Abstract
Although development of first-generation thrombopoietic growth factors (recombinant human thrombopoietin [TPO] and pegylated recombinant human megakaryocyte growth and development factor [PEG-rHuMGDF]) was stopped due to development of antibodies to PEG-rHuMGDF, nonimmunogenic second-generation thrombopoietic growth factors with unique pharmacologic properties have been developed. TPO peptide mimetics contain TPO receptor-activating peptides inserted into complementarity-determining regions of Fab (Fab 59), attached to the IgG Fc region (AMG 531), or pegylated (Peg-TPOmp). Orally available, TPO nonpeptide mimetics (eltrombopag, AKR-501) bind and activate the TPO receptor by a mechanism different from TPO and may have an additive effect to TPO. TPO agonist antibodies are monoclonal antibodies activating the TPO receptor but modified in size [TPO minibodies; ie, VB22B sc(Fv)2] or immunoglobuln type (domain subclass-converted TPO agonist antibodies; ie, MA01G4G344). All second-generation thrombopoietic growth factors stimulate growth of TPO-dependent cell lines via JAK2/STAT signaling pathways and increase platelet counts in animals. When tested in healthy humans, TPO peptide and nonpeptide mimetics produced a dose-dependent rise in platelet count. AMG 531 and eltrombopag markedly increase platelet counts in patients with immune thrombocytopenic purpura, without significant adverse effects. One or more second-generation thrombopoietic growth factors should soon be clinically available for treating thrombocytopenic disorders.
Introduction
Following the purification and cloning of human thrombopoietin (TPO) in 1994, 2 recombinant thrombopoietin molecules, recombinant human thrombopoietin (rhTPO) and pegylated human recombinant megakaryocyte growth and development factor (PEG-rHuMGDF), underwent extensive clinical studies in a wide range of thrombocytopenic disorders.1–6 However, this development activity came to an abrupt end in 1998 when some patients paradoxically developed thrombocytopenia as a result of treatment with PEG-rHuMGDF.7,8 Autoantibodies formed against PEG-rHuMGDF and cross-reacted with and neutralized endogenous TPO, producing thrombocytopenia in healthy human subjects. Further development of PEG-rHuMGDF was stopped, and, although it had been associated with no such problems, rhTPO did not undergo any further development.
Given the clinical benefit demonstrated in studies with these first-generation molecules, recent efforts have been directed toward the development of thrombopoietic growth factors that are potent stimulators of platelet production but are not antigenic. A variety of new thrombopoietic growth factors have been developed that have unique properties not found in the recombinant thrombopoietins. This review seeks (1) to discuss the first-generation thrombopoietic growth factors, (2) to assess the lessons learned from the clinical studies done with the first-generation thrombopoietic growth factors,(3) to describe the structure and function of the new thrombopoietic growth factors, (4) to evaluate the potential utility of these new thrombopoietic growth factors in treating thrombocytopenic disorders, and (5) to consider the potential risk of thrombopoietic growth factor therapy.
First-generation thrombopoietic growth factors
The first-generation thrombopoietic growth factors were recombinant proteins based on the then emerging understanding of the structure and function of TPO (Table 1). TPO is a 332-amino acid (95 kDa) glycoprotein that contains 2 domains: a receptor-binding domain (residues 1-153) that shows considerable homology to erythropoietin and a carbohydrate-rich domain (residues 154-332) that is highly glycosylated and is important in maintaining protein stability (Figure 1).1,5,9 Crystal structure data show that TPO has an antiparallel 4-helix bundle fold structure and binds to the thrombopoietin receptor with a 1:2 stoichiometry and binding constants of 3.33 × 10−9 M and 1.1 × 10−6 M.10
Thrombopoietic growth factors
| First-generation thrombopoietic growth factors |
| Recombinant human thrombopoietins |
| rhTPO |
| PEG-rHuMGDF |
| Recombinant TPO fusion proteins |
| Promegapoietin (TPO/IL3 fusion protein) |
| Second-generation thrombopoietic growth factors |
| TPO peptide mimetics |
| Fab 59 |
| AMG 531 |
| Peg-TPOmp |
| TPO nonpeptide mimetics |
| Eltrombopag (SB497115, Promacta) |
| AKR-501 |
| TPO agonist antibodies |
| Minibodies [VB22B sc(Fv)2] |
| Domain subclass-converted TPO agonist antibodies (MA01G4G344) |
| First-generation thrombopoietic growth factors |
| Recombinant human thrombopoietins |
| rhTPO |
| PEG-rHuMGDF |
| Recombinant TPO fusion proteins |
| Promegapoietin (TPO/IL3 fusion protein) |
| Second-generation thrombopoietic growth factors |
| TPO peptide mimetics |
| Fab 59 |
| AMG 531 |
| Peg-TPOmp |
| TPO nonpeptide mimetics |
| Eltrombopag (SB497115, Promacta) |
| AKR-501 |
| TPO agonist antibodies |
| Minibodies [VB22B sc(Fv)2] |
| Domain subclass-converted TPO agonist antibodies (MA01G4G344) |
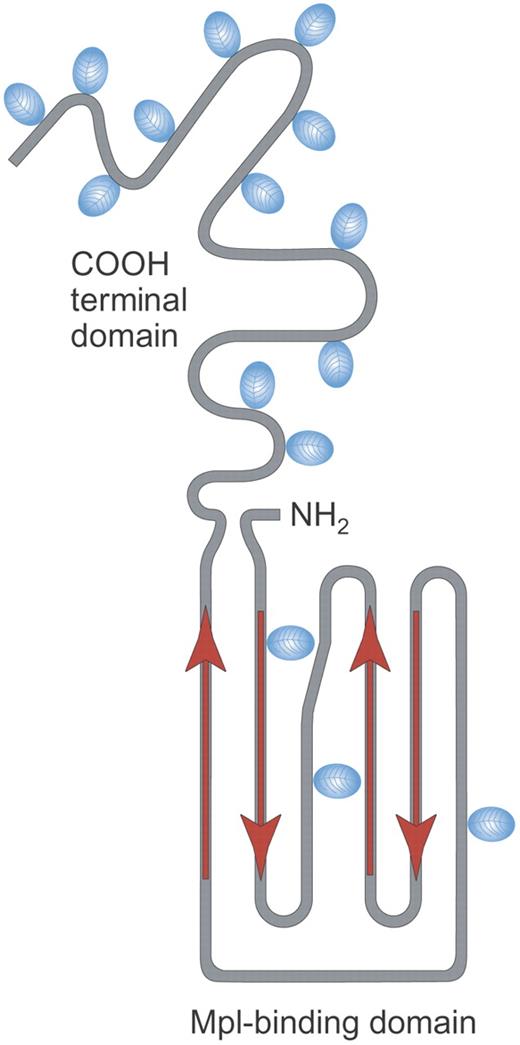
Structure of rhTPO. Recombinant human TPO (rhTPO) is a fully glycosylated TPO made in Chinese hamster ovary (CHO) cells. It contains the TPO receptor binding domain (Mpl-binding domain) and the carbohydrate-rich COOH terminal domain. Red arrows indicate alpha helical areas; blue ovals denote areas of glycosylation. Illustration by Paulette Dennis.
Structure of rhTPO. Recombinant human TPO (rhTPO) is a fully glycosylated TPO made in Chinese hamster ovary (CHO) cells. It contains the TPO receptor binding domain (Mpl-binding domain) and the carbohydrate-rich COOH terminal domain. Red arrows indicate alpha helical areas; blue ovals denote areas of glycosylation. Illustration by Paulette Dennis.
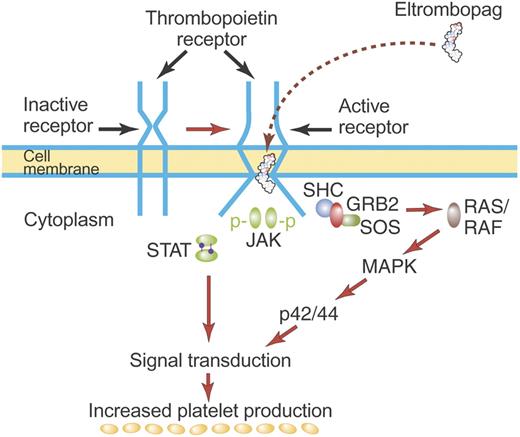
The TPO receptor, c-Mpl, is a typical hematopoietic cytokine receptor and contains 2 cytokine receptor homology modules (CRMs).11 Biochemical and crystallographic data show that TPO binds only the distal CRM (CRM 1) and thereby initiates signal transduction (Figure 2).10,12 In the absence of the distal CRM, c-Mpl becomes active, suggesting that the distal CRM functions as an inhibitor of c-Mpl until relieved by TPO binding.13 This may be similar to the erythropoietin receptor where the CRM domains preform an inactive dimeric receptor in which the intracellular regions are sufficiently distant one from the other to prevent phosphorylation and activation of JAK2.14,15 Subsequent binding of erythropoietin to the preformed dimeric receptor changes the structure of the dimeric receptor and initiates signal transduction. Whether TPO causes dimerization of the thrombopoietin receptor or simply stabilizes preformed dimers is uncertain.
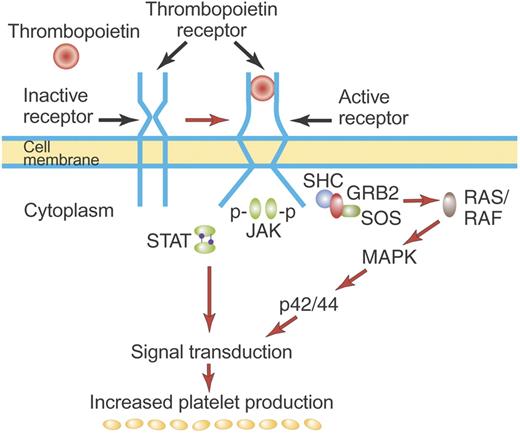
Mechanism of activation of the TPO receptor by TPO. TPO binds the distal CRM (CRM 1) of the inactive TPO (c-Mpl) receptor and creates an activated receptor that initiates many downstream signal transduction events (see “First-generation thrombopoietic growth factors”). Although drawn as such here, the existence of preformed inactive dimers of the TPO receptor has not yet been convincingly demonstrated. Illustration by Paulette Dennis.
Mechanism of activation of the TPO receptor by TPO. TPO binds the distal CRM (CRM 1) of the inactive TPO (c-Mpl) receptor and creates an activated receptor that initiates many downstream signal transduction events (see “First-generation thrombopoietic growth factors”). Although drawn as such here, the existence of preformed inactive dimers of the TPO receptor has not yet been convincingly demonstrated. Illustration by Paulette Dennis.
TPO is synthesized in the liver and is secreted without a storage form into the circulation.3,16 Whether other tissues produce physiologically relevant amounts of TPO is unclear: estimates of the relative amount of TPO produced by the liver range from 62% to 100%.17–20 TPO then binds to the TPO receptors on hematopoietic stem cells, progenitor cells, and platelets.21–23 On binding to the megakaryocyte progenitor TPO receptor, TPO initiates a number of signal transduction events (Figure 2): JAK2 and STAT5 phosphorylation leading to cell proliferation, MAPK activation promoting differentiation events, and activation of antiapoptotic pathways that promote cellular viability.24 Although TPO promotes the viability of pluripotential stem cells and early progenitor cells of all lineages, a major effect is to promote the viability and growth of megakaryocyte colony-forming cells (Meg-CFCs) and early megakaryocyte progenitors. In so doing, TPO specifically increases the production of mature megakaryocytes and platelets.
In normal physiology, TPO is constitutively produced and enters the circulation where it binds TPO receptors on megakaryocytes and platelets, is internalized, and degraded.22,23,25,26 In situations in which platelet production is reduced or the megakaryocyte mass is decreased, circulating levels of thrombopoietin rise in an apparent effort to stimulate platelet production.3,6,27 There is no sensing system of the platelet mass such as that which has been demonstrated for the regulation of renal erythropoietin production by the mass of red blood cells.
Of the first-generation thrombopoietic growth factors, only rhTPO and PEG-rHuMGDF have undergone clinical development. A number of other recombinant thrombopoietic proteins, such as the TPO/IL3 fusion protein (promegapoietin), were potent stimulators of megakaryocyte growth but did not undergo further clinical development.28
Recombinant human thrombopoietin (rhTPO)
rhTPO has the same amino acid structure as TPO and was a glycosylated protein produced in CHO cells (Figure 1). The recombinant form had a slightly lower molecular weight (90 kDa) than TPO (95 kDa), presumably because of the different glycosylation pattern produced by CHO cells. rhTPO was administered intravenously, had a half-life of 30 to 40 hours, and markedly increased platelet production when given to patients with cancer. After a single dose of rhTPO, there was a dose-dependent rise in platelet counts that began 5 days after its administration and peaked some 10 to 14 days later.29,30
Pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF)
PEG-rHuMGDF was composed of the amino-terminal 163 amino acids of human TPO (the 4 α-helical regions that bind the TPO receptor) coupled to a 20-kDa polyethylene glycol moiety. This truncated, nonglycosylated version of TPO was produced in Escherichia coli and then pegylated on the amino terminus to produce a molecule whose molecular weight was approximately 60 kDa and which had a half-life of 25 to 35 hours.31 The pegylation step was crucial; in its absence the rHuMGDF was biologically inactive because of a very short circulatory half-life. PEG-rHuMGDF was administered subcutaneously and produced a dose-dependent rise in platelet count in healthy persons.32 As with rhTPO, the platelet count rise began 5 days after the first injection and peaked some 10 to 14 days later.
Lessons learned from the first-generation thrombopoietic growth factors
After administration of either PEG-rHuMGDF or rhTPO to patients who received a stem cell transplant there was no effect upon the platelet count nadir, time to platelet count recovery to 20 × 109/L, or number of platelet transfusions.33 It is unclear whether this lack of response was due to the lack of target bone marrow cells, already high levels of TPO, or other unidentified factors. Nonetheless, despite numerous studies in which both the dose and schedule of the thrombopoietic growth factors were altered, there appeared to be no benefit.34,35 The only clinical benefit shown with thrombopoietic growth factors in stem cell transplantation occurred when the stem cell donor was treated with rhTPO.36,37 Administration of rhTPO increased the yield of CD34+ cells, and this produced a small, statistically significant reduction in time to neutrophil recovery, platelet transfusions, and red blood cell transfusions when the TPO-stimulated stem cells were used for autologous stem cell transplantation.
In patients undergoing induction chemotherapy or remission consolidation for acute leukemia, there was again no significant effect of thrombopoietic growth factors on the nadir platelet count, time to platelet count recovery, or platelet transfusions.38,39 Administration before, during, or after chemotherapy did not alter this outcome. When bone marrow biopsies were analyzed, patients who received the thrombopoietic growth factors appeared to have a marked stimulation of megakaryocytes, but as with the patients who received a stem cell transplant, they experienced only a postrecovery rebound thrombocytosis.40
A wide range of studies in patients receiving nonmyeloablative cancer chemotherapy has been conducted. In brief, these studies showed that treatment with either PEG-rHuMGDF or rhTPO increased the nadir platelet count and shortened the duration of thrombocytopenia.41–43 In gynecologic oncology patients undergoing chemotherapy, there was a marked decrease in the number of platelet transfusions.30 Different dosing schemes have been tested with no particular regimen associated with a strikingly different benefit.44 In one clinical trial, a single dose of PEG-rHuMGDF given right after chemotherapy was as good as multiple doses given for 10 days after chemotherapy. In all studies rhTPO and PEG-rHuMGDF were well tolerated and devoid of significant adverse events.
In 2 reported studies, 4 of 5 patients with immune thrombocytopenic purpura (ITP) showed improvement in their platelet counts when given PEG-rHuMGDF. Three of 4 Japanese patients with ITP given PEG-rHuMGDF daily for 7 days had a significant rise in platelet count.45 One patient with cyclic immune thrombocytopenia responded to twice weekly treatment with PEG-rHuMGDF for more than 6 years.46
The final 2 treatment studies with first-generation thrombopoietins were conducted with healthy human subjects. In one, platelet apheresis donors were treated with a single dose of PEG-rHuMGDF and were subjected to a standardized apheresis 14 days later.32,47 By the day of apheresis there was a dose-dependent peak platelet count for the placebo, 1 μg/kg, and 3 μg/kg cohorts of 252 × 109/L, 360 × 109/L, and 599 × 109/L, respectively; platelet apheresis yields showed a similar response of 3.7 × 1011, 5.5 × 1011, and 11.2 × 1011, respectively. No adverse events were noted for the donors, and the apheresis product showed normal platelet aggregation in vitro and was hemostatically active in vivo.32,47 Such TPO-generated platelets have also been cryopreserved and later transfused into their donors after chemotherapy.48
In the largest study with any thrombopoietic growth factor, paid healthy volunteers were given up to 3 doses of 3 μg/kg PEG-rHuMGDF (538 subjects) or placebo (528 subjects) every 28 days.6,7 Treatment with PEG-rHuMGDF produced a declining peak platelet count of 589 × 109/L, 506 × 109/L, and 494 × 109/L for cycles 1 to 3, respectively, compared with the baseline counts of 240 × 109/L. Unfortunately, 13 subjects treated with PEG-rHuMGDF (0 of 210 after 1 dose, 2 of 204 after 2 doses, and 11 of 124 after 3 doses) developed thrombocytopenia and antibody against PEG-rHuMGDF that cross-reacted with endogenous thrombopoietin. It is these subjects that prompted the discontinuation of further human studies of the first-generation thrombopoietic growth factors.
Second-generation thrombopoietic growth factors
Motivated by the antibody problems with PEG-rHuMGDF as well as by the desire to develop forms of therapy that might be administered orally or produced more efficiently, a number of novel thrombopoietic growth factors have been developed in the past 7 years (Table 1). These include TPO peptide mimetics, TPO nonpeptide mimetics, and TPO agonist antibodies. All of these bind to and activate the TPO receptor in different ways. All have been engineered to have unique pharmacologic attributes.
TPO peptide mimetics
One general approach to developing new thrombopoietic growth factors has been to screen peptide libraries for sequences that stimulate the growth of TPO-dependent cell lines and that lack sequence homology with TPO (Table 1). Cwirla et al49 identified one 14-amino acid peptide (Ile-Glu-Gly-Pro-Thr-Leu-Arg-Gln-Trp-Leu-Ala-Ala-Arg-Ala) that bore no sequence homology with TPO and yet bound the TPO receptor with high affinity. Chemical dimerization of this peptide markedly increased its biologic activity to a level the same as for rhTPO, demonstrating the need for a bivalent structure for TPO agonists. Subsequent studies suggested that antibodies made against such mimetics would not cross-react with human TPO.50 Although equipotent with rhTPO in cell-based assays, such linear peptides were too short-lived in the circulation to have any therapeutic effect. This led to efforts to prolong their biologic activity by insertion into human Fab and Fc constructs or by pegylation.
Fab 59.
Frederickson et al51 have recently described a rationally designed TPO peptide mimetic (Fab 59) made by engrafting 2 of the 14-amino acid TPO peptides described in the paragraph above into a fully human Fab, one peptide into the heavy chain complementarity-determining region 3 (HC-CDR3) and the other into the light chain CDR2 (Figure 3). The CDR regions were chosen because they are highly variable in nature, and insertion of a peptide would not be expected to stimulate autoantibodies.
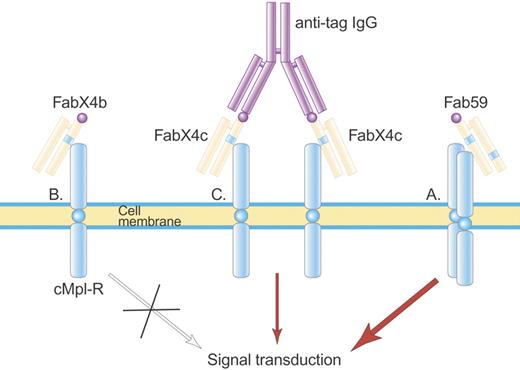
Structure of Fab 59. Fab 59 (A) is a fully human Fab containing one TPO agonist peptide (blue stripe) in the heavy-chain CDR3 and a second TPO agonist peptide in the light-chain CDR2, which activate the TPO receptor. In contrast, a related Fab, FabX4b (B), containing only one TPO agonist peptide, is not able to activate the TPO receptor until made dimeric by an anti-tag IgG antibody (C) that binds the hemagglutinin (HA) tag (purple ball) on FabX4c. Illustration by Paulette Dennis.
Structure of Fab 59. Fab 59 (A) is a fully human Fab containing one TPO agonist peptide (blue stripe) in the heavy-chain CDR3 and a second TPO agonist peptide in the light-chain CDR2, which activate the TPO receptor. In contrast, a related Fab, FabX4b (B), containing only one TPO agonist peptide, is not able to activate the TPO receptor until made dimeric by an anti-tag IgG antibody (C) that binds the hemagglutinin (HA) tag (purple ball) on FabX4c. Illustration by Paulette Dennis.
In developing this TPO agonist, 2 important principles were uncovered. First, the amino acids flanking the peptide strongly affected its ability to bind the TPO receptor. When the TPO peptide was inserted into HC-CDR3, TPO receptor binding was maximal when there was a proline at the flanking position immediately downstream from the peptide. The second principle was that the positions of the 2 peptides within the antibody framework were critical. When the 2 peptides were more separated as in the different arms of an IgG or an F(ab′)2, agonist activity was less than when both peptides were in the same Fab.
Fab 59 was equipotent to rhTPO in stimulating the growth of TPO-dependent cell lines but was 30-fold less active than rhTPO in increasing the platelet count when injected into mice, possibly because of differences in binding to the murine TPO receptor. No autoantibodies formed against TPO. No human studies have been reported.
AMG 531.
AMG 531 is another rationally designed TPO peptide agonist that is a “peptibody” composed of 2 disulphide-bonded human IgG1 κ-HC constant regions (an Fc fragment) each of which is covalently bound at residue 228 with 2 identical peptide sequences linked via polyglycine.52,53 The peptide component was selected by screening libraries of peptides that had no sequence homology with human TPO to find one with a tertiary structure that would allow it to bind to and activate the human TPO receptor. The Fc component extends the half-life of the molecule in the circulation. The 60-kDa AMG 531 binds the endothelial FcRn salvage receptor and undergoes endothelial recirculation; it is removed by the reticuloendothelial system.
In vitro AMG 531 bound to the human TPO receptor and competed with rhTPO for binding. AMG 531 induced phosphorylation of JAK2 and STAT5 in TPO-dependent cell lines, promoted the growth of TPO-dependent cell lines and megakaryocyte colony-forming cells (Meg-CFCs), and increased megakaryocyte ploidy and maturation in vitro.54 Studies in rhesus monkeys showed that single doses gave a dose-dependent rise in platelet count starting on day 5 and peaking at day 7 to 9; doses as low as 0.5 mg/kg increased the platelet count 1.5-fold. Drug terminal half-lives ranged from 110 to 160 hours. There was no thrombocytopenia or formation of antibody against TPO.
Based on pharmacokinetic modeling from the rhesus monkey studies, phase 1 studies in healthy humans started with a single dose of 10 μg/kg intravenously (1/50 of the minimally active dose in monkeys).52 Unexpectedly, this produced peak platelet counts well greater than 1000 × 109/L. In retrospect this robust effect in humans was probably due to the much higher affinity of AMG 531 for the human TPO receptor than the monkey TPO receptor. Subsequent doses from 0.1 to 2.0 μg/kg demonstrated a dose-dependent rise in platelet count, starting at day 5 and peaking at days 12 to 15. A clinically effective dose of 1 μg/kg was identified that increased the platelet count 2-fold. No subject developed antibodies to TPO; intravenous and subcutaneous routes produced identical responses.
Phase 1 to 3 trials have been completed with AMG 531 and are discussed under “Clinical studies with the second-generation thrombopoietin.” In its current formulation, AMG 531 is administered as a subcutaneous injection every week.
Peg-TPOmp.
Pegylation has been one other approach to increasing the half-life of TPO peptide agonists.55 Preliminary reports of one such molecule, Peg-TPOmp, show that it is active at picomolar concentrations in cell-based assays. In vivo it increased platelet production in mice and dogs. When single doses were administered intravenously to healthy male subjects at doses of 0.375 to 3 μg/kg, there was a dose-dependent peak platelet count on day 10 to 12 that ranged from 1.4-fold to 3.2-fold above baseline. Interestingly, there was a dose-dependent rise in the endogenous TPO levels, suggesting that the PEG-TPOmp altered normal TPO clearance. There were no adverse events, and the platelets showed normal function in aggregation experiments. No other human study information is currently available.
TPO nonpeptide mimetics
A second approach to the development of new thrombopoietic growth factors has been to screen large libraries of small nonpeptide molecules for the ability to stimulate reporter genes, such as STAT, in TPO-dependent cell lines. Using such an approach Duffy56 reported the identification of 4-diazo-3-hydroxy-1-naphthalenesulfonic acids as TPO mimics. Modification of the core structure resulted in the development of (5-oxo-1,5-dihydropyrazol-4-ylidene)hydrazines that had potent TPO activity in cell-based assays. Further work showed that thiosemicarbazone and naphtho[1,2-d]imidazole structures shared characteristics that gave TPO agonist activity: lipophilic group at one end, acidic group on the other end, and a metal chelate group in the center.57–59 To improve oral bioavailability, subsequent series of biphenyl carboxylates were developed.60 A large number of TPO nonpeptide mimetics have thus been identified by at least 6 different research groups. Most are still in preclinical development, but 2 have been optimized for their pharmacologic characteristics and tested in humans: eltrombopag and AKR-501 (Table 1).
Before reviewing in detail these 2 TPO nonpeptide mimetics, it is important to describe 3 apparent characteristics of many of the TPO nonpeptide mimetics: (1) they are highly species specific, (2) they activate the TPO receptor in a manner that differs from rhTPO, and (3) they have an effect additive to TPO.
The species restriction is a characteristic of many of the TPO nonpeptide mimetics, and this has often limited their preclinical evaluation only to toxicology testing. Except for a few studies in nonobese diabetic severe combined immunodeficient (NOD/SCID) mice engrafted with human CD34+ cells, most efficacy studies have been done only in humans. For example, eltrombopag and its precursors activate cells carrying the human and chimp but not the rat, mouse, ferret, or cynomolgus monkey TPO receptor.59,61 To understand this species difference, the CRM1, CRM2, transmembrane (TM), and cytoplasmic domains of the human and cynomolgus monkey TPO receptors were swapped. Eltrombopag activated cells carrying the intact human TPO receptor but not the intact cynomolgus TPO receptor. When the monkey TM domain replaced the human TM domain, the human TPO receptor could no longer be activated; inserting the human TM domain into the monkey TPO receptor allowed it now to be activated by eltrombopag. This was due mostly to a single amino acid difference in the TM domain of the TPO receptor. Human and chimpanzee TPO receptors have a histidine at residue 499, whereas all the other species have a leucine. Changing this histidine to a leucine in the human TPO receptor causes loss of activity; changing the cynomolgus monkey TPO receptor leucine to a histidine makes the receptor now responsive to the mimetic.
These results suggest a model wherein binding of TPO nonpeptide mimetics to the TPO receptor either induces dimerization of the TPO receptor or directly activates the signal transduction mechanism (Figure 4). Histidine 499 (and probably also threonine 496) in the transmembrane region is important either as a mediator of this effect or as the actual binding site of the mimetic. Furthermore, there does not seem to be any competition for binding with rhTPO. Together these findings suggest that many TPO nonpeptide mimetics bind the TPO receptor at a distance from the binding site for TPO and appear to initiate signal transduction by a mechanism different from rhTPO.
Mechanism of activation of TPO receptor by some TPO nonpeptide mimetics. TPO nonpeptide mimetics such as eltrombopag activate the TPO receptor by a mechanism different from TPO (see “TPO nonpeptide mimetics”). Although drawn here to suggest direct binding of eltrombopag to the TM region of the TPO receptor, eltrombopag may bind elsewhere on the TPO receptor but may have its effect mediated by unique structures in the TM region. Most TPO nonpeptide mimetics do not compete for binding with rhTPO and have a biologic effect additive to that of rhTPO. Illustration by Paulette Dennis.
Mechanism of activation of TPO receptor by some TPO nonpeptide mimetics. TPO nonpeptide mimetics such as eltrombopag activate the TPO receptor by a mechanism different from TPO (see “TPO nonpeptide mimetics”). Although drawn here to suggest direct binding of eltrombopag to the TM region of the TPO receptor, eltrombopag may bind elsewhere on the TPO receptor but may have its effect mediated by unique structures in the TM region. Most TPO nonpeptide mimetics do not compete for binding with rhTPO and have a biologic effect additive to that of rhTPO. Illustration by Paulette Dennis.
It is therefore not surprising that the effect of many of these TPO nonpeptide mimetics is additive to the effect of rhTPO in cell-based assays. When human CD34+ cells were cultured in the presence of maximal amounts of rhTPO, the addition of AKR-501 resulted in a 66% increase in production of CD41+ cells.62 In mice engrafted with human fetal liver CD34+ cells, human platelet production stimulated by rhTPO could be increased further by the addition of a TPO nonpeptide mimetic.63
Eltrombopag.
Eltrombopag (SB497115, Promacta) is an orally available, hydrazone small (MW = 546 D) molecule (Figure 5).59,60,64–67 In vitro it stimulates the growth of TPO-dependent cell lines via JAK2 and STAT signaling pathways and stimulates isolated human CD34+ cells to become megakaryocytes and produce platelets.59
Structure of eltrombopag. Eltrombopag [3′-{N′-[1-(3,4-Dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydropyrazol-4-ylidene]hydrazino}-2′-hydroxybiphenyl-3-carboxylic acid] has an acidic (COOH) group at one end, lipophilic (CH3) groups at the other end, and a metal chelate group in the center, which create a potent, orally available TPO nonpeptide agonist. Illustration by Paulette Dennis.
Structure of eltrombopag. Eltrombopag [3′-{N′-[1-(3,4-Dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydropyrazol-4-ylidene]hydrazino}-2′-hydroxybiphenyl-3-carboxylic acid] has an acidic (COOH) group at one end, lipophilic (CH3) groups at the other end, and a metal chelate group in the center, which create a potent, orally available TPO nonpeptide agonist. Illustration by Paulette Dennis.
In phase 1 studies in healthy male humans, single oral doses of eltrombopag did not increase the platelet count. However, when subjects received doses of 5, 10, 20, 30, 50, or 75 mg daily for 10 days, a dose-dependent rise in platelet count was seen at doses of 30 mg or more; platelet counts increased approximately 1.3-, 1.4-, and 1.5-fold over baseline for the 30-, 50-, and 75-mg doses, respectively.68 For subjects at the 75-mg dose, the platelet count rise started on day 7 and peaked at day 16. The subjects had no adverse effects.
Clinical studies in ITP, liver disease, and chemotherapy-induced thrombocytopenia have been conducted as described under “Clinical studies with the second-generation thrombopoietin.” Eltrombopag is currently being administered as a once-daily oral pill taken on an empty stomach. Its oral absorption is affected by concomitant administration of some other medications and foodstuffs.
AKR-501.
AKR-501 (formerly YM 477) is a second orally active TPO nonpeptide agonist identified through screening of small molecule libraries for the ability to activate a reporter molecule in TPO-dependent cell lines. Lead compounds were initially identified and then optimized for their biologic effect and pharmaceutical properties. In vitro AKR-501 stimulated the growth of TPO-dependent cell lines, increased the growth of megakaryocytes from human CD34+ cells, and activated only the human and chimp TPO receptors. AKR-501 increased the production of human platelets in mice into which human fetal liver CD34+ cells were engrafted.63
Single and multiple oral doses have been tested in healthy human subjects.62 In a single-dose study, cohorts of 9 subjects each were randomly assigned to receive doses of 1, 3, 10, 20, 50, 75, and 100 mg or placebo. Unlike for eltrombopag, single doses of 20 mg or more produced a rise in platelet count as high as 1.75 times the baseline platelet count. Daily oral doses of 3 mg, 10 mg, or 20 mg for 14 days increased the peak platelet count by 1.3-, 2.25-, and 2.8-fold, respectively. None of the subjects experienced any adverse effects. AKR-501 was well absorbed and exhibited dose linear phamacokinetics with a serum half-life of approximately 16 hours.
The single and multiple dose studies in healthy humans suggest that AKR-501 may be moderately more active than eltrombopag. AKR-501 is formulated as a once-daily pill. Clinical studies in ITP, liver disease, and chemotherapy are planned.
TPO agonist antibodies
Therapeutic monoclonal antibodies have been widely used to target various cell receptors, invariably to inhibit or kill the associated cell. In general, therapeutic monoclonal antibodies are well tolerated, nonimmunogenic, and have a long circulating half-life. Agonist antibodies against cell receptors have not yet entered clinical practice and present some challenges for development, especially because the size of the intact immunoglobulin molecule may sterically hinder receptor binding and dimerization. Nonetheless, monoclonal antibodies against the TPO receptor can be readily identified after injecting mice with purified human c-Mpl or cell lines expressing human c-Mpl. These intact monoclonal antibodies usually have high affinity for c-Mpl but usually do not stimulate the growth of TPO-dependent cell lines very well. However, when further genetically engineered to optimize their size and/or bivalent receptor binding properties, such monoclonal antibodies become potent TPO agonists (Table 1).
TPO minibodies [VB22B sc(Fv)2].
Monoclonal antibodies that bound c-Mpl have been genetically engineered to be TPO agonist “minibodies.” The heavy chain variable region (VH) and light chain variable region (VL) were isolated and genetically modified to create small bivalent antibody fragments comparable in size to an Fab fragment.69 In so doing, the binding affinity for c-Mpl was retained, but the smaller molecule had a markedly increased agonist activity. One formulation, VB22B sc(Fv)2, bound to and activated a TPO-dependent cell line just as well as did rhTPO (Figure 6). VB22B sc(Fv)2 also induced phosphorylation of JAK2, STAT5, and c-Mpl just like rhTPO. When injected into cynomolgus monkeys, VB22B sc(Fv)2 had a half-life of 8 to 9 hours, increased the platelet count, and did not cause antibody formation (despite the species differences).
Structure of VB22B sc(Fv)2 minibody. A noncovalent (55 kDa) “diabody” (middle of figure) can be generated by taking the VH and VL regions of a whole anti-Mpl monoclonal IgG (left) and coupling them via a 5-amino acid linker (GGGGS). VB22B sc(Fv)2 is made by binding one VH and one VL fragment together with a 15-amino acid linker [(GGGGS)3] and then binding 2 of these constructs together with the same 15-amino acid linker (middle). Both the diabody and the VB22Bsc(Fv)2 bind and activate the TPO receptor (right). Illustration by Paulette Dennis.
Structure of VB22B sc(Fv)2 minibody. A noncovalent (55 kDa) “diabody” (middle of figure) can be generated by taking the VH and VL regions of a whole anti-Mpl monoclonal IgG (left) and coupling them via a 5-amino acid linker (GGGGS). VB22B sc(Fv)2 is made by binding one VH and one VL fragment together with a 15-amino acid linker [(GGGGS)3] and then binding 2 of these constructs together with the same 15-amino acid linker (middle). Both the diabody and the VB22Bsc(Fv)2 bind and activate the TPO receptor (right). Illustration by Paulette Dennis.
Domain subclass-converted TPO agonist antibodies (MA01G4G344).
The development of a second TPO agonist antibody suggests that it may not be just size but also the structure of the immunoglobulin agonist that is important for activating the TPO receptor.70 MA01 is an IgG1 monoclonal antibody that was cloned from human antibody-producing mice immunized with human Mpl-expressing cells. MA01 weakly stimulated the growth of a TPO-dependent cell line. When the constant heavy chain (CH) region of MA01 was converted to IgG4 (MA01G4) to reduce antibody-dependent cellular toxicity and complement-dependent cytotoxicity, there was no effect on binding affinity or agonist activity. However, when the hinge region (CH2-CH3) was converted from IgG1 to IgG3 (MA01G4G344), agonist activity increased 10-fold with no change in binding affinity (Figure 7). MA01G4G344 stimulated a TPO-dependent cell line and increased human Mk-CFCs in vitro; a single injection increased the platelet count in human c-Mpl transgenic mice for longer than 1 month.
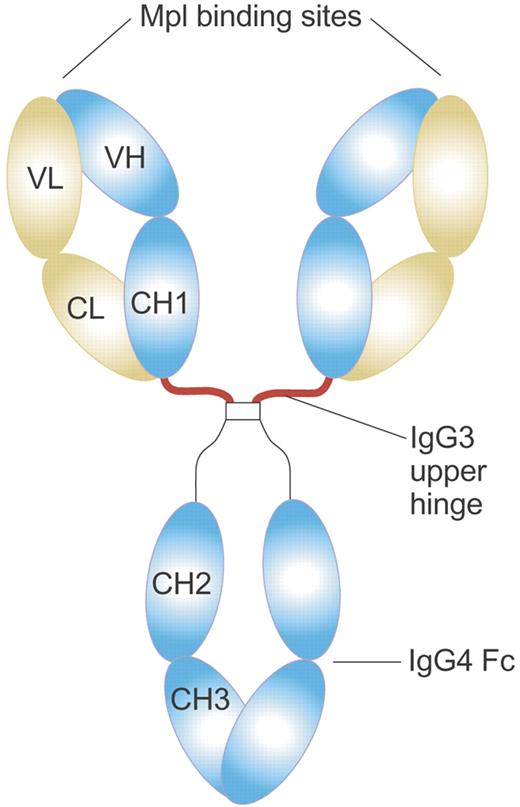
Structure of MA01G4G344 TPO agonist antibody. MA01G4G344 is a novel anti-Mpl agonist antibody that has an upper hinge region of human IgG3 and the other parts of human IgG4. The IgG3 upper hinge improved agonist activity of the whole IgG. The EC50 value of MA01G4344 in the UT7/TPO proliferation assay was 0.01 to 0.1 nM, comparable to rhTPO. Illustration by Paulette Dennis.
Structure of MA01G4G344 TPO agonist antibody. MA01G4G344 is a novel anti-Mpl agonist antibody that has an upper hinge region of human IgG3 and the other parts of human IgG4. The IgG3 upper hinge improved agonist activity of the whole IgG. The EC50 value of MA01G4344 in the UT7/TPO proliferation assay was 0.01 to 0.1 nM, comparable to rhTPO. Illustration by Paulette Dennis.
Studies in humans have not been reported for either the TPO minibody or the domain subclass-converted TPO agonist antibody.
Clinical studies with the second-generation thrombopoietins
The second-generation thrombopoietins have so far been studied primarily in thrombocytopenic patients with ITP or liver disease, conditions in which the bone marrow cellularity is usually normal. Studies in myelodysplastic syndrome (MDS) and chemotherapy-induced thrombocytopenia are under way but with no results yet reported.
Both AMG 531 and eltrombopag have been studied in patients with ITP. In most studies, patients with ITP were eligible if platelet counts were less than 30 × 109/L and they had failed at least one prior treatment regimen. In a phase 1 study with AMG 531, 7 of 12 patients had their platelet count double and increase above 50 × 109/L after 2 doses of 3, 6, or 10 μg/kg. The peak platelet count was dose dependent: 163 × 109/L, 309 × 109/L, and 746 × 109/L for the 3, 6, and 10 μg/kg cohorts, respectively.53 In a phase 2 study, AMG 531 (1 or 3 μg/kg) or placebo were injected weekly for 6 weeks. Twelve of 16 patients treated with AMG 531 had their platelet counts double and rise to greater than 50 × 109/L. Mean peak platelet counts were 135 × 109/L, 241 × 109/L, and 81 × 109/L for the 1 μg/kg, 3 μg/kg, and placebo groups, respectively.53 Results of a recently completed, randomized, placebo-controlled phase 3 study of AMG 531 versus placebo for 6 months in patients with ITP with and without splenectomies have not yet been released.
More than 100 subjects from the AMG 531 phase 1 to 3 studies have enrolled in an open-label study of long-term administration of AMG 531. Recent analysis of 36 subjects, most treated for more than 48 weeks, showed that they had a stable mean platelet count of greater than 100 × 109/L, were on a stable weekly dose, and half were able to stop other concomitant immunosuppressive drugs.71
In all 3 studies, AMG 531 was well tolerated. The major reported complication of therapy was mild headache in half of the persons on the day of treatment. On stopping therapy, all of the subjects had their platelet counts return to baseline. A few had a rebound worsening of their baseline thrombocytopenia lasting for up to 2 weeks. The only potential long-term complication that was identified was increased bone marrow reticulin in 2 of more than 100 patients.
Eltrombopag has shown significant activity in patients with ITP as well. In a randomized, double-blind, placebo-controlled phase 2 trial, subjects were given daily oral treatment with placebo or eltrombopag at doses of 30 mg, 50 mg, or 75 mg for 6 weeks.72 Platelet counts rose to greater than 50 × 109/L in approximately 12%, 27%, 65%, and 86%, and the median peak platelet counts were 16 × 109/L, 26 × 109/L, 128 × 109/L, and 183 × 109/L for the placebo, 30 mg, 50 mg, and 75 mg cohorts, respectively. There was a trend toward fewer bleeding events on therapy in those receiving the 50- mg and 75-mg doses.72 On stopping eltrombopag, the platelet count returned to baseline in most of these patients. No patient reported any significant adverse event from therapy. A phase 3 clinical trial is currently under way to assess the benefit of eltrombopag versus placebo during 6 months of treatment.
Patients with chronic hepatitis C liver disease and platelets 20 to 70 × 109/L have been treated for 4 weeks with placebo or eltrombopag at doses of 30 mg, 50 mg, or 75 mg daily in a blinded, randomized phase 2 study.73 The median platelet count at week 4 was 53 × 109/L, 137 × 109/L, 214 × 109/L, and 209 × 109/L with 0%, 75%, 74%, and 95% having platelets 100 × 109/L or greater, respectively. None of these patients with liver disease seemed to have any worsening of their liver function tests. Patients were then eligible to undergo interferon treatment along with the eltrombopag, and 12 weeks of therapy were successfully completed by 6%, 36%, 53%, and 65% of the subjects, respectively.
Potential risks of thrombopoietic growth factor therapy
Table 2 lists the potential complications of therapy with thrombopoietic growth factors. Both the first- and second-generation thrombopoietic growth factors can readily increase platelet counts to levels considerably above normal. Data from studies of the first-generation thrombopoietic growth factors showed that thrombocytosis did not increase the rate of thrombosis, even when patients had cancer.30,41,43 This conclusion must be tempered, however, because these thrombopoietic growth factor studies excluded patients with active cardiac or cerebrovascular disease or a history of thrombosis. In studies with extravascular shunts in baboons, a rise in platelet count was associated with a proportional increase in platelet deposition in the extravascular shunt.74–76 Conceivably in persons with active cardiovascular or cerebrovascular disease, raising the platelet count too high might pose a potential risk of thrombosis.
Potential adverse consequences of thrombopoietic growth factor treatment
| Thrombocytosis |
| Thrombosis |
| Stimulation of tumor cell growth |
| Stimulation of leukemia cell growth |
| Interactions with other cytokines |
| Autoantibody formation |
| Stem cell depletion |
| Reduction in threshold for platelet activation |
| Increased bone marrow reticulin (positive reticulin stain) |
| Increased bone marrow collagen (positive trichrome stain) |
| Rebound worsening of thrombocytopenia upon stopping treatment |
| Thrombocytosis |
| Thrombosis |
| Stimulation of tumor cell growth |
| Stimulation of leukemia cell growth |
| Interactions with other cytokines |
| Autoantibody formation |
| Stem cell depletion |
| Reduction in threshold for platelet activation |
| Increased bone marrow reticulin (positive reticulin stain) |
| Increased bone marrow collagen (positive trichrome stain) |
| Rebound worsening of thrombocytopenia upon stopping treatment |
Although none of the thrombopoietic growth factors directly activates platelets, the threshold for activation by specific platelet activators (ADP, collagen, and thrombin receptor agonist peptide) is lower when rhTPO, PEG-rHuMGDF, or AMG 531 are present at significant concentrations.74,75,77 In the absence of these growth factors, platelets generated by these growth factors had a normal threshold for activation.32 It is doubtful that this effect has much clinical importance because clinical studies with the first-generation thrombopoietic growth factors did not show any increase in thrombosis. Furthermore, treatment of baboons with PEG-rHuMGDF did not increase fibrin deposition in extravascular shunts despite marked increases in platelet count.75,76 Finally, in the rabbit carotid artery model, PEG-rHuMGDF did not alter “cyclic flow reduction,” a very sensitive test of platelet-dependent thrombosis.74 The TPO nonpeptide mimetics appear to lack this effect on the platelet activation threshold; there is no data available for the TPO agonist antibodies.
Despite that cells in many hematopoietic malignancies contain the TPO receptor, there was no evidence that administration of first-generation thrombopoietic growth factors to patients with acute myeloid leukemia (AML) or MDS increased the blast count or affected treatment response.38,78
Finally, the only unanswered issue for the second-generation thrombopoietic growth factors is whether long-term administration might increase marrow reticulin (positive reticulin stain) or possibly even lead to collagen deposition (positive trichrome stain). Administration of very high doses of PEG-rHuMGDF to mice for 7 to 10 days produced reversible bone marrow reticulin.79 Furthermore, overexpression of thrombopoietin in mice by transplantation of c-Mpl transfected bone marrow cells caused extensive bone marrow fibrosis, osteosclerosis, and extramedullary hematopoiesis like the human disease chronic idiopathic myelofibrosis.80–83 These and other data suggest that when megakaryocyte production is stimulated, there is a reversible increase in reticulin deposition, presumably because of the TGF-β being released by the stimulated megakaryocytes.79,84
In humans given rhTPO and PEG-rHuMGDF there has been little clinical evidence for bone marrow fibrosis, but, given the brevity of most exposures and the lack of routine bone marrow analysis, this problem was not fully explored. In the only study in which bone marrow analysis was performed, most patients treated with rhTPO had transient increase in bone marrow reticulin. Serial analyses of bone marrow and peripheral blood were conducted in 9 patients who received rhTPO after AML induction therapy and in 8 patients undergoing the same AML induction treatment but without rhTPO treatment.40 Eight of the 9 rhTPO-treated patients and 5 of the 8 control patients had increased bone marrow cellularity; 8 of 9 rhTPO-treated patients and 2 of 6 control patients had increased bone marrow reticulin staining. All these morphologic findings resolved within 42 days of the last dose of rhTPO.
As mentioned above, 2 subjects who received AMG 531 developed mild or moderate increased reticulin in the bone marrow but without evidence for increased collagen fibrosis (no trichrome staining) and with normal cytogenetics.53 Both subjects were asplenic and were receiving relatively high doses of AMG 531 (> 10 μg/kg), with minimal or no response. Fourteen weeks after discontinuation of therapy, a second bone marrow biopsy done in one of the subjects showed improvement in the reticulin deposition. A follow-up bone marrow biopsy was not done in the other subject. Longitudinal human studies are under way to assess the presence of reticulin before and after exposure to AMG 531. Whether eltrombopag or AKR-501 might also produce this finding has not yet been assessed by bone marrow examination.
Conclusions
Although development of the first-generation thrombopoietic growth factors was stopped by the development of autoantibodies, studies with them indicated several thrombocytopenic disorders in which such growth factors might be effective. Now a number of second-generation thrombopoietic growth factors have been developed that are potent stimulators of platelet production and are devoid of any apparent immunogenicity. Two of these, AMG 531 and eltrombopag, are currently in the final phases of clinical development and have been shown to be highly effective in the treatment of ITP. Eltrombopag has also been shown to increase the platelet count in thrombocytopenic patients with hepatitis C and allow them to undergo effective antiviral therapy. Adverse effects of these drugs appear to be minimal, although long-term exposure studies need to be continued. Hopefully, within the next 18 months, one or both of these molecules will be clinically available to treat ITP and possibly other thrombocytopenic disorders. If they lack significant adverse effects, they might also be considered for use in increasing the yield from platelet apheresis donors.
Although studies with the first-generation thrombopoietic growth factors suggest the clinical areas in which drugs with an identical mechanism of action such as AMG-531 may be effective (ITP, nonmyeloablative chemotherapy, platelet apheresis donors) or ineffective (AML treatment, stem cell transplantation), such predictions may not be true for the TPO nonpeptide mimetics. The TPO nonpeptide mimetics have a unique mechanism of activation of the TPO receptor, and their effect is often additive to that of rhTPO. Because endogenous TPO levels are usually high in the clinical settings (AML treatment, stem cell transplantation) where rhTPO and PEG-rHuMGDF failed, use of the TPO nonpeptide mimetics might have an effect that is additive to the endogenous TPO and produce an increase in platelet count.
Development of other TPO nonpeptide mimetics such as AKR-501 and some TPO agonist antibodies is rapidly progressing. Ongoing studies should soon help determine the utility of all of these thrombopoietic growth factors in the treatment of thrombocytopenic patients.
Acknowledgments
I thank the following colleagues for their provision of preliminary data and structural information: Janet L. Nichol (Amgen, Thousand Oaks, CA), Dr Richard J. Frankovitch and Julian Jenkins (GlaxoSmithKline, Collegeville, PA), Drs Hiroshi Miyazaki and Masayuki Kai (Kirin Pharmaceutical Research Laboratories, Takasaki, Japan), Dr Katherine S. Bowditch (Alexion Antibody Technologies, San Diego, CA), Dr Christopher J. Molloy (Johnson & Johnson Pharmaceutical Research and Development, Spring House, PA), Dr Robert S. Desjardins (AkaRx, Paramus, NJ), and Dr Masayuki Tsuchiya, Chugai Pharmaceutical, Shizuoka, Japan).
This work was supported by the National Institutes of Health (grants HL72299 and HL82889).
National Institutes of Health
Authorship
Contribution: D.J.K. conceived and wrote this article.
Conflict-of-interest disclosure: D.J.K. has been a consultant to Amgen, Kirin, Pfizer, Genentech, Pharmacia, AkaRx, GlaxoSmithKline, Alexion, and Ligand. He has no commercial interest to report.
Correspondence: David J. Kuter, Hematology Division, Yawkey 7940, 55 Fruit St, Boston, MA 02114; e-mail: kuter.david@MGH.harvard.edu

![Figure 5. Structure of eltrombopag. Eltrombopag [3′-{N′-[1-(3,4-Dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydropyrazol-4-ylidene]hydrazino}-2′-hydroxybiphenyl-3-carboxylic acid] has an acidic (COOH) group at one end, lipophilic (CH3) groups at the other end, and a metal chelate group in the center, which create a potent, orally available TPO nonpeptide agonist. Illustration by Paulette Dennis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-10-019315/4/m_zh80110703760005.jpeg?Expires=1765030078&Signature=SKXFOANchsDl829TAl73bbyBHw1w4Sy-3lMuL02Zyr8SbOj8y9BEJf304wvQ-rlsLcURRdjpZt7h9FSil8b2JeBnsLxVVEGnSh3usDYE1RqKXXRPjmTvawGqz724qPjKWSfD5QS9wJ~P2iqQIe7hyU6xpS9CnLkwKMX139N0qEiBLn916jbGeLsLi~lH5ToCaE~G7~6NgL6ncd1UkRJ~RvTf~t-esKdgRZ0lSaEkW5FzzgrVHdA9h6ntTDV0HmT1XOTWX1Y57tN3c7Sb~Mt~vJmVOpogUZx0fwUSSibzK9pB4RW13cyMSprJVIrwjP2MrhjLcXaVPhNLSOfjAJG4AA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Structure of VB22B sc(Fv)2 minibody. A noncovalent (55 kDa) “diabody” (middle of figure) can be generated by taking the VH and VL regions of a whole anti-Mpl monoclonal IgG (left) and coupling them via a 5-amino acid linker (GGGGS). VB22B sc(Fv)2 is made by binding one VH and one VL fragment together with a 15-amino acid linker [(GGGGS)3] and then binding 2 of these constructs together with the same 15-amino acid linker (middle). Both the diabody and the VB22Bsc(Fv)2 bind and activate the TPO receptor (right). Illustration by Paulette Dennis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-10-019315/4/m_zh80110703760006.jpeg?Expires=1765030078&Signature=l-wqYV0iVwQZwS0OAdUrW95ixcbcQ18L~9HaE9c5aqOa4Q3j1iQiJOjLUZWsMvxlyCTyzMADRmolNGQBvu4imfZfnQojSOPP0acYRSiZLj-VPfS71oepd4bGQdzlCIz~d~Y~aB1EyKTe61aY5QpGBgrwP9gU8KjuHLaTcb7Cn-gGbaYwwAr50B~h5Sy5AaSsZx6~9QrU1z7UsZNCCgOAF-3uE0GhFSr1BPe46LH4tDM7LVACp8b8lcOUSswEKusvyzMKv5780qXVoogVWie7X-WBNYZDBUktRPEoD1FTbH-~FbzB-x5lTM1T8xRAr-ZfPZ7Yu5lN3-60hx6hQP~eMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)