Abstract
The extensive exploitation of the antitumor effect of donor lymphocytes infused after allogeneic hematopoietic stem-cell transplantation (allo-HSCT) is limited by the risk of graft-versus-host disease (GvHD). To overcome this limitation, we investigated the therapeutic potential of donor lymphocytes engineered with the suicide gene thymidine kinase of herpes simplex virus (TK) in 23 patients experiencing recurrence of hematologic malignancies after allo-HSCT. Long-term follow-up of infused patients included analysis of engraftment of genetically engineered lymphocytes, in vivo assessment of antitumor effect, and control of GvHD by ganciclovir. All 17 patients evaluable for engraftment and graft-versus-leukemia (GvL) had circulating TK+ cells detectable beginning at a median time of 18 days. Eleven patients (65%) experienced a substantial clinical benefit resulting in 6 (35%) complete remissions and 5 (29%) partial responses. The antitumor effect tightly correlated with the in vivo expansion of TK+ cells. Seven patients received ganciclovir, resulting in elimination of TK+ cells and effective and selective treatment of GvHD. Immunization against HSV-TK was observed in 7 patients but did not preclude an effective GvL. These data validate the feasibility, safety, and efficacy of TK+ cells in the context of allografting and represent the basis for a broader application of this technology.
Introduction
The curative potential of allogeneic hematopoietic stem-cell transplantation (allo-HSCT) strongly relies upon the immune response against host antigens mediated by donor T lymphocytes as effectors of antitumor immune surveillance.1 The graft-versus-leukemia (GvL) effect is further exploited by the use of delayed infusions of donor lymphocytes (DLIs).2 DLI has become the first treatment option for tumor relapse3,4 and other complications related to the immunosuppressive status of patients that received transplants such as reactivation of cytomegalovirus (CMV) and Epstein-Barr virus–induced posttransplantation lymphoproliferative disorders (EBV-PTLDs).5–7 However, the therapeutic impact of donor lymphocytes is limited by the risk of graft-versus-host disease (GvHD). Approximately 55% to 60% of patients treated with bulk doses of DLI for recurrent leukemia develop GvHD.8
Different strategies aimed to control GvHD while preserving GvL have been attempted. The escalating dose regimen has a proven equal GvL rate with a significant reduction in GvHD in indolent diseases.9,10 However, the dose of DLI required for a therapeutic GvL differs among different malignancies and depends upon the tumor mass at the time of infusion. In addition, it remains to be determined whether limiting the T-cell dose will compromise the remission stability. The therapeutic potential of other approaches, such as the infusions of CD8+-depleted T cells11 and the transfer of leukemia-reactive effectors and T cells specific for hematopoietic-restricted minor histocompatibility antigens, has been proposed and may significantly reduce the risk of GvHD.12–14 However, the close association of GvL and GvHD suggests that a significant component of GvL is represented by alloreactivity itself. Consequently, any form of T-cell depletion, in particular, selective depletion of alloreactive cells, may result in some degree of reduction of GvL.
We previously reported that the genetic manipulation of a polyclonal population of donor lymphocytes with a “suicide gene” enables their selective elimination and results in the resolution of GvHD.15,16 Although different suicide genes have been described,17–20 the thymidine kinase of herpes simplex virus (TK) was the first used in clinical trials15,16,21–23 and has proved to offer the most effective suicide machinery.
In a pilot study involving the use of TK-transduced donor lymphocytes for in vivo modulation of donor antitumor immunity in patients with recurrent disease after allo-HSCT, we showed the feasibility of the procedure, the maintenance of immune competence of genetically modified lymphocytes, and the efficacy of their in vivo selective elimination with abrogation of GvHD.15,16 Despite this encouraging initial experience and a positive feedback in the field of transplantation,24–27 dissemination of the technology has proceeded at a low pace mainly due to the complexities associated with the ex vivo manipulation of donor lymphocytes and with the difficulties in implementing standardized gene-transfer procedures. This may represent the most likely cause of the variability in the clinical outcome of different studies.15,16,21–23
It is now clear that exploitation of this technology requires definition of rigorous conditions for upscale production of TK+ cells under Good Manufacturing Practice (GMP) conditions and the establishment of a direct correlation with clinical efficacy of the TK-DLI strategy used in extended clinical trials. In Europe, several groups are engaged in this effort in the context of the Suicide Gene Therapy in Stem Cell Transplantation Working Group that operates under the sponsorship of the European Union (www.sgtinsct.org). A first success of this effort was reported by Tiberghien and colleagues (Sauce et al28,29 ), who demonstrated that only specific culture and selection conditions are able to preserve EBV-reactive T cells in the population of genetically modified lymphocytes.
Here, we show the feasibility, safety, and efficacy of the TK strategy in 23 patients who relapsed after allo-HSCT from an HLA-identical sibling donor. More specifically, we demonstrate the feasibility of a standardized production of engineered donor lymphocytes in GMP conditions; the efficacy of TK+ cells in mediating antitumor responses able to induce persistent remission in patients with disease relapse after allo-HSCT for acute and chronic leukemia, non-Hodgkin lymphoma (NHL), and multiple myeloma (MM); and the safety of the overall procedure. Additionally, we confirm the efficiency of the TK suicide machinery in selectively eliminating engineered lymphocytes and controlling GvHD. T-cell transduction with suicide gene–encoding vectors is easily reproducible in GMP structures specialized in cell and gene therapy, relatively short time-consuming, and highly cost-effective.
Patients, materials, and methods
Patient characteristics and treatment regimen
Use of the TK technology in the context of allo-HSCT has been approved by the Italian Ministry of Health and the status of orphan drug has been granted by European Agency for the Evaluation of Medicinal Products (EMEA). This study was approved by the San Raffaele Scientific Institute Institutional Review Board and informed consent was obtained from donors and recipients. Of 30 patients enrolled with relapsed disease after HSCT for hematologic malignancies, 23 were infused with TK+ cells. Seven patients could not be infused for reasons associated with time constrains of cell preparation in relation to the speed of disease progression. The characteristics of the 23 patients infused and of the 7 patients who failed to receive TK cells are detailed in Table 1.
Patient characteristics
| Patient no. . | Age, y/sex . | Disease . | Type of transplant . | Disease status at HSCT . | Time from HSCT to REL, wk . | Time from relapse to TK cell infusions or death*, wk . |
|---|---|---|---|---|---|---|
| 1 | 30/F | DLBCL | MR | CR2 | 7 | 2 |
| 2 | 51/M | CML | MR | CP-CML | 28 | 11 |
| 3 | 18/F | CML | MR | AP-CML | 84 | 192 |
| 4 | 41/F | AML | MR | CR1 | 45 | 1 |
| 5 | 57/M | AML | MR | CR1 | 14 | 1 |
| 6 | 25/M | CML | MR | CP-CML | 5 | 1 |
| 7 | 22/F | AML | MMR | REL | 24 | 2 |
| 8 | 17/F | CMML | MR | CP-CMML | 19 | 4 |
| 9 | 19/M | ALL | MMR | CR4 | 12 | < 1 |
| 10 | 50/F | AML | MMR | REL | 11 | 2 |
| 11 | 35/F | AML | MMR | REL | 6 | 0 |
| 12 | 55/F | AML | MR | REL | 6 | < 1 |
| 13 | 45/F | AML | MR | CR1 | 5 | 1 |
| 14 | 45/M | AML | MR | REL | 4 | 3 |
| 15 | 28/F | ALL | MMR | REL | 6 | < 1 |
| 16 | 47/M | FCL | MR | REL | 10 | 3 |
| 17 | 48/F | MM | MR | PR | 50 | 2 |
| 18 | 30/F | HD | MR | REL | 26 | 95 |
| 19 | 37/F | AML | MR | REL | 4 | < 1 |
| 20 | 47/F | MM | MR | PR | 8 | 270 |
| 21 | 56/F | CML | MR | CP-CML | 102 | 1 |
| 22 | 28/F | HD | MR | REL | 14 | 2 |
| 23 | 58/M | MCL | MR | PR | 7 | 17 |
| 24 | 36/M | ALL | MR | REL | 18 | 6* |
| 25 | 50/M | ALL | MR | CR2 | 10 | 2* |
| 26 | 49/M | NHL | MR | CR3 | 22 | 2* |
| 27 | 49/F | ALL | MR | REL | 4 | < 1* |
| 28 | 52/M | AML | MR | CR2 | 122 | 2* |
| 29 | 29/M | ALL | MR | REL | 2 | 2* |
| 30 | 37/F | AML | MR | REL | 4 | 1* |
| Patient no. . | Age, y/sex . | Disease . | Type of transplant . | Disease status at HSCT . | Time from HSCT to REL, wk . | Time from relapse to TK cell infusions or death*, wk . |
|---|---|---|---|---|---|---|
| 1 | 30/F | DLBCL | MR | CR2 | 7 | 2 |
| 2 | 51/M | CML | MR | CP-CML | 28 | 11 |
| 3 | 18/F | CML | MR | AP-CML | 84 | 192 |
| 4 | 41/F | AML | MR | CR1 | 45 | 1 |
| 5 | 57/M | AML | MR | CR1 | 14 | 1 |
| 6 | 25/M | CML | MR | CP-CML | 5 | 1 |
| 7 | 22/F | AML | MMR | REL | 24 | 2 |
| 8 | 17/F | CMML | MR | CP-CMML | 19 | 4 |
| 9 | 19/M | ALL | MMR | CR4 | 12 | < 1 |
| 10 | 50/F | AML | MMR | REL | 11 | 2 |
| 11 | 35/F | AML | MMR | REL | 6 | 0 |
| 12 | 55/F | AML | MR | REL | 6 | < 1 |
| 13 | 45/F | AML | MR | CR1 | 5 | 1 |
| 14 | 45/M | AML | MR | REL | 4 | 3 |
| 15 | 28/F | ALL | MMR | REL | 6 | < 1 |
| 16 | 47/M | FCL | MR | REL | 10 | 3 |
| 17 | 48/F | MM | MR | PR | 50 | 2 |
| 18 | 30/F | HD | MR | REL | 26 | 95 |
| 19 | 37/F | AML | MR | REL | 4 | < 1 |
| 20 | 47/F | MM | MR | PR | 8 | 270 |
| 21 | 56/F | CML | MR | CP-CML | 102 | 1 |
| 22 | 28/F | HD | MR | REL | 14 | 2 |
| 23 | 58/M | MCL | MR | PR | 7 | 17 |
| 24 | 36/M | ALL | MR | REL | 18 | 6* |
| 25 | 50/M | ALL | MR | CR2 | 10 | 2* |
| 26 | 49/M | NHL | MR | CR3 | 22 | 2* |
| 27 | 49/F | ALL | MR | REL | 4 | < 1* |
| 28 | 52/M | AML | MR | CR2 | 122 | 2* |
| 29 | 29/M | ALL | MR | REL | 2 | 2* |
| 30 | 37/F | AML | MR | REL | 4 | 1* |
REL indicates, relapse; F, female; DLBCL, diffuse large B-cell lymphoma; MR, matched related; CR, complete remission; M, male; CML, chronic myeloid leukemia; CP-CML, chronic-phase CML; AP-CML, accelerated-phase CML; AML, acute myelogenous leukemia; MMR, mismatched related; CMML, chronic myelomonocytic leukemia; ALL, acute lymphoblastic leukemia; FCL, follicular cell lymphoma; MM, multiple myeloma; PR, partial remission; HD, Hodgkin lymphoma; and MCL, mantle cell lymphoma.
Time from relapse to death.
TK+ cells were prepared and cryopreserved for clinical use. TK+ cells were infused by intravenous injection in normal saline solution and 4% human serum albumin after premedication with clorpheniramine. To define the optimal dose for antitumor activity, the first 11 patients received escalating doses of donor lymphocytes, beginning at 105 cells/kg. Escalating doses were given monthly in the absence of GvHD to reach a total dose of 1 × 108 cells/kg.16 Since escalating infusions were found to contribute to immunization against the transgene product in some patients (see Table 6), starting from patient 12 patients received a single dose of 1 × 108 TK+ cells/kg. Patients surviving 30 days in the absence of ganciclovir (GCV) administration were evaluable for engraftment of TK+ cells and for the analysis of GvL, the main end point of the study. Occurrence of GvHD following administration of genetically engineered donor lymphocytes was also analyzed in all patients who survived more than 90 days after infusion.3
Following TK+ cell infusion, foscarnet was used in CMV pre-emptive treatment.
Production of genetically modified donor lymphocytes
Donor peripheral-blood mononuclear cells (PBMCs) were collected by leukapheresis prior to mobilization of hematopoietic precursors to avoid the immune-modulatory effect of G-CSF.30 PBMCs were purified by Fycoll-Hypaque gradient centrifugation (Lymphoprep; Nycomed, Pharma AS, Oslo, Norway). The immunophenotype of PBMCs was analyzed on a fluorescence-activated cell sorter (FACS) analyzer (Becton Dickinson, Milan, Italy) by staining with PE- and FITC-conjugated antibodies specific for CD2, CD19, CD14, CD3, CD56, CD4, and CD8 (Becton Dickinson).
The first patients received donor lymphocytes genetically modified by the SFCMM-2 retroviral vector encoding for the HSV-TK/Neo fusion suicide gene and the ΔLNGFR selectable gene.16 From patient 12, the neo-less SFCMM-3 vector encoding for the suicide gene (HSV-TK) and the gene for the selectable cell-surface marker (low-affinity nerve growth factor receptor, truncated of the intracytoplasmic domain: ΔLNGFR) was used. This vector switch was justified by the evidence of higher GCV sensitivity of SFCMM-3–transduced lymphocytes31 and to reduce the chances of in vivo immunization against transgene products.
The following transduction protocol was scaled-up and performed under GMP to maximize preservation of repertoire and effector function of transduced lymphocytes. PBMCs were cultured at 1 × 106 cells/mL following activation in RPMI1640 supplemented with 5% autologous plasma, phytohemagglutinin-L (2 μg/mL; Boehringer Mannheim-Roche GmbH, Mannheim, Germany), and 100 U/mL recombinant human interleukin-2 (rhIL-2; EuroCetus Italia, Milan, Italy). Transduction was carried out by 72-hour cocultivation with semiconfluent lethally irradiated packaging cells (100 Gy; GIL RAD, Gilardoni, Mandello, Italy) on the first day of stimulation in the presence of polybrene (8 μg/mL). Cytometric evaluation of transduction efficiency was performed by staining with 20.4 mouse anti–human ΔLNGFR monoclonal antibody (mAb) followed by FITC-conjugated goat anti–mouse IgG1. To calculate transduction efficiency, the negative control (cells stained with FITC-conjugated goat anti–mouse IgG1) was subtracted. To evaluate potential nonspecific binding of the anti-LNGFR antibody, PBMCs from a healthy donor were simultaneously stained with the same antibodies and analyzed by FACS. Following transduction, lymphocytes were selected for the expression of the surface marker ΔLNGFR with a mouse anti–human ΔLNGFR mAb (Boehringer Mannheim-Roche) and goat anti–mouse IgG1–coated magnetic beads (Dynabeads M-450; Dynal AS, Oslo, Norway).16,31 Following selection, lymphocytes were maintained ex vivo for a total of 14 days to reacquire resting state and subsequently frozen.32 At the end of the procedure, TK+ cells were analyzed for expression of ΔLNGFR, CD14, CD2, CD19, CD3, CD56, CD4, and CD8 for vitality, absence of adventitious agents including mycoplasma, absence of replication-competent retrovirus (RCR) by molecular and cellular tests, and absence of IL-2–independent growth. Only gene-modified cells with vitality greater than 80% and purity greater than 85% and meeting all the other specifications were infused. In extensive ex vivo and in vitro testing, this method proved to offer adequate preservation of effector function in both anti-allo and antiviral activity (see Marktel32 ). Before infusion, a sample of transduced cells was cultured in the absence and in the presence of IL-2 (600 UI/mL). Cells were counted every 3 to 4 days, up to 30 days to verify IL-2 dependency.33
Ex vivo detection of engineered T cells
Engraftment of TK+ cells was documented weekly for the first month after infusion, monthly for the first year, and every 6 months thereafter. Cytofluorimetric analyses were performed to define the frequency of cells expressing ΔLNGFR (sensitivity 0.5%) and to characterize their immunophenotypic profile by double staining with anti-CD3, anti-CD4, and anti-CD8 antibodies. Frequency of circulating TK+ cells was also analyzed by a semiquantitative polymerase chain reaction (PCR) for HSV-TK31 (sensitivity 10−4). Aliquots of serum, plasma, mononuclear cells, and bioptic samples collected at specific time points were frozen for future analysis. After ganciclovir administration, samples of PBMCs were obtained every 3 days to monitor the in vivo elimination of TK+ cells.
Clinical outcome
Disease status was assessed through examination of marrow aspirates and biopsies and cytogenetic and molecular analyses. Imaging studies were performed when indicated. Clinical response was classified according to the European Group for Blood and Marrow Transplantation (EBMT) criteria. For patients with chronic myeloid leukemia (CML), hematologic remission was defined as normalization of blood counts, differentials, and marrow cellularity. Cytogenetic response was defined as absence of the Philadelphia (Ph) chromosome by G-banding cytogenetics on at least 30 metaphases. Molecular remission was defined as 2 consecutive negative nested reverse transcriptase–PCRs (RT-PCRs) for BCR-ABL. For patients with multiple myeloma, partial response was defined as a decrease in disease-related immunoglobulins greater than 50%; complete response as negative immunofixation. In patients with lymphoma, partial response was classified as 50% or more reduction in tumor size with disappearance of tumor-related symptoms, whereas complete response was classified as disappearance of all measurable disease and disease-related symptoms. In patients with acute myeloid leukemia, complete responses including cytogenetic and molecular remissions were defined according to revised International Working Group criteria.34 The The World Health Organization (WHO) adverse events grading was used to evaluate treatment toxicities.
Ex vivo isolation of CMV-specific genetically modified T cells
In addition to antitumor activity, the potential of TK+ cells to provide rapid immune reconstitution against viral antigens in the posttransplantation period was analyzed as an additional end point of the study. In vivo expansion of TK+ cells specific for CMV was investigated in selected HLA-A0201+ patients. PBMCs isolated ex vivo and TK+ cells infused to the same patient were stimulated in vitro as previously reported.32 Briefly, 5.6 × 106 PHA-activated donor lymphocytes were pulsed with 40 μM HLA-A0201–restricted pp65495-503 peptide (PRIMM, Milan, Italy), derived from the CMV structural protein pp65, in 1 mL of IMDM in the presence of 2 μg/mL β2-microglobulin (Sigma Chemical, Milan, Italy) for 4 hours at room temperature. Cells were irradiated at 60 Gy (GIL RAD), washed, and cultured with patient's PBMCs at a responder-stimulator (R/S) ratio of 1.5:1. At day 3, a final concentration of 10 U/mL of rhIL-2 was added to each culture. Effector cells were tested 10 days later in a cold inhibition 51Cr-release assay. To this purpose, pp65495-503-pulsed cells and unpulsed HLA-A0201 TAP-deficient cells were labeled with 51Cr and used as targets.
Treatment of GvHD
Acute and chronic GvHD developed after TK-DLI was graded according to standard criteria.35 If grade II GvHD or higher occurred, ganciclovir at 10 mg/kg/d was administered for 14 days (or shorter if all GvHD signs and symptoms regressed). Treatment with immunosuppressive agents was intended to be started if no evidence of GvHD regression was observed after 1 week of treatment with ganciclovir or in the case of GvHD progression after 4 days of GCV treatment.
Ex vivo detection of transgene-specific immune responses
When disappearance of TK+ cells below the level of PCR sensitivity was observed in the absence of GCV treatment, the possibility of an immune response to the transgene was investigated ex vivo. PBMCs were cultured at 1 × 106 cells/mL in IMDM supplemented with 10% autologous serum in the presence of irradiated (50 Gy) donor-derived SFCMM-2– or SFCMM-3–transduced lymphocytes at an R/S ratio of 1:1. At day 3, a final concentration of 10 U/mL of rhIL-2 was added to each culture. Ten days later, effector cells were tested against the same stimulators in a standard 51Cr-release assay.
Results
Feasibility and safety of production and use of TK-transduced donor lymphocytes
Among the 30 patients enrolled in this study, 23 (77%) were infused with TK+ cells, whereas 7 patients failed to receive the cells due to progressive disease. A single transduction procedure under GMP was sufficient to produce TK+ cells for each patient. Median transduction efficiency was 39.5% (range, 10%-80%), median expansion at the end of production was 7-fold (range, 0.3-10.8), and final purity for ΔLNGFR selection was 94% (88%-100%), as detailed in Table 2. This result represents a significant improvement over previously reported methods of cell manipulation16,31 in terms of homogeneity of the final product, short time required for the overall procedure, and preservation of the immune potential of transduced cells.32 The immunophenotype of TK+ cells is detailed in Table 3. TK+ cells expressed the panlymphocytic CD2 marker and the T-cell marker CD3, whereas B lymphocytes (CD19+) and monocytes (CD14+) were lost during culture. As previously reported,32 an inversion of the CD4+/CD8+ ratio was usually observed in TK+ cells. Median proportions of CD4+ and CD8+ cells were 24% (range, 11%-82%) and 72.5% (range, 18%-87%), respectively.
Preparation of genetically modified donor lymphocytes
| Patient no. . | Transduction efficiency, % . | ΔLNGFR selection efficiency . | Final N of ΔLNGFR+ cells, × 109 . | Yield at the end of production . | |
|---|---|---|---|---|---|
| Yield . | Purity, % . | ||||
| 1 | 15 | NA | 100 | 0.1 | 0.9 |
| 2 | 17 | NA | 96 | 3.285 | 2.7 |
| 3 | 23 | NA | 98 | 0.2 | 1.1 |
| 4 | 23 | NA | 91 | 0.2 | 0.3 |
| 5 | 16 | NA | 95 | 0.465 | 1.7 |
| 6 | 24 | NA | 86 | 0.081 | 0.7 |
| 7 | 25 | NA | 91 | 1.570 | 4.5 |
| 8 | 50 | NA | 96 | 1.870 | 10.8 |
| 9 | 10 | NA | 95 | NA | NA |
| 10 | 20 | NA | 90 | NA | NA |
| 11 | 75 | 0.41 | 97 | 6.5 | NA |
| 12 | 36 | 0.36 | 92 | 3.9 | 1.6 |
| 13 | 49 | 0.77 | 92 | 13.3 | 5.0 |
| 14 | 70 | 0.72 | 95 | 27.0 | 6.5 |
| 15 | 80 | 0.72 | 96 | 12.0 | 8.1 |
| 16 | 52 | 0.69 | 94 | 26.2 | 8 |
| 17 | 35 | 0.8 | 95 | 19.0 | 6.6 |
| 18 | 36 | 0.31 | 93 | 5.3 | 2.6 |
| 19 | 43 | 0.74 | 96 | 19.9 | 6.6 |
| 20 | 45 | 0.57 | 95 | 29.3 | 9.5 |
| 21 | 23 | 0.39 | 94 | 3.8 | 1.2 |
| 22 | 20 | 1.2 | 88 | 7.7 | 10.4 |
| 23 | 32 | 0.57 | 89 | 5.0 | 6 |
| Median | 39.5 | 0.7 | 94 | 12.7 | 7 |
| Range | 10-80 | 0.31-1.2 | 88-100 | 0.081-29.3 | 0.3-10.8 |
| Patient no. . | Transduction efficiency, % . | ΔLNGFR selection efficiency . | Final N of ΔLNGFR+ cells, × 109 . | Yield at the end of production . | |
|---|---|---|---|---|---|
| Yield . | Purity, % . | ||||
| 1 | 15 | NA | 100 | 0.1 | 0.9 |
| 2 | 17 | NA | 96 | 3.285 | 2.7 |
| 3 | 23 | NA | 98 | 0.2 | 1.1 |
| 4 | 23 | NA | 91 | 0.2 | 0.3 |
| 5 | 16 | NA | 95 | 0.465 | 1.7 |
| 6 | 24 | NA | 86 | 0.081 | 0.7 |
| 7 | 25 | NA | 91 | 1.570 | 4.5 |
| 8 | 50 | NA | 96 | 1.870 | 10.8 |
| 9 | 10 | NA | 95 | NA | NA |
| 10 | 20 | NA | 90 | NA | NA |
| 11 | 75 | 0.41 | 97 | 6.5 | NA |
| 12 | 36 | 0.36 | 92 | 3.9 | 1.6 |
| 13 | 49 | 0.77 | 92 | 13.3 | 5.0 |
| 14 | 70 | 0.72 | 95 | 27.0 | 6.5 |
| 15 | 80 | 0.72 | 96 | 12.0 | 8.1 |
| 16 | 52 | 0.69 | 94 | 26.2 | 8 |
| 17 | 35 | 0.8 | 95 | 19.0 | 6.6 |
| 18 | 36 | 0.31 | 93 | 5.3 | 2.6 |
| 19 | 43 | 0.74 | 96 | 19.9 | 6.6 |
| 20 | 45 | 0.57 | 95 | 29.3 | 9.5 |
| 21 | 23 | 0.39 | 94 | 3.8 | 1.2 |
| 22 | 20 | 1.2 | 88 | 7.7 | 10.4 |
| 23 | 32 | 0.57 | 89 | 5.0 | 6 |
| Median | 39.5 | 0.7 | 94 | 12.7 | 7 |
| Range | 10-80 | 0.31-1.2 | 88-100 | 0.081-29.3 | 0.3-10.8 |
Transduction efficiency was measured as the percentage of ΔLNGFR+ cells detected by FACS analysis 4 days after transduction. Yield after ΔLNGFR selection was calculated as the ratio of ΔLNGFR+ cells obtained after immune selection and the number of ΔLNGFR+ cells present after transduction. Purity of immune selection was measured as the percentage of ΔLNGFR+ cells detected by FACS analysis 4 days after selection. Yield at the end of production is calculated as the ratio of ΔLNGFR+ cells present at the end of the manipulation and the number of PBMCs present at the beginning of the overall procedure.
NA indicates data not available.
Immunophenotypic characterization of genetically modified donor lymphocytes
| Patient no. . | CD2 . | CD3 . | CD4 . | CD8 . | CD56 . | CD14 . | CD19 . |
|---|---|---|---|---|---|---|---|
| 1 | NA | 98 | 82 | 18 | NA | NA | NA |
| 2 | NA | NA | 30 | 62 | NA | NA | NA |
| 3 | NA | 99 | 16 | 86 | NA | NA | NA |
| 4 | NA | 91 | 40 | 53 | 8 | NA | NA |
| 5 | NA | 60 | 39 | 41 | NA | NA | NA |
| 6 | NA | 100 | 40 | 55 | NA | NA | NA |
| 7 | NA | 100 | 40 | 60 | NA | NA | NA |
| 8 | NA | 99 | 19 | 83 | NA | NA | NA |
| 9 | NA | 81 | 19 | 81 | NA | NA | NA |
| 10 | 99 | 93 | 15 | 73 | 12 | NA | 0 |
| 11 | 99 | 98 | 48 | 82 | 11 | 0.1 | 0.2 |
| 12 | 99 | 98 | 47 | 52 | 6 | 0.20 | 0.6 |
| 13 | 95.8 | 99 | 14 | 86 | 3 | 0.3 | 0.6 |
| 14 | 99.2 | 99 | 14 | 86 | 12 | 0.4 | 0.2 |
| 15 | 99 | 99 | 28 | 72 | 11 | 0.2 | 0 |
| 16 | 99 | 98 | 31 | 71 | 12 | 1 | 1.8 |
| 17 | 99.3 | 98 | 38 | 60 | 19 | 0.3 | 0.2 |
| 18 | 99 | 98 | 11 | 85 | 15 | 0 | 0 |
| 19 | 99.1 | 97.9 | 42 | 57 | 1 | 0.4 | 2.1 |
| 20 | 99 | 100 | 12 | 87 | 11 | 0.4 | 3.8 |
| 21 | 99 | 83 | 14 | 68 | 9 | 1.2 | 0.9 |
| 22 | 99.8 | 98 | 27 | 73 | 2 | 0.1 | 0.3 |
| 23 | 99 | 99 | 21 | 78 | 10 | 0.1 | 0.3 |
| Median | 99 | 98 | 24 | 72.5 | 10.5 | 0.3 | 0 |
| Range | 95.8-99.8 | 81-100 | 11-82 | 18-87 | 1-19 | 0.1-1.2 | 0-3.8 |
| Patient no. . | CD2 . | CD3 . | CD4 . | CD8 . | CD56 . | CD14 . | CD19 . |
|---|---|---|---|---|---|---|---|
| 1 | NA | 98 | 82 | 18 | NA | NA | NA |
| 2 | NA | NA | 30 | 62 | NA | NA | NA |
| 3 | NA | 99 | 16 | 86 | NA | NA | NA |
| 4 | NA | 91 | 40 | 53 | 8 | NA | NA |
| 5 | NA | 60 | 39 | 41 | NA | NA | NA |
| 6 | NA | 100 | 40 | 55 | NA | NA | NA |
| 7 | NA | 100 | 40 | 60 | NA | NA | NA |
| 8 | NA | 99 | 19 | 83 | NA | NA | NA |
| 9 | NA | 81 | 19 | 81 | NA | NA | NA |
| 10 | 99 | 93 | 15 | 73 | 12 | NA | 0 |
| 11 | 99 | 98 | 48 | 82 | 11 | 0.1 | 0.2 |
| 12 | 99 | 98 | 47 | 52 | 6 | 0.20 | 0.6 |
| 13 | 95.8 | 99 | 14 | 86 | 3 | 0.3 | 0.6 |
| 14 | 99.2 | 99 | 14 | 86 | 12 | 0.4 | 0.2 |
| 15 | 99 | 99 | 28 | 72 | 11 | 0.2 | 0 |
| 16 | 99 | 98 | 31 | 71 | 12 | 1 | 1.8 |
| 17 | 99.3 | 98 | 38 | 60 | 19 | 0.3 | 0.2 |
| 18 | 99 | 98 | 11 | 85 | 15 | 0 | 0 |
| 19 | 99.1 | 97.9 | 42 | 57 | 1 | 0.4 | 2.1 |
| 20 | 99 | 100 | 12 | 87 | 11 | 0.4 | 3.8 |
| 21 | 99 | 83 | 14 | 68 | 9 | 1.2 | 0.9 |
| 22 | 99.8 | 98 | 27 | 73 | 2 | 0.1 | 0.3 |
| 23 | 99 | 99 | 21 | 78 | 10 | 0.1 | 0.3 |
| Median | 99 | 98 | 24 | 72.5 | 10.5 | 0.3 | 0 |
| Range | 95.8-99.8 | 81-100 | 11-82 | 18-87 | 1-19 | 0.1-1.2 | 0-3.8 |
Immunophenotype of TK+ cells analyzed at the end of the procedure by FACS analysis immediately before cryopreservation.
NA indicates data not available.
Analyses of all the cell preparations for the clinical application did not reveal alterations in the proliferative capacity of T cells.32 All cultures remained strictly dependent on IL-2 for growth and survival.33 Assays for detection of RCR were negative in all tested samples. No local or systemic toxicity related to the gene-transfer procedure was observed in this clinical trial. Within 43 infusions, the only exception was an acute cell lysis syndrome observed immediately after the second infusion of TK+ cells in patient 20 correlated with the development of a cytotoxic immune response to the transgene.
Engraftment, expansion, and antitumor activity of TK+ lymphocytes
Engraftment of TK+ cells was assessed by defining the proportion of ΔLNGFR+ cells on circulating peripheral-blood leukocytes (PBLs) and by PCR quantization of the HSV-TK transgene on DNA extracted from circulating PBMCs. Seventeen (74%) of 23 treated patients were evaluable for engraftment. Three patients were considered not evaluable due to early death (patients 4, 6, and 15) and 3 patients were considered not evaluable because ganciclovir was administered early after TK-DLI (patients 9, 14, and 19). As detailed in Table 4, all evaluable patients had detectable circulating TK+ cells within a median time of 2.5 weeks (range, 1-6 weeks). In 3 patients, TK+ cells could be detected only at low levels by PCR; in all other patients, gene-modified cells were detected by FACS analysis for ΔLNGFR expression. Within these patients, median peak of circulating TK+ cells was 66 cells/μL (8-166 cells/μL). No significant differences were observed in the total number and phenotype of infused TK+ cells between patients who achieved consistent levels and patients with low levels of circulating TK+ cells, suggesting that this was not a relevant variable in the expansion observed in vivo in the majority of patients. The in vivo expansion of TK+ cells significantly correlated with the antitumor activity observed after infusion of genetically engineered cells (see below).
Graft-versus-tumor effect of suicide gene-modified T cells
| Patient no. . | Disease . | TK+ cells infused, 106/kg . | Peak of TK+ cells/μL (% of PBLs)* . | Outcome after TK-DLI† . | GvHD after TK-DLI† . | GvHD response after GCV treatment . | OS, wk after TK-DLI . |
|---|---|---|---|---|---|---|---|
| 1 | EBV+PTLD | 1.5 | 166 (13) | CR | Y | Y | 13‡ |
| 2 | CML | 38.6 | 115 (4.9) | PR | Y | Y | 89 |
| 3 | CML | 0.5 | 39 (3.3) | PR | N | NA | 104 |
| 4 | AML | 3.3 | NE | NE | NE | NA | NE |
| 5 | AML | 5.2 | 12 (1.9) | NR | N | NA | 12 |
| 6 | EBV+PTLD | 0.5 | NE | NE | NE | NA | NE |
| 7 | AML | 11.1 | 37.4 (3.4) | CR§ | N | NA | 85‡ |
| 8 | CMML | 20.0 | 75 (11.9) | CR | Y | Y | 556‖ |
| 9 | ALL | 1.0 | NE | NE | NE | E | NE |
| 10 | AML | 1.0 | 10 (6) | NR | NE | NA | 8 |
| 11 | AML | 110.0 | 103 (3.2) | PR | N | NA | 10 |
| 12 | AML | 24.0 | 115 (17) | NE¶ | NE | NA | 8 |
| 13 | AML | 10.0 | 8 (1.7) | NR | NE | E | 10 |
| 14 | AML | 80.0 | NE | NE | NE | E | NE |
| 15 | ALL | 100.0 | NE | NE | NE | NA | NE |
| 16 | FCL | 280.0 | 56 (4) | CR | N | NA | 471‖ |
| 17 | MM | 47.0 | 56 (1) | CR | N | NA | 166 |
| 18 | HD | 80.0 | PCR+ | NR | NE | NA | 6 |
| 19 | AML | 145.0 | NE | NE | NE | E | NE |
| 20 | MM | 300.0 | PCR+ | PR | N | NA | 76 |
| 21 | CML | 50.0 | PCR+ | CR | N | NA | 405‖ |
| 22 | HD | 110.0 | 77 (2.4) | NR | Y | NA | 15 |
| 23 | MCL | 120.0 | 120 (9.7) | PR | NE | NA | 4 |
| Patient no. . | Disease . | TK+ cells infused, 106/kg . | Peak of TK+ cells/μL (% of PBLs)* . | Outcome after TK-DLI† . | GvHD after TK-DLI† . | GvHD response after GCV treatment . | OS, wk after TK-DLI . |
|---|---|---|---|---|---|---|---|
| 1 | EBV+PTLD | 1.5 | 166 (13) | CR | Y | Y | 13‡ |
| 2 | CML | 38.6 | 115 (4.9) | PR | Y | Y | 89 |
| 3 | CML | 0.5 | 39 (3.3) | PR | N | NA | 104 |
| 4 | AML | 3.3 | NE | NE | NE | NA | NE |
| 5 | AML | 5.2 | 12 (1.9) | NR | N | NA | 12 |
| 6 | EBV+PTLD | 0.5 | NE | NE | NE | NA | NE |
| 7 | AML | 11.1 | 37.4 (3.4) | CR§ | N | NA | 85‡ |
| 8 | CMML | 20.0 | 75 (11.9) | CR | Y | Y | 556‖ |
| 9 | ALL | 1.0 | NE | NE | NE | E | NE |
| 10 | AML | 1.0 | 10 (6) | NR | NE | NA | 8 |
| 11 | AML | 110.0 | 103 (3.2) | PR | N | NA | 10 |
| 12 | AML | 24.0 | 115 (17) | NE¶ | NE | NA | 8 |
| 13 | AML | 10.0 | 8 (1.7) | NR | NE | E | 10 |
| 14 | AML | 80.0 | NE | NE | NE | E | NE |
| 15 | ALL | 100.0 | NE | NE | NE | NA | NE |
| 16 | FCL | 280.0 | 56 (4) | CR | N | NA | 471‖ |
| 17 | MM | 47.0 | 56 (1) | CR | N | NA | 166 |
| 18 | HD | 80.0 | PCR+ | NR | NE | NA | 6 |
| 19 | AML | 145.0 | NE | NE | NE | E | NE |
| 20 | MM | 300.0 | PCR+ | PR | N | NA | 76 |
| 21 | CML | 50.0 | PCR+ | CR | N | NA | 405‖ |
| 22 | HD | 110.0 | 77 (2.4) | NR | Y | NA | 15 |
| 23 | MCL | 120.0 | 120 (9.7) | PR | NE | NA | 4 |
Y indicates yes; N, no; NA, data not available; NE, not evalulable; and E, elimination of transduced cells with ganciclovir treatment, in the absence of GvHD. Additional abbreviations are explained in Table 1.
Peak of circulating TK+ cells is reported as the highest level of TK+ cells quantified by FACS for ΔLNGFR expression. In case of FACS negativity, the peak of TK+ cells was quantified by PCR for the HSV-TK transgene.
Responses after TK-DLI are evaluated according to standard criteria. GvHD was diagnosed according to Glucksberg criteria.
Death in CR.
Patient received debulking chemotherapy with ARA-C 100 mg/ms × 7 days + daunorubicine 20 mg/kg × 3 days and obtained hematologic remission.
Alive in CR.
Patient not evaluable for GvL; however, a marked antiviral response with clearance of CMV reactivation was observed.
Among 23 patients infused with TK+ cells, 17 (74%) were evaluable for antitumor response (Tables 4–5). Nonevaluable patients were affected by rapidly progressive malignancies (4 acute myeloid leukemia [AML], 1 acute lymphoblastic leukemia [ALL], 1 EBV+PTLD) and died of disease progression within the first 4 weeks from the infusion. Among patients affected by less aggressive malignancies, all subjects infused were evaluable for antitumor response. Six of the 17 evaluable patients (35%) achieved a complete response after infusion of TK-DLI, 5 patients (29%) obtained a partial response, and 6 patients (35%) had progressive disease. Three of 6 patients who achieved a complete response are long-term survivors in sustained complete response. In line with results obtained with the infusion of unmodified donor lymphocytes,2–4 clinical responses were observed after infusion of TK-DLIs in the absence of any chemotherapeutic treatment in patients affected by chronic leukemias, MM, and NHL. On the contrary, successful treatment of acute leukemia could be obtained only in 1 patient (patient 7) after tumor debulking with chemotherapeutic agents.
Disease response after TK-DLI according to diagnosis
| Diagnosis . | No. of infused patients . | No. of evaluable patients . | No. in complete remission . | No. in partial remission . |
|---|---|---|---|---|
| CML/CMML | 4 | 4 | 2 | 2 |
| AML | 9 | 5 | 1* | 1 |
| ALL | 2 | 1 | 0 | 0 |
| NHL | 4 | 3 | 2 | 1 |
| HD | 2 | 2 | 0 | 0 |
| MM | 2 | 2 | 1 | 1 |
| Total | 23 | 17 | 6 | 5 |
| Diagnosis . | No. of infused patients . | No. of evaluable patients . | No. in complete remission . | No. in partial remission . |
|---|---|---|---|---|
| CML/CMML | 4 | 4 | 2 | 2 |
| AML | 9 | 5 | 1* | 1 |
| ALL | 2 | 1 | 0 | 0 |
| NHL | 4 | 3 | 2 | 1 |
| HD | 2 | 2 | 0 | 0 |
| MM | 2 | 2 | 1 | 1 |
| Total | 23 | 17 | 6 | 5 |
Patients were evaluable for graft-versus-tumor (GvT) activity if surviving more than 30 days after infusion of TK-DLI. Clinical response was graded according to criteria described in “Patients, materials, and methods”.
Abbreviations are explained in Table 1.
Patient received debulking chemotherapy.
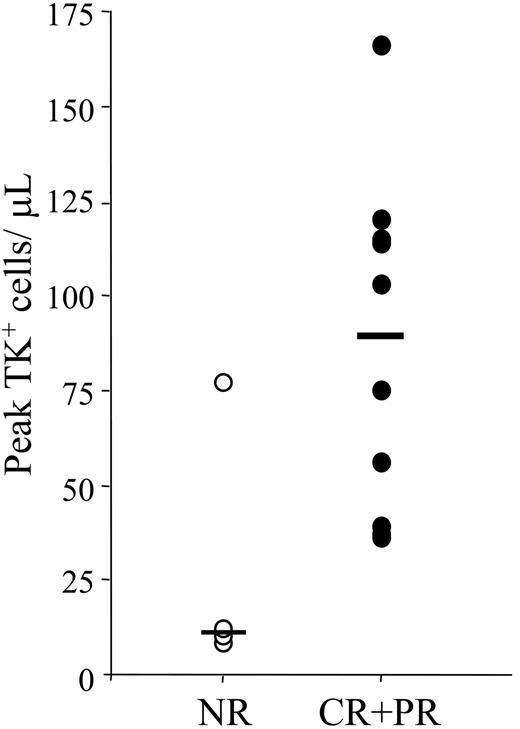
Although the level of TK+ lymphocyte expansion varied significantly among different patients, we observed a direct correlation between the antitumor effect and the peak levels of TK+ cells in the circulation (Figure 1), as well as with the in vivo expansion of transduced cells. An estimate of TK+ cell expansion was calculated, assuming that 2% of the total pool of lymphocytes circulates in the blood.36 Although the median time to maximal TK cell expansion did not vary in responders versus nonresponders (24 and 28 days after TK cell infusion, respectively), the median expansion was different in the 2 patient populations: 23.9 in patients who achieved complete remission (CR) and 4.6 in patients with partial response, reflecting median peaks of circulating TK+ cells of 65.5 and 109 cells/μL, respectively. In nonresponders, median expansion was 3.3, reflecting a peak of circulating TK+ cells of only 11 cells/μL. These data strongly suggest that antigenic stimulation rather than homeostatic proliferation drives in vivo expansion of TK+ cells and that only when antigenic stimulation is effective in promoting T-cell expansion does the patient achieve a clinical response. In accordance with this hypothesis, time kinetics of expansion of genetically modified cells correlated with time kinetics of clinical responses. Representative kinetics of antitumor responses for 2 patients affected by chronic leukemia (patient 8; Figure 2) and multiple myeloma (patient 17; Figure 3) are plotted.
Correlation between peak levels of TK+ cells and clinical response. TK+ circulating cells were monitored periodically by FACS analysis as ΔLNGFR-expressing cells. In patients with circulating TK cells detectable by FACS, peak of TK+ circulating cells was measured as the highest level of ΔLNGFR+ cells achieved in each patient (absolute numbers/μL). Evaluable patients are grouped as responders (CR+PR; ●60; n = 10) and nonresponders (NR; ○; n = 4). Medians are indicated. The difference between the 2 groups is statistically significant with P < .05 (2-tailed Wilcoxon test).
Correlation between peak levels of TK+ cells and clinical response. TK+ circulating cells were monitored periodically by FACS analysis as ΔLNGFR-expressing cells. In patients with circulating TK cells detectable by FACS, peak of TK+ circulating cells was measured as the highest level of ΔLNGFR+ cells achieved in each patient (absolute numbers/μL). Evaluable patients are grouped as responders (CR+PR; ●60; n = 10) and nonresponders (NR; ○; n = 4). Medians are indicated. The difference between the 2 groups is statistically significant with P < .05 (2-tailed Wilcoxon test).
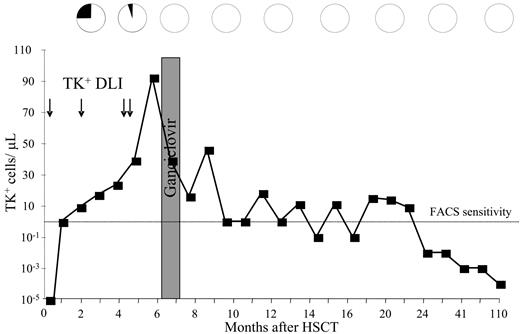
Kinetics of disease response in a patient with CMML following TK+ DLI. Patient 8, affected by chronic myelomonocytic leukemia (CMML) in relapse after allogeneic HSCT, was treated with multiple TK+ DLI (arrows). Absolute numbers of circulating TK+ cells, quantified by FACS analysis, are shown as ■. Below the level of FACS sensitivity, quantitative PCR values are reported. Percentage of malignant cells in the bone marrow (black sectors of pies) was assessed by analyses of host karyotype and quantification of myelodysplastic precursors. Disease response strictly correlated with the kinetics of expansion of TK+ cells and persisted after ganciclovir administration for cGvHD. TK+ cells were detectable by PCR during a complete remission long-term follow-up up to 110 months.
Kinetics of disease response in a patient with CMML following TK+ DLI. Patient 8, affected by chronic myelomonocytic leukemia (CMML) in relapse after allogeneic HSCT, was treated with multiple TK+ DLI (arrows). Absolute numbers of circulating TK+ cells, quantified by FACS analysis, are shown as ■. Below the level of FACS sensitivity, quantitative PCR values are reported. Percentage of malignant cells in the bone marrow (black sectors of pies) was assessed by analyses of host karyotype and quantification of myelodysplastic precursors. Disease response strictly correlated with the kinetics of expansion of TK+ cells and persisted after ganciclovir administration for cGvHD. TK+ cells were detectable by PCR during a complete remission long-term follow-up up to 110 months.
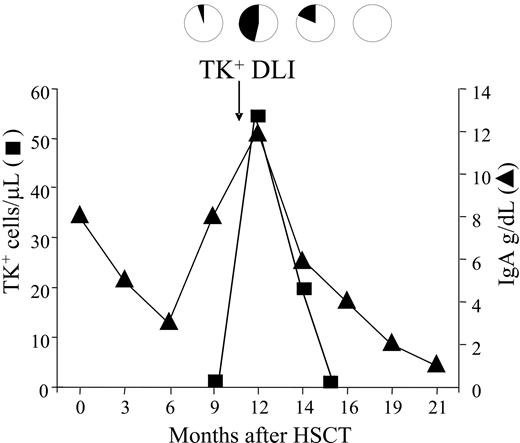
Kinetics of disease response in a patient with multiple myeloma following TK+ DLI. Patient 17 affected by IgA-secreting multiple myeloma was treated with a single infusion of TK+ DLI. Absolute numbers of circulating TK+ cells, quantified by FACS analysis, are shown as ■. Percentage of malignant cells in the bone marrow (black sectors of pies) was assessed by quantification of monoclonal plasma cells in the bone marrow. Disease status was also monitored by the levels of serum monoclonal IgA (▴) and confirmed by IgAκ immunofixation (not shown). Clearance of all parameters of disease closely followed the peak of circulating TK+ cells.
Kinetics of disease response in a patient with multiple myeloma following TK+ DLI. Patient 17 affected by IgA-secreting multiple myeloma was treated with a single infusion of TK+ DLI. Absolute numbers of circulating TK+ cells, quantified by FACS analysis, are shown as ■. Percentage of malignant cells in the bone marrow (black sectors of pies) was assessed by quantification of monoclonal plasma cells in the bone marrow. Disease status was also monitored by the levels of serum monoclonal IgA (▴) and confirmed by IgAκ immunofixation (not shown). Clearance of all parameters of disease closely followed the peak of circulating TK+ cells.
The main causes for abrupt disappearance of circulating TK+ cells were ganciclovir administration (patients 1, 2, and 13) or development of anti-TK immune responses (patients 2, 7, 16, 17, 20, and 21). In the absence of these events, the rapid expansion of TK+ cells in vivo was always followed by a slow, progressive decrease in the number of circulating TK+ cells that could be followed long-term by PCR analysis. The progressive shrink in the population of TK+ lymphocytes surviving in vivo confirms that genetically engineered cells transduced by the SFCMM-3 retroviral vector do not show an “abnormal” proliferative/survival potential.
In the patients who presented with abrupt disappearance of TK+ cells, this event was permanent. The disappearance of genetically modified cells, in the absence of ganciclovir administration, suggested the occurrence of an immune response against vector-encoded antigens.37 To demonstrate and characterize this immune response, T cells from the patients showing disappearance from circulation of TK+ cells were stimulated in vitro with vector-transduced donor T cells. The presence of cytotoxic T cells specific for the transduced targets could be documented in all patients (see accompanying article by Traversari et al,38 beginning on page 4708). The lytic activity of the SFCMM2/SFCMM3-specific T cells derived from the patients was restricted to genetically modified cells, as demonstrated by resistance of the untransduced donor targets to lysis. On the contrary, immunologic studies performed on patients with detectable circulating TK+ cells showed the absence of transgene-specific effectors. The factors associated with the immune response against the transgenes were (i) time between HSCT and TK-DLI resulting in anti-TK immune response (average 95 weeks in immunized patients [range, 24-278 weeks] versus 43 weeks in nonimmunized patients [range, 6-276 weeks]); and (ii) number of circulating CD4+ cells at the time of TK-DLI (median count of 281 cells/μL [range 60-656 cells/μL] in immunized patients and 114 cells/μL [range, 0-400 cells/μL] in nonimmunized patients).
The clinical outcome of patients immunized against HSV-TK (Table 6) strictly correlated with the level of GvL obtained before immunization: patients who obtained a complete response before developing an immune response against the transgenes remained in complete response for at least 1 year and up to 10 years, despite circulating TK+ cells under the level of detection in all patients but one (patient 8; Figure 2). In this patient, genetically engineered cells remained detectable by PCR for more than 10 years. Clinical benefits of TK-DLI were completely lost in patients who had achieved only partial response before the development of immunity to transgene products. This suggests that, once achieved, clinical remission can also be maintained after apparent clearance of effectors. The mechanism underlying this event is still under investigation: it may reflect complete clearance of leukemic cells or persistence of low numbers of effectors patrolling peripheral tissues.
Characteristics and clinical outcome of patients who developed an immune response against transgene products
| Patient no. . | CD4+ cells at infusion, cells/μL . | Time between HSCT and infusion, wk . | Multiple infusions . | Clinical outcome after TK-DLI . | Clinical outcome after immunization against transgenes (wk) . |
|---|---|---|---|---|---|
| 2 | 234 | 53 | Y | PR | NR |
| 7 | 107 | 73 | Y | CR | CR (49) |
| 8 | 60 | 24 | Y | CR | CR (269) |
| 16 | 270 | 67 | Y | CR | CR (328) |
| 17 | 442 | 52 | N | CR | CR (100) |
| 20 | 656 | 278 | Y | PR | NR |
| 21 | 200 | 120 | N | CR | CR (238) |
| Average for nonimmunized patients | 96 | 36 | 4/11 Y | NA | NA |
| Patient no. . | CD4+ cells at infusion, cells/μL . | Time between HSCT and infusion, wk . | Multiple infusions . | Clinical outcome after TK-DLI . | Clinical outcome after immunization against transgenes (wk) . |
|---|---|---|---|---|---|
| 2 | 234 | 53 | Y | PR | NR |
| 7 | 107 | 73 | Y | CR | CR (49) |
| 8 | 60 | 24 | Y | CR | CR (269) |
| 16 | 270 | 67 | Y | CR | CR (328) |
| 17 | 442 | 52 | N | CR | CR (100) |
| 20 | 656 | 278 | Y | PR | NR |
| 21 | 200 | 120 | N | CR | CR (238) |
| Average for nonimmunized patients | 96 | 36 | 4/11 Y | NA | NA |
The reported parameters were measured at the infusion inducing immunity for patients 2, 7, 8, and 16, and at the time of the first infusion for all the other patients. Clinical outcome after TK-DLI was measured at the time of detection of immunization against transgene products.
NA indicates not applicable. Additional abbreviations are explained in Table 1.
Early reconstitution of antiviral immunity by donor TK+ cells
In addition to the antitumor potential of TK-DLI, the main end point of the study, the ability of TK+ cells to provide antiviral responses after T-cell–depleted (TCD) HSCT was evaluated, as an additional end point, in 10 patients who received TK-DLI early after transplantation. All patients were profoundly immunodepressed and had viral reactivations (in 8 patients CMV, in 2 EBV) at the time of infusion. As previously reported, in 1 patient (patient 1) with an aggressive EBV+ lymphoproliferative disease, a rapid and potent immune response to EBV, resulting in complete response, occurred after TK-DLI.16 Within the 8 patients with CMV reactivation, 5 achieved a documented TK+ cell engraftment and 4 of them did not require any further CMV treatment, suggesting that immune response was efficiently controlling CMV infection. Three patients experienced persisting CMV antigenemia and required GCV treatment within the first month after infusion, before documented TK+ cell engraftment was achieved.
Direct evidence of antiviral immune response mediated by TK+ lymphocytes was documented in an HLA-A0201 patient (patient 12). This patient had CMV reactivation and no detectable circulating CD3+ cells at the time of infusion of the TK-DLI. A rapid expansion of gene-modified cells was observed in this patient within 20 days after infusion. Circulating T cells reached a peak of 115 cells/μL; 100% of T cells were TK+, suggesting that transduced lymphocytes can immune reconstitute patients in the absence of any helper function provided by nonmanipulated T cells. Complete clearance of CMV antigenemia was observed in this patient in the absence of antiviral treatment. As shown in Figure 4A, kinetics of CMV clearance strictly followed kinetics of immune reconstitution, suggesting an immune control. To demonstrate this hypothesis, lymphocytes harvested at the time of CMV clearance from patient 12 were stimulated in vitro with irradiated autologous PBMCs pulsed with the pp65495-503-immunodominant peptide of CMV.39 After a single round of in vitro stimulation, specific lysis of HLA-A0201 cells pulsed with the peptide was observed (Figure 4B). Strikingly, lysis mediated by effectors harvested from the patient was higher than lysis mediated by cells sampled at the time of infusion, suggesting that an expansion of CMV-specific genetically modified precursors occurred in vivo upon antigenic stimulation.
Reconstitution of anti-CMV protective immunity by TK+ DLI. (A) Patient 12 experienced a CMV reactivation syndrome with high titers of viremia, as detected by quantitative PCR (shaded area). TK+ DLI was followed by a rapid expansion of gene-modified cells, as measured by FACS analysis for ΔLNGFR expression, which peaked at 115 cells/μL after 20 days. At this time point nearly all circulating CD3+ cells (●) were TK+ (▴). Concomitantly, CMV viremia dropped and all clinical signs of CMV disease disappeared. (B) At a time corresponding to the peak of TK+ cells in the circulation, PBMCs were harvested (■) and stimulated with irradiated autologous PBMCs pulsed with the CMV-immunodominant pp65495-503 peptide. For comparison, a sample of preinfusion TK+ cells (□) was also stimulated and tested. After 7 days, specific lysis of the 2 effector populations was measured against HLA-A0201+ T2 cells pulsed with the pp65495-503 peptide in a chromium-release assay. No lysis of unpulsed T2 cells was observed.
Reconstitution of anti-CMV protective immunity by TK+ DLI. (A) Patient 12 experienced a CMV reactivation syndrome with high titers of viremia, as detected by quantitative PCR (shaded area). TK+ DLI was followed by a rapid expansion of gene-modified cells, as measured by FACS analysis for ΔLNGFR expression, which peaked at 115 cells/μL after 20 days. At this time point nearly all circulating CD3+ cells (●) were TK+ (▴). Concomitantly, CMV viremia dropped and all clinical signs of CMV disease disappeared. (B) At a time corresponding to the peak of TK+ cells in the circulation, PBMCs were harvested (■) and stimulated with irradiated autologous PBMCs pulsed with the CMV-immunodominant pp65495-503 peptide. For comparison, a sample of preinfusion TK+ cells (□) was also stimulated and tested. After 7 days, specific lysis of the 2 effector populations was measured against HLA-A0201+ T2 cells pulsed with the pp65495-503 peptide in a chromium-release assay. No lysis of unpulsed T2 cells was observed.
In vivo abrogation of GvHD and elimination of TK+ cells by ganciclovir treatment
Four (30%) of 12 patients evaluable for GvHD developed this complication. Three patients experienced acute GvHD (grade I, II-III, III) involving the skin (patients 1 and 22) and the liver (patient 2), whereas 1 patient developed chronic extensive GvHD not preceded by acute GvHD (patient 8). Three of 4 patients were successfully treated with GCV. In the 2 patients affected by acute GvHD, GCV treatment resulted in complete clinical remission while in the patient affected by chronic extensive GvHD, GCV treatment resulted in a significant improvement of lung and skin signs.16 Patient 22 was successfully treated with local steroids for aGvHD grade I. Following GCV administration, a search for HSV-TK–spliced forms40 was performed. In all cases, the spliced form could not be detected.
Four additional patients required ganciclovir to treat CMV reactivation. In all patients, the selective elimination of transduced cells was documented in terms of absence of engraftment of TK+ cells (patients 9 and 14) or elimination of circulating TK+ cells within a few days from the first GCV administration (patients 13 and 19). This observation represents an additional, though not deliberate, demonstration of the effectiveness of the suicide gene machinery in the in vivo elimination of TK-transduced lymphocytes.
Discussion
We have previously shown that the TK suicide gene is an efficient tool for controlling GvHD in the context of donor lymphocytes infused following HSCT for hematologic malignancies.16 In the present study, we validated the GvL activity of TK+ cells in an extended clinical series of patients affected by disease relapse after HSCT. Rate of responses observed are comparable to those obtained using unmanipulated donor lymphocytes reported from large series of studies,3,4,9,10,41–43 confirming that an appropriate ex vivo manipulation for retroviral vector–mediated gene transfer does not affect the GvL effect provided by donor lymphocytes. The demonstration of the antitumor potential of donor TK+ cells, the primary end point of this study, is further supported by the strong correlation between the degree of in vivo expansion of TK+ cells and clinical response. Indeed, the peak level of circulating TK+ cells was the single most important variable able to predict clinical response. This, together with a closely overlapping time kinetics of expansion of genetically engineered cells and the kinetics of disease remission, strongly suggests that TK+ DLI has a curative potential.
Preservation of GvL after transduction was questioned by some of the initial clinical studies that included in vivo long-term culture of donor lymphocytes associated with transduction and the negative selection of transduced cells by neomycin. We believe that those technical limitations have now been overcome by crucial technological improvements that include (1) setup of large-scale production systems with a single transduction procedure; (2) positive immune selection resulting in a short-time procedure; and (3) improved standardization of the final product. As a result, engraftment of TK+ cells has been documented in all patients evaluable long enough after infusion. Detection of circulating TK+ cells required a median time of 2 to 3 weeks, suggesting that infused TK+ cells undergo a first wave of extravascular sequestration and are subsequently redistributed in the blood stream. After the peak, circulating TK+ lymphocytes progressively declined but lasted up to 416 weeks after infusion. Genetically engineered cells that expanded in vivo provided GvL activity not only against tumors known to respond to unmodified donor lymphocytes but also against tumors reported as poor responders to conventional DLI, such as multiple myeloma and lymphoma. This encouraging observation may represent the building ground to develop more effective genetically modified and/or activated effectors able to increase the antitumor potential of DLI.
The immune potential of TK+ cells was also proven as the ability to reconstitute antiviral immunity, suggesting that this technology could find specific application in transplantation settings where delayed or incomplete immune reconstitution may affect morbidity and mortality.
Finally, despite many preclinical models showing a reduction in the alloreactive potential of genetically modified lymphocytes, in our study the immune competence of TK+ cells was demonstrated by the occurrence of GvHD in 4 (30%) of 13 evaluable patients, a percentage not significantly lower than the one reported after escalating doses of unmanipulated DLI.9,10 Most importantly, in this study, 7 patients received ganciclovir treatment resulting in selective elimination of TK+ cells and in specific treatment of GvHD in all patients, confirming the potential of the TK technology.
The long-term follow-up of patients enrolled in this study confirmed a stable transgenes expression and GCV sensitivity of engineered lymphocytes31,32 and a favorable safety profile of the technology used.44
Immunization against epitopes encoded by the HSV-TK transgene has emerged as a major limitation of this strategy. The clinical protocol modifications introduced to reduce the risks of immunization (single-dose infusion, vector modification) failed in preventing the immune elimination of TK+ cells. Patients infused late after transplantation at a stage of a complete immune reconstitution have been identified as high risk for this complication. Nonetheless, even TK-immunized patients could benefit from TK+ DLI, with prolonged tumor regressions observed after immune elimination of TK+ cells.
The encouraging results obtained in this trial represent the basis for the exploitation of the TK technology in novel transplantation settings, such as reduced-toxicity allo-HSCT or HSCT from mismatched donors, in which delayed immune reconstitution and/or high risk of GvHD represent a major limitation. The use of highly immunosuppressive regimens with low myeloablative activity to reduce transplantation-related mortality (TRM) resulted in excellent rates of engraftment with minimal nonhematologic toxicity in the context of both related and unrelated HSCT. In this context, GvHD still represents a major complication, with a 50% to 60% probability of grade II-IV acute GvHD, 45% to 60% probability of extensive chronic GvHD, and 20% to 40% probability of related deaths.43,45 This complication steadily increases with long-term follow-up and may offset any benefits in terms of reduced TRM and relapse-free survival. Nonmyeloablative regimens including alemtuzumab, ATG, or ex vivo T-cell depletion are highly effective in preventing GvHD.46 However, the reduced antitumor activity of such protocols may require the early use of DLI to promote GvL and limit infectious morbidity. The timing and dosage that can be safely administered remain poorly defined, particularly in the reduced-intensity setting where the early administration of DLI in combination with the persistence of host antigen-presenting cells could favor the development of GvHD.47 A program of escalating DLI after alemtuzumab-based nonmyeloablative regimen was complicated by 41% GvHD and 20% mortality attributed to GvHD.48 Thus the application of TK+ cells in this transplantation setting could allow the infusion of high numbers of donor effectors, allowing a selective modulation of alloreactivity and overall improvement of response rates. HSC transplants from haploidentical donors could also benefit from the TK technology. Here, failure to immune reconstitute and development of severe opportunistic infections represent major causes of mortality.49,50 Therefore, the development of effective antiviral immune responses by TK+ cells could have a major impact on overall survival. In such alternative transplantation settings, clinical trials based on the TK technology have been prompted by the preliminary dissemination of the results described in the present study. A series of clinical protocols have been approved by regulatory authorities in Europe, the United States, Israel, and Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are deeply indebted to the nursing staffs. We are grateful to Hans Kolb and Jane Apperley for the helpful discussions during the study design and patient care. This work was supported by the European Community (Biomed contract no. CT97-2074, QLK3-CT-2001-01265); the Cariplo Foundation; the Italian Association for Cancer Research (AIRC); the Italian Ministry of Health; and the Italian Ministry of University and Research (Ricerca Finalizzata).
The current address for Paolo Corradini is as follows: Department of Hematology, Istituto Nazionale Tumori, University of Milan, Italy.
Authorship
Contribution: F.C., C. Bonini, C.T., and C. Bordignon wrote the paper; S.M., A.B., Z.M., M.S., C. Bonini, C. Benati, and M.P. performed laboratory research; F.C., C. Bonini, C.T., and C. Bordignon designed and discussed experiments; and F.C., C. Bonini, E.Z., P.S., M. Bernardi, A.P., J.P., S.R., L.C., M. Bregni, and P.C. performed clinical research.
Conflict-of-interest disclosure: C. Bordignon, F.C., and C. Bonini have declared a financial interest in MolMed Spa, whose potential product (TR-DLI) was studied in the present work. S.C.G., H.M.v.d.B., and J.G.v.d.B. have received unrestricted research/educational funding for various projects at the Van Creveldklinik from the following companies: Bayer, Baxter, ZLB Behring, Novo Nordisk, and Wyeth.
Correspondence: Claudio Bordignon, Istituto Scientifico San Raffaele, Via Olgettina 58, Milan, Italy; e-mail: c.bordignon@hsr.it.