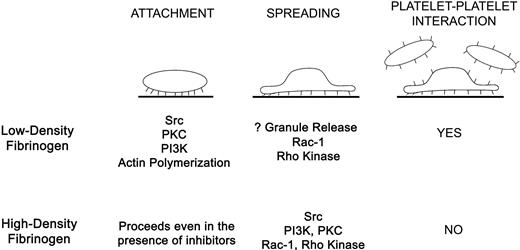
Binding of αIIbβ3 to immobilized fibrinogen during platelet adhesion results in outside-in signaling,2,12 initiated perhaps by ligand-induced structural rearrangements between the β3 βA (βI-like) and hybrid domains.3 Our data indicate that the surface concentration of fibrinogen affects both platelet adhesion and αIIbβ3-initiated outside-in signaling, resulting in differences in Ca2+ signaling, signal transduction mechanisms, morphology, and inside-out signaling (Figure 7). Using TIR-FM, we found that interactions of αIIbβ3 with low-density fibrinogen lead to biphasic morphologic changes during platelet attachment, characterized by the sequential development of filopodia and lamellipodia and resulting in extensive spreading. Increases in intracellular Ca2+ occur rapidly in some platelets, with about half of them ultimately developing sustained Ca2+ oscillations. Actin polymerization and Src activation are essential for platelet attachment and full spreading, whereas Rac-1, PKC, and PI3K contribute partially. Activation of luminal αIIbβ3 receptors and recruitment of additional layers of platelets are specific for platelets adhering to low-density fibrinogen. Of interest, even though we observed a decrease in platelet spreading when either apyrase or a Rac-1 inhibitor was used, there was no effect on the degree of FAK phosphorylation, suggesting that on low-density fibrinogen FAK activation requires ADP or Rac-1 to achieve platelet spreading or that FAK activation is largely independent of the degree of platelet spreading. All of our observations on platelet adhesion to low-density fibrinogen are consistent with a model in which initial αIIbβ3 interaction with fibrinogen results in outside-in signaling followed by inside-out signaling, luminal αIIbβ3 activation, and cytoskeletal rearrangements, with only modest granule release.
Summary of differences in platelets signaling and spreading during αIIbβ3-mediated interaction with low- versus high-density fibrinogen. Attachment of platelets to low-density fibrinogen requires activity of Src, PKC, and PI3 kinases and actin polymerization. Full platelet spreading depends on Rac-1 and Rho kinase. Platelet adhesion to low-density fibrinogen further leads to inside-out signaling, resulting in αIIbβ3 activation and recruitment of additional platelets on top of the adherent platelets. In contrast, platelet attachment to high-density fibrinogen is possible even in the presence of inhibitors of Src, PKC, PI3-kinase, and actin polymerization. Platelet spreading depends on Src, PI3K, PKC, Rac-1, and Rho kinase activation. Few platelets are recruited to the adherent platelets indicating inadequate inside-out signaling or inhibition of luminal αIIbβ3 activation.
Summary of differences in platelets signaling and spreading during αIIbβ3-mediated interaction with low- versus high-density fibrinogen. Attachment of platelets to low-density fibrinogen requires activity of Src, PKC, and PI3 kinases and actin polymerization. Full platelet spreading depends on Rac-1 and Rho kinase. Platelet adhesion to low-density fibrinogen further leads to inside-out signaling, resulting in αIIbβ3 activation and recruitment of additional platelets on top of the adherent platelets. In contrast, platelet attachment to high-density fibrinogen is possible even in the presence of inhibitors of Src, PKC, PI3-kinase, and actin polymerization. Platelet spreading depends on Src, PI3K, PKC, Rac-1, and Rho kinase activation. Few platelets are recruited to the adherent platelets indicating inadequate inside-out signaling or inhibition of luminal αIIbβ3 activation.
In contrast, αIIbβ3 interactions with high-density fibrinogen lead to an earlier onset of lamellipodia formation and a longer period of filopodia formation, resulting in a prolonged period when both processes occur simultaneously. Despite these active processes, platelet spreading is significantly reduced. Increases in intracellular Ca2+ occur less frequently and Ca2+ oscillations are sustained in only a small percentage of platelets. None of the signal transduction inhibitors used alone inhibited platelet attachment but PKC, PI3K, Src, and to some extent Rac-1, all contributed to platelet spreading. In contrast to platelets on low-density fibrinogen, inhibitors that decrease platelet spreading also decrease FAK phosphorylation. Time-lapse TIR-FM and studies with αIIbβ3 antagonists demonstrate that αIIbβ3 interactions with high-density fibrinogen are more dynamic and reversed more easily compared to low-density fibrinogen. In addition, adhesion to high-density fibrinogen is associated with even less granule release and does not lead to activation of luminal αIIbβ3 receptors.
Our studies with conformation-specific mAbs demonstrate that αIIbβ3 receptors exist in different conformations on the surface of a single platelet. Time-lapse TIR-FM of platelets stained with fluorescently labeled AP5, which preferentially reacts with ligand-bound receptors, suggests that initially receptors on the entire basal surface engage the ligand, but with increasing spreading only receptors at the edge of the platelets remain engaged. The interactions with ligands are more dynamic on high-density fibrinogen, perhaps because more ligand molecules than receptors are available.21 On low-density fibrinogen, biphasic morphologic changes occur during platelet attachment, where formation of filopodia is followed by formation of lamellipodia, resulting in anisotropic spreading. Similar observations have been made with endothelial cells, which tended to spread anisotropically on low-density and isotropically on high-density RGD-containing gels.45
The more intense PAC-1 staining of platelets on low-density fibrinogen indicates that these platelets have more available activated αIIbβ3 receptors. Since platelets on low-density, but not high-density, fibrinogen can recruit additional layer of platelets, we conclude that at least some of these activated receptors are on the luminal surface. The much greater initiation of sustained intracellular Ca2+ fluxes in platelets on low-density fibrinogen is likely to explain the greater αIIbβ3 activation since calcium signaling is associated with αIIbβ3 activation, platelet aggregation, and thrombus formation and propagation.46,47
The effect of ligand density on signaling, mobility, and motility has been studied in other cells with similar findings, namely, low ligand density results in greater mobility and high ligand density results in inhibition of signaling.48–50 Random migration of αIIbβ3-expressing CHO cells on fibrinogen was inhibited at ligand-coating concentrations above approximately 5 μg/mL, a value comparable to the division we found between low- and high-density fibrinogen.50 Cox et al48 found similar ligand density dependence of α5β1-mediated adhesion of CHO cells to fibronectin and αMβ2-mediated adhesion of neutrophils to fibrinogen. Moreover, they observed less spreading and polarization of CHO cells on high fibronectin densities and concluded that high ligand densities induce a stop signal(s) for cell migration and polarization. The interplay of Rho family GTPases has been suggested to have a role in this process as Rac-1 is inhibited at high ligand densities.48 Similarly, in our studies, the morphology of platelets adhering to low-density fibrinogen in the presence of a Rac-1 inhibitor resembled the morphology of control platelets on high-density fibrinogen, suggesting that Rac GTPase may not be fully activated during adhesion to high-density fibrinogen. Ligand density has also been shown to affect activation of FAK; however, in these studies increased fibronectin density led to increased α5β1-mediated FAK phosphorylation.51,52
The effects of fibrinogen density and/or surface orientation appear to be especially important in the thrombogenicity of artificial surfaces. Fibrinogen preferentially adsorbs from plasma and mediates platelet interactions with artificial surfaces.11,53 Platelet recruitment, however, does not necessarily correlate with the total amount of surface-bound fibrinogen, but rather with the amount of fibrinogen that can be recognized by specific antibodies.11,54–56 We and others previously suggested that most fibrinogen molecules are oriented vertically on the surface when immobilized at high density and horizontally at low density.57–60 Thus, on high-density fibrinogen, the αIIbβ3 recognition sites on the γ-chain C-termini are likely to extend out from the surface, whereas on low-density fibrinogen the horizontal orientation is likely to reduce access to the γ-chain C-termini and/or induce conformational changes by multiple contacts between fibrinogen molecules and the surface. This may expose regions that are cryptic in solution61–63 and make them available for the interaction with the integrin, maybe contributing to the unique outside-in signaling observed on low-density fibrinogen. It has been recently proposed that both the γ-chain C-terminal sequence and the RGD sequence(s) are needed for maximal spreading of αIIbβ3-expressing CHO cells and platelets on fibrinogen.40
It is also possible that fibrinogen adsorption to the surface at low density results in the arrangement of the ligands in the form of clusters rather than as evenly spaced ligands. Clustering of the receptors mediating adhesion may facilitate lateral interactions among αIIbβ3 receptors64 and facilitate focal adhesion formation, the initiation of signal transduction, platelet activation, and spreading.65 However, imaging with TIR-FM suggests that there is no obvious difference in cluster size of αIIbβ3 integrins on low- versus high-density fibrinogen. Immobilizing fibrinogen at high density is associated with a thicker ring of AP5 staining, suggesting that a larger number of αIIbβ3 receptors are engaged in interaction with fibrinogen, and it is possible that this initiates an inhibitory signal that counterbalances any activating signals that are generated.48
In conclusion, our data demonstrate that the morphologic differences in platelet adhesion to low- and high-density fibrinogen are paralleled by differences in signal transduction, indicating that the αIIbβ3 outside-in signaling mechanisms are fundamentally different. These data need to be considered when interpreting data on platelet adhesion to fibrinogen in vitro and in vivo. Moreover, the ability to modify platelet adhesion to a synthetic surface so that the platelet does not recruit additional layers of platelets has the potential to improve the biocompatibility of materials that come in contact with blood. In vivo, depending on the nature and extent vascular injury, the density of immobilized fibrinogen may range up to the high-density values defined in our studies, suggesting that our observations may also be relevant to both hemostasis and thrombosis.5
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grant 19278 from the National Heart, Lung, and Blood Institute; by a General Clinical Research Center grant (M01-RR00102) and a Clinical and Translational Science Award (UL1-RR024143) from the National Center for Research Resources at the NIH; grant BES-0322867 from NSF; grant 1P20GM072015-01 from NIH; and by funds from Stony Brook University.
The confocal microscopy was performed at the Bio-Imaging Resource Center at Rockefeller University with help from Dr Alison J. North and TIRF imaging was performed in the laboratory of Dr Sanford M. Simon. We are grateful to Dr JiHong Li, Rockefeller University, for her excellent technical assistance; Dr Carol Bodian, Mount Sinai School of Medicine, New York, NY, for her help with statistical analysis; Mr Heikki Väänänen, Mount Sinai School of Medicine, New York, NY, for help with the time-lapse phase-contrast microscopy experiments; and Dr Peter J. Newman, Blood Research Institute, the Blood Center of Southeastern Wisconsin, Milwaukee, WI, for AP5 antibody. Antibody 7H2 was produced at National Cell Culture Center, Minneapolis, MN.
National Institutes of Health
Authorship
Contribution: M.J. and J.K.J. designed the research, performed the studies, analyzed the data, and wrote the paper; B.S.C. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markéta Jiroušková, Rockefeller University, Box 309, 1230 York Ave, New York, NY 10021; e-mail: marketa@rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal