Abstract
The somatic JAK2 valine-to-phenylalanine (V617F) mutation has been detected in up to 90% of patients with polycythemia and in a sizeable proportion of patients with other myeloproliferative disorders such as essential thrombocythemia and idiopathic myelofibrosis. Suppressor of cytokine signaling 3 (SOCS3) is known to be a strong negative regulator of erythropoietin (EPO) signaling through interaction with both the EPO receptor (EPOR) and JAK2. We report here that JAK2 V617F cannot be regulated and that its activation is actually potentiated in the presence of SOCS3. Instead of acting as a suppressor, SOCS3 enhanced the proliferation of cells expressing both JAK2 V617F and EPOR. Additionally, although SOCS1 and SOCS2 are degraded in the presence of JAK2 V617F, turnover of SOCS3 is inhibited by the JAK2 mutant kinase and this correlated with marked tyrosine phosphorylation of SOCS3 protein. We also observed constitutive tyrosine phosphorylation of SOCS3 in peripheral blood mononuclear cells (PBMCs) derived from patients homozygous for the JAK2 V617F mutant. These findings suggest that the JAK2 V617F has overcome normal SOCS regulation by hyperphosphorylating SOCS3, rendering it unable to inhibit the mutant kinase. Thus, JAK2 V617F may even exploit SOCS3 to potentiate its myeloproliferative capacity.
Introduction
The somatic valine-to-phenylalanine (V617F) mutation in JAK2 has been associated with a variety of myeloproliferative disorders (MPD), including polycythemia vera (PV), essential thrombocythemia (ET), and idiopathic myelofibrosis (IMF).1–5 In wild-type JAKs the JH2 domain inhibits the JH1 kinase domain through interactions at 2 interfaces, with the region containing V617 being predicted to preserve the inactive conformation of the activation loop.6 The V617F mutation might alter this conformation and perhaps stabilize the activation loop in an active state, or it may prevent access of other proteins to the catalytic domain. The V617 residue of JAK2 is conserved in the JH2 domain of JAK1 and TYK2, whereas in JAK3 it is replaced by methionine. Like JAK2 V617F, analogous mutations in JAK1 or TYK2 also results in their constitutive activation.7 Janus kinases require the JH2 pseudokinase domain for normal physiologic activation of the JH1 catalytic domain. Therefore, it seems that the V617F mutation may disrupt the putative inhibition of the catalytic domain by the pseudokinase domain and create a constitutively activated kinase. However, the Janus kinases are also potently regulated by the suppressor of cytokine signaling (SOCS) proteins that are thought to bind to the JH1 catalytic loop and target the kinases for degradation. Whether SOCS can regulate the JAK2 V617F mutant has not been explored.8
SOCS1 and SOCS3 bind to the catalytic groove of JAK2 via their kinase inhibitory region (KIR) to inhibit catalytic activity.8 Both of these SOCS proteins can also target TEL-JAK2 and wild-type JAK2 for ubiquitination and degradation via their SOCS box ECS ubiquitin E3 ligase interaction motif.9,10 Additionally, SOCS3 binds via its SH2 domain to tyrosine residues of the EPOR: Y401, where STAT5 and SHP-2 also bind, and Y429/Y431, where SHP-1 also binds.11 The ability of SOCS3 to interact with both the EPOR and JAK2, may increase the inhibitory effect of SOCS3 on the EPO signaling pathway. Indeed, the inhibitory effect of SOCS3 is greatly enhanced in the presence of a cognate receptor, as has been reported for interleukin 2 receptor β (IL-2Rβ)12 and GP130 signaling,13 suggesting that receptor interaction may be essential for SOCS3-mediated inhibition. Lu and colleagues14 have suggested that JAK2 V617F specifically contributes to diseases of myeloid lineage as these cells express homodimeric type I cytokine receptors which can interact with and be inhibited by SOCS3, whereas lymphocytes do not.
Here we show that SOCS3 was unable to regulate EPO signaling in the presence of the MPD-associated JAK2 mutant. SOCS3 was unable to block proliferation in cells expressing JAK2 V617F and indeed growth was somewhat enhanced, suggesting SOCS3 could potentiate its activation. Although SOCS1 and SOCS2 were degraded in the presence of the JAK2 mutant, SOCS3 was not. Instead, SOCS3 was strongly tyrosine phosphorylated and the JAK2 mutant was stabilized in its presence. Therefore, not only is SOCS3 unable to regulate JAK2 V617F, but it may actually contribute to the pathogenesis of the diseases associated with this mutation.
Materials and methods
Cells and transfections
Ba/F3-SOCS3 cells11 were retrovirally infected with EPOR pMX-IRES-GFP, JAK2 V617F pMX-IRES-CD4, or JAK2 WT pMX-IRES-CD4.2 293T cells were transfected with 2 μg SOCS1 pME18S Flag, SOCS2 pME18S Flag, SOCS3 pME18S Flag, JAK2 WT pRK, and JAK2 V617F pRK as appropriate using FuGENE (Roche Diagnostics, West Sussex, United Kingdom).6 All cell lines were maintained as described.12
RT-PCR analysis
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of SOCS expression in 293T transfectants was carried out as described.15
Immunoprecipitations and Western blotting
Ba/F3 and 293T cells were lysed with RIPA16 and Brij 9717 buffers, respectively. Lysates were immunoprecipitated with anti-JAK2 (Upstate Biotechnology, Lake Placid, NY) or anti-SOCS3 clone 008 (Fusion Antibodies, Belfast, Northern Ireland) as required. Western blots were probed with antiphosphotyrosine clone 4G10, anti-JAK2 (Upstate Biotechnology), anti-Flag M2 (Sigma Aldrich, Dorset, United Kingdom), or anti-SOCS3 clone 008.
Proliferation assay
Ba/F3 cell populations were seeded at a density of 1 × 105 per mL and grown in RPMI-1640 containing 5% fetal calf serum (FCS) and 5 U/mL EPO in the presence or absence of tetracycline (4 μg/mL). Viable cells were counted by trypan blue exclusion every 24 hours or measured by MTT assay as described.17 Statistical analysis was carried out using a Student t test.
Isolation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll density gradient centrifugation from consenting healthy donors or patients with PV who gave informed consent in accordance with the Declaration of Helsinki. Approval for these studies was obtained from the Belfast City Hospital Trust Institutional Review Board. The isolated PBMCs were washed twice with phosphate-buffered saline (PBS) and lysed in Brij 97 buffer. Whole cell lysate (100 μg) was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) while the remainder was immunoprecipitated with anti-SOCS3 clone 008. Western blots were probed with antiphosphotyrosine clone 4G10 and reprobed with anti-SOCS3.
Results
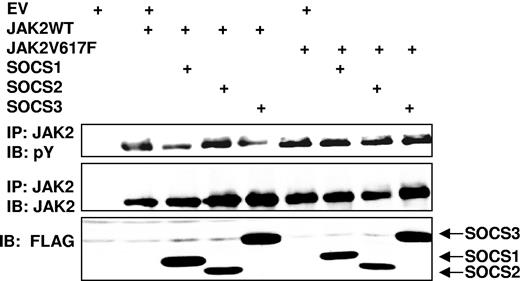
The V617F mutation in JAK2 leads to a dysregulated kinase. Since SOCS proteins negatively regulate JAK activity, we hypothesized that altered SOCS-mediated control of JAK2 V617F may contribute to its dysregulation. We explored this by coexpressing in 293T cells SOCS1, SOCS2, or SOCS3 with either JAK2 WT or JAK2 V617F (Figure 1). Although both SOCS1 and SOCS3 could inhibit wild-type JAK2 phosphorylation, the ability of these SOCS proteins to reduce JAK2 phosphorylation was attenuated by the V617F mutation (Figure 1).
Aberrant regulation of JAK2 V617F by SOCS proteins. 293T cells were transfected with JAK2 or JAK2 V617F alone, in combination with SOCS1, SOCS2, or SOCS3, as indicated. Lysates were immunoprecipitated (IP) with JAK2 antibody and the complexes immunoblotted (IB) with phosphotyrosine (pY) antibody (top panel). The blot was stripped and reprobed for JAK2 (middle panel). SOCS protein expression is also shown (bottom panel).
Aberrant regulation of JAK2 V617F by SOCS proteins. 293T cells were transfected with JAK2 or JAK2 V617F alone, in combination with SOCS1, SOCS2, or SOCS3, as indicated. Lysates were immunoprecipitated (IP) with JAK2 antibody and the complexes immunoblotted (IB) with phosphotyrosine (pY) antibody (top panel). The blot was stripped and reprobed for JAK2 (middle panel). SOCS protein expression is also shown (bottom panel).
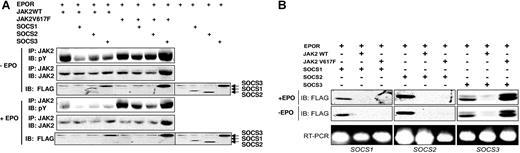
A recent report has concluded that the EPOR is required for transformation by JAK2 V617F.14 We therefore coexpressed SOCS1, SOCS2, or SOCS3 with the EPOR and either JAK2 WT or JAK2 V617F (Figure 2A). Although SOCS1, SOCS2, and SOCS3 could inhibit JAK2 WT phosphorylation, they were unable to block JAK2 V617F phosphorylation, particularly in the presence of EPO stimulation (Figure 2A). Interestingly, SOCS3-expressing cells consistently showed higher levels of phosphorylated JAK2 V617F with or without EPO stimulation and this was accompanied by an accumulation of JAK2 V617F protein.
SOCS3 enhances JAK2 V617F phosphorylation and fails to be degraded. (A) 293T cells were transfected with combinations of EPOR, JAK2, or JAK2 V617F, and FLAG-tagged SOCS1, SOCS2, or SOCS3 as indicated. Cells were treated for 30 minutes with EPO (50 U/mL) (+EPO, top 3 panels) or left untreated (−EPO, bottom 3 panels). Lysates were immunoblotted with phosphotyrosine antibody then stripped and reprobed with JAK2 antibody (top panels) or anti-FLAG to detect SOCS proteins. (B) Cell lysates from panel A were simultaneously analyzed for SOCS protein and mRNA levels. Whole-cell lysates were immunoblotted with FLAG antibody to detect SOCS proteins, while RT-PCR was performed on the total RNA using primer pairs specific for the SOCS genes, and products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide (bottom panels).
SOCS3 enhances JAK2 V617F phosphorylation and fails to be degraded. (A) 293T cells were transfected with combinations of EPOR, JAK2, or JAK2 V617F, and FLAG-tagged SOCS1, SOCS2, or SOCS3 as indicated. Cells were treated for 30 minutes with EPO (50 U/mL) (+EPO, top 3 panels) or left untreated (−EPO, bottom 3 panels). Lysates were immunoblotted with phosphotyrosine antibody then stripped and reprobed with JAK2 antibody (top panels) or anti-FLAG to detect SOCS proteins. (B) Cell lysates from panel A were simultaneously analyzed for SOCS protein and mRNA levels. Whole-cell lysates were immunoblotted with FLAG antibody to detect SOCS proteins, while RT-PCR was performed on the total RNA using primer pairs specific for the SOCS genes, and products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide (bottom panels).
SOCS proteins can inhibit JAK activity by functioning as E3 ubiquitin ligases via SOCS box–mediated interaction with Elongin C and its partner Elongin B.18 SOCS proteins are degraded in conjunction with the JAK thereby allowing this negative feedback to be terminated.19,20 To determine whether loss of JAK2 phosphorylation coincided with the degradation of SOCS, lysates from Figure 2A were simultaneously analyzed for SOCS protein and mRNA levels (Figure 2B). Although loss of SOCS1 and SOCS2 protein was observed in the presence of both JAK2 WT and the JAK2 mutant, loss of SOCS3 only occurred in the presence of wild-type JAK2 (Figure 2A-B). Moreover, SOCS3 protein was actually stabilized when coexpressed with JAK2 V617F and appeared as a doublet, which may be due to posttranslational modification of SOCS3 by the mutant JAK2. This altered SOCS expression most likely resulted from protein turnover as no change in mRNA levels was observed (Figure 2B). These results suggest that SOCS3 is unable to inactivate mutant JAK2 and as a result fails to undergo degradation itself.
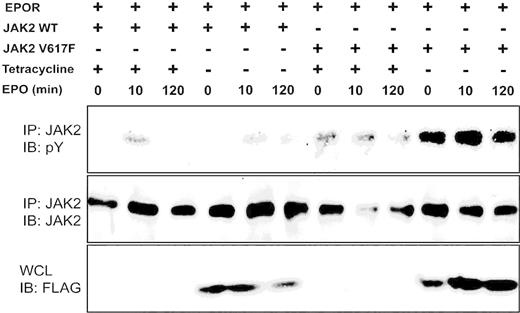
It has previously been demonstrated that the expression of JAK2 V617F can transform the murine IL-3–dependent hematopoietic cell line Ba/F3 to exhibit autonomous growth in the absence of cytokine stimulation2,3 and this effect could be significantly enhanced by coexpression of a type I cytokine receptor.14 We therefore created Ba/F3 cells expressing EPOR with either JAK2 WT or JAK2 V617F, in which SOCS3 expression was regulated by the removal of tetracycline (Figure 3). The levels of SOCS3 expressed in these cells upon withdrawal from tetracycline are comparable with the levels of endogenous SOCS3 induced in Ba/F3 cells upon IL-3 stimulation.17 Similar to our results in coexpression studies, SOCS3 protein was effectively degraded in the presence of JAK2 WT and inhibited JAK2 WT phosphorylation. However, when expressed with JAK2 V617F, SOCS3 failed to undergo degradation and actually enhanced the phosphorylation of the mutant JAK2 (Figure 3).
SOCS3 differentially affects growth signals from JAK2 V617F. Ba/F3 cells expressing EPOR with either JAK2WT or JAK2 V617F and SOCS3 regulated by tetracycline removal (-) were incubated in cytokine-free medium for 4 hours, stimulated with EPO (100 U/mL) for 10 minutes, washed and incubated for 120 minutes in cytokine-free medium. Lysates were immunoprecipitated (IP) with JAK2 antibody and blotted with phosphotyrosine antibody (top panel). The blot was stripped and reprobed with JAK2 antibody (middle panel). SOCS3 expression was determined by probing of corresponding whole-cell lysates (WCLs) with FLAG antibody (bottom panel).
SOCS3 differentially affects growth signals from JAK2 V617F. Ba/F3 cells expressing EPOR with either JAK2WT or JAK2 V617F and SOCS3 regulated by tetracycline removal (-) were incubated in cytokine-free medium for 4 hours, stimulated with EPO (100 U/mL) for 10 minutes, washed and incubated for 120 minutes in cytokine-free medium. Lysates were immunoprecipitated (IP) with JAK2 antibody and blotted with phosphotyrosine antibody (top panel). The blot was stripped and reprobed with JAK2 antibody (middle panel). SOCS3 expression was determined by probing of corresponding whole-cell lysates (WCLs) with FLAG antibody (bottom panel).
We then analyzed the effect of sustained SOCS3 expression and enhanced JAK2 V617F phosphorylation on cell proliferation in these cells (Figure 4A). While SOCS3 was a potent inhibitor of proliferation in cells expressing EPOR alone or expressing both EPOR and JAK2, induction of SOCS3 in cells expressing EPOR and JAK2 V617F did not inhibit proliferation (Figure 4A). To further analyze the effect of SOCS3 on JAK2 V617F–mediated growth we created Ba/F3 cells stably expressing JAK2 V617F with either EPOR or SOCS3 alone and with both EPOR and SOCS3 (Figure 4B). In this setting, SOCS3 again enhanced JAK2 V617F–induced cell growth in the presence of EPOR with or without EPO stimulation as demonstrated by both trypan blue exclusion and MTT assays (Figure 4B). This experiment also demonstrated that the EPOR was critical for the transformation, as cells expressing JAK2 V617F alone or SOCS3 and JAK2 V617F without EPOR show significantly less proliferation than those expressing all 3 proteins.
SOCS3 enhances JAK2 V617F–induced proliferation. (A) Ba/F3 cells expressing EPOR (top graph), EPOR and wild-type JAK2 (middle graph), or EPOR and JAK2 V617F (bottom graph) in which SOCS3 was absent due to the presence of tetracycline (□) or in which expression of SOCS3 was induced by the removal of tetracycline (■) were incubated in the presence of 5 U/mL EPO. Viable cell numbers determined by trypan blue exclusion. Significant differences between samples at each time point are indicated: *P < .05, **P < .01. (B) Ba/F3 cells stably expressing JAK2 V617F alone, or in combination with EPOR and/or SOCS3 as indicated were incubated in the presence of 5 U/mL EPO (top panels) or in the absence of cytokine (bottom panel), and viable cell numbers determined by trypan blue exclusion (top and bottom panels) or MTT assay (middle panel). Significant differences of samples to JAK2 V617F + EPOR + SOCS3 sample at each time point are indicated: *P < .05, **P < .01. Error bars represent standard deviation (SD).
SOCS3 enhances JAK2 V617F–induced proliferation. (A) Ba/F3 cells expressing EPOR (top graph), EPOR and wild-type JAK2 (middle graph), or EPOR and JAK2 V617F (bottom graph) in which SOCS3 was absent due to the presence of tetracycline (□) or in which expression of SOCS3 was induced by the removal of tetracycline (■) were incubated in the presence of 5 U/mL EPO. Viable cell numbers determined by trypan blue exclusion. Significant differences between samples at each time point are indicated: *P < .05, **P < .01. (B) Ba/F3 cells stably expressing JAK2 V617F alone, or in combination with EPOR and/or SOCS3 as indicated were incubated in the presence of 5 U/mL EPO (top panels) or in the absence of cytokine (bottom panel), and viable cell numbers determined by trypan blue exclusion (top and bottom panels) or MTT assay (middle panel). Significant differences of samples to JAK2 V617F + EPOR + SOCS3 sample at each time point are indicated: *P < .05, **P < .01. Error bars represent standard deviation (SD).
To investigate a possible mechanism by which V617F-induced cell growth is enhanced in the presence of SOCS3, we analyzed the effect of JAK2 WT and JAK2 V617F on SOCS3 phosphorylation. 293T cells were transfected with EPOR and SOCS3 alone or in combination with JAK2 WT or JAK2 V617F and treated with and without EPO. Analysis of lysates with anti-FLAG antibodies revealed that SOCS3 was degraded in the presence of wild-type JAK2, whereas JAK2 V617F had a much reduced effect on SOCS3 protein levels. Following immunoprecipitation with anti-SOCS3, tyrosine phosphorylated bands corresponding to JAK2 were observed, indicating that SOCS3 can associate with both wild-type and V617F JAK2. On EPO stimulation, a slight increase in phosphorylation of SOCS3 and JAK2 as well as association of SOCS3 with JAK2 was observed. However, compared with the levels of phosphorylated SOCS3 detected with wild-type JAK2, expression of JAK2 V617F caused a massive accumulation of tyrosine phosphorylated SOCS3 with or without EPO treatment.
We next investigated the effect of the JAK2 mutant on endogenous SOCS3 in 293T cells. Following transfection with EPOR, JAK2 WT, or JAK2 V617F alone or with FLAG-SOCS3 as a control, cell lysates were immunoprecipitated with anti-SOCS3 and immunoblotted with a phosphotyrosine-specific antibody (Figure 5B). Although we were unable to detect endogenous tyrosine phosphorylated SOCS3 in the presence of wild-type JAK2, expression of JAK2 V617F caused an accumulation of phosphorylated endogenous SOCS3. This result reinforces our observations that the V617F mutation induces robust SOCS3 tyrosine phosphorylation compared with wild-type JAK2.
SOCS3 is hyperphosphorylated by JAK2 V617F. (A) 293T cells were transfected with EPOR and SOCS3 alone or in combination with JAK2 WT or JAK2 V617F. Cells were treated with or without EPO (50 U/mL) for 15 minutes prior to lysis. Whole-cell lysates were immunoblotted with anti-JAK2 to detect JAK2 or anti-FLAG to detect SOCS3. The remaining lysates were immunoprecipitated with anti-SOCS3 clone 008 and resulting precipitates were immunoblotted with antiphosphotyrosine (4G10). (B) 293T cells were transfected with EPOR and JAK2, V617F, or FLAG-SOCS3 alone or combinations of JAK2 SOCS3 or V617F SOCS3 and analyzed as in panel A. (C) PBMCs were isolated from blood samples from a control patient and 2 individual patients with PV (patient nos. 1 and 2) homozygous for JAK2 V617F by Ficoll density gradient centrifugation. Cells were washed twice with PBS before being lysed in Brij buffer. Whole-cell lysates and anti-SOCS3 immunoprecipitates were immunoblotted with antiphosphotyrosine and reprobed with anti-SOCS3.
SOCS3 is hyperphosphorylated by JAK2 V617F. (A) 293T cells were transfected with EPOR and SOCS3 alone or in combination with JAK2 WT or JAK2 V617F. Cells were treated with or without EPO (50 U/mL) for 15 minutes prior to lysis. Whole-cell lysates were immunoblotted with anti-JAK2 to detect JAK2 or anti-FLAG to detect SOCS3. The remaining lysates were immunoprecipitated with anti-SOCS3 clone 008 and resulting precipitates were immunoblotted with antiphosphotyrosine (4G10). (B) 293T cells were transfected with EPOR and JAK2, V617F, or FLAG-SOCS3 alone or combinations of JAK2 SOCS3 or V617F SOCS3 and analyzed as in panel A. (C) PBMCs were isolated from blood samples from a control patient and 2 individual patients with PV (patient nos. 1 and 2) homozygous for JAK2 V617F by Ficoll density gradient centrifugation. Cells were washed twice with PBS before being lysed in Brij buffer. Whole-cell lysates and anti-SOCS3 immunoprecipitates were immunoblotted with antiphosphotyrosine and reprobed with anti-SOCS3.
An important extension of our hypothesis was to establish whether patients harboring a somatic JAK2 mutation also had constitutively phosphorylated SOCS3. PBMCs and neutrophils were isolated from healthy donors and patients with PV homozygous for the V617F mutation (all of whom had signed informed consent). Cells were lysed, immunoprecipitated with anti-SOCS3, and immunoblotted with a phosphotyrosine antibody. Compared with the control, each patient with PV showed greater levels of phosphorylated SOCS3 in both PBMCs (Figure 5C) and neutrophils (data not shown). This suggests that homozygous patients with the JAK2 V617F mutation also have constitutively phosphorylated SOCS3.
Discussion
In this paper we report that the JAK2 V617F mutant cannot be regulated by SOCS1, SOCS2, or SOCS3. We find that SOCS3 not only fails to inhibit the mutant kinase but potentiates its phosphorylation, its expression levels and the proliferation of cells expressing JAK2 V617F. Compellingly, JAK2 V617F enhances SOCS3 tyrosine phosphorylation in coexpression studies and, more importantly, cells from patients with the PV mutant JAK2 also exhibit increased levels of tyrosine-phosphorylated SOCS3. These unexpected observations suggest that SOCS3 may potentiate mutant JAK2-induced proliferation by stabilizing the protein and thus prolonging signaling.
Our findings indicate that SOCS3 plays a central role in modulating the activity of JAK2 V617F. As expected we found that SOCS3 blocked EPO responses and was degraded in the presence of wild-type JAK2. However, SOCS3 was not degraded in the presence of JAK2 V617F and was unable to inhibit the phosphorylation of the JAK2 V617F mutant. This inability to inhibit JAK2 V617F phosphorylation was independent of EPO stimulation but was clearly augmented in the presence of the EPOR.
Strikingly, cell transformation by JAK2 V617F is inhibited by overexpression of the wild-type JAK2.2 This competition can be demonstrated at the level of cell proliferation and also STAT5 and STAT3 activation2 and may be explained by competition between JAK2 and JAK2 V617F for limiting amounts of type I cytokine receptors. Cells that undergo mitotic recombination and lose wild-type JAK23 would acquire a significant proliferative advantage. However, mitotic recombination is a late event in MPD. For the initial phase of MPDs, our results may explain why in a heterozygous context, expression of JAK2 V617F succeeds to transform cells: low levels of signaling by JAK2 V617F would induce SOCS3, which would presumably knock down wild-type JAK2 signaling and not signaling by JAK2 V617F. Further experiments are required to test whether other mutations in JH2 that may activate JH1 (ie, E665K) would also lead to this particular interaction with SOCS3. Were this not the case, then activation of JAK2 V617F signaling by SOCS3 may help explain why the V617F is so prevalent in MPDs.
We have previously identified SOCS3 tyrosine phosphorylation on residues Y204 and Y221 in response to many cytokines, including EPO.20 Mutation of these residues creates a much more stable protein,19 suggesting that tyrosine phosphorylation enhances degradation. Interestingly, we also found that while SOCS3 is tyrosine phosphorylated and degraded rapidly in the presence of wild-type JAK2, an accumulation of phosphorylated SOCS3 occurred in the presence of the JAK2 V617F and SOCS3 protein was stabilized, indicating a more complex mechanism controlling SOCS protein degradation.
Tyrosine phosphorylation is known to regulate the interaction of SOCS3 with the E3 ligase component Elongin C.19 SOCS3 is not unique in this regard, as serine phosphorylation can regulate the stability of SOCS1 and its capacity to interact with Elongin C. For example, v-ABL–mediated phosphorylation of SOCS1 disrupts interaction with the Elongin BC complex, thus stabilizing SOCS1 and blocking JAK degradation,21 which can contribute to the transformation process. Similarly, phosphorylation of SOCS1 by the PIM family of kinases blocks the SOCS1–Elongin C interaction and may inhibit the ability of SOCS1 to target active JAKs to the proteasome.22 Treatment of SOCS1 with λ phosphatase (which removes all phosphate groups) restores the interaction of SOCS1 with Elongin C.22 These findings coupled with ours suggest that phosphorylation not only controls the interaction with the ECS E3 ligase, but may also suggest that it regulates E3 ligase activity. Moreover, in some pathologies such as v-ABL or JAK2 V617F transformation aberrant SOCS phosphorylation may block assembly of the E3 ligase and its activation resulting in JAK stabilization.
Myeloproliferative disorders are associated with myeloid cells expressing the type I cytokine receptors EPO, TPO, and G-CSFR, which have been shown to play a role in the pathogenesis of diseases associated with the JAK2 V617F mutation.14 For each of these homodimeric receptors, SOCS3 is able to interact with the receptor via an SH2 domain–phosphotyrosine interaction and presumably with the JAK2 kinase associated with each. Furthermore, SOCS3 can inhibit STAT activation by these receptors, perhaps via inhibition and degradation of JAKs, although it has also emerged that SOCS3 can shuttle the G-CSF receptor through the endosomal pathway.23 We have investigated the mechanism by which SOCS3 is degraded in the presence of wild-type JAK2 and EPOR. Treatment of 293T cells with the lysosomal inhibitor E64D for 2 hours, resulted in rescue of SOCS3 and phosphorylated JAK2 levels (Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article). This suggests that like G-CSF, SOCS3 may direct EPOR and associated JAK2 to the lysosome for degradation. The current investigation also establishes that the EPOR contributes to the dysregulation of the JAK2 mutant facilitating both the inhibition of wild-type JAK2 by SOCS3 as well as the ability of JAK2 V617F to recruit SOCS3. Since the JAK2 V617F mutation is associated with a range of MPDs it would be interesting to determine whether SOCS3 can enhance proliferation in the presence of other relevant type I cytokine receptors.
SOCS3 was originally reported to regulate cytokine signaling in the same way as SOCS1 by binding to the activation loop of JAKs thereby inhibiting any further activation. However, many reports have shown that SOCS3 inhibits cytokine signaling by binding cytokine receptors at a putative immunoreceptor tyrosine-based inhibitory motif (ITIM).11,13 Mutation of the EPOR ITIM reduces the inhibitor effect of SOCS3, suggesting that it interacts with both JAK2, probably via its kinase inhibitory region (KIR), and the EPOR. This may explain why SOCS3 blunts the EPO response so strongly and why SOCS1 does not enhance the JAK2 V617F response. Moreover, our observations show clearly that while SOCS3 is not degraded by JAK2 V617F, SOCS1 is.
In conclusion, our results indicate that the MPD-associated JAK2 V617F mutation cannot be regulated by SOCS3, which instead may act to stabilize the mutant kinase and enhance transformation. These observations imply that in PV both the mutant JAK2 V617F and EPOR responses cannot be controlled by the main regulator of this pathway, SOCS3, most likely contributing to the development of disease.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Prof Terry Lappin for providing us with EPO and Mr Gerry Clarke for his assistance with cell sorting. This work was supported by grants from the Biotechnology and Biological Sciences Research Council (no. 81/C17 863; M.B.H., J.E., Y.S., and J.A.J.); the Wellcome Trust (no. 07 034/Z/03/Z); the Research and Development Office at Health and Personal Social Services (HPSS) (no. RRG9.6 RSG/1960/02); a Télévie PhD fellowship of the Fonds National de la Recherche Scientifique (FNRS) Belgium (J.S.); a Deakin University International Study Program Award (A.C.W.); La Ligue Nationale contre le Cancer (équipe libellisée 2003; W.V.); and the Fédération Belge contre le Cancer, FNRS, Belgium (Mandat d'impulsion), the “Maggy and Robert de Hovre” Foundation, the Fonds Speciaux de Recherche of the Université Catholique de Louvain, and the Christian de Duve Institute of Cell Pathology (S.N.C.). W.V. is supported by an interface contract between INSERM and the Institut Gustave Roussy (IGR). S.N.C. is a Research Associate of FNRS, Belgium.
Authorship
Contribution: M.B.H., J.E., Y.S., and A.C.W. designed and performed research and wrote the paper; J.S. performed research; W.V. and S.N.C. contributed to writing the paper and provided vital reagents; M.J.P. and M.F.M. also provided reagents; and J.A.J. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James A. Johnston, Centre for Cancer Research and Cell Biology, 2nd Fl, Whitla Medical Bldg, 97 Lisburn Rd, Belfast BT9 7BL, Northern Ireland; e-mail: jim.johnston@qub.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal