Abstract
Antineutrophil cytoplasmic antibodies (ANCAs) with specificity for proteinase 3 (PR3) are central to a form of ANCA-associated vasculitis. Membrane PR3 (mPR3) is expressed only on a subset of neutrophils. The aim of this study was to determine the mechanism of PR3 surface expression on human neutrophils. Neutrophils were isolated from patients and healthy controls, and hematopoietic stem cells from cord blood served as a model of neutrophil differentiation. Surface expression was analyzed by flow cytometry and confocal microscopy, and proteins were analyzed by Western blot experiments. Neutrophil subsets were separated by magnetic cell sorting. Transfection experiments were carried out in HEK293 and HL60 cell lines. Using neutrophils from healthy donors, patients with vasculitis, and neutrophilic differentiated stem cells we found that mPR3 display was restricted to cells expressing neutrophil glycoprotein NB1, a glycosylphosphatidylinositol (GPI)–linked surface receptor. mPR3 expression was decreased by enzymatic removal of GPI anchors from cell membranes and was absent in a patient with paroxysmal nocturnal hemoglobinuria. PR3 and NB1 coimmunoprecipitated from and colocalized on the neutrophil plasma membrane. Transfection with NB1 resulted in specific PR3 surface binding in different cell types. We conclude that PR3 membrane expression on neutrophils is mediated by the NB1 receptor.
Introduction
Antineutrophil cytoplasmic autoantibodies (ANCAs) are central in the ANCA-associated vasculitis syndromes. The neutrophil serine protease proteinase 3 (PR3) is a major autoantigen for ANCA.1 Binding of PR3-ANCA to cytokine-primed neutrophils results in respiratory burst activity, increased adhesion, degranulation, and dysregulated apoptosis of neutrophils in vitro.2 No suitable animal model for PR3-ANCA–associated vasculitis is available. However, Pfister et al3 recently demonstrated increased dermal inflammation in mice after passive PR3-ANCA transfer. PR3 has also been termed myeloblastin because of its early expression and importance in neutrophil development.4 PR3 is stored in primary and secondary granules and is also found in secretory vesicles.5 Although all neutrophils contain intracellular PR3, only a subset displays the protein on the plasma membrane allowing neutrophil-ANCA interaction.6 Cytokine-priming increases the amount of mPR3 without affecting the percentage of mPR3-positive cells. In the general population, this percentage varies from 0 to 100%. The percentage of mPR3-expressing cells is stable in a given person and strongly influenced by genetic variance.6–8 The mean percentage of mPR3-positive neutrophils is increased in patients with ANCA-associated vasculitis, and a higher percentage predicts an unfavorable clinical course.9–12 In contrast to the importance of mPR3, little is known about the mechanism of its membrane display. Hydrophobic insertion has been proposed in a system of reconstituted lipid bilayers.13 mPR3 associates with lipid raft proteins, such as integrins and the Fcγ-receptor.14 However, how PR3 is physically presented and how the unique feature of a fixed percentage of mPR3-positive neutrophils is mediated is not known.
The human neutrophil antigen NB1, also known as CD177, human neutrophil antigen 2A, or polycythemia rubra vera-1 (PRV1), is an approximately 50-kDa glycoprotein. The molecule was first discovered in patients with alloimmune neonatal neutropenia and was later implicated in the pathophysiology of PRV.15,16 NB1 belongs to the urokinase plasminogen activator receptor/CD59/Ly-6 snake toxin superfamily.17 These receptors are inserted into the plasma membrane via a glycosylphosphatidylinositol (GPI) linker. Similar to PR3, NB1 shows a bimodal expression pattern with expression on 0% to 100% of neutrophils in the population.18 We encountered a patient with PRV who kindled our interest in this protein. Our findings caused us to test the hypothesis that NB1 serves as the receptor for PR3 neutrophil membrane display.
Patients, materials, and methods
Patients and probands
Neutrophils were obtained from healthy volunteers. Patients with ANCA-associated vasculitis were recruited from our inpatient and outpatient departments. They were diagnosed according to the criteria of the Chapel Hill Consensus Conference and the American College of Rheumatology.19,20 Umbilical cord blood was obtained from healthy term deliveries at this hospital. The study was carried out according to the principles of the Declaration of Helsinki and written informed consent was obtained from all subjects or caretakers prior to the studies after approval by the Institutional Review Board of the Charité University Medicine Berlin (Zentrale Ethikkommission.)
Antibodies
PR3 was detected using CLB12.8. MAB (CLB, Amsterdam, Netherlands), 4A5 and 6A6 (Wieslab, Lund, Sweden), and MCPR3-2 (Dianova, Hamburg, Germany). The NB1 antibody was clone MEM166 (BD Pharmingen, San Diego, CA; BioLegend, San Diego, CA). Anti-CD11b, CD18, and CD66b were from Immunotech (Krefeld, Germany) and CD16b was from Cymbus Biotech (Hants, United Kingdom). Anti-MPO was from Acris Antibodies (Hiddenhausen, Germany), and antielastase and secondary FITC and PE-conjugated F(ab) fragments of goat anti–mouse IgG were from DAKO (Hamburg, Germany). Cy5-conjugated donkey anti–mouse IgG was from Jackson Immunoresearch (Newmarket, United Kingdom), HRP-labeled goat anti–mouse IgG was from Santa Cruz Biotechnology (Santa Cruz, CA).
Neutrophil isolation and separation of mPR3- and mNB1-positive and -negative cells
Neutrophils were isolated from heparinized whole blood by red blood cell sedimentation with dextran 1%, followed by Ficoll-Hypaque density gradient centrifugation and hypotonic erythrocyte lysis as described.7 Cell viability was detected in every cell preparation by trypan blue exclusion and exceeded 99%. The neutrophil percentage in the suspension was greater than 95% by Wright-Giemsa staining. For separation of subpopulations, isolated neutrophils were stained for PR3 (CLB12.8) or NB1 (MEM166), respectively, labeled with rat anti–mouse IgG microbeads (Miltenyi Biotech, Bergisch-Gladbach, Germany), and separated with magnetic cell sorting LD columns (Miltenyi Biotech) according to the manufacturer's instructions as described.21 Purity was assessed by flow cytometry and was 90% ± 5%.
Isolation and culture of hematopoietic CD34+ progenitors from umbilical cord blood
Procedures were essentially as described.11 Mononuclear cells from heparin-anticoagulated cord blood were obtained by centrifugation over a LSM1077 (PAA, Pasching, Austria) gradient (800g, 30 minutes). Cells were washed and stained with direct CD34+ progenitor isolation kit (Miltenyi Biotech) and sorted according to the manufacturer's instructions. Cells were cultivated in stem span serum-free medium (Stem Cell Technologies, Vancouver, Canada) supplemented with penicillin/streptomycin, 100 ng/mL SCF, 20 ng/mL TPO, and 50 ng/mL FLT3-L (PeproTech, London, United Kingdom) for expansion. Neutrophil differentiation was in RPMI with 10% FCS and 10 ng/mL G-CSF (PeproTech). Light microscopy was performed using a Zeiss Axioplan II microscope equipped with a Plan Apochromat 100×/1.4 oil-immersion objective lens, and image acquisition was performed with a Sensicam 120 using Axiovision 3.0. software (all from Carl Zeiss, Jena, Germany).
Flow cytometry
Flow cytometry was used as described previously to evaluate the membrane protein expression.21 If indicated, cells were stimulated with 2 ng/mL TNF-α (Genzyme, Rüsselsheim, Germany) or 10−9 10−8, and 10−6 M N-formyl-Met-Leu-Phe (fMLP; Sigma, Deisenhofen, Germany) for 20 minutes at 37°C. Samples were incubated on ice for antibody binding, washed, and counted using a FACScan (Becton Dickinson, Heidelberg, Germany). Ten thousand events per sample were collected and analyzed with Cell-quest Pro software (Becton Dickinson).
Cell lysis, SDS-PAGE, Western Blot, and immunoprecipitation
Cells were incubated on ice in lysis buffer (20 mM Tris HCl pH 8.8, 138 mM NaCl, 10% Glycerol, 2 mM EDTA, 1% Triton X-100, 1% NP40 supplemented with proteinase inhibitors [10 μg/mL Quercetin, 10 μg/mL Leupeptin, 0.1 mM Aprotinin, 5 mM iodoacetamid), 0.2 mM Na3VO4, 20 mM NaF, and 1 mM PMSF) for 10 minutes, insoluble material was pelleted, and samples were boiled without β-mercaptoethanol and run in 10% SDS–polyacrylamide gel electrophoresis (PAGE) gels. Protein was transferred to PVDF-WB membranes (Roche, Mannheim, Germany), and detected by enhanced chemiluminescence–WB reagent (Pierce, Rockford, IL) on hyperfilm (GE Healthcare, Chalfont St Giles, United Kingdom). For immunoprecipitation, the supernatant of 107 phospholipase C–treated neutrophils was precleared with protein-A–sepharose beads (GE Healthcare), rotated overnight with 5 μg antibody at 4°C, followed by the precipitation with sepharose for 2 hours. Samples were spun down, washed extensively, boiled, and subjected to SDS-PAGE.
Cleavage of glycosylphosphatidylinositol (GPI) linkers by phospholipase C
Phospholipase C from Bacillus cereus was obtained from Sigma. Cells were incubated with 0.1 U/mL at 37°C for 2 hours.
Confocal microscopy
Live cells were analyzed immediately after staining with FITC- or Cy5-labeled antibodies using a Zeiss LSM 510 Meta mounted on an Axiovert 200M using a 63 × phase-contrast plan-apochromat oil objective (numerical aperture [NA] 1.4) at room temperature. Acquisition settings for all images were UV/488/543/633, and specific parameters for the fluorophores were FITC excitation at 488 nm light, detected with a 500 to 530 bandpass filter; Cy5 excited at 633 nm and detected with a 650 nm longpass filter. Image acquisition was performed sequentially in the line modus to allow for detection of colocalization despite the rapid membrane movements. For line scan analysis, the membrane surfaces of 10 optical sections from each staining were selected and analyzed counterclockwise from the indicated starting points after using bandpass-filtered, color-adjusted, and merged images with the program WCIF-Image J Collection.
Vectors, cloning, and sequencing
NB1 was removed from clone pCMV-SPORT6-CD177 (RZPD, Berlin, Germany) by digestion with EcoRI and NotI (NEB, Frankfurt, Germany) and inserted into pcDNA4 (Invitrogen, Karlsruhe, Germany). pcDNA4–β-galactosidase (Invitrogen) was used for control transfection. All plasmids were sequenced using ABI PRISM 377 DNA sequencer (Applied Biosystems, Darmstadt, Germany) prior to use. Plasmids were amplified in Escherichia coli One Shot Top (Invitrogen) and purified by endotoxin-free Qiagen Maxi-Prep kit (Qiagen, Hilden, Germany).
Cell culture and transfection
HEK293 cells were cultivated in DMEM; HL60 cells were cultivated in RPMI supplemented with 10% FCS, L-glutamine, and penicillin/streptomycin (Biochrom, Berlin, Germany). HEK293 cells were transfected with FugeneHD transfection kit (Roche, Indianapolis, IN) and used 48 to 72 hours after transfection; HL60 cells were transfected using Amaxa Cell Line Nucleofactor Kit V (Amaxa Biosystems, Cologne, Germany) according to the manufacturer's instructions. Transfection efficiency was determined in each experiment and was greater than 80% for HEK293 cells and approximately 50% for HL60 cells. NB1- and control plasmid–transfected cells were incubated with 1 μg/mL (HEK293) or 5 μg/mL (HL60) purified neutrophil Proteinase 3 (The Binding Site, Birmingham, United Kingdom) and equimolar concentrations of myeloperoxidase (MPO) and human neutrophil elastase (HNE; Calbiochem, Nottingham, United Kingdom) for 2 hours on ice, washed, and stained for flow cytometry.
Statistics
Statistics were calculated using Stat view 4.5 (Abacus Concepts, Berkeley, CA). Correlations are given with 95% confidence interval. Values are given with SEM. For comparison of 2 groups, 2-sided t test was applied. P values less than .05 were considered significant and are indicated by asterisks.
Results
Membrane PR3 expression highly correlates with membrane NB1 expression
In previous studies, we had analyzed the neutrophil membrane expression of various molecules, including potential receptors, such as β2-integrins and Fcγ receptors.21 However, none of these molecules showed a bimodal surface expression pattern characteristic for PR3. The GPI-linked neutrophil glycoprotein NB1 has variable expression levels across the general population.17 Therefore, we tested for NB1 and PR3 membrane expression in parallel. Figure 1 shows expression in 2 healthy persons, one with 90% mPR3 expression (Figure 1A-B) and one negative for mPR3 (Figure 1C). The mNB1 percentages were identical, namely 90% and 0%. The mean fluorescence for both proteins was increased after TNF-α priming in the 90% positive person (Figure 1A). We observed also a concentration-dependent mNB1 and mPR3 increase in response to fMLP (1B). fMLP (10−9 M) increased the MFI for NB1 to 170% ± 35% and for PR3 to 141% ± 15%. fMLP at 10−8 M resulted in further MFI increase to 230% ± 32% for NB1 and to 223% ± 52% for PR3. The values for fMLP at 10−6 M were an MFI increase to 265% ± 29% for NB1 and to 287% ± 38% for PR3 (n = 3). However, stimulation did not affect the percentage of either mPR3 or mNB1-positive cells. Identical results were obtained with 4 different mAbs to PR3 (data not shown). By flow cytometry, we studied mPR3 and mNB1 expression in a total of 51 healthy donors and found a correlation of r = 0.996 (Figure 1D). The same association was found in 11 patients with ANCA-associated vasculitis (r = 0.999; Figure 1E).
Membrane PR3 expression highly correlates with membrane expression of granulocyte antigen NB1. Live neutrophils were surface stained with monoclonal antibodies. Fluorescence was measured by flow cytometry. Percentages of membrane PR3- and NB1-positive cells were identical in donors with different percentages of membrane-positive cells (A-B, 90%;C, 0%). MFI of both proteins increased after stimulation with TNF-α (A; bold line for cells stimulated with buffer control and dotted line for TNF-α stimulation) with fMLP (bold line for buffer control, dashed line for 10−6 M fMLP). In contrast, the NB1- and PR3-positive percentage remained unchanged, and no increase in the expression of either molecule occurred with TNF-α when cells from an mPR3/NB1-negative donor were used (C). (D) The correlation is shown of PR3 and NB1 percentages in 51 healthy donors (r = 0.996, P < .001). (E) The values are given for patients with ANCA-associated vasculitis (r = 0.999, P < .001).
Membrane PR3 expression highly correlates with membrane expression of granulocyte antigen NB1. Live neutrophils were surface stained with monoclonal antibodies. Fluorescence was measured by flow cytometry. Percentages of membrane PR3- and NB1-positive cells were identical in donors with different percentages of membrane-positive cells (A-B, 90%;C, 0%). MFI of both proteins increased after stimulation with TNF-α (A; bold line for cells stimulated with buffer control and dotted line for TNF-α stimulation) with fMLP (bold line for buffer control, dashed line for 10−6 M fMLP). In contrast, the NB1- and PR3-positive percentage remained unchanged, and no increase in the expression of either molecule occurred with TNF-α when cells from an mPR3/NB1-negative donor were used (C). (D) The correlation is shown of PR3 and NB1 percentages in 51 healthy donors (r = 0.996, P < .001). (E) The values are given for patients with ANCA-associated vasculitis (r = 0.999, P < .001).
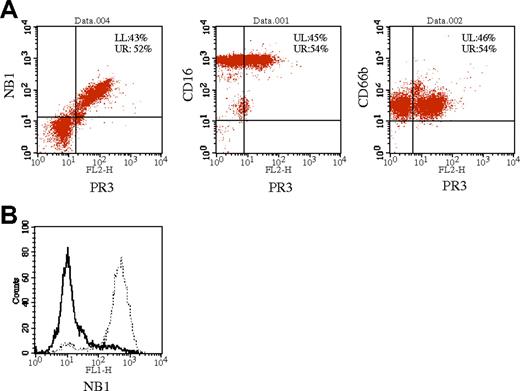
Membrane PR3-positive and membrane NB 1-positive neutrophils are identical
Double staining was performed to analyze whether PR3 and NB1 were expressed on the surface of the same neutrophil subset. Cells staining positive for NB1 and PR3 were identical (Figure 2A). Stainings for the GPI-linked surface molecules CD66b and CD16b served as controls and showed no difference in fluorescence intensity between the mPR3-positive and -negative subsets (Figure 2A). As a second approach, we separated mPR3-positive and -negative neutrophils by magnetic cell sorting and determined mNB1 expression in these physically separated cell populations. NB1 was only detected on the cells positively sorted for mPR3 (Figure 2B). In contrast, no NB1 membrane staining was observed on neutrophils without mPR3 expression. Together, both experimental approaches independently demonstrate identity of mPR3 and mNB1-expressing neutrophils.
Membrane PR3-positive and NB1-positive neutrophils are identical. Neutrophils were stained with anti-PR3 and PE-labeled secondary antibody (x-axis) and FITC-labeled antibodies against NB1, CD16b, and CD66b (A). Measurements were taken after the appropriate compensation. Percentages of cells in the quadrants are noted. (B) Neutrophil membrane PR3-positive and -negative subpopulations were separated by magnetic cell sorting and stained with directly labeled anti-NB1 antibody. mPR3-negative cells do not stain for NB1 (bold line), whereas mPR3-positively sorted cells are recognized by the anti-NB1 antibody (dotted line).
Membrane PR3-positive and NB1-positive neutrophils are identical. Neutrophils were stained with anti-PR3 and PE-labeled secondary antibody (x-axis) and FITC-labeled antibodies against NB1, CD16b, and CD66b (A). Measurements were taken after the appropriate compensation. Percentages of cells in the quadrants are noted. (B) Neutrophil membrane PR3-positive and -negative subpopulations were separated by magnetic cell sorting and stained with directly labeled anti-NB1 antibody. mPR3-negative cells do not stain for NB1 (bold line), whereas mPR3-positively sorted cells are recognized by the anti-NB1 antibody (dotted line).
Membrane PR3 expression is restricted to cells with NB1
We next assayed intracellular PR3 and NB1 by Western blot in neutrophils sorted for membrane expression of either PR3 or NB1. We detected PR3 in all neutrophil lysates, independent of cell membrane expression (Figure 3). In contrast, NB1 was only found in lysates of neutrophils selected for positive membrane staining for PR3 or NB1. Western blot also demonstrated that there was no cross-reactivity of the used antibodies. These data support the hypothesis that the presence of NB1 is the determinate of membrane PR3 expression by acting as a receptor for display.
Membrane PR3 expression is restricted to cells with NB1 protein. Cells sorted for mPR3 (n = 5, A) and mNB1 (n = 3, B) expression (purity, 90% ± 5%) were subjected to immunoblot analysis for PR3 and NB1. Corresponding optical densitometry showed no significant difference in whole-cell PR3 content in the membrane-positive and -negative subpopulations. However, NB1 was restricted to mPR3- and mNB1-positive cells (*P < .05). Error bars indicate ± SEM.
Membrane PR3 expression is restricted to cells with NB1 protein. Cells sorted for mPR3 (n = 5, A) and mNB1 (n = 3, B) expression (purity, 90% ± 5%) were subjected to immunoblot analysis for PR3 and NB1. Corresponding optical densitometry showed no significant difference in whole-cell PR3 content in the membrane-positive and -negative subpopulations. However, NB1 was restricted to mPR3- and mNB1-positive cells (*P < .05). Error bars indicate ± SEM.
Membrane NB1 and membrane PR3 expression correlate in stem-cell–derived neutrophils
To study mPR3 and mNB1 expression during neutrophil development, we used a model of in vitro differentiation from umbilical CD34+ hematopoietic stem cells as described earlier.11 Differentiation was assessed by morphology and neutrophil surface marker expression (Figure 4). Identical mPR3- and mNB1-positive percentages were detected after 7 days of differentiation. These experiments indicate that differentiation of neutrophils, even under in vitro conditions, results in coexpression of PR3 and NB1 on a proportion of cells. Furthermore, they strengthen the contention that the GPI-linked NB1 receptor molecule is causally involved in mPR3 presentation.
NB1 and PR3 surface expression in an in vitro model of neutrophil differentiation. CD34+ stem cells were isolated from umbilical cord blood, expanded, and differentiated into neutrophils. Morphology is shown using Wright-Giemsa staining and microscopy. Surface expression of differentiation markers was assessed by flow cytometry. Seven days of differentiation resulted in identical PR3 and NB1 percentages as shown for a typical of 10 independent experiments.
NB1 and PR3 surface expression in an in vitro model of neutrophil differentiation. CD34+ stem cells were isolated from umbilical cord blood, expanded, and differentiated into neutrophils. Morphology is shown using Wright-Giemsa staining and microscopy. Surface expression of differentiation markers was assessed by flow cytometry. Seven days of differentiation resulted in identical PR3 and NB1 percentages as shown for a typical of 10 independent experiments.
Loss of GPI linkers from plasma membrane of neutrophils results in decreased mPR3 expression
NB1 is a GPI-linked protein and can be removed from the cell membrane by cleaving the glucosamine moiety of the GPI linker from its lipid membrane anchor using phospholipase C.22 Neutrophils treated with phospholipase C showed a reduction of mNB1 expression in flow cytometry (Figure 5A-B). This reduction was accompanied by decreased mPR3 expression, whereas the control marker CD18 was unaffected. In addition, we studied neutrophils from a patient with paroxysmal nocturnal hemoglobinuria (PNH), a rare acquired defect in the formation of GPI linkers.23 These patients show a variable decrease of GPI-linked proteins on their neutrophils.24 In parallel to a healthy donor we document that the neutrophils of the patient with PNH had a major decrease in a number of GPI-linked proteins (Figure 5C). No NB1 was detected on their surface, although intracellular NB1 was present in Western blot (data not shown). Similar to the NB1 results, PR3 was also absent from the cell surface. These experiments further suggest NB1 as a receptor that is necessary for PR3 membrane presentation.
PR3 neutrophil membrane display depends on GPI linkers. Neutrophils subjected to GPI-anchor cleavage by phospholipase C showed decreased membrane NB1 and PR3 expression (A, dotted lines) as compared with control cells (bold lines). The mean change of fluorescence in 7 independent experiments was −43% ± 4% for NB1 and −33% ± 12% for PR3, P < .05. Error bars indicate SEM; *P < .05; **P < .01. (B). The β2-integrin CD18, a marker of cell activation, remained unchanged (6 ± 6). (C) NB1 and several other GPI-linked proteins are expressed on neutrophils from a patient with PNH in comparison to a healthy control subject. Isotype control antibody staining is shown by the thin line and the GPI-linked molecule staining by bold lines. PNH is characterized by an acquired deficiency in GPI-linker synthesis. Membrane display of GPI-linked proteins was strongly decreased, with mNB1 and mPR3 expression even being absent in the patient was PNH.
PR3 neutrophil membrane display depends on GPI linkers. Neutrophils subjected to GPI-anchor cleavage by phospholipase C showed decreased membrane NB1 and PR3 expression (A, dotted lines) as compared with control cells (bold lines). The mean change of fluorescence in 7 independent experiments was −43% ± 4% for NB1 and −33% ± 12% for PR3, P < .05. Error bars indicate SEM; *P < .05; **P < .01. (B). The β2-integrin CD18, a marker of cell activation, remained unchanged (6 ± 6). (C) NB1 and several other GPI-linked proteins are expressed on neutrophils from a patient with PNH in comparison to a healthy control subject. Isotype control antibody staining is shown by the thin line and the GPI-linked molecule staining by bold lines. PNH is characterized by an acquired deficiency in GPI-linker synthesis. Membrane display of GPI-linked proteins was strongly decreased, with mNB1 and mPR3 expression even being absent in the patient was PNH.
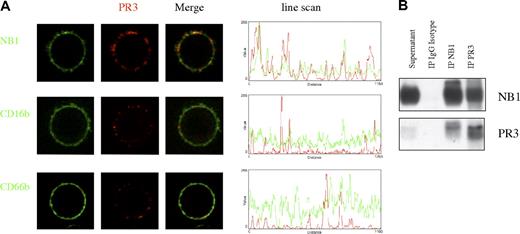
PR3 and NB1 colocalize on and are coimmunoprecipitated from the neutrophil plasma membrane
Live-cell confocal microscopy shows that PR3 and NB1 were not only localized on the same cell but also colocalized on the plasma membrane (Figure 6A; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We did not observe similar proximity between mPR3 and CD66b or CD16b. We were also able to coimmunoprecipitate NB1 and PR3 from the supernatant of neutrophils that were treated with phospholipase C to mobilize GPI-linked proteins from the plasma membrane (Figure 6B). These results demonstrate physical interaction of PR3 and NB1 on the neutrophil plasma membrane as expected for a receptor-ligand pair.
PR3 and NB1 colocalize on and coimmunoprecipitate from the neutrophil plasma membrane. Confocal microscopy of live neutrophils showed colocalization of PR3- and NB1-stained spots on the plasma membrane in comparison to CD66b and CD16b (A). Line scans of fluorescence intensity indicate colocalization of peak color intensities. Immunoprecipitation and Western blot analysis of PR3 and NB1 was performed from the supernatant of TNF-α–primed, phospholipase C–treated neutrophils. This procedure resulted in specific coimmunoprecipitation of PR3 after NB1 precipitation and vice versa (B).
PR3 and NB1 colocalize on and coimmunoprecipitate from the neutrophil plasma membrane. Confocal microscopy of live neutrophils showed colocalization of PR3- and NB1-stained spots on the plasma membrane in comparison to CD66b and CD16b (A). Line scans of fluorescence intensity indicate colocalization of peak color intensities. Immunoprecipitation and Western blot analysis of PR3 and NB1 was performed from the supernatant of TNF-α–primed, phospholipase C–treated neutrophils. This procedure resulted in specific coimmunoprecipitation of PR3 after NB1 precipitation and vice versa (B).
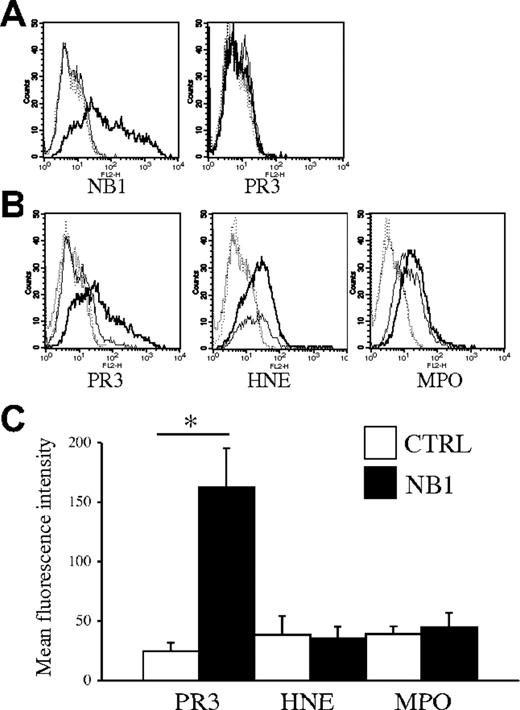
NB1 transfection results in NB1 membrane expression and enables PR3 binding
NB1 transfected into HEK293 cells resulted in correct-sized protein expression in Western blot analysis (data not shown), and surface NB1 expression was demonstrated by flow cytometry (Figure 7A). NB1-transfected cells that were incubated with purified human PR3 showed markedly elevated PR3 surface display compared with mock-transfected cells. HEK293 cells were incubated with control proteins at equimolar concentrations in parallel. We selected the second major ANCA antigen myeloperoxidase (MPO) and human neutrophil elastase (HNE), another neutrophil protease of similar size as PR3. Our data show that neither MPO nor HNE surface display was increased by NB1 expression (Figure 7B). Similar results were obtained after NB1 transfection into HL60 cells, a myeloblastic cell line (Figure S2). These data demonstrate that NB1 confers the ability to display PR3 on the plasma membrane.
Ectopic NB1 expression confers PR3 surface binding. Transfection of a NB1 expression plasmid into HEK293 cells resulted in NB1 surface expression (A; bold line for NB1-transfected cells, thin line for ctrl-transfected cells, isotype control staining for ctrl- and NB1-transfection is depicted as dotted lines). Monoclonal anti-PR3 antibodies did not bind to any of these cells as shown for CLB12.8. (B) NB1 transfected (bold line) and mock-transfected cells (thin line) were incubated on ice for 2 hours with 1 μg/mL purified PR3 or equimolar concentrations of neutrophil elastase (HNE) or myeloperoxidase (MPO), washed and stained for surface display. (C) Binding of PR3 was specifically elevated in NB1-transfected cells (▪) as compared with mock-transfected (□) cells (MFI, 163 ± 33 versus 25 ± 7; *P < .05; n = 5 independent transfections). HNE and MPO binding was unaffected by NB1 transfection (MFI: HNE, 36 ± 10 versus 39 ± 16, and MPO, 45 ± 12 versus 39 ± 6; n = 3 independent transfections). Error bars indicate SEM.
Ectopic NB1 expression confers PR3 surface binding. Transfection of a NB1 expression plasmid into HEK293 cells resulted in NB1 surface expression (A; bold line for NB1-transfected cells, thin line for ctrl-transfected cells, isotype control staining for ctrl- and NB1-transfection is depicted as dotted lines). Monoclonal anti-PR3 antibodies did not bind to any of these cells as shown for CLB12.8. (B) NB1 transfected (bold line) and mock-transfected cells (thin line) were incubated on ice for 2 hours with 1 μg/mL purified PR3 or equimolar concentrations of neutrophil elastase (HNE) or myeloperoxidase (MPO), washed and stained for surface display. (C) Binding of PR3 was specifically elevated in NB1-transfected cells (▪) as compared with mock-transfected (□) cells (MFI, 163 ± 33 versus 25 ± 7; *P < .05; n = 5 independent transfections). HNE and MPO binding was unaffected by NB1 transfection (MFI: HNE, 36 ± 10 versus 39 ± 16, and MPO, 45 ± 12 versus 39 ± 6; n = 3 independent transfections). Error bars indicate SEM.
Discussion
Our study is the first to show that NB1, a GPI-linked receptor, is necessary for the presentation of the ANCA autoantigen PR3 on the membrane surface of human neutrophils. Autoantibody binding to mPR3 is characteristic for ANCA-associated vasculitis25 and results in cell activation with release of reactive oxygen species and tissue-toxic granule proteins.2,26 Experimental data suggest that mPR3-positive neutrophils show a stronger respiratory burst activity when incubated with PR3-ANCA than mPR3-negative cells from the same donor.21 Understanding how PR3 is displayed on the neutrophil could have mechanistic and therapeutic importance.
The mechanism of PR3 presentation on the cell surface has not yet been defined. On endothelial cells, a 111-kDa protein was suggested to serve as a PR3 receptor, but the nature of this molecule has not been shown.27 In neutrophils, an association of mPR3 with the β2-integrins CD18 and CD11b and with the Fcγ-receptor IIIB in lipid rafts was described.14,28 However, none of these molecules show a bimodal membrane phenotype that is characteristic for mPR3. Our studies demonstrate that mNB1 expression has characteristics similar to PR3. Both molecules show bimodal expression patterns, and cytokine-priming increases the amount of surface expression without changing the percentage of positive cells. Similar findings were recently described in a prepublished manuscript by Bauer et al.29 In addition, we show by several independent methods in isolated blood neutrophils and in vitro–differentiated stem cell–derived neutrophils that the neutrophil subset that expresses surface NB1 and PR3 is the same.
NB1 is a neutrophil surface receptor that was described originally as a neutrophil alloantigen. Similar to mPR3, the percentage of mNB1-positive neutrophils ranges from 0% to 100%. The percentage was stable in healthy persons and did not change with G-CSF administration.30,31 NB1 polymorphisms were described, and defective splicing was proposed as a mechanism explaining the phenotype in 0% NB1-expressing persons.32,33 Acquired anti-NB1 antibodies in a NB1-deficient mother during a previous pregnancy were responsible for neonatal neutropenia after consecutive pregnancies.15 A second disease association was found with PRV, where the NB1 mRNA expression was increased in peripheral blood neutrophils.34 The protein was therefore also named PRV1 and only later found to be an allelic variant of NB1.35 NB1 belongs to the same family of GPI-linked molecules as the receptor of another serine-protease, namely urokinase-type plasminogen activator receptor (uPAR).
Live-cell confocal microscopy and coprecipitation from the membrane was used to demonstrate that both molecules indeed physically interacted. Other GPI-linked receptors, such as CD16b and CD66b, did not demonstrate the same degree of colocalization. Enzymatic removal of GPI linkers off the neutrophil membrane resulted in decreased mPR3 expression. In addition, we had the opportunity to study neutrophils from a patient with PNH, an acquired clonal loss of GPI linker synthesis.24 Our patient showed the expected reduction of CD66b and CD16b, and even loss of NB1 with a parallel lack of mPR3 expression on neutrophils. To provide firm evidence that NB1 expression confers the capability to express mPR3, we transfected different cell lines with NB1. Transgenic NB1 membrane expression resulted in the ability to bind exogenous human PR3, whereas the control proteins HNE and MPO did not show specific binding.
Our data establish the NB1 receptor as the mechanism of mPR3 display. Our findings also raise a number of new and interesting questions. We show that exogenous PR3 binds to mNB1 and also find a parallel membrane up-regulation of both molecules in response to proinflammatory degranulation-inducing mediators. However, whether NB1 and PR3 are also translocated as a complex from intracellular pools, such as secondary granules or secretory vesicles is currently unknown. Both granule types were shown to contain NB1 and PR3. Furthermore, it is also conceivable that PR3 and NB1 translocate independently and associate on the membrane. Failed efforts of coimmunoprecipitation from secondary vesicles (S.v.V., unpublished data, April-July, 2006) favor the latter explanation, but more detailed investigations are needed to clarify intracellular association.
No physiologic ligand of NB1 has been described, and little is known on signal transduction by this molecule. However, anti-NB1 antibodies were shown to induce respiratory burst activity in primed neutrophils, similar to PR3-ANCA.36 In fact, we obtained data indicating that anti-NB1 mAb result in phosphorylation of p38 MAPK, ERK, and PI3K/Akt (data not shown). Other investigators and our own group showed previously that all 3 of these pathways control ANCA-induced neutrophil activation.37–39 Possibly, additional molecules are recruited to the NB1-PR3 complex before neutrophils are activated. The uPAR is a well-studied member of the same family that plays an important role in coagulation and cell migration.40 For signaling, uPAR associates with integrins as was shown in epithelial and mesenchymal cells.41,42 β2-Integrins are important in neutrophil signaling; their association with mPR3 was reported earlier.14,26,43 It is tempting to speculate that a complex of PR3 and NB1 with integrins and possibly Fcγ-receptor forms on PR3-ANCA binding and mediates PR3-ANCA–induced neutrophil activation.28,44 This issue will be the subject of further investigations that will require a neutrophil model system that can be successfully transfected and subjected to activation studies.
In summary, we show that PR3 is presented on human neutrophils by the surface receptor NB1. This finding identifies NB1 as a potential therapeutic target in ANCA-associated vasculitis.
Authorship
Contribution: S.v.V. designed and performed research and wrote the paper; G.T. and C.E. performed research; M.W. and M.C.C. supervised and designed research; and F.C.L. and R.K. designed research and wrote the paper. All authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ralph Kettritz, Nephrology and Hypertension Section, Franz-Volhard-Klinik, Wiltbergstrasse 50, 13125 Berlin, Germany; e-mail: kettritz@charite.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by InnoRegio 03 I 4509B, ProFIT (R.K.), and the European Union ESF program (G.T.).





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal