Abstract
Chronic autoimmune thrombocytopenic purpura (AITP) is associated with autoantibodies specific for platelet membrane components, often including glycoprotein GPIIIa. T helper (Th) cells reactive with GPIIIa, which are capable of driving the autoantibody response, are activated in AITP, and the aim here was to map the epitopes that they recognize. Peripheral blood mononuclear cells (PBMCs) were obtained from 31 patients with AITP and 30 control donors and stimulated with a panel of 86 overlapping synthetic 15-mer peptides spanning the complete sequence of GPIIIa. One or more peptides elicited recall proliferation by PBMCs from 28 of the patients, and, typically, multiple sequences were stimulatory. In contrast, responses in healthy control donors were rare (chi-square test = 115.967; P ≤ .001). It was confirmed that the proliferating PBMCs from patients were cells of the CD3+CD4+ helper phenotype that were MHC class II restricted. Despite variation between different cases of AITP, particular sequences were commonly recognized with PBMCs from 24 patients (77%) responding to 1 or more of the 4 most dominant peptides. Mapping such dominant autoreactive helper epitopes is the first step in the development of new approaches to the treatment of AITP, based on the use of peptides to tolerize Th cells specific for platelet glycoproteins.
Introduction
Chronic autoimmune thrombocytopenic purpura (AITP) is a bleeding disorder characterized by the production of autoantibodies that mediate platelet destruction.1,2 The clinical signs include petechial hemorrhages, hemorrhagic bullae on mucous membranes, gingival or gastrointestinal bleeding, menorrhagia, retinal hemorrhages, and, most seriously, intracranial hemorrhage. Current therapeutic strategies for AITP rely on nonspecific immunosuppressive agents,3,4 or intravenous immunoglobulin or anti-D,5 with refractory cases undergoing splenectomy to remove a major site of autoantibody production and platelet destruction.6 Unfortunately, the results of these approaches are frequently unsatisfactory.3–6 A fuller understanding of the pathogenesis of AITP is therefore required to develop safe, effective treatments that specifically inhibit the disease process.
A major focus of research into the pathogenesis of AITP has been the characterization of the autoantibody response.1,7–10 Platelet membrane glycoprotein IIb/IIIa (GPIIb/IIIa) has emerged as the major autoantigen that is bound by pathogenic autoantibodies from most patients.8 Other platelet antigens that can be targeted, but less frequently, include glycoproteins GPIb/IX, GPIa/IIa, and GPV.7–10 Although this progress in determining the specificities of the autoantibodies has led to novel diagnostic assays for AITP,9,10 the mechanisms underlying the loss of self-tolerance remain to be elucidated.
The vast majority of IgG responses are driven by CD4+ helper T (Th) cells, including the production of pathogenic antibodies in murine models of autoimmune blood cell destruction.11,12 Human AITP is no exception, because the disease is associated with loss of peripheral T-cell tolerance and the development of recall helper responses to platelet autoantigens.13–17 Peripheral blood Th cells from patients with AITP, in comparison with those from healthy controls, exhibit accelerated proliferation when stimulated in vitro with fragments of purified17 or recombinant18 GPIIb/IIIa, indicative of prior activation in vivo. These memory Th cells are capable of driving anti-GPIIb/IIIa IgG synthesis by peripheral blood B cells from patients in vitro,17,18 with the spleen as the primary site for the autoreactive B cells to receive such help in vivo.19 T cells in AITP may, in addition to providing help for the autoantibody response, also contribute directly to platelet destruction.20 In response to the accumulating evidence that Th cells represent potential therapeutic targets, a small number of patients with AITP have been treated with a humanized monoclonal antibody that blocks the helper costimulatory molecule, CD40 ligand (CD154).21 The effects were to reduce both the frequency and in vitro collaboration of peripheral blood Th and B cells responsive to GPIIb/IIIa, and, in some cases, treatment was associated with increased platelet counts.21 Any such immune inhibition may be only temporary and not necessarily limited to the pathogenic response, but it should be possible to develop longer-lasting and more-selective immunotherapy by tolerizing the Th cells specific for platelet autoantigen, once the helper response has been sufficiently well characterized.11
CD4+ Th cells recognize short peptides that have been processed and displayed bound to MHC class II molecules by antigen-presenting cells (APCs).22 Antigen-specific tolerance can be induced in vivo by synthetic peptides containing dominant helper epitopes, if administered appropriately in soluble form, particularly via mucosal surfaces in the nose or gut.23 There are now many examples of successful treatments based on this approach for inhibiting animal models of immune-mediated disease, including autoimmune hemolytic anemia12 and responses to blood group antigens,24 and human trials are under way in patients with allergy,25 rheumatoid arthritis,26 and type 1 diabetes.27 To exploit a similar strategy in AITP, it will first be necessary to map peptides that contain the dominant Th epitopes from platelet autoantigens. Initial screening of large recombinant fragments has identified some of the regions of GPIIb/IIIa in which such epitopes may be located,18 but the entire sequence of neither molecule has yet been tested, and the precise peptides that are recognized have still to be defined.
The aim of the current work was to map the fine specificity of platelet-specific Th cells from patients with chronic AITP. Our approach, which has proved successful for other antigens,28–30 was to screen a panel of short, overlapping peptides spanning the entire sequence of platelet glycoprotein for the ability to stimulate recall responses by peripheral blood Th cells. We focused on responses to one major autoantigenic molecule, GPIIIa, which is known to contain important B- and T-cell determinants.18,31 The results identify GPIIIa peptides that contain epitopes recognized by autoreactive Th cells from patients with AITP, and which are candidate tolerogens for specific immunotherapy of the disease.
Patients, materials, and methods
Patients and control subjects
Approval for the study was received from the Grampian Local and Regional Ethics Committee (number 00/0052). Informed written consent was obtained from all patients and healthy controls, in accordance with the Declaration of Helsinki. Samples of whole blood were obtained from 31 patients (21 women and 10 men) with AITP, who attended the outpatient hematology clinic at Aberdeen Royal Infirmary. The details of the patients, who are all North European whites, are summarized in Table 1. The diagnosis of AITP was made by exclusion of other causes of thrombocytopenia and in compliance with the British Committee for Standards in Haematology Guideline.4 The majority (29 of 31) of the patients were being treated with immunosuppressive drugs at the time of sampling, and 8 had undergone splenectomy.
Clinical details of patients with AITP
| Patient with AITP . | Sex . | Age at diagnosis, y . | Disease duration, y . | Platelet count at diagnosis, × 109/L . | Treatment during course of disease . | |
|---|---|---|---|---|---|---|
| Corticosteroids . | Splenectomy . | |||||
| AITP1 | F | 52 | 12 | 24 | PDL, AZP, DAP, IVIg | Yes |
| AITP2 | F | 55 | 4 | 2 | PDL, AZP | No |
| AITP3 | M | 60 | 3 | 5 | PDL, IVIg | No |
| AITP4 | M | 59 | 3 | 6 | PDL, IVIg | No |
| AITP5 | M | 64 | 2 | 2 | PDL | No |
| AITP6 | F | 83 | 4 | 5 | PDL, AZP, DAP | No |
| AITP7 | F | 35 | 7 | 3 | PDL, IVIg | No |
| AITP8 | M | 68 | 2 | 5 | PDL, DAP | No |
| AITP9 | F | 75 | 7 | 5 | PDL | No |
| AITP10 | F | 53 | 7 | 5 | PDL, IVIg | Yes |
| AITP11 | F | 31 | 2 | 4 | PDL | No |
| AITP12 | F | 61 | 11 | 3 | PDL, AZP | Yes |
| AITP13 | M | 54 | 2 | 2 | PDL | No |
| AITP14 | F | 31 | 1 | 68 | PDL, AZP, MYC | No |
| AITP15 | M | 79 | 2 | 5 | PDL, DAP, IVIg | Yes |
| AITP16 | M | 51 | 2 | 71 | None | No |
| AITP17 | F | 71 | 5 | 126 | PDL | No |
| AITP18 | F | 38 | 23 | 61 | PDL | No |
| AITP19 | M | 66 | 3 | 54 | PDL | No |
| AITP20 | F | 60 | 8 | 106 | None | No |
| AITP21 | F | 58 | 19 | 30 | PDL, AZP, DAP, IVIg | No |
| AITP22 | F | 74 | 2 | 20 | PDL | No |
| AITP23 | F | 69 | 7 | 6 | PDL, IVIg | Yes |
| AITP24 | F | 51 | 2 | 5 | PDL | Yes |
| AITP25 | F | 25 | 2 | 32 | PDL | No |
| AITP26 | M | 51 | 1 | 5 | PDL | No |
| AITP27 | F | 65 | 2 | 62 | PDL | No |
| AITP28 | M | 24 | 1 | 10 | PDL | No |
| AITP29 | F | 52 | 2 | 9 | PDL, DAP, IVIg, Cyclosporine | Yes |
| AITP30 | F | 76 | 4 | 121 | PDL | No |
| AITP31 | F | 45 | 1 | 36 | PDL, IVIg | Yes |
| Patient with AITP . | Sex . | Age at diagnosis, y . | Disease duration, y . | Platelet count at diagnosis, × 109/L . | Treatment during course of disease . | |
|---|---|---|---|---|---|---|
| Corticosteroids . | Splenectomy . | |||||
| AITP1 | F | 52 | 12 | 24 | PDL, AZP, DAP, IVIg | Yes |
| AITP2 | F | 55 | 4 | 2 | PDL, AZP | No |
| AITP3 | M | 60 | 3 | 5 | PDL, IVIg | No |
| AITP4 | M | 59 | 3 | 6 | PDL, IVIg | No |
| AITP5 | M | 64 | 2 | 2 | PDL | No |
| AITP6 | F | 83 | 4 | 5 | PDL, AZP, DAP | No |
| AITP7 | F | 35 | 7 | 3 | PDL, IVIg | No |
| AITP8 | M | 68 | 2 | 5 | PDL, DAP | No |
| AITP9 | F | 75 | 7 | 5 | PDL | No |
| AITP10 | F | 53 | 7 | 5 | PDL, IVIg | Yes |
| AITP11 | F | 31 | 2 | 4 | PDL | No |
| AITP12 | F | 61 | 11 | 3 | PDL, AZP | Yes |
| AITP13 | M | 54 | 2 | 2 | PDL | No |
| AITP14 | F | 31 | 1 | 68 | PDL, AZP, MYC | No |
| AITP15 | M | 79 | 2 | 5 | PDL, DAP, IVIg | Yes |
| AITP16 | M | 51 | 2 | 71 | None | No |
| AITP17 | F | 71 | 5 | 126 | PDL | No |
| AITP18 | F | 38 | 23 | 61 | PDL | No |
| AITP19 | M | 66 | 3 | 54 | PDL | No |
| AITP20 | F | 60 | 8 | 106 | None | No |
| AITP21 | F | 58 | 19 | 30 | PDL, AZP, DAP, IVIg | No |
| AITP22 | F | 74 | 2 | 20 | PDL | No |
| AITP23 | F | 69 | 7 | 6 | PDL, IVIg | Yes |
| AITP24 | F | 51 | 2 | 5 | PDL | Yes |
| AITP25 | F | 25 | 2 | 32 | PDL | No |
| AITP26 | M | 51 | 1 | 5 | PDL | No |
| AITP27 | F | 65 | 2 | 62 | PDL | No |
| AITP28 | M | 24 | 1 | 10 | PDL | No |
| AITP29 | F | 52 | 2 | 9 | PDL, DAP, IVIg, Cyclosporine | Yes |
| AITP30 | F | 76 | 4 | 121 | PDL | No |
| AITP31 | F | 45 | 1 | 36 | PDL, IVIg | Yes |
PDL indicates prednisolone; AZP, azathioprine; DAP, dapsone; IVIg, intravenous immunoglobulin; MYC, mycophenlate mofetil.
Samples of whole blood for peripheral blood mononuclear cell (PBMC) isolation were also taken from 25 healthy control blood donors (18 women and 7 men). None was on any medication. PBMC samples from a further group of 5 patients with aplastic anemia (4 men and 1 woman) were included as disease controls, because this condition responds to immunosuppression and is considered to have an autoimmune basis,32 and patients also have low platelet counts.
Platelet recovery and preparation of eluates
Platelets from patients with AITP and controls were isolated by differential centrifugation of anticoagulated (citrate-phosphate-dextrose) blood. Anti-body was eluted from the surface of platelets as described by Hürlimann-Forster et al9 and stored at −80°C until used.
Detection of antiplatelet autoantibodies against GPIIb/IIIa from serum and platelet eluates of patients with AITP and controls
Anti-GPIIb/IIIa autoantibody concentrations in sera and eluates were measured by enzyme-linked immunoabsorbent assay (ELISA) using published methods.33,34 Briefly, samples were screened in duplicate wells of microtiter plates coated with purified GPIIb/IIIa. Background binding was determined by incubating each sample in uncoated wells, and control samples positive and negative for antibody were also included. Absorbance was read at 540 nm using a multiscan plate reader (Labsystems, Helsinki, Finland). Specific optical densities (ODs) greater than 0.1 and greater than 0.05 were interpreted as positive results for serum and eluate samples, respectively (determined from the mean of healthy control samples + 2 SD).
HLA class II DNA typing using PCR-SSP
Genomic DNA preparation from the whole blood of patients with AITP and controls and HLA class II typing was carried using polymerase chain reaction with sequence-specific priming (PCR-SSP) as reported elsewhere.34 Visual interpretation of positive bands after gel electrophoresis were confirmed using HELMBERG SCORE software v3.000T (provided by Dr W. Helmberg, Institute for Transfusion Medicine, University of Graz, Austria).
Preparation of antigens and mitogens
The human platelet membrane GPIIIa amino acid sequence reported by Frachet et al35 (GeneBank Accession no. M35999) was synthesized (Pepceuticals, Nottingham, United Kingdom) as a complete panel of 86 15-mer peptides, overlapping by 5 to 10 amino acids (Table 3). Peptide purity was monitored by amino acid analysis and mass spectrometry as reported previously.28–30 The peptides were used for stimulation of T cells at the previously determined optimum concentration of 20 μg/mL in culture.28–30
The antigen mycobacterial purified protein derivative (PPD; Statens Serumintitut, Copenhagen, Denmark) was added to cultures at 20μg/mL to stimulate positive control recall T-cell responses.36 Concanavalin A (Con A; Sigma, Poole, Dorset, United Kingdom) was used at 20 μg/mL as a positive control T-cell mitogen.
Isolation of peripheral blood mononuclear cells (PBMCs)
Mononuclear cells were recovered from anticoagulated samples of peripheral blood from patients with AITP and control donors by density gradient centrifugation (Lymphoprep; Nycomed, Roskilde, Denmark). Cell viability determined by trypan blue exclusion was greater than 90% in all samples.
T-cell proliferation assay
Assays of T-cell proliferation were carried out as described elsewhere29,30,34 under culture conditions designed to favor responses by previously activated T cells, rather than primary responses.28–30,36 Briefly, PBMCs were cultured at 1.25 × 106 cells per mL in Alpha Modification of Eagle Medium (Sigma) supplemented with 5% autologous serum. Synthetic GPIIIa peptides or control stimuli were added to cultures, which were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. T-cell proliferation was estimated from the incorporation of 3H-thymidine in triplicate 100-μL samples withdrawn from the cultures 5 days after stimulation, when recall responses peak.28–30,36 Results are presented either as the mean counts per minute (CPM) ± SD of the triplicate samples, or as a stimulation index (SI) expressing the ratio of mean CPM in stimulated versus unstimulated control cultures. An SI greater than 3 is interpreted as a positive response.37
Flow cytometric characterization of lymphocytes responding to stimulation
As previously described,34 cultures of unstimulated PBMCs, and those proliferating in response to peptides, were analyzed for expression of the T-cell marker CD3, the T-helper marker CD4, and the activation marker CD71 by 3-color flow cytometry. All antibodies and control immunoglobulins were supplied by Beckman Coulter (Bucks, United Kingdom). A total of 10 000 cells per sample were counted using an Epics XL cytometer (Beckman Coulter), and the results were analyzed with Expo 32 software (Beckman Coulter).
HLA restriction of PBMCs proliferating in response to GPIIIa peptides
Prediction of peptide-binding motifs for HLA-DR molecules
Protein sequences were entered into ProPred predictive software (http://www.imtech.res.in/raghava/propred/),38 which is based on quantitative matrices derived by Stumiolo et al.39 An algorithm allows the sequences to be scanned for motifs predicted to have high affinity for binding to many of the commonly expressed HLA-DR molecules.
Statistical analysis
Nonparametric chi-square and Fisher exact tests were used for statistical analysis, with P less than .05 considered to represent significance.
Results
Mapping peptides derived from the GPIIIa sequence that stimulate proliferation by PBMCs from patients with AITP or healthy controls
The prime aim was to identify the peptide sequences from GPIIIa that contain Th epitopes. PBMCs were obtained from the group of 31 patients with AITP (clinical details summarized in Table 1) and from 25 healthy control blood donors. A panel of 86 synthetic overlapping 15-mer peptides, spanning the entire sequence of the platelet GPIIIa (Table 2) was screened for the ability to stimulate the proliferation of PBMCs from each of the patients and controls. The platelet glycoprotein-responsive Th cells that are associated with AITP have previously been shown to be activated in vivo,17,18 as would be expected for autoaggressive lymphocytes of pathogenic relevance. Therefore, to map the epitopes recognized by these cells, the culture conditions were based on those previously designed to favor fast-developing recall, rather than slower primary, responses.28–30,36
Amino acid sequences of the panel of overlapping, synthetic GPIIIa peptides spanning the entire length of the GPIIIa molecule34
| Peptide no. . | Amino acid sequence . | GPIIIa residues . |
|---|---|---|
| 1 | GPNICTTRGVSSCQQ | 1-15 |
| 2 | TTRGVSSCQQCLAVS | 6-20 |
| 3 | SSCQQCLAVSPMCAW | 11-25 |
| 4 | CLAVSPMCAWCSDEA | 16-30 |
| 5 | PMCAWCSDEALPLGS | 21-35 |
| 6 | CSDEALPLGSPRCDL | 26-40 |
| 7 | LPLGSPRCDLKENLL | 31-45 |
| 8 | PRCDLKENLLKDNCA | 36-50 |
| 9 | KENLLKDNCAPESIE | 41-55 |
| 10 | KDNCAPESIEFPVSE | 46-60 |
| 11 | PESIEFPVSEARVLE | 51-65 |
| 12 | FPVSEARVLEDRPLS | 56-70 |
| 13 | ARVLEDRPLSDKGSG | 61-75 |
| 14 | DRPLSDKGSGDSSQV | 66-80 |
| 15 | DKGSGDSSQVTQVSP | 71-85 |
| 16 | DSSQVTQVSPQRIAL | 76-90 |
| 17 | TQVSPQRIALRLRPD | 81-95 |
| 18 | QRIALRLRPDDSKNF | 86-100 |
| 19 | RLRPDDSKNFSIQVR | 91-105 |
| 20 | DSKNFSIQVRQVEDY | 96-110 |
| 21 | SIQVRQVEDYPVDIY | 101-115 |
| 22 | PVDIYYLMDLSYSMK | 111-125 |
| 23 | SYSMKDDLWSIQNLG | 121-135 |
| 24 | IQNLGTKLATQMRKL | 131-145 |
| 25 | QMRKLTSNLRIGFGA | 141-155 |
| 26 | IGFGAFVDKPVSPYM | 151-165 |
| 27 | VSPYMYISPPEALEN | 161-175 |
| 28 | EALENPCYDMKTTCL | 171-185 |
| 29 | KTTCLPMFGYKHVLT | 181-195 |
| 30 | KHVLTLTDQVTRFNE | 191-205 |
| 31 | TRFNEEVKKQSVSRN | 201-215 |
| 32 | SVSRNRDAPEGGFDA | 211-225 |
| 33 | GGFDAIMQATVCDEK | 221-235 |
| 34 | CDEKIGWRNDASHL | 231-245 |
| 35 | DASHLLVFTTDAKTH | 241-255 |
| 36 | DAKTHIALDGRLAGI | 251-265 |
| 37 | RLAGIVQPNDGQCHV | 261-275 |
| 38 | GQCHVGSDNHYSAST | 271-285 |
| 39 | YSASTTMDYPSLGLM | 281-295 |
| 40 | SLGLMTEKLSQKNIN | 219-305 |
| 41 | QKNINLIFAVTENVV | 301-315 |
| 42 | TENVVNLYQNYSELI | 311-325 |
| 43 | YSELIPGTTVGVLSM | 321-335 |
| 44 | GVLSMDSSNVLQLIV | 331-345 |
| 45 | LQLIV DAYGK IRSKV | 341-355 |
| 46 | IRSKV ELEVR DLPEE | 351-365 |
| 47 | DLPEELSLSFNATCL | 361-375 |
| 48 | NATCLNNEVIPGLKS | 371-385 |
| 49 | PGLKSCMGLKIGDTV | 381-395 |
| 50 | IGDTVSFSIEAKVRG | 391-405 |
| 51 | AKVRGCPQEKEKSFT | 401-415 |
| 52 | EKSFTIKPVGFKDSL | 411-425 |
| 53 | FKDSLIVQVTFDCDC | 421-435 |
| 54 | FDCDCACQAQAEPNS | 431-445 |
| 55 | AEPNSHRCNNGNGTF | 441-455 |
| 56 | GNGTFECGVCRCGPG | 451-465 |
| 57 | RCGPGWLGSQCECSE | 461-475 |
| 58 | CECSE EDYRP SQQDE | 471-485 |
| 59 | SQQDECSPREGQPVC | 481-495 |
| 60 | GQPVCSQRGECLCGQ | 491-505 |
| 61 | CLCGQCVCHSSDFGK | 501-515 |
| 62 | SDFGKITGKYCECDD | 511-525 |
| 63 | CECDDFSCVRYKGEM | 521-535 |
| 64 | YKGEMCSGHGQCSCG | 531-545 |
| 65 | QCSCGDCLCDSDWTG | 541-555 |
| 66 | SDWTGYYCNCTTRTD | 551-565 |
| 67 | TTRTDTCMSSNGLLC | 561-575 |
| 68 | NGLLCSGRGKCECGS | 571-585 |
| 69 | CECGSCVCIQPGSYG | 581-595 |
| 70 | PGSYGDTCEKCPTCP | 591-605 |
| 71 | CPTCPDACTFKKECV | 601-615 |
| 72 | KKECVECKKFDRGAL | 611-625 |
| 73 | DRGALHDENTCNRYC | 621-635 |
| 74 | CNRYCRDEIESVKEL | 631-645 |
| 75 | SVKELKDTGKDAVNC | 641-655 |
| 76 | DAVNCTYKNEDDCVV | 651-665 |
| 77 | DDCVVRFQYYEDSSG | 661-675 |
| 78 | EDSSGKSILYVVEEP | 671-685 |
| 79 | VVEEPECPKGPDILV | 681-695 |
| 80 | PDILVVLLSVMGAIL | 691-705 |
| 81 | MGAILLIGLAALLIW | 701-715 |
| 82 | ALLIWKLLITIHDRK | 711-725 |
| 83 | IHDRKEFAKFEEERA | 721-735 |
| 84 | EEERARAKWDTANNP | 731-745 |
| 85 | TANNPLYKEATSTFT | 741-755 |
| 86 | KEATSTFTNITYRGT | 748-762 |
| Peptide no. . | Amino acid sequence . | GPIIIa residues . |
|---|---|---|
| 1 | GPNICTTRGVSSCQQ | 1-15 |
| 2 | TTRGVSSCQQCLAVS | 6-20 |
| 3 | SSCQQCLAVSPMCAW | 11-25 |
| 4 | CLAVSPMCAWCSDEA | 16-30 |
| 5 | PMCAWCSDEALPLGS | 21-35 |
| 6 | CSDEALPLGSPRCDL | 26-40 |
| 7 | LPLGSPRCDLKENLL | 31-45 |
| 8 | PRCDLKENLLKDNCA | 36-50 |
| 9 | KENLLKDNCAPESIE | 41-55 |
| 10 | KDNCAPESIEFPVSE | 46-60 |
| 11 | PESIEFPVSEARVLE | 51-65 |
| 12 | FPVSEARVLEDRPLS | 56-70 |
| 13 | ARVLEDRPLSDKGSG | 61-75 |
| 14 | DRPLSDKGSGDSSQV | 66-80 |
| 15 | DKGSGDSSQVTQVSP | 71-85 |
| 16 | DSSQVTQVSPQRIAL | 76-90 |
| 17 | TQVSPQRIALRLRPD | 81-95 |
| 18 | QRIALRLRPDDSKNF | 86-100 |
| 19 | RLRPDDSKNFSIQVR | 91-105 |
| 20 | DSKNFSIQVRQVEDY | 96-110 |
| 21 | SIQVRQVEDYPVDIY | 101-115 |
| 22 | PVDIYYLMDLSYSMK | 111-125 |
| 23 | SYSMKDDLWSIQNLG | 121-135 |
| 24 | IQNLGTKLATQMRKL | 131-145 |
| 25 | QMRKLTSNLRIGFGA | 141-155 |
| 26 | IGFGAFVDKPVSPYM | 151-165 |
| 27 | VSPYMYISPPEALEN | 161-175 |
| 28 | EALENPCYDMKTTCL | 171-185 |
| 29 | KTTCLPMFGYKHVLT | 181-195 |
| 30 | KHVLTLTDQVTRFNE | 191-205 |
| 31 | TRFNEEVKKQSVSRN | 201-215 |
| 32 | SVSRNRDAPEGGFDA | 211-225 |
| 33 | GGFDAIMQATVCDEK | 221-235 |
| 34 | CDEKIGWRNDASHL | 231-245 |
| 35 | DASHLLVFTTDAKTH | 241-255 |
| 36 | DAKTHIALDGRLAGI | 251-265 |
| 37 | RLAGIVQPNDGQCHV | 261-275 |
| 38 | GQCHVGSDNHYSAST | 271-285 |
| 39 | YSASTTMDYPSLGLM | 281-295 |
| 40 | SLGLMTEKLSQKNIN | 219-305 |
| 41 | QKNINLIFAVTENVV | 301-315 |
| 42 | TENVVNLYQNYSELI | 311-325 |
| 43 | YSELIPGTTVGVLSM | 321-335 |
| 44 | GVLSMDSSNVLQLIV | 331-345 |
| 45 | LQLIV DAYGK IRSKV | 341-355 |
| 46 | IRSKV ELEVR DLPEE | 351-365 |
| 47 | DLPEELSLSFNATCL | 361-375 |
| 48 | NATCLNNEVIPGLKS | 371-385 |
| 49 | PGLKSCMGLKIGDTV | 381-395 |
| 50 | IGDTVSFSIEAKVRG | 391-405 |
| 51 | AKVRGCPQEKEKSFT | 401-415 |
| 52 | EKSFTIKPVGFKDSL | 411-425 |
| 53 | FKDSLIVQVTFDCDC | 421-435 |
| 54 | FDCDCACQAQAEPNS | 431-445 |
| 55 | AEPNSHRCNNGNGTF | 441-455 |
| 56 | GNGTFECGVCRCGPG | 451-465 |
| 57 | RCGPGWLGSQCECSE | 461-475 |
| 58 | CECSE EDYRP SQQDE | 471-485 |
| 59 | SQQDECSPREGQPVC | 481-495 |
| 60 | GQPVCSQRGECLCGQ | 491-505 |
| 61 | CLCGQCVCHSSDFGK | 501-515 |
| 62 | SDFGKITGKYCECDD | 511-525 |
| 63 | CECDDFSCVRYKGEM | 521-535 |
| 64 | YKGEMCSGHGQCSCG | 531-545 |
| 65 | QCSCGDCLCDSDWTG | 541-555 |
| 66 | SDWTGYYCNCTTRTD | 551-565 |
| 67 | TTRTDTCMSSNGLLC | 561-575 |
| 68 | NGLLCSGRGKCECGS | 571-585 |
| 69 | CECGSCVCIQPGSYG | 581-595 |
| 70 | PGSYGDTCEKCPTCP | 591-605 |
| 71 | CPTCPDACTFKKECV | 601-615 |
| 72 | KKECVECKKFDRGAL | 611-625 |
| 73 | DRGALHDENTCNRYC | 621-635 |
| 74 | CNRYCRDEIESVKEL | 631-645 |
| 75 | SVKELKDTGKDAVNC | 641-655 |
| 76 | DAVNCTYKNEDDCVV | 651-665 |
| 77 | DDCVVRFQYYEDSSG | 661-675 |
| 78 | EDSSGKSILYVVEEP | 671-685 |
| 79 | VVEEPECPKGPDILV | 681-695 |
| 80 | PDILVVLLSVMGAIL | 691-705 |
| 81 | MGAILLIGLAALLIW | 701-715 |
| 82 | ALLIWKLLITIHDRK | 711-725 |
| 83 | IHDRKEFAKFEEERA | 721-735 |
| 84 | EEERARAKWDTANNP | 731-745 |
| 85 | TANNPLYKEATSTFT | 741-755 |
| 86 | KEATSTFTNITYRGT | 748-762 |
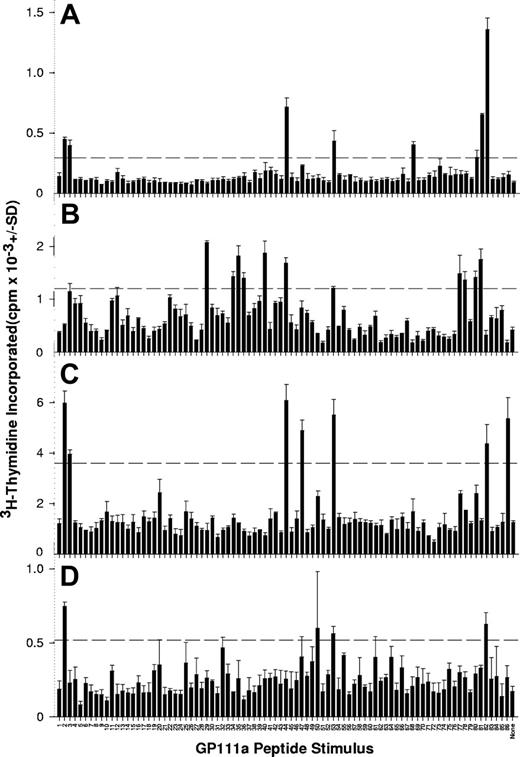
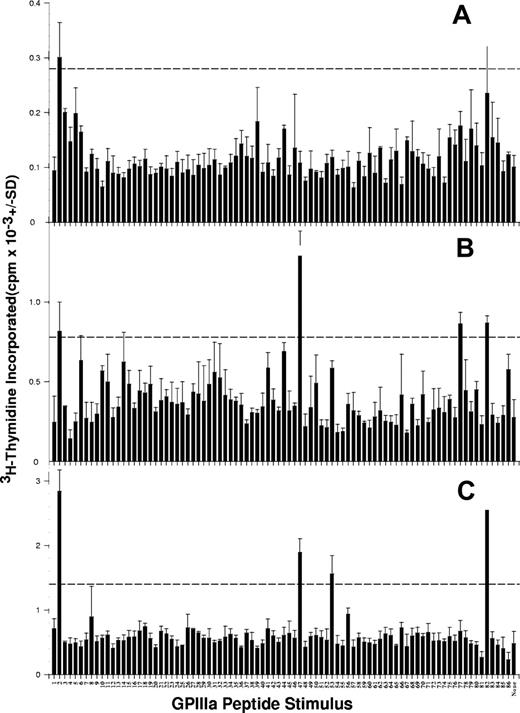
Representative results from 4 patients with AITP, demonstrating GPIIIa peptides that elicit PBMC proliferation, are illustrated in Figure 1, and the stimulatory peptides for each of the 31 patients are listed in Table 3. It can be seen that PBMCs from all but 3 patients responded to at least one member of the peptide panel, and that, typically, multiple sequences induced proliferation.
PBMCs from patients with AITP proliferate in response to peptides from the sequence of GPIIIa. PBMCs were isolated from representative patients AITP1 (A), AITP8 (B), AITP10 (C), and AITP20 (D) tested for the ability to proliferate against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
PBMCs from patients with AITP proliferate in response to peptides from the sequence of GPIIIa. PBMCs were isolated from representative patients AITP1 (A), AITP8 (B), AITP10 (C), and AITP20 (D) tested for the ability to proliferate against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
Summary of GPIIIa peptides eliciting PBMC proliferation from AITP patients in vitro
| Patient with AITP . | Platelet count at testing, × 109/L . | HLA-DR type DRB1* . | HLA-DQ type DQB1* . | Anti-GPIIb/IIIa status . | Stimulatory peptides, SI > 3 . | |
|---|---|---|---|---|---|---|
| Serum . | Eluate . | |||||
| AITP1 | 10 | 03/11 | 02/03 | Pos | wPos | 2*, 3, 44*, 53*, 68, 81, 82* |
| AITP2 | 102 | 07/11 | 02/03 | Pos | wPos | 42, 49, 50, 58, 60, 67, 70*, 71, 72, 73, 74, 77*, 78, 80, 81 |
| AITP3 | 3 | 01/01 | 05/05 | Pos | Pos | 2*, 44*, 50, 80, 82* |
| AITP4 | 14 | 01/04 | 03/05 | Neg | NT | 72, 82* |
| AITP5 | 163 | 03/04 | 02/03 | Neg | Pos | 82* |
| AITP6 | 33 | 03/07 | 02/03 | Neg | NT | None |
| AITP7 | 3 | 03/03 | 02/02 | Pos | Pos | 8, 11, 14, 15, 35, 40, 47*, 56, 70*, 82* |
| AITP8 | 16 | 03/07 | 02/02 | Neg | NT | 29, 34, 35, 36, 40, 44*, 5*, 77*, 78, 80, 81 |
| AITP9 | 338 | 15/15 | 06/06 | Neg | Pos | 2*, 6, 7, 14, 15, 30, 46, 47*, 53*, 82* |
| AITP10 | 394 | 01/03 | 02/05 | Pos | wPos | 2*, 3, 44*, 47*, 53*, 82* |
| AITP11 | 177 | 04/13 | 03/06 | Pos | Pos | 2*, 32, 47*, 53*, 77*, 82*, 86 |
| AITP12 | 170 | 03/04 | 02/03 | Pos | Pos | 9, 17, 31, 53*, 81, 82* |
| AITP13 | 127 | NT | NT | Pos | Neg | 69 |
| AITP14 | 49 | NT | NT | Neg | Pos | 54, 83 |
| AITP15 | 60 | 11/13 | 03/06 | Pos | Neg | 70* |
| AITP16 | 104 | 11/15 | 06/06 | Pos | Pos | 3, 47*, 68, 74, 77, 82* |
| AITP17 | 163 | 01/03 | 02/05 | Neg | wPos | None |
| AITP18 | 62 | 13/13 | 03/06 | Pos | NT | 2*, 44*, 53*, 82* |
| AITP19 | 152 | 15/15 | 06/06 | Pos | Pos | 2*, 47*, 48, 52, 82* |
| AITP20 | 61 | 04/15 | 03/06 | Pos | Pos | 2*, 47*, 50, 53*, 70*, 82* |
| AITP21 | 7 | 0103/15 | 05/06 | Pos | Pos | 1, 2*, 29, 34, 44*, 47*, 49, 81, 82* |
| AITP22 | 76 | 15/15 | 06/06 | Neg | Pos | 2*, 47*, 53*, 77*, 82* |
| AITP23 | 5 | 07/15 | 02/06 | Pos | Pos | 5, 30, 31, 36, 47*, 54, 60, 61, 86 |
| AITP24 | 163 | 01/07 | 03/03 | Pos | wPos | 2*, 3, 44*, 47*, 53*, 56, 70*, 82* |
| AITP25 | 76 | NT | NT | Pos | Neg | 82* |
| AITP26 | NT | NT | NT | Neg | Pos | 2*, 47*, 53*, 62, 63 |
| AITP27 | 95 | NT | NT | Pos | Neg | None |
| AITP28 | 260 | NT | NT | Pos | Neg | 4, 7, 9, 11, 21, 23, 24, 43, 57, 63, 69, 70* |
| AITP29 | 217 | NT | NT | Pos | Pos | 5, 9, 17, 32, 33, 36, 38, 40, 52, 53*, 57, 70* |
| AITP30 | 121 | NT | NT | Neg | Neg | 2*, 20, 41, 44*, 47*, 53*, 76 |
| AITP31 | 327 | NT | NT | Pos | Neg | 2*, 37, 47*, 65, 82* |
| Patient with AITP . | Platelet count at testing, × 109/L . | HLA-DR type DRB1* . | HLA-DQ type DQB1* . | Anti-GPIIb/IIIa status . | Stimulatory peptides, SI > 3 . | |
|---|---|---|---|---|---|---|
| Serum . | Eluate . | |||||
| AITP1 | 10 | 03/11 | 02/03 | Pos | wPos | 2*, 3, 44*, 53*, 68, 81, 82* |
| AITP2 | 102 | 07/11 | 02/03 | Pos | wPos | 42, 49, 50, 58, 60, 67, 70*, 71, 72, 73, 74, 77*, 78, 80, 81 |
| AITP3 | 3 | 01/01 | 05/05 | Pos | Pos | 2*, 44*, 50, 80, 82* |
| AITP4 | 14 | 01/04 | 03/05 | Neg | NT | 72, 82* |
| AITP5 | 163 | 03/04 | 02/03 | Neg | Pos | 82* |
| AITP6 | 33 | 03/07 | 02/03 | Neg | NT | None |
| AITP7 | 3 | 03/03 | 02/02 | Pos | Pos | 8, 11, 14, 15, 35, 40, 47*, 56, 70*, 82* |
| AITP8 | 16 | 03/07 | 02/02 | Neg | NT | 29, 34, 35, 36, 40, 44*, 5*, 77*, 78, 80, 81 |
| AITP9 | 338 | 15/15 | 06/06 | Neg | Pos | 2*, 6, 7, 14, 15, 30, 46, 47*, 53*, 82* |
| AITP10 | 394 | 01/03 | 02/05 | Pos | wPos | 2*, 3, 44*, 47*, 53*, 82* |
| AITP11 | 177 | 04/13 | 03/06 | Pos | Pos | 2*, 32, 47*, 53*, 77*, 82*, 86 |
| AITP12 | 170 | 03/04 | 02/03 | Pos | Pos | 9, 17, 31, 53*, 81, 82* |
| AITP13 | 127 | NT | NT | Pos | Neg | 69 |
| AITP14 | 49 | NT | NT | Neg | Pos | 54, 83 |
| AITP15 | 60 | 11/13 | 03/06 | Pos | Neg | 70* |
| AITP16 | 104 | 11/15 | 06/06 | Pos | Pos | 3, 47*, 68, 74, 77, 82* |
| AITP17 | 163 | 01/03 | 02/05 | Neg | wPos | None |
| AITP18 | 62 | 13/13 | 03/06 | Pos | NT | 2*, 44*, 53*, 82* |
| AITP19 | 152 | 15/15 | 06/06 | Pos | Pos | 2*, 47*, 48, 52, 82* |
| AITP20 | 61 | 04/15 | 03/06 | Pos | Pos | 2*, 47*, 50, 53*, 70*, 82* |
| AITP21 | 7 | 0103/15 | 05/06 | Pos | Pos | 1, 2*, 29, 34, 44*, 47*, 49, 81, 82* |
| AITP22 | 76 | 15/15 | 06/06 | Neg | Pos | 2*, 47*, 53*, 77*, 82* |
| AITP23 | 5 | 07/15 | 02/06 | Pos | Pos | 5, 30, 31, 36, 47*, 54, 60, 61, 86 |
| AITP24 | 163 | 01/07 | 03/03 | Pos | wPos | 2*, 3, 44*, 47*, 53*, 56, 70*, 82* |
| AITP25 | 76 | NT | NT | Pos | Neg | 82* |
| AITP26 | NT | NT | NT | Neg | Pos | 2*, 47*, 53*, 62, 63 |
| AITP27 | 95 | NT | NT | Pos | Neg | None |
| AITP28 | 260 | NT | NT | Pos | Neg | 4, 7, 9, 11, 21, 23, 24, 43, 57, 63, 69, 70* |
| AITP29 | 217 | NT | NT | Pos | Pos | 5, 9, 17, 32, 33, 36, 38, 40, 52, 53*, 57, 70* |
| AITP30 | 121 | NT | NT | Neg | Neg | 2*, 20, 41, 44*, 47*, 53*, 76 |
| AITP31 | 327 | NT | NT | Pos | Neg | 2*, 37, 47*, 65, 82* |
NT indicates not tested; Pos, positive reaction; wPos, weak positive reaction; Neg, negative reaction; SI, stimulation index.
Immunodominant peptides.
The presence of antiplatelet antibodies reactive with GPIIb/IIIa was confirmed in 27 of the 31 patients with AITP (Table 3). The persons generating anti-GPIIb/IIIa included 21 patients with serum antibodies, 14 of whom also had platelet-bound antibodies demonstrated after elution, plus a further 6 with no detectable serum antibodies but positive eluates. All 8 patients who had undergone splenectomy had persisting antibodies. Comparison with the results of PBMC stimulation reveals that the vast majority of the patients with AITP (25 of 31) had both anti-GPIIb/IIIa antibody and proliferative responses against GPIIIa peptides. This association between detectable anti-GPIIb/IIIa and peptide responsiveness is not absolute because, for example, 3 of the 4 antibody-negative patients did show PBMC proliferation to peptides. However, in these cases it was possible to screen only sera for anti-GPIIb/IIIa, and the testing of platelet eluates was often necessary to detect the antibody. Table 3 also illustrates that there is no simple relationship between the number, or the identities, of the stimulatory peptides and the platelet count of the patients with AITP at the time of sampling.
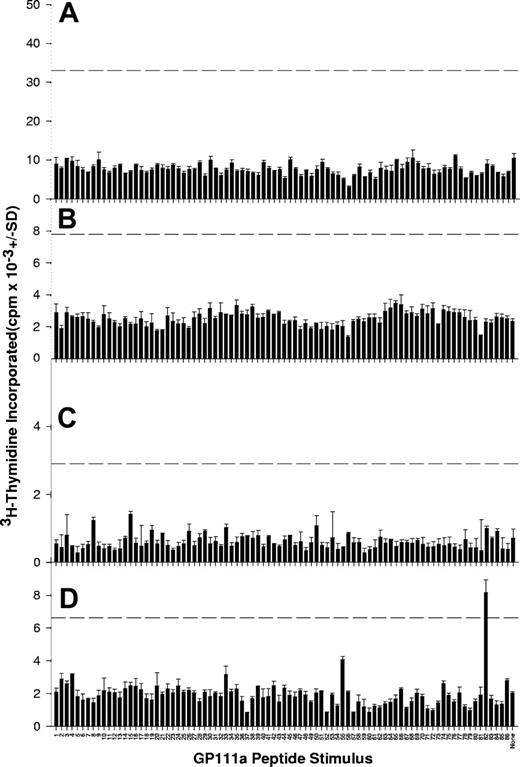
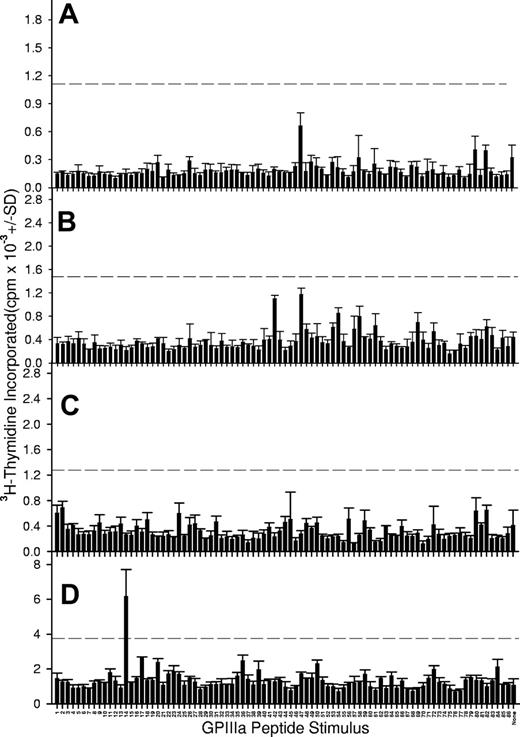
In contrast to the results obtained in patients with AITP, responses were rarely seen when the peptide panel was used to stimulate PBMCs from healthy control donors. Examples of results from the control group are depicted in Figure 2, with the data summarized in Table 4. No anti-GPIIb/IIIa antibodies were detected in serum or platelet eluate samples from this group. PBMCs from only 9 of the control donors demonstrated proliferation to any of the peptide panel, and, in each of these cases, responsiveness was limited to 1 or 2 sequences. It should be noted that PBMCs from all patients and control donors proliferated normally when stimulated with the control recall antigen mycobacterial PPD (Figure 3) or the mitogen Con A (results not shown), indicating that any lack of response to the GPIIIa peptide panel is specific and not attributable to a general loss of immune function or lymphocyte viability. The background levels of proliferation in the absence of antigen or mitogen are generally higher in the control donors than in the patients with AITP, reflecting the effects of disease state and immunosuppressive treatment.28,30 The difference in the total number of peptide responses in the patient and control groups was highly significant (a total of 178 responses to peptides in 31 patients versus 12 in 25 healthy donors, chi-square test = 115.967; P < .001), consistent with the view that recall Th responses specific for platelet glycoprotein are associated with AITP.17,18 To confirm that responsiveness to GPIIIa epitopes is not a feature of immune-mediated disease in general, or of low platelet counts, PBMCs from a group of 5 patients with aplastic anemia were also stimulated with the peptide panel (results summarized in Table 5, with representative examples illustrated in Figure 4). It can be seen that, as in the healthy donors, responses to GPIIIa peptides in this disease control group are very infrequent.
PBMCs from healthy control donors rarely proliferate when stimulated with peptides from the GPIIIa sequence. Shown here are proliferative responses of PBMCs from representative healthy control donors C5 (A), C6 (B), C8 (C), and C17 (D) against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
PBMCs from healthy control donors rarely proliferate when stimulated with peptides from the GPIIIa sequence. Shown here are proliferative responses of PBMCs from representative healthy control donors C5 (A), C6 (B), C8 (C), and C17 (D) against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
Summary of GPIIIa peptides eliciting PBMC proliferation from healthy controls in vitro
| Control donor . | HLA-DR type DRB1* . | HLA-DQ type DQB1* . | Anti-GPIIb/IIIa status . | Stimulatory peptides, SI > 3 . | |
|---|---|---|---|---|---|
| Serum . | Eluate . | ||||
| C1 | 04/07 | 02/03 | NT | NT | None |
| C2 | 01/11 | 03/05 | Neg | Neg | None |
| C3 | 04/15 | 03/06 | Neg | Neg | None |
| C4 | 04/1325 | 02/02 | Neg | Neg | None |
| C5 | 13/15 | 06/06 | Neg | Neg | None |
| C6 | 07/11 | 02/02 | Neg | Neg | None |
| C7 | 03/15 | 02/06 | Neg | Neg | 75, 82* |
| C8 | 01/15 | 05/06 | NT | NT | 85 |
| C9 | 07/15 | 02/06 | Neg | Neg | None |
| C10 | 15/15 | 06/06 | Neg | Neg | None |
| C11 | 04/15 | 03/06 | Neg | Neg | 72 |
| C12 | 08/15 | 04/06 | Neg | Neg | 55, 82* |
| C13 | 03/03 | 02/02 | NT | NT | 12 |
| C14 | 04/07 | 03/03 | NT | NT | 82* |
| C15 | 13/15 | 03/06 | Neg | Neg | 11 |
| C16 | 07/15 | 02/06 | Neg | Neg | None |
| C17 | 04/13 | 03/06 | Neg | Neg | None |
| C18 | 01/04 | 03/05 | Neg | Neg | None |
| C19 | 03/11 | 02/03 | Neg | Neg | None |
| C20 | 01/14 | 05/05 | Neg | Neg | None |
| C21 | 03/03 | 0201/0202 | Neg | Neg | None |
| C22 | 01/03 | 02/05 | Neg | NT | 60 |
| C23 | 03/15 | 02/06 | Neg | Neg | None |
| C24 | 04/07 | 02/03 | Neg | Neg | 45, 73 |
| C25 | 15/15 | 06/06 | Neg | Neg | None |
| Control donor . | HLA-DR type DRB1* . | HLA-DQ type DQB1* . | Anti-GPIIb/IIIa status . | Stimulatory peptides, SI > 3 . | |
|---|---|---|---|---|---|
| Serum . | Eluate . | ||||
| C1 | 04/07 | 02/03 | NT | NT | None |
| C2 | 01/11 | 03/05 | Neg | Neg | None |
| C3 | 04/15 | 03/06 | Neg | Neg | None |
| C4 | 04/1325 | 02/02 | Neg | Neg | None |
| C5 | 13/15 | 06/06 | Neg | Neg | None |
| C6 | 07/11 | 02/02 | Neg | Neg | None |
| C7 | 03/15 | 02/06 | Neg | Neg | 75, 82* |
| C8 | 01/15 | 05/06 | NT | NT | 85 |
| C9 | 07/15 | 02/06 | Neg | Neg | None |
| C10 | 15/15 | 06/06 | Neg | Neg | None |
| C11 | 04/15 | 03/06 | Neg | Neg | 72 |
| C12 | 08/15 | 04/06 | Neg | Neg | 55, 82* |
| C13 | 03/03 | 02/02 | NT | NT | 12 |
| C14 | 04/07 | 03/03 | NT | NT | 82* |
| C15 | 13/15 | 03/06 | Neg | Neg | 11 |
| C16 | 07/15 | 02/06 | Neg | Neg | None |
| C17 | 04/13 | 03/06 | Neg | Neg | None |
| C18 | 01/04 | 03/05 | Neg | Neg | None |
| C19 | 03/11 | 02/03 | Neg | Neg | None |
| C20 | 01/14 | 05/05 | Neg | Neg | None |
| C21 | 03/03 | 0201/0202 | Neg | Neg | None |
| C22 | 01/03 | 02/05 | Neg | NT | 60 |
| C23 | 03/15 | 02/06 | Neg | Neg | None |
| C24 | 04/07 | 02/03 | Neg | Neg | 45, 73 |
| C25 | 15/15 | 06/06 | Neg | Neg | None |
NT indicates not tested; Neg, negative reaction; SI, stimulation index.
Immunodominant peptides.
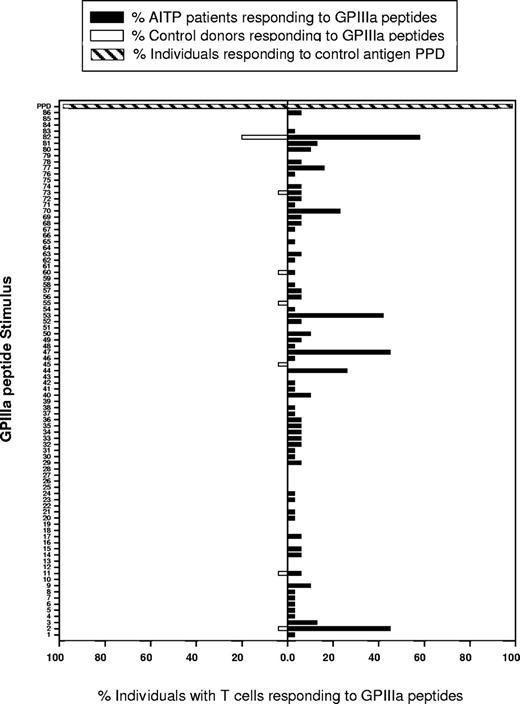
Particular dominant peptides from GPIIIa stimulate T cells from many patients with AITP to proliferate. Shown here are the proportions of patients with AITP (▪) and healthy control donors (□) whose PBMCS proliferated in response to each of the 86 peptides from the panel spanning GPIIIa. PBMCS from all persons in both groups responded to stimulation with the control recall antigen mycobacterial PPD (▨).
Particular dominant peptides from GPIIIa stimulate T cells from many patients with AITP to proliferate. Shown here are the proportions of patients with AITP (▪) and healthy control donors (□) whose PBMCS proliferated in response to each of the 86 peptides from the panel spanning GPIIIa. PBMCS from all persons in both groups responded to stimulation with the control recall antigen mycobacterial PPD (▨).
Summary of GPIIIa peptides eliciting PBMC proliferation from disease control donors in vitro
| Patient control . | Sex . | Age at testing, y . | Clinical disease . | Platelet count at testing, × 109/L . | Stimulatory peptides, SI > 3 . |
|---|---|---|---|---|---|
| C26 | M | 54 | Aplastic anemia | 4 | None |
| C27 | M | 56 | Aplastic anemia | 49 | None |
| C28 | M | 64 | Aplastic anemia | 3 | None |
| C29 | M | 24 | Aplastic anemia | 4 | 14 |
| C30 | F | 69 | Aplastic anemia | 23 | 82* |
| Patient control . | Sex . | Age at testing, y . | Clinical disease . | Platelet count at testing, × 109/L . | Stimulatory peptides, SI > 3 . |
|---|---|---|---|---|---|
| C26 | M | 54 | Aplastic anemia | 4 | None |
| C27 | M | 56 | Aplastic anemia | 49 | None |
| C28 | M | 64 | Aplastic anemia | 3 | None |
| C29 | M | 24 | Aplastic anemia | 4 | 14 |
| C30 | F | 69 | Aplastic anemia | 23 | 82* |
Immunodominant peptides.
PBMCs from disease control donors rarely proliferate when stimulated with peptides from the GPIIIa sequence. Shown here are proliferative responses of PBMCS from representative patients with aplastic anemia C26 (A), C27 (B), C28 (C), and C29 (D) against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
PBMCs from disease control donors rarely proliferate when stimulated with peptides from the GPIIIa sequence. Shown here are proliferative responses of PBMCS from representative patients with aplastic anemia C26 (A), C27 (B), C28 (C), and C29 (D) against the panel of 86 peptides spanning the GPIIIa molecule. The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
Distribution of stimulatory peptides on the platelet GPIIIa
Despite variation between patients with AITP in the profile of GPIIIa peptides that elicited PBMC proliferation (Table 3), particular peptides were identified as dominant, because they stimulated responses in a high proportion of cases. These dominant sequences are shown in Figure 3, which summarizes the number of patients in which each peptide induced proliferation. The 4 most dominant peptides are 2 (aa6-20), 47 (aa361-375), 53 (aa421-435), and 82 (aa711-725), with 24 patients (77%) showing PBMC responses to at least 1 of these sequences, and 13 (42%) to 3 or more. A further 3 peptides, 44 (aa331-345), 70 (aa591-605), and 77 (aa661-675), exhibited a lower level of dominance, with each stimulating proliferation by PBMCs from at least 5 (15%) patients.
Analysis of the GPIIIa peptides eliciting the relatively rare responses by control donor PBMCs (Tables 4–5; Figure 3) reveals that they include only 1 of the 7 sequences identified as dominant in patients with AITP. This peptide, 82 (aa711-725), was the only member of the entire panel to stimulate PBMCs from more than one control donor. Thus, compared with those from patients with AITP, the responses of healthy control PBMCs to GPIIIa sequences are not only infrequent, but they generally target sporadic peptides that differ from those commonly recognized in AITP.
Variation over time in the pattern of GPIIIa peptides that stimulate responses
Longitudinal studies of patients with chronic autoimmune diseases other than AITP28,40,41 demonstrate changes over time in the identities of autoantigen-derived peptides recognized by autoaggressive Th cells. To establish whether the same is true for AITP, serial PBMC samples taken over periods of weeks or months from patients (n = 10) were screened for responsiveness to the GPIIIa peptide panel. Figure 5 depicts a typical set of results, where the GPIIIa peptides were tested against PBMCs taken from patient AITP22 on 3 different occasions over 56 weeks. The dominant peptide 2 (aa6-20) elicited proliferation from all samples, whereas responsiveness to dominant sequences 47 (aa361-375), 53 (aa421-435), and 82 (aa711-725) was initially absent but appeared at later time points, and proliferation to the lower-ranking dominant peptide 77 (aa661-675) was seen only in the second sample. It should be noted that these differences are consistent across all replicate cultures set up from each sample, and therefore do not represent chance interwell variation. These results from patient AITP22, and the other examples, illustrate a complex, dynamic pattern of responsiveness, with some peptides persistently stimulating PBMC proliferation, and others eliciting responses that fluctuate over time. Such evolution of the fine specificity of the immune response does not directly correlate with the clinical course of disease, because there is no relationship between the changes over time in the identities of the stimulatory peptides, and the platelet count of the patients with AITP (Figure 5).
The pattern of GPIIIa peptides that stimulate PBMCs from patients with AITP to proliferate can evolve over time. Proliferative responses of PBMCs from a representative patient (AITP22) against the panel of 86 peptides spanning the GPIIIa molecule were compared on 3 different occasions, at presentation (A; platelet count 76 × 109/L), then after 44 weeks (B; platelet count 54 × 109/L), and 56 weeks (C; platelet count 84 × 109/L). The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
The pattern of GPIIIa peptides that stimulate PBMCs from patients with AITP to proliferate can evolve over time. Proliferative responses of PBMCs from a representative patient (AITP22) against the panel of 86 peptides spanning the GPIIIa molecule were compared on 3 different occasions, at presentation (A; platelet count 76 × 109/L), then after 44 weeks (B; platelet count 54 × 109/L), and 56 weeks (C; platelet count 84 × 109/L). The dashed horizontal line denotes the level of proliferation taken as representing a significant positive response (SI > 3).
Characterization of the phenotype of PBMCs that proliferate in response to GPIIIa peptides
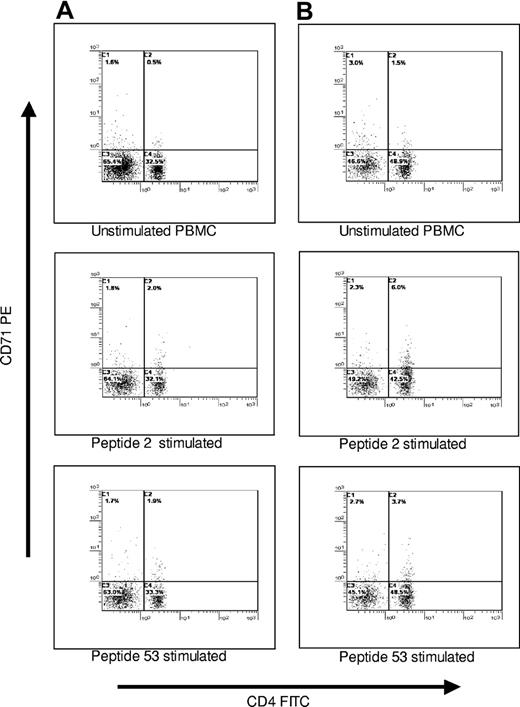
To confirm that the PBMCs proliferating against GPIIIa peptides were of the CD3+CD4+ Th phenotype, selected cultures were analyzed by multicolor flow cytometry. Responding cells were labeled with antibody to the activation marker CD71, and the Th subset was identified by counterstaining with anti-CD3 and anti-CD4. Representative results (n = 6) from 2 patients with AITP are shown in Figure 6. It can be seen that, as expected, the background level of CD71 expression in control, resting cultures was very low, and there was a small increase (1.5%-3.8%) in numbers of activated CD71+ cells after stimulation with dominant peptides 2 (aa6-20) or 53 (aa421-435). The size of this expansion is typical of the responses to antigen made by specific lymphocytes within a polyclonal population, and the vast majority (88%-100%) of the cells that up-regulated CD71 as a result of the peptide stimulation were CD3+CD4+.
PBMCs from patients with AITP that respond to GPIIIa peptides are predominantly of the helper phenotype. PBMCs from patients AITP10 (A) and AITP18 (B) were either left unstimulated in culture or incubated with GPIIIa peptides 2 or 53 that induce proliferative responses in these patients, before being stained for CD4 expression and the activation marker CD71. Results are shown gated on the CD3+ population.
PBMCs from patients with AITP that respond to GPIIIa peptides are predominantly of the helper phenotype. PBMCs from patients AITP10 (A) and AITP18 (B) were either left unstimulated in culture or incubated with GPIIIa peptides 2 or 53 that induce proliferative responses in these patients, before being stained for CD4 expression and the activation marker CD71. Results are shown gated on the CD3+ population.
Role of HLA class II in responses of PBMCs from patients with AITP and control donors
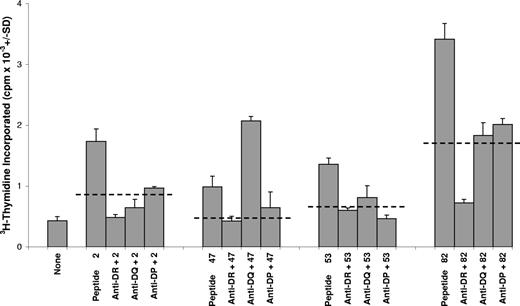
To demonstrate functionally that the lymphocytes responding to GPIIIa peptides came from the Th subset, which is restricted by MHC class II molecules, blocking antibodies specific for anti–HLA-DP, -DQ, and -DR were tested for the ability to inhibit the responses. Dominant peptides 2(aa6-20), 47 (aa361-375), 53 (aa421-435), and 82 (aa711-725) were selected for these experiments and used to stimulate PBMCs from 4 patients with AITP, in the presence or absence of anti-DP, -DQ, or -DR. Representative results from one patient are illustrated in Figure 7. Each example of peptide-induced proliferation was blocked by at least one of the antibodies, of which anti-DR was consistently the most potent, inhibiting 15 of the 16 responses tested.
The proliferation of T cells from patients with AITP against GPIIIa peptides is dependent on HLA-class II molecules. Cultures of PBMCs from a representative patient (AITP9) were stimulated with dominant GPIIIa peptides 2, 47, 53, or 82, and class II restricted responses were blocked by addition of antibody specific for HLA-DR,-DQ, or -DP. For each peptide stimulus, the dashed horizontal lines denote the level of inhibition taken as significant (> 50%). Similar results were obtained with PBMCs from another 3 patients.
The proliferation of T cells from patients with AITP against GPIIIa peptides is dependent on HLA-class II molecules. Cultures of PBMCs from a representative patient (AITP9) were stimulated with dominant GPIIIa peptides 2, 47, 53, or 82, and class II restricted responses were blocked by addition of antibody specific for HLA-DR,-DQ, or -DP. For each peptide stimulus, the dashed horizontal lines denote the level of inhibition taken as significant (> 50%). Similar results were obtained with PBMCs from another 3 patients.
HLA type is one of the factors that can influence predisposition to particular immune-mediated diseases. The panels of patients with AITP and healthy controls were typed for HLA-DR and HLA-DQ polymorphic β-chain genes (Tables 3–4), and the results were compared with published data from the general UK population.42 The most common alleles at each locus among patients were, respectively, DRB1*03 and DRB1*15, and DQB1*03 and DQB1*06, but there were no significant positive or negative associations with the disease or the ability of particular sequences to stimulate proliferation.
Table 6 demonstrates that the dominant peptides are located throughout different domains of GPIIIa, including the transmembrane/cytoplasmic area,43 reflecting the fact that T cells, unlike pathogenic antibody, are not limited to the recognition of epitopes accessible on the intact cell. The selection of dominant helper epitopes in autoimmune disease may be also determined by different criteria from those that shape the fine specificity of conventional responses by CD4+ T cells to foreign antigens.44 In particular, the major self-epitopes may be dominant because of a lack of tolerance in the corresponding Th cell repertoire, rather than because they are contained in the most efficiently presented peptides that exhibit high affinity for their restricting elements.30,45,46 To test whether this is true for AITP, a web-based algorithm (http://www.imtech.res.in/raghava/propred/)38,39 was used to predict the motifs within the sequence of GPIIIa that have high affinity for a comprehensive panel of HLA-DR molecules, including all those expressed by the patients with AITP. The results in Table 6 reveal that 3 of the 7 dominant GPIIIa peptides were predicted not to have high affinity for any of the class II molecules evaluated. Of the 4 dominant peptides computed to be displayed at high levels by particular HLA-DR molecules, only peptide 82 (aa711-725) showed a correlation (chi-square test = 10; P < .05) between the ability to stimulate Th responses and the expression of the relevant class II type by AITP patients. Thus, with the exception of peptide 82 (aa711-725), the vast majority of interactions between the dominant GPIIIa peptides and their restricting MHC molecules in patients with AITP are predicted to be of low affinity.
Summary of predicted motifs in dominant GPIIIa peptides for binding to HLA-DR molecules and responsiveness of PBMC from AITP patients
| Dominant peptide no. . | Position on GPIIIa* . | HLA-DR molecules bound with high affinity† . | Patients with AITP with PBMC response to peptide . | |
|---|---|---|---|---|
| High-affinity DR expressed . | No high-affinity DR expressed . | |||
| 2 (aa6-20) | PSI domain | None | None | AITP1, AITP3, AITP9, AITP10, AITP11, AITP18, AITP19, AITP20, AITP21,AITP22, AITP24 |
| 44 (aa331-345) | Spanning βA and hybrid domain | DR04, DR07 | AITP8, AITP24 | AITP1, AITP3, AITP10, AITP18, AITP21, |
| 47 (aa361-375) | Hybrid domain | None | None | AITP7, AITP9, AITP10, AITP11, AITP16, AITP19, AITP20, AITP21, AITP22, AITP23, AITP24, AITP26 |
| 53 (aa421-435) | Spanning hybrid and PSI domain | DR04, DR13 | AITP11, AITP-12, AITP18, AITP20 | AITP1, AITP8, AITP9, AITP10, AITP22, AITP24 |
| 70 (aa591-605) | EGF-like domain | None | None | AITP2, AITP7, AITP15, AITP20, AITP24 |
| 77 (aa661-675) | EGF-like domain | DR04, DR15 | AITP11, AITP16, AITP22 | AITP2, AITP8 |
| 82 (aa711-725) | βTD domain (transmembrane/cytoplasmic) | DR01, DR08, DR11, DR13, DR15 | ‡AITP1, AITP3, AITP4, AITP5, AITP9, AITP10, AITP11, AITP16, AITP18, AITP19, AITP20, AITP21, AITP22, AITP24 | ‡AITP7, AITP12 |
| Dominant peptide no. . | Position on GPIIIa* . | HLA-DR molecules bound with high affinity† . | Patients with AITP with PBMC response to peptide . | |
|---|---|---|---|---|
| High-affinity DR expressed . | No high-affinity DR expressed . | |||
| 2 (aa6-20) | PSI domain | None | None | AITP1, AITP3, AITP9, AITP10, AITP11, AITP18, AITP19, AITP20, AITP21,AITP22, AITP24 |
| 44 (aa331-345) | Spanning βA and hybrid domain | DR04, DR07 | AITP8, AITP24 | AITP1, AITP3, AITP10, AITP18, AITP21, |
| 47 (aa361-375) | Hybrid domain | None | None | AITP7, AITP9, AITP10, AITP11, AITP16, AITP19, AITP20, AITP21, AITP22, AITP23, AITP24, AITP26 |
| 53 (aa421-435) | Spanning hybrid and PSI domain | DR04, DR13 | AITP11, AITP-12, AITP18, AITP20 | AITP1, AITP8, AITP9, AITP10, AITP22, AITP24 |
| 70 (aa591-605) | EGF-like domain | None | None | AITP2, AITP7, AITP15, AITP20, AITP24 |
| 77 (aa661-675) | EGF-like domain | DR04, DR15 | AITP11, AITP16, AITP22 | AITP2, AITP8 |
| 82 (aa711-725) | βTD domain (transmembrane/cytoplasmic) | DR01, DR08, DR11, DR13, DR15 | ‡AITP1, AITP3, AITP4, AITP5, AITP9, AITP10, AITP11, AITP16, AITP18, AITP19, AITP20, AITP21, AITP22, AITP24 | ‡AITP7, AITP12 |
From structural analysis of β3 integrin.43
Predicted using the Propred algorithm (http://www.imtech.res.in/raghava/propred/).38,39
Significant association between response to peptide 82 and expression of HLA-DR molecules to which peptide predicted to bind with high affinity (chi-square test = 10; P < .05).
Discussion
This report identifies the peptide epitopes from the platelet autoantigen GPIIIa that are recognized by Th cells from patients with AITP and describes 7 dominant sequences. Autoreactive Th cells specific for platelet glycoprotein are known to be activated in AITP,11–19 but this is the first time that peptides driving the response have been mapped. The results not only provide further insight into the mechanisms of disease but open the way for novel forms of peptide immunotherapy23–27 for AITP that selectively target the pathogenic Th cells.
PBMCs from almost all patients with AITP proliferated against members of a peptide panel spanning the sequence of GPIIIa, and such responses are strongly associated with the disease because they were rarely exhibited by samples from healthy or disease control donors. The culture conditions were biased in favor of supporting accelerated recall responses by Th cells that have previously been activated in vivo as part of the disease process and not by naive Th cells.28–30,36 The vast majority of the patients with AITP had both anti-GPIIb/IIIa antibodies and PBMCs that mount recall proliferation to GPIIIa peptides, strengthening the view1,13–21 that the pathogenic B cell response is dependent on T-cell help specific for the same autoantigenic complex. The small number of patients with AITP with PBMCs responsive to GPIIIa peptides, but no detectable anti-GPIIb/IIIa antibodies, may reflect the limited serologic assays that could be performed in these cases. As with other autoantigens,28,30 the relatively rare and weak responses to GPIIIa peptides observed in control donors could well represent cross-reactivity with environmental antigens,48 particularly given the limited sequence homology between different peptides necessary for T-cell cross-reactivity.48 It was confirmed by flow cytometric analysis that the cells from patients with AITP that responded in vitro to immunodominant GPIIIa peptides were of the CD3+CD4+ Th phenotype, and the ability of anti-HLA antibodies consistently to block the proliferation verified that they were MHC class II–restricted cells. DR appears to be the principal restricting locus, but the effects of the blocking antibodies suggest that DP and DQ molecules may also compete for presentation of particular GPIIIa peptides. The peptide-specific Th cells identified here therefore resemble those previously reported to react with purified GPIII,17 or large recombinant fragments,18 which have been activated in vivo in patients with AITP but not control donors and which are predominantly DR-restricted.
The results reveal a number of features of the Th cells specific for GPIIIa in AITP, which are consistent with a pathogenic role, because they are characteristic of the autoaggressive responses seen in other examples of human and experimental autoimmune disease. First, multiple peptides from GPIIIa stimulated proliferation by Th cells from most patients with AITP, a diversity of fine specificity that mirrors the results of epitope mapping studies in human type 1 diabetes,49 rheumatoid arthritis,50 autoimmune glomerulonephritis,30 multiple sclerosis,40,41 and autoimmune hemolytic anemia (AIHA).28 Animal models of these diseases, including AIHA in the NZB mouse, reveal that such diversity may follow the phenomenon of epitope spreading.12,51 This occurs when the autoimmune helper response initially targets very few, or only one, self-determinant(s), but further Th clones with new specificities for the same, or associated, autoantigens are recruited over time as pathology develops.44 The second, related feature of GPIIIa recognition that resembles other autoaggressive responses28,40,41 is the variation, seen in individual patients with AITP over time, in the peptides that induce proliferation by peripheral blood Th cells in vitro. Such gain or loss of stimulation by peptides can reflect changes in the frequency of the corresponding Th cells in the circulation, attributable to the respective effects of epitope spreading and clonal exhaustion.28,40,41,51 Similar diversity, plasticity, evolution and cyclical changes in self-recognition have been reported for Th cells reactive with autoantigen in other diseases, including AIHA28 and multiple sclerosis,40,41 and, as here, do not necessarily correlate with the severity or clinical course of disease.
The third finding in AITP, consistent with other autoimmune diseases,11,23,28,30 is that, despite the variation between cases in the patterns of stimulatory GPIIIa peptides, particular sequences are dominant and stimulate responses in many patients. We identified 7 such peptides distributed throughout GPIIIa, numbers 2 (aa6-20), 44 (aa331-345), 47 (aa361-375), 53 (aa421-435), 70 (aa591-605), 77 (aa661-675), and 82 (aa711-725). The question arises as to why these peptides should contain dominant epitopes. When considering conventional immune responses to foreign antigens, the dominant Th epitopes can often be predicted because of their ability to bind well to the restricting MHC molecules.38,39 However, the same is not true of many autoimmune diseases, where lack of tolerance in the helper compartment, whether mediated by deletion, anergy, or regulation, is a prime factor in the selection of dominant helper epitopes, rather than high affinity for the restricting class II molecules.30,44–47 Indeed, there are well-characterized examples where inefficient presentation of self-peptides contributes crucially to the failure to tolerize the corresponding repertoire and allows the persistence of potentially autoaggressive Th cells that can be activated to drive disease.30,45–47 AITP fits with this pattern, because many of the dominant peptides fail to exhibit high-predicted affinity for any HLA-DR molecules from an extensive panel. Furthermore, with the exception of peptide 82 (aa711-725), for any of the dominant sequences that do carry an HLA-DR binding motif, there is no correlation in different patients between the expression of the respective class II molecule and the stimulation of responses. The likely low affinity of most of the dominant GPIIIa peptides for their restricting MHC molecules would lead to poor presentation and could account for the escape of the corresponding Th cells from mechanisms that purge the immune repertoire of potentially autoaggressive lymphocytes.36,44–47 These Th cells would then be available to be activated in disease by events such as stimulation with higher avidity cross-reactive microbial antigens, or increased production and display of the dominant GPIIIa peptides following changes in antigen presentation in vivo.44 Th cells that recognize peptide 82 (aa711-725) may survive not because of poor binding and display of the sequence by restricting MHC molecules but because of “destructive processing” by enzymes that cleave the sequence within APCs,45 although it should be noted that this peptide may be of less pathogenic relevance because it is the only dominant sequence to induce proliferation by Th cells from control donors.
A previous study of Japanese patients with AITP by Kuwana et al18 examined Th proliferative responses to 3 large recombinant fragments of GPIIIa, aa22-262, aa254-462, and aa708-762, spanning much, but not all, of the antigen sequence. Helper epitopes could be demonstrated in each fragment, consistent with the multiple stimulatory peptides mapped here, but there are differences between the results of the 2 approaches. Not all the dominant peptides identified in the current study correspond to stimulatory recombinant sequences, and none is contained within the fragment most frequently recognized by Th cells from the Japanese patients, which spans the amino terminal portion of the molecule from residues 22 to 262.18 There are several explanations for these apparent discrepancies. First, there are gaps in the extent of the recombinant fragments tested by Kuwana et al18 compared with the complete, overlapping peptide panel screened here. In particular, the recombinant fragments omitted the sequences of 3 of the dominant peptides, 2 (aa6-20), 70 (aa591-605), and 77 (aa661-675), and so would be unable to stimulate the corresponding Th cells. It is also relevant that our patient group was drawn from a different, Northern European white population. The importance of ethnic origin in influencing Th responses is well known and exemplified by the positive associations between the presence of anti-GPIIIa autoantibodies in Japanese patients and inheritance of HLA-DRB1*0405 or DRB1*0401,52 correlations that are not replicated in the current study population. The final explanation relates to the need for processing of large proteins or fragments into peptides in order for Th recognition to take place.22 Th cells from patients with AITP do not respond in vitro to purified GPIIIa but only to enzyme-treated or recombinant fragments,17,18 indicating that the relevant epitopes are to some degree cryptic in vitro and not necessarily available from the antigen in its native conformation. Hence, it is plausible that at least some of the dominant peptides identified here may, in turn, be processed inefficiently in vitro from the recombinant fragments, which would therefore stimulate fewer responses than expected in comparison with the peptide panel. The stimulatory peptides may nonetheless be relevant to disease, in the different processing environment in vivo.44
The need for specific, effective, and safe treatment for patients with chronic AITP may be met by the development of peptide immunotherapy to re-induce Th tolerance to the platelet glycoproteins. It has been shown from animal models of other autoimmune diseases11,12,24 that peptides containing dominant Th cell epitopes can prevent responses to the corresponding antigen when given in soluble form without adjuvant or if administered by a tolerogenic mucosal route. Importantly, induction to tolerance to only one dominant epitope, particularly if mediated by active immune regulation, can ablate responsiveness to the entire autoantigen from which it is derived and also to other, associated antigens by a process of bystander suppression.23 The strategy of “therapeutic vaccination” to reinstate tolerance with peptides is showing promise in a number of early human trials of immune-mediated disease,23,25–27 and the identification of a small number of dominant GPIIIa sequences that are recognized by autoreactive Th cells from most patients with AITP opens the door to this approach in AITP.
Authorship
Contribution: H.S. performed research, analyzed data, and wrote the paper; H.G.W. collected and analyzed data; S.J.U. designed research, collected and analyzed data, and wrote the paper; and R.N.B. designed research, collected and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
R.N.B. and S.J.U. contributed equally to this work.
Correspondence: Robert N. Barker, Department of Medicine and Therapeutics, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD United Kingdom; e-mail: r.n.barker@abdn.ac.uk
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Scottish National Blood Transfusion Service and the government of Swaziland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal