Abstract
Intravenous immunoglobulin (IVIg) preparations are increasingly used for therapy of several neuroimmunologic diseases. IVIg therapy is considered safe, although serious side effects like aseptic meningitis, cerebral vasospasm, or ischemic encephalopathy have been reported. These side effects are frequently associated with neutrophilic pleocytosis in the cerebrospinal fluid (CSF), suggesting a neutrophil-mediated mechanism. To elucidate the potential role of neutrophil activation, we analyzed IVIg preparations from 5 different commercial sources for the presence of antineutrophil cytoplasmic antibody (ANCA)–like immunoglobulins against ethanol-fixed peripheral-blood neutrophils, purified human antigens, and a panel of human and nonhuman tissues. All IVIg batches tested (n = 13) contained atypical ANCAs (IgG titer up to 1:2048, IgA up to 1:512). Moreover, all preparations were capable of inducing hydrogen peroxide production in TNFα-primed human neutrophils, with a significant correlation (P < .005) between atypical ANCA titers in IVIg preparations and neutrophil activation. Fc-mediated binding and activation was ruled out by the use of IVIg-F(ab′)2 fragments. Our findings strongly suggest that in vivo activation of TNFα-primed neutrophils by atypical ANCAs of IVIg may contribute to the side effects of IVIg therapy and for the first time demonstrate that the activation of neutrophil granulocytes by IVIg occurs in an Fc receptor (FcR)–independent, hence antigen-dependent, way.
Introduction
Intravenous immunoglobulins (IVIg's) are increasingly used for the therapy of several neuroimmunologic diseases such as Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MNN), and many others.1–8 Generally, IVIg therapy is considered safe.9–12 There are some mild common side effects like headache, fever, and chills, which are mainly transitory in nature and often related to the speed of infusion.4,13–15 However, potentially serious side effects such as ischemic encephalopathy,16–19 cerebral infarction,20–27 and aseptic meningitis28–33 have been reported in patients treated with IVIg. These side effects are not uncommon. Sekul et al28 reported aseptic meningitis to occur in up to 11% of patients treated with high-dose (2 g/kg) IVIg, and Sztajzel et al19 found subclinical cerebral vasospasm to occur in 1 of 10 patients treated with IVIg. Frequently, these IVIg side effects are associated with an aseptic neutrophilic pleocytosis in cerebrospinal fluid (CSF), suggesting a neutrophil-mediated pathogenic mechanism.16,29,34
In an earlier study, we had reported on a GBS patient who developed cerebral vasospasm with encephalopathy, which was associated with neutrophilic pleocytosis in CSF, after IVIg therapy.16 As this preparation contained high titers of atypical antineutrophil cytoplasmic antibodies (ANCAs), we wanted to clarify the types and titers of ANCA-like immunoglobulins contained in this and other preparations of IVIg's, the neutrophil activating potential of these preparations, and the causal relationship between ANCA titer and neutrophil activation.
Materials and methods
IVIg samples and controls
In addition to 1 IVIg batch that had caused cerebral vasospasm, ischemic encephalopathy, and neutrophil pleocytosis in 1 of our patients,16 a total of 12 additional batches of 5 different commercially available IVIg preparations were analyzed in this study (A1-A5, B1-2, C1-2, D1-3, E1; Table 1)
List of intravenous immunoglobulin preparations analyzed in this study, in alphabetical order
| IVIg preparation . | Manufacturer . |
|---|---|
| Endobulin S/D | Baxter, Heidelberg, Germany |
| Intraglobin F | Biotest, Dreieich, Germany |
| Pentaglobin | Biotest Pharma, Dreieich, Germany |
| Sandoglobulin | Novartis Pharma, Nürnberg, Germany |
| Venimmun | Behring AG, Marburg, Germany |
| Venimmun N | Aventis Behring, Marburg, Germany |
| IVIg preparation . | Manufacturer . |
|---|---|
| Endobulin S/D | Baxter, Heidelberg, Germany |
| Intraglobin F | Biotest, Dreieich, Germany |
| Pentaglobin | Biotest Pharma, Dreieich, Germany |
| Sandoglobulin | Novartis Pharma, Nürnberg, Germany |
| Venimmun | Behring AG, Marburg, Germany |
| Venimmun N | Aventis Behring, Marburg, Germany |
Freeze-dried preparations (samples A1-5, B1-2, E1) were reconstituted according to the manufacturer's instructions using aqua ad injectionem as a solvent (final Ig concentration: 50 mg/mL). Ready-for-use IVIg solutions (samples C1-2, D1-2) were used as provided by the manufacturers at a final Ig concentration of 50 mg/mL, if otherwise not stated.
Two additional samples of batch A4 were reconstituted using a sterile solution of highly purified and pasteurized human albumin (Grifols, Langen, Germany; final albumin concentration: 50 mg/mL; final IVIg concentration: 50 mg/mL) and pooled serum of healthy donors (n = 5; final albumin concentration: 50 mg/mL; final IVIg concentration: 50 mg/mL), respectively.
cANCA-positive serum (1:512) obtained from a patient suffering from Wegener granulomatosis was used as positive control. Pooled serum of healthy donors (n = 5) and human albumin (50 mg/mL) were used as negative controls.
Preparation of total IgG
Total IgG was purified from batch A4 with protein A–Sepharose CL-4B (Amersham, Freiburg, Germany). IVIg was loaded onto a protein A–Sepharose CL-4B column and washed 3 times with an excess of Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS; pH 7.5) or TBS containing 2 M NaCl, respectively. Bound IgG was eluted with 0.2 M glycine containing 0.5 M NaCl (pH 2.3) and neutralized immediately with 1 M Tris base (pH 9). The eluted IgG was dialyzed against 0.1 M sodium acetate buffer (pH 4.5), and IgG concentration (11.42 mg/mL) was determined by spectrophotometry from the absorbance at 280 nm using an extinction coefficient of 1.3 L × g−1 × cm−1.
Preparation of F(ab′)2 fragments
Purified total IgG derived from batch A4 was incubated at 37°C with pepsin (5 mg/100 mg IgG; Sigma, St Louis, MO) for 24 hours. Reaction was stopped using Tris-HCl-buffer, pH 7.0 (TBS). Fragments were purified by affinity chromatography by repeated passage through a protein A–Sepharose column. The pass was collected and F(ab′)2 fragments were precipitated with 50% (NH4)SO4 and dissolved in TBS. The resulting solution was dialyzed against TBS. Concentration of F(ab′)2 fragments [IVIg-F(ab′)2; 14 mg/mL] was assessed by spectrophotometry (extinction at 280 nm; extinction coefficient 1.3 L × g−1 × cm−1) and affirmed by the Biuret method.
By NuPAGE Bis-Tris/MES electrophoresis (running time 1 h 17 min, 150 V; start: 70 mA, end: 40 mA; InVitrogen, Karlsruhe, Germany) and simple blue staining, a distinct band was detected at about 100 kDa (LWM calibration kit; Amersham); no bands were detected above or below 100 kDa (calibration range, 14 to 220 kDa).
In order to achieve an IVIg sample containing the same molar concentration of antibodies as contained in the 14 mg/mL F(ab′)2 sample, 1 probe of batch A4 was diluted to 21 mg/mL IgG in accordance with the specific molecular weights of IgG and F(ab′)2 (150 kDa and ∼100 kDa).
Indirect immunofluorescence assay
Titer and type of ANCA-like immunoglobulins contained in the IVIg preparations were assessed by indirect immunofluorescence (Leica DM RXE conventional fluorescence microscope, Leica IM software version 4.0.85; Leica Microsystems, Heidelberg, Germany) using BIOCHIP Mosaics (Euroimmun, Luebeck, Germany) coated with sedimented ethanol-fixed human peripheral-blood granulocytes. The slides were supplemented with BIOCHIP mosaics consisting of acetone-fixed human epithelial cells (HEp-2) and tissue sections of primate liver, rat liver, rat kidney, rat stomach, and primate heart.
The IVIg preparations were analyzed in dilutions of 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, 1:2048, and 1:4096 on granulocytes, primate liver tissue sections, and human epithelial cells (HEp-2). Bound antibody was detected with FITC-conjugated rabbit antibodies to human IgG, IgA, or IgM (Euroimmun). Conjugates were diluted 1:5 in PBS containing 0.2% (vol/vol) Tween-20. F(ab′)2 fragments were analyzed in dilutions of 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, and 1:2048 on granulocytes. As secondary antibody, FITC-conjugated F(ab′)2 fragment–specific goat anti–human IgG was used (Jackson Laboratories, West Grove, PA). Conjugates were diluted 1:100 in sterile PBS containing 0.2% (vol/vol) Tween-20.
The ANCA pattern was classified in accordance with the recommendations of the international consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies35,36 : classical ANCA (cANCA; cytoplasmic fluorescence with central or interlobular accentuation); atypical classical ANCA (cANCA [atypical], acANCA; a “flat” cytoplasmic fluorescence lacking central interlobular accentuation); perinuclear ANCA (pANCA; perinuclear cytoplasmic fluorescence with or without nuclear extension); and atypical ANCA (aANCA; including all fluorescence patterns other than those described as cANCA, acANCA, or pANCA).
The discrimination of ANCA pattern was enhanced by confocal laser microscopy analysis (Leica TCS confocal light microscope, Leica TCS NT Software v1.6.587; Leica Microsystems, Heidelberg, Germany). Primate liver tissue sections, human epithelial cells (Hep-2), and formaldehyde-fixed granulocytes were used to detect antinuclear antibodies (ANAs), which can hamper the diagnosis of perinuclear and atypical ANCAs. Sections of rat liver, rat kidney, rat stomach, primate heart, and Hep-2 were analyzed in dilutions of 1:30, 1:60, 1:120, 1:240, 1:480, 1:960, 1:1920, and 1:3840 in order to detect autoantibodies such as antimitochondrial antibodies (AMAs), antiribosomal antibodies, and anti–smooth muscle antibodies (ASMAs) directed against the target antigen actin, which are able to interfere with the diagnosis of neutrophil cytoplasmic antibodies.37 Autoantibodies were detected using FITC-conjugated goat anti–human IgG, IgA, and IgM antibodies (Euroimmun) diluted 1:5 in PBS containing 0.2% (vol/vol) Tween-20.
ELISA
The presence of antibodies against the 7 most common granule-associated ANCA antigens (proteinase 3, myeloperoxidase, bactericidal/permeability increasing protein, lactoferrin, lysozyme, human leukocyte elastase, and cathepsin G) was studied by means of semiquantitative indirect enzyme-linked immunosorbent assay (ELISA; Euroimmun) according to the manufacturer's instructions.
Briefly, polystyrene microplate strips were coated with purified biochemically characterized antigens. Samples were diluted 1:100 in PBS containing 0.1% casein. Attached antibodies were detected with peroxidase (POD)–labeled rabbit anti–human IgG antibodies (dilution 1:100). Bound detection antibodies were made visible using a tetramethyl-benzidine/H202 mixture.
Oxidative-burst assay
The oxidative-burst (OB) reaction was assessed by flow cytometry measuring the oxidation of dihydrorhodamine 123 by hydrogen peroxide in tumor necrosis factor α (TNFα)–primed human peripheral-blood neutrophils as described earlier.38
Briefly, peripheral-blood leukocytes (PBLs) of 5 healthy male donors with normal C-reactive protein (CRP), leukocyte, and granulocyte counts were separated by Ficoll density gradient. Cells were then primed with 1 ng/mL human recombinant TNFα (R&D Systems, Wiesbaden, Germany) for 15 minutes and exposed to IVIg at 0.25, 2.5, and 25 mg/mL for 35 to 125 minutes and to F(ab′)2 fragments at 1.6 mg/mL for 100 minutes at 37°C. PBLs were loaded with nonfluorescent dihydrorhodamine 123 (Molecular Probes, Leiden, Netherlands) and 5-(and-6)-carboxy seminaphthorhodafluor-1-acetoxymethyl ester (SNARF-1/AM; Molecular Probes) and incubated for another 25 minutes. Then, the reaction was stopped on ice and the oxidation of dihydrorhodamine 123 to green fluorescent rhodamine 123 (510/530 nm) was measured by flow cytometry. Live granulocytes were gated using a combination of sideward scatter (SSC), rhodamine 123 fluorescence (FL1), and SNARF-1 esterase activity (FL3).
Phorbol-12-myristate-13-acetate (PMA; Sigma, Deisenhofen, Germany) was dissolved in N, N-dimethylformamide (DMF; Sigma-Aldrich, Munich, Germany) to yield a stock solution of 1 mM in DMF. Working concentrations of 10 μM PMA were prepared with PBS and used as a positive control stimulus. As negative controls, PBS, PBS plus TNFα, and PBS plus IVIg were assessed.
Statistical analysis
Correlations between titer levels and mean fluorescent intensity of rhodamine 123 in about 2000 granulocytes exposed to various IVIg concentrations (0.25, 2.5, and 25 mg/mL) were assessed by calculating the Spearman rank correlation coefficient and respective P values. All P values are 2-sided and subject to a significance level of 5%. Computations were carried out with the SPSS version 11.5 (SPSS, Chicago, IL).
Results
Presence of ANCA-like immunoglobulins in IVIg preparations
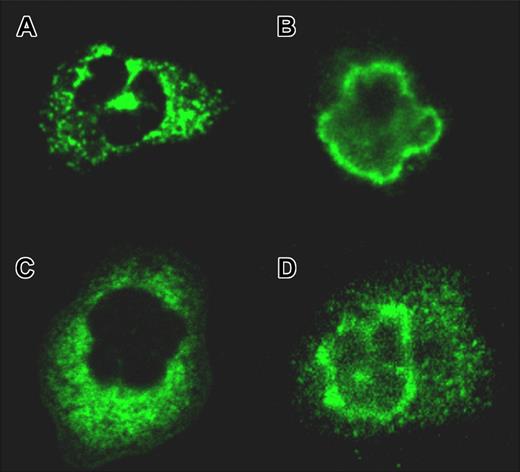
All IVIg preparations and all batches tested contained ANCA-like immunoglobulins. Most preparations contained atypical ANCA (aANCA) as shown in Figure 1D. Some batches contained atypical cANCA (acANCA; Table 2; Figure 1C). Since batch A5 was associated with neutrophilic pleocytosis and cerebral vasospasm in 1 of our patients, we tested 4 other batches of the same preparation (A1-4), all containing high titers of aANCA, with IgG up to 1:2048, IgA up to 1:512, and IgM up to 1:64. All 4 other IVIg preparations tested (B-E) also contained aANCA, albeit with lower titers ranging from 1:32 to 1:256 (Table 2).
ANCA patterns as identified by confocal microscopy. (A) Classical ANCA (patient 1: diagnosis, Wegener granulomatosis; IIF, cANCA-IgG 1:1024; ELISA, anti-PR3 positive). (B) Perinuclear ANCA (patient 2: diagnosis, microscopic polyangiitis; IIF, pANCA-IgG 1:256; ELISA, anti-MPO positive). (C) Atypical cANCA contained in an intravenous immunoglobulin preparation (preparation E1, IgG titer 1:256). (D) Atypical ANCA (preparation A1, IgG titer 1:1024). A Leica TCS NT confocal light microscope with a 63×/1.32 NA oil PL APO 1.32-0.6 lens was used.
ANCA patterns as identified by confocal microscopy. (A) Classical ANCA (patient 1: diagnosis, Wegener granulomatosis; IIF, cANCA-IgG 1:1024; ELISA, anti-PR3 positive). (B) Perinuclear ANCA (patient 2: diagnosis, microscopic polyangiitis; IIF, pANCA-IgG 1:256; ELISA, anti-MPO positive). (C) Atypical cANCA contained in an intravenous immunoglobulin preparation (preparation E1, IgG titer 1:256). (D) Atypical ANCA (preparation A1, IgG titer 1:1024). A Leica TCS NT confocal light microscope with a 63×/1.32 NA oil PL APO 1.32-0.6 lens was used.
Titers and types of antineutrophil cytoplasmic antibodies (ANCAs) and titers of antinuclear antibodies (ANAs) and smooth muscle antibodies (ASMAs) contained in intravenous immunoglobulin preparations as demonstrated by IIF
| . | ANCA IgG . | ANCA IgA . | ANCA IgM . | ANA IgG . | ASMA IgG . |
|---|---|---|---|---|---|
| A1 | 1:1024* | 1:512* | 1:16* | 1:120† | 1:240‡ |
| A2 | 1:1024* | 1:512* | 1:64* | 1:60† | 1:240‡ |
| A3 | 1:512* | 1:512* | 1:32* | 1:60† | 1:240‡ |
| A4 | 1:512* | 1:256* | 1:32* | 1:30† | 1:120‡ |
| A5 | 1:2048* | NT | NT | NT | NT |
| B1 | 1:32* | – | – | 1:120† | – |
| B2 | 1:128* | 1:8* | – | 1:60† | – |
| C1 | 1:64* | – | – | 1:30† | – |
| C2 | 1:64* | NT | NT | NT | NT |
| D1 | 1:64§ | 1:128* | 1:256§ | 1:120† | – |
| D2 | 1:64* | NT | NT | NT | NT |
| D3 | 1:256* | NT | NT | NT | NT |
| E1 | 1:256* | 1:16* | – | 1:240† | – |
| . | ANCA IgG . | ANCA IgA . | ANCA IgM . | ANA IgG . | ASMA IgG . |
|---|---|---|---|---|---|
| A1 | 1:1024* | 1:512* | 1:16* | 1:120† | 1:240‡ |
| A2 | 1:1024* | 1:512* | 1:64* | 1:60† | 1:240‡ |
| A3 | 1:512* | 1:512* | 1:32* | 1:60† | 1:240‡ |
| A4 | 1:512* | 1:256* | 1:32* | 1:30† | 1:120‡ |
| A5 | 1:2048* | NT | NT | NT | NT |
| B1 | 1:32* | – | – | 1:120† | – |
| B2 | 1:128* | 1:8* | – | 1:60† | – |
| C1 | 1:64* | – | – | 1:30† | – |
| C2 | 1:64* | NT | NT | NT | NT |
| D1 | 1:64§ | 1:128* | 1:256§ | 1:120† | – |
| D2 | 1:64* | NT | NT | NT | NT |
| D3 | 1:256* | NT | NT | NT | NT |
| E1 | 1:256* | 1:16* | – | 1:240† | – |
NT indicates not tested; –, negative.
Atypical ANCA (aANCA).
Granular pattern.
Nonactin type.
Atypical cANCA (acANCA).
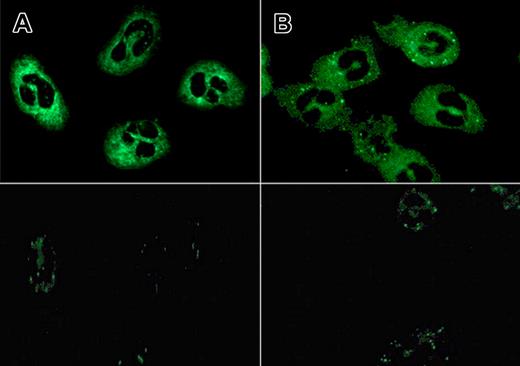
With IVIg-F(ab′)2 fragments—prepared from batch A4, which resulted in a titer of 1:512 (Table 2)—as a primary antibody, an aANCA-IgG titer of 1:128 was obtained (Figure 2). Titer results did not depend on which secondary antibody, FITC-conjugated rabbit antibodies to human IgG or FITC-conjugated goat antibodies to human IgG F(ab′)2 fragment, was used. A titer of 1:128 was also found when using equimolar concentrations of IVIg-IgG and F(ab′)2 (21 mg/mL and 14 mg/mL, respectively). As a positive control, serum obtained from a patient with Wegener granulomatosis was used, resulting in an ANCA titer of 1:512 (granular cytoplasmic fluorescence), regardless of which of the 2 secondary antibodies was used.
ANCA patterns found with IVIg and IVIg-F(ab′)2 fragments using equimolar concentrations. Flat atypical cANCA pattern identified with IVIg (21 mg/mL; batch A4; titer 1:128; panel A) and IVIg-F(ab′)2 fragments (14 mg/mL; prepared from batch A4; titer 1:128; panel B) as primary antibody, respectively, and FITC-conjugated goat anti–human anti-F(ab′)2 as secondary antibody (top panel) and corresponding negative controls (pooled serum of healthy donors, n = 5; diluted 1:8, bottom panel). A Leica DM RXE conventional fluorescence microscope with a 40×/0.75 PL APO ∞ −0, 17 lens was used.
ANCA patterns found with IVIg and IVIg-F(ab′)2 fragments using equimolar concentrations. Flat atypical cANCA pattern identified with IVIg (21 mg/mL; batch A4; titer 1:128; panel A) and IVIg-F(ab′)2 fragments (14 mg/mL; prepared from batch A4; titer 1:128; panel B) as primary antibody, respectively, and FITC-conjugated goat anti–human anti-F(ab′)2 as secondary antibody (top panel) and corresponding negative controls (pooled serum of healthy donors, n = 5; diluted 1:8, bottom panel). A Leica DM RXE conventional fluorescence microscope with a 40×/0.75 PL APO ∞ −0, 17 lens was used.
Blocking of ANCA reactivity
In order to assess the effect of serum protein on the binding capacity of IVIg, 1 sample of batch A4 (titer 1:512) was reconstituted with pooled serum from healthy donors as a solvent (final IVIg concentration: 50 mg/mL; final albumin concentration: 50 mg/mL), leading to a reduced titer of 1:128. To assess whether blocking of ANCA binding was mediated by albumin, another sample of batch A4 was reconstituted in a solution containing 50 mg/mL albumin, again resulting in a reduced titer of 1:128. By contrast, the pooled serum sample at the 1:8 dilution, like albumin alone, resulted in some background staining but did not generate a specific ANCA pattern (Figure 2).
Cell-type specificity of ANCA staining
Using BIOCHIP mosaics as described in “Indirect immunofluorescence assay,” no cytoplasmic fluorescence was detected on eosinophilic granulocytes, basophilic granulocytes, or lymphocytes by indirect immunofluorescence with any of the IVIg batches tested. Very faint fluorescence, however, was seen with monocytes.
ANAs, which may hamper the diagnosis of pANCAs and aANCAs, were found in all batches tested with titers ranging from 1:30 to 1:240 (Table 2). ASMAs of the actin type, which may mimic acANCA staining, however, were not found in any of the preparations, whereas nonactin-type ASMAs (not interfering with ANCA staining) were identified in 3 of 8 batches tested (A1-3, titer 1:240; Table 2). In addition, when testing the IVIg preparation with the highest ANCA titer (preparation A), no antineutrophil alloantibodies, especially anti-Mart or anti-NB1, which may also mimic the ANCA pattern, were found (data obtained by Prof Dr Bux, Giessen, Germany; not shown).
Search for target antigens by ELISA
By ELISA technique, antibodies against cathepsin G, BPI, lactoferrin, and human leukocyte elastase were detected in some of the preparations. No reactivity was detected against proteinase-3, myeloperoxidase, or lysozyme. With some of the batches tested, a combination of several ANCA reactivities was found. ANCA titers and ANCA profiles were shown to vary not only from preparation to preparation but also from batch to batch, corresponding to the varying composition of donors (Tables 2–3).
ANCA-IgG antigen profiles found with different IVIg preparations by ELISA testing
| . | ANCA IgG . | PR3 . | MPO . | LF . | HLE . | CG . | BPI . | LZ . |
|---|---|---|---|---|---|---|---|---|
| A1 | 1:1024 | − | − | − | − | − | + | − |
| A2 | 1:1024 | − | − | + | − | + | + | − |
| A3 | 1:512 | − | − | − | − | − | − | − |
| A4 | 1:512 | − | − | − | − | − | − | − |
| A5 | 1:2048 | − | − | NT | NT | NT | NT | NT |
| B1 | 1:32 | − | − | − | + | + | ++ | − |
| B2 | 1:128 | − | − | + | − | ++ | + | − |
| C1 | 1:64 | − | − | − | − | + | + | − |
| C2 | 1:64 | − | − | NT | NT | NT | NT | NT |
| D1 | 1:64 | − | − | − | − | − | − | − |
| D2 | 1:64 | − | − | NT | NT | NT | NT | NT |
| D3 | 1:256 | − | − | NT | NT | NT | NT | NT |
| E1 | 1:256 | − | − | − | + | + | ++ | − |
| . | ANCA IgG . | PR3 . | MPO . | LF . | HLE . | CG . | BPI . | LZ . |
|---|---|---|---|---|---|---|---|---|
| A1 | 1:1024 | − | − | − | − | − | + | − |
| A2 | 1:1024 | − | − | + | − | + | + | − |
| A3 | 1:512 | − | − | − | − | − | − | − |
| A4 | 1:512 | − | − | − | − | − | − | − |
| A5 | 1:2048 | − | − | NT | NT | NT | NT | NT |
| B1 | 1:32 | − | − | − | + | + | ++ | − |
| B2 | 1:128 | − | − | + | − | ++ | + | − |
| C1 | 1:64 | − | − | − | − | + | + | − |
| C2 | 1:64 | − | − | NT | NT | NT | NT | NT |
| D1 | 1:64 | − | − | − | − | − | − | − |
| D2 | 1:64 | − | − | NT | NT | NT | NT | NT |
| D3 | 1:256 | − | − | NT | NT | NT | NT | NT |
| E1 | 1:256 | − | − | − | + | + | ++ | − |
PR3 indicates proteinase 3; MPO, myeloperoxidase; LF, lactoferrin; HLE, human leukocyte elastase; CG, cathepsin G; BPI, bactericidal/permeability increasing protein; LZ, lysozyme; −, negative; +, weakly positive; NT, not tested; ++, positive; and +++, strongly positive as defined by OD values.
Induction of oxidative burst by IVIg
All preparations tested were capable of inducing an oxidative-burst reaction. However, activation of neutrophils by IVIg did occur only after prestimulation with TNFα; a significant burst was not induced by stimulation with TNFα alone or by IVIg alone (Figures 3–4).
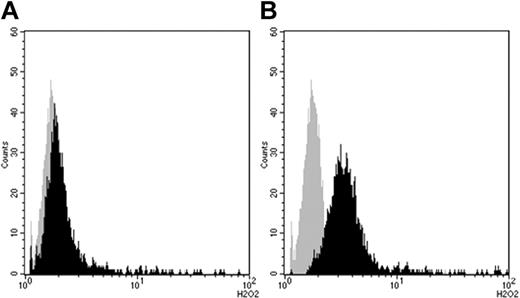
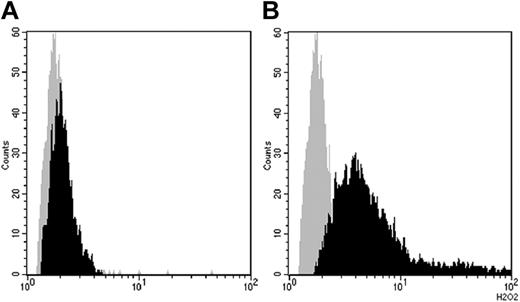
Hydrogen peroxide production (mean of fluorescence intensity; arbitrary units) in primed human peripheral-blood neutrophils before (gray curve) and after (black curve) stimulation with IVIg assessed by flow cytometry measuring the oxidation of dihydrorhodamine 123 to rhodamine 123 (x-axis; arbitrary units). (A) Oxidative burst induced by batch B1 (IgG ANCA 1:32, mean 1.72). (B) Oxidative burst induced by IVIg A1 (IgG ANCA 1:1024, mean 4.96). Incubation time 100 minutes, IVIg concentration 2.5 mg/mL in both experiments. Cells were prestimulated with TNFα (1 ng/mL) for 15 minutes.
Hydrogen peroxide production (mean of fluorescence intensity; arbitrary units) in primed human peripheral-blood neutrophils before (gray curve) and after (black curve) stimulation with IVIg assessed by flow cytometry measuring the oxidation of dihydrorhodamine 123 to rhodamine 123 (x-axis; arbitrary units). (A) Oxidative burst induced by batch B1 (IgG ANCA 1:32, mean 1.72). (B) Oxidative burst induced by IVIg A1 (IgG ANCA 1:1024, mean 4.96). Incubation time 100 minutes, IVIg concentration 2.5 mg/mL in both experiments. Cells were prestimulated with TNFα (1 ng/mL) for 15 minutes.
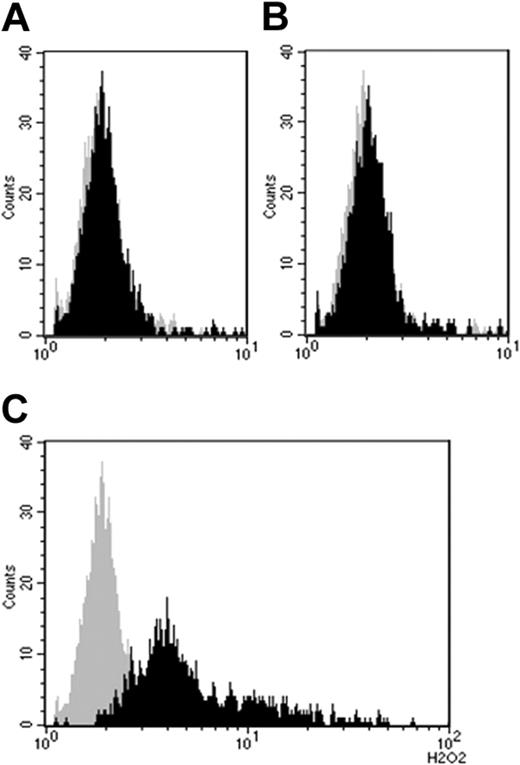
Burst induction by IVIg required prestimulation with TNFα. (A) Gray curve indicates PBS only; black curve, PBS + TNFα. (B) Gray curve indicates PBS; black curve, PBS + IVIg (batch A4). (C) Gray curve indicates PBS; black curve, PBS + TNFα + IVIg (batch A4). IVIg concentration 2.5 mg/mL, incubation time 80 minutes.
Burst induction by IVIg required prestimulation with TNFα. (A) Gray curve indicates PBS only; black curve, PBS + TNFα. (B) Gray curve indicates PBS; black curve, PBS + IVIg (batch A4). (C) Gray curve indicates PBS; black curve, PBS + TNFα + IVIg (batch A4). IVIg concentration 2.5 mg/mL, incubation time 80 minutes.
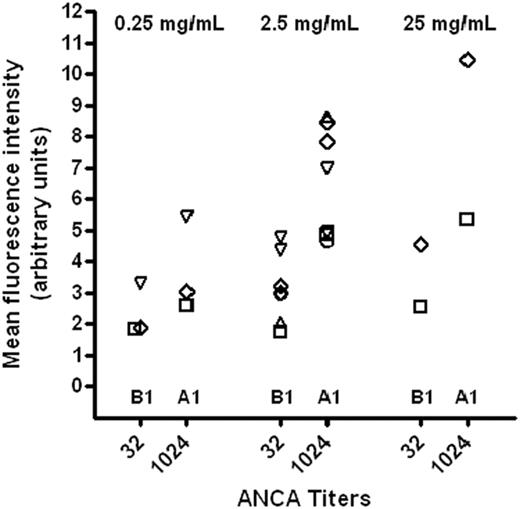
The extent of hydrogen peroxide production varied widely between preparations and batches. However, the preparation with the highest ANCA titer (A1) always induced the highest burst, and the preparation with the lowest ANCA titer (B1) always induced the lowest oxidative burst. These titer-related differences were independent of the IVIg concentration used (0.25, 2.5, 25 mg/mL), incubation time (60, 80, 100, 120, 150 min), or granulocyte donor and were reproducible in 12 of 12 trials (Figure 5).
Scatter plot showing mean fluorescence intensities of rhodamine 123 after repeated stimulation with batch B1 (ANCA IgG titer 1:32) and batch A1 (ANCA IgG titer 1:1024), respectively, employing varying IVIg concentrations (0.25, 2.5, and 25 mg/mL) and incubation times (60 ○, 80 ▿, 100 ⋄, 120 ▵, and 150 □ min). With both preparations, a dose-dependent effect of IVIg on granulocytic H202 production was seen.
Scatter plot showing mean fluorescence intensities of rhodamine 123 after repeated stimulation with batch B1 (ANCA IgG titer 1:32) and batch A1 (ANCA IgG titer 1:1024), respectively, employing varying IVIg concentrations (0.25, 2.5, and 25 mg/mL) and incubation times (60 ○, 80 ▿, 100 ⋄, 120 ▵, and 150 □ min). With both preparations, a dose-dependent effect of IVIg on granulocytic H202 production was seen.
Stimulation with F(ab′)2 fragments (prepared from batch A4) resulted in H2O2 production of similar intensity when used in a comparable concentration (Figure 6). As with intact IVIg, IVIg-F(ab′)2 was capable of activating neutrophils only after prestimulating neutrophils with TNFα (not shown).
Hydrogen peroxide production by neutrophil granulocytes stimulated with IVIg-F(ab′)2. (A) Gray curve indicates TNFα + PBS; black curve, TNFα + IVIg (batch B1). (B) Gray curve indicates TNFα + PBS; black curve, TNFα + IVIg-F(ab′)2 (batch A4). IVIg concentration 2.5 mg/mL, IVIg(Fab′)2 concentration 1.6 mg/mL, incubation time 80 minutes. Cells were prestimulated with TNFα (1 ng/mL) for 15 minutes.
Hydrogen peroxide production by neutrophil granulocytes stimulated with IVIg-F(ab′)2. (A) Gray curve indicates TNFα + PBS; black curve, TNFα + IVIg (batch B1). (B) Gray curve indicates TNFα + PBS; black curve, TNFα + IVIg-F(ab′)2 (batch A4). IVIg concentration 2.5 mg/mL, IVIg(Fab′)2 concentration 1.6 mg/mL, incubation time 80 minutes. Cells were prestimulated with TNFα (1 ng/mL) for 15 minutes.
Correlation of oxidative burst and ANCA titers
Total ANCA titers, as determined by indirect immunofluorescence (IIF), were correlated with the amount of rhodamine 123 that was formed in IVIg-exposed TNFα-primed granulocytes by the oxidation of dihydrorhodamine inside the cells. Oxidation of dihydrorhodamine indicated the formation of hydrogen peroxide and hence the IVIg-induced burst reaction. Comparing the ANCA titers of 5 preparations (A1, B1, C1, D1, E1) with their oxidative-burst–inducing activity, we found a Spearman correlation coefficient of 0.829 (P = .04) when stimulation was done with 2.5 mg/mL IVIg (corresponding to low-dose IVIg therapy) and 25 mg/mL IVIg (corresponding to high-dose IVIg therapy) and a coefficient of 0.943 (P = .005) when stimulation was done with 0.25 mg/mL IVIg. Overall correlation, disregarding IVIg concentrations and incubation times, was 0.829 (P = .04).
Discussion
Our study for the first time demonstrates that IVIg preparations contain naturally occurring antibodies that bind to and activate TNFα-stimulated neutrophil granulocytes in an antigen-dependent way. The titers of these antineutrophil antibodies varied considerably among IVIg preparations and correlated to the ability of IVIg to induce an oxidative burst in vitro. Activation of an oxidative burst in neutrophils by IVIg, however, required TNFα priming and most likely a stimulus-dependent exposure and up-regulation of surface target antigens. In subsequent experiments we explored the possibility that aggregated or multivalent immunoglobulins contained in these preparations directly activated granulocytes via Fc receptors. Using IVIg-derived F(ab′)2 fragments, lacking the Fc region of IgG, unspecific binding of aggregated IgG, dimers, or oligomers to Fc receptors was excluded. IVIg-F(ab′)2 preparations still contained significant titers against neutrophils (1:128) which equaled those of intact IVIg's when the same molar concentrations were compared. Moreover, both IVIg-F(ab′)2 and IVIg preparations acted on TNFα-primed neutrophil granulocytes and triggered an oxidative burst in primed neutrophils. Remarkably, 1 commercial IVIg is manufactured in a way that Fc domains of IgG show reduced receptor binding affinities.39 Preparation nevertheless displayed the most potent oxidative-burst–inducing effects in 12 of 12 experiments, reinforcing that Fcγ-receptor cross-linking is not responsible for these adverse effects on neutrophils.
Previously, 2 groups reported that staining of neutrophilic granulocytes with IVIg preparations can generate an atypical cytoplasmic pattern as seen with aANCA and cANCA sera from patients with rare autoimmune conditions.40,41 Our study confirms and extends these initial observations and reveals a high frequency of naturally occurring antineutrophil antibodies. Indeed, all 13 IVIg batches tested were positive for ANCA. The issue as to how these antibodies interacted with neutrophils, however, was not addressed by the previous study. In particular, it remained unclear whether IVIg's interacted with neutrophils via their antigen-combining sites or via their Fc receptor–binding domain. This latter possibility, binding of IVIg-associated antibodies to neutrophils via Fc receptors, was suggested40,42 but does not explain the binding of F(ab′)2 fragments that we prepared from IVIg preparations.
In order to elucidate the specificity of IVIg-associated self-reactive antibodies, we evaluated the binding of IVIg preparations for the first time on a BIOCHIP mosaic covering a broad range of antigens on all types of peripheral-blood cells and various human tissue sections. These analyses were also done with F(ab′)2 fragments and show that 2 IVIg preparations (B and E) contained significant reactivities with BPI and cathepsin G, 2 well-known human neutrophil antigens, whereas other preparations at the most showed borderline reactivities. In contrast, batch A4, which elicited the largest oxidative burst, and batch A5, which induced cerebral vasospasm with encephalopathy in a documented patient,16 did not react with any of the neutrophil antigens tested. Hence, our initial findings obtained by the indirect immunofluorescence test are not simply explained by a cross-reactivity with a single abundant antigen from neutrophils. While ANCA-like staining of neutrophils by IVIg was most striking and significant, we also noticed faint cytoplasmic staining of mononuclear cells (ie, monocytes), suggesting that their respective target antigens are also expressed, though at much lower concentrations, by monocytes.43 Similar differential staining of both monocytes and neutrophils occurs with ANCAs from patients with Wegener granulomatosis and microscopic polyangiitis.44 Our observation that 3 of 9 batches did not show any reactivity in the available ELISAs, but showed clear signals on ethanol-fixed neutrophils, implies that the proteins from granulocytes so far tested do not comprise all antigenic structures that interact with IVIg preparations.
The anticipated broader and variable specificity of neutrophil-reactive antibodies in pooled immunoglobulins has prompted us to consider them as a subgroup of naturally occurring antibodies (NAbs). NAbs are produced by B-1 cells prior to and independent of external antigenic stimulation. Since B-1 cells are positively selected during the neonatal period, a high proportion of NAbs are self-reactive. Human NAbs have been previously described and are directed to exoplasmic, cytoplasmic, and cytoskeletal proteins.45–47 Many of these antibodies, at least in the human but not the mouse, are of the IgG rather than the IgM class and serve a general tissue homeostatic purpose under physiologic conditions.45–51 Under certain conditions (eg, in ischemia/reperfusion injury), NAbs can have a devastating effect when a comparatively large portion of cells exposes endogenous antigens simultaneously that are otherwise not exposed or are very slowly exposed. In a mouse model of ischemia/reperfusion injury, NAbs of the IgM class have been shown to stimulate complement deposition and to contribute to tissue damage.52
While self-reactivity of naturally occurring antibodies in unfractionated serum is low and is probably masked by circulating serum proteins, pooling and processing of immunoglobulins has been shown to unmask some reactivities against self-antigens. After intravenous infusions of high IVIg doses, these reactivities may not be fully blocked by endogenous proteins of the recipient and may, therefore, bind to their cognate antigens.53 Consistent with these considerations, we found that ANCA binding to ethanol-fixed neutrophils was blocked markedly when IVIg was reconstituted at a final concentration of 50 mg/mL in pooled human serum instead of aqua ad injectionem, resulting in significantly lower ANCA titers (1:128 versus 1:512, batch A4). The same was noticed when IVIg was reconstituted in a human serum albumin (HSA) solution, indicating that albumin reduced the binding of antibodies to antigens on neutrophil surfaces.
Clinical applications of IVIg are regarded as a safe and generally well-tolerated treatment modality. Despite considerable improvements in the generation of IVIg preparations, a variety of adverse effects have been reported.9–13,16–34,41,54–56 Some of these side effects have been ascribed to the low content of IgG oligomers (aggregates) and dimers in the preparations that increase upon storage. Activation of the respiratory burst, generation of PAF, and degranulation of neutrophils can be triggered by IgG dimers and oligomers via Fcγ RIIa receptors57,58 without complexation to antigens and are thus believed to trigger some of the known clinical side effects.41 Ayliffe et al,40 however, reported on a patient who developed recurrent retinal vasculitis and panuveitis after repeated infusion of an ANCA-positive IVIg preparation. These complications improved after infusion of an ANCA-negative IVIg preparation, suggesting that antibody interactions with endogenous ANCA antigens occurred in this patient after IVIg infusions.
In view of these previous observations and in the light of the present study, Fcγ-receptor cross-linking alone in response to low amounts of aggregated, dimerized, or multivalent immunoglobulins is not expected to generate the clinical side effects of IVIg preparations as discussed. In our view, neutrophil antigens targeted by naturally occurring antibodies are most likely implicated in neutrophil activation by IVIg preparations, which moreover requires neutrophil priming (eg, by TNFα), and exposure of surface accessible epitopes. The direct interactions of IVIg antibodies with neutrophils thus share several features with classical PR3- and MPO-specific ANCAs, which contribute to the manifestations of Wegener granulomatosis and microscopic polyangiitis, respectively.
Whereas the content of dimers and oligomers in IVIg preparations can be minimized by optimization of storage conditions and purification procedures, genuine interactions between naturally occurring antibodies and antigens that are generated by neutrophils or externalized to neutrophil membranes cannot easily be prevented in vivo. Under certain conditions, when neutrophils are systemically primed or locally recruited into tissues, neutrophil-reactive antibodies of IVIg preparations may become pathogenic and may cause some of the side effects like aseptic meningitis in IVIg-treated patients. Pre-existing alterations of the cerebral microvasculature or site-specific alterations of endothelial cells may contribute to the preferential recruitment of neutrophils to the meninges. Alterations of the vascular blood brain barrier (BBB) have been demonstrated in various neurologic conditions frequently treated with IVIg including multiple sclerosis,59 Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and other polyneuropathies60 but also in migraine,60 which has previously been reported to be a risk factor for IVIg-associated aseptic meningitis28 ; BBB dysfunction is also discussed in systemic lupus with central nervous system (CNS) involvement.61 In addition, alterations of the chemokine and cytokine profiles linked to the underlying autoimmune condition or the IVIg therapy itself may be involved in priming and recruitment of neutrophils. However, since postmortem or bioptical studies have never been performed in patients with IVIg-associated side effects such as aseptic meningitis28 or cerebral vasospasm,16 it remains uncertain whether neutrophil recruitment is indeed limited to the CNS. Other organs and tissues may be subclinically affected as well.
Carefully designed clinical studies are now needed to clarify the clinical impact of our findings and to answer the question of whether specific precautions in blood donor recruitment, in the selection of patients for IVIg therapy, or in the manufacturing process are most promising to reduce the side effects of IVIg therapy. The priming state of neutrophils and the titers of IVIg antibodies that react with neutrophils from the patient may increase the risk of IVIg treatments.
Authorship
Contribution: S.J., R.V., P.E., D.E.J., and R.H. designed the study. S.J., P.E., M.W., M.H.A., and B.H.B. performed the experiments. S.J., P.E., M.W., S.W., and R.V. analyzed the data. S.J., R.V., D.E.J., and R.H. wrote the manuscript. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond Voltz, Department of Palliative Medicine, University of Cologne, D-50924 Cologne, Germany; e-mail: raymond.voltz@uk-koeln.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank O. Bieberle, I. Eckerlein, E. Eisl, R. Herbst, M. Hoffmann, and H. Pahl for expert technical assistance and F. Hoffmann MD (Munich) and James P. Chalcroft PhD for valuable advice and discussion.
This work was supported by Deutsche Forschungsgemeinschaft (SFB571; R.H., R.V., and D.E.J.), European Neurological Society (ENS; S.J.), and the Hermann und Lilly Schilling Stiftung (R.H., R.V.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal