Abstract
GATA-3 and T-box expressed in T cells (T-bet) play central roles in Th-cell development and function. Consistently, studies in mice document their selective expression in Th1 and Th2 cells, respectively. In contrast, it is not clear whether these genes are regulated in human Th cells. Here we show that T-bet expression is polarized to a comparable degree in human and mouse Th-cell cultures, while only mouse GATA3 is subject to substantial regulation. This did not reflect differential skewing efficiency in human versus mouse cultures, as these contained similar frequencies of IFN-γ– and IL-4–producing cells. However, GATA-3 was expressed at significantly higher levels in human IL-4–producing cells enriched via capture with monoclonal antibodies (mAbs) against the PGD2 receptor, CRTH2, the best selective Th2-cell surface marker to date. Along with increased IL-4 and GATA-3, CRTH2+ Th cells isolated from Th2-skewed cultures or the circulating memory pool exhibited markedly decreased IFN-γ and T-bet expression. Thus, the human GATA-3 gene is not regulated in response to polarizing signals that are sufficient to direct Th2-specific expression in mouse cells. This postulates the involvement of an additional level of complexity in the regulation of human GATA-3 expression and stresses the existence of nontrivial differences in the regulation of human versus mouse T-cell function.
Introduction
T-cell receptor (TCR) ligation by the peptide–major histocompatibility complex (MHC) class II induces the clonal expansion of naive Th cells and their differentiation into at least 2 subtypes of effector cells: Th1, which produces IFN-γ and plays a crucial role in immune responses against intracellular pathogens, and Th2, which expresses IL-4, IL-5, and IL-13 and is involved in antibody (Ab)–mediated responses to extracellular parasites. An imbalance in the relative proportion and function of either Th-cell population—as determined by the complex interaction of the genetic background and environmental factors, including exposure to certain infectious agents—has been implicated in the development of abnormal immune responses and the onset of autoimmune and allergic conditions (reviewed by Wills-Karp et al1 )
Besides the intrinsic predisposition of naive Th cells to acquire a Th1 or Th2 phenotype, which is thought to be controlled by genetic and stochastic variables, a host of extrinsic factors influences Th-cell differentiation through the regulation of dendritic-cell function, and thereby the antigen load, and the exposure to polarizing costimulatory signals and cytokines (eg, IL-12).2,3 Transcriptional and epigenetic mechanisms play eminent roles in the coordinate regulation of the molecular programs that bring to selective activation or repression of Th-cell–polarized genes. Specifically, T-box expressed in T cells (T-bet), also known as T-box 21 (Tbx21), plays a central role in Th1-cell development and selectively induces the expression of such Th1-restricted genes as IFN-γ and the β subunit of the IL-12 receptor (IL-12Rβ) (reviewed by Szabo et al4 ). Conversely, GATA-3 has been characterized as the master switch factor for Th2 cells, because it induces, by different mechanisms, the coordinate activation of the IL-4, IL-5, and IL-13 genes.5 At least as importantly, T-bet and GATA-3 contribute to the definition of a stable (ie, inheritable) polarized cytokine phenotype, also by promoting the irreversible silencing of type 2 and type 1 genes, respectively.3–5
In line with this notion, studies of in vitro–polarized murine Th cells have consistently documented the selective transcription of the T-bet and GATA-3 genes in Th1 and Th2 cells, respectively.6–8 Thus, these genes appear to be subject to tight regulation by developmental signals, particularly those delivered through the IL-12 and IL-4 receptors. Their expression pattern has rapidly become an integral part of the “Th1/Th2 paradigm” and a surrogate marker of polarization in studies of Th-cell phenotype, function, and involvement in human disease.9–12 In this context, over the past decade much attention has focused on the possibility that Th-cell polarization might be reflected in the surface phenotype as well, a notion carrying both conceptual and practical implications. A few recent studies have identified certain chemoattractant receptors as being preferentially expressed on Th1 or Th2 cells. Specifically, the chemokine receptors CCR5 and CXCR3 have been most often detected on Th1 cells, while CCR4 and CCR8 are preferentially expressed on Th2 cells.13,14
The PGD2 receptor DP-2 (CD294), also referred to as chemoattractant receptor expressed on Th2 cells (CRTH2) or G protein–coupled receptor-44 (GPR-44), has recently emerged as a consistent, predictive marker of Th2-cell function, IL-4 production, and Th2 bias in basic and translational studies.15–18 CRTH2 ligation by PGD2 can enhance Th2-cell function and signaling through CRTH2 perhaps contributing to Th2-cell differentiation.19,20 However, while this molecule is clearly Th2-restricted in the human species, no such bias is apparent in murine Th cells.20,21 This does not seem to reflect a general failure to transcribe the CRTH2 gene in the mouse strains studied so far—the molecule is indeed expressed at high levels on both human and mouse eosinophils22 —and points to substantial differences in the mechanisms of polarized gene expression in Th cells from either species.
While much of our current knowledge of Th-cell differentiation and function comes from studies in established mouse models, few studies to date have been aimed at comparing human and mouse Th cells, and the results are not always consistent (reviewed by Mestas and Hughes12 ). In particular, contrasting data in the recent literature do not allow a definitive conclusion to be reached as to whether transcription of the T-bet and GATA-3 genes is regulated in differentiating human Th cells. In this study we show a comparable degree of polarization of the IL-4, IFN-γ, CRTH2, and T-bet genes in cells from unrelated human donors and 2 mouse strains. In contrast, although GATA-3 is expressed, as expected, at markedly higher levels in mouse Th2 than Th1 cultures, its expression is regulated similarly in human Th1- and Th2-skewed cells. However, we detected significantly higher levels of GATA-3 transcripts in the CRTH2-expressing subset of human Th2 cultures, in parallel with increased IL-4 expression and decreased IFN-γ and T-bet expression. We infer that the human GATA-3 gene is not as strictly regulated as is its mouse counterpart in response to Th-polarizing signals. Increased transcription of GATA3 in CRTH2+ cells is indeed associated with a more pronounced Th2 phenotype, but its unrestricted expression in human Th cultures speaks against its characterization as an exclusive downstream target of IL-12 and IL-4 signaling and a consistent molecular marker of human Th-cell polarization.
Materials and methods
Human Th-cell separation and culture
This study was conducted in accordance with federal regulations on human subject protection with informed consent and approved by a Johns Hopkins Medicine institutional review board (IRB). Twenty donors in apparent good health (16 females and 11 males aged 19 to 49 years; mean ± SEM, 30.5 ± 2.1) with no history of asthma or atopy were recruited from the Johns Hopkins Asthma and Allergy outpatient clinic and personnel. Sixty milliliters of anticoagulated blood were diluted 1:3 with sterile PBS and peripheral-blood mononuclear cells (PBMCs) separated on Ficoll-Hypaque density gradients (GE Healthcare, Piscataway, NJ). Naive Th cells were purified to 95% to 99% by negative selection using the StemSep Human Naive CD4+ T-Cell Enrichment Cocktail (StemCell Technologies, Vancouver, BC, Canada) and LS magnetic-activated cell separation (MACS) columns (Miltenyi Biotec, Auburn, CA) and then cultured for 1 week in RPMI 1640 (Invitrogen, Carlsbad, CA), 10% FBS (Hyclone, Logan, UT), 40 IU/mL rIL-2 (Biological Resources Branch [BRB] Preclinical Repository, National Cancer Institute [NCI], Frederick, MD), 1% l-glutamax (Invitrogen), 0.1 mg/mL Primocin (Amaxa, Gaithersburg, MD), and anti-CD3/anti-CD28–coated beads (0.5 per cell; Dynabeads CD3/CD28 T-Cell Expander; Invitrogen). Th1- and Th2-skewing conditions consisted respectively of 5 ng/mL rIL-12 (R&D Systems, Minneapolis, MN) and 5 μg/mL anti–IL-4 monoclonal antibody (mAb) (clone MP4-25D2; eBioscience, San Diego, CA) and of 50 ng/mL rIL-4 (R&D Systems) and 10 μg/mL anti–IFN-γ mAb (NIB42; eBioscience).23
Enrichment and culture of CRTH2-sorted Th cells
CRTH2+ cells were enriched by positive selection with immunomagnetic beads. Th cells were incubated for 30 minutes with an unconjugated anti-CRTH2 mAb (BM16; BD Biosciences, San Diego, CA), extensively washed, and then incubated for an additional 30 minutes with sheep anti–rat IgG Ab-coated beads for separation against a magnetic concentrator (Dynal MPC-1; Invitrogen). Purity of the recovered cells, as verified by the appreciation of bead-associated cells in the resulting preparations, was consistently more than 95% from Th2-skewed cultures and more than 70% from negatively selected peripheral-blood CD4+ T cells. This was confirmed in preparations obtained by selection with PE-labeled anti-CRTH2 and LS columns (CD294 MicroBead Kit; Miltenyi Biotec). As reported,18 CRTH2+ cells from both sources were uniformly CD45RO+CD25+ (not shown). After CRTH2+ cell removal, CD4+CD45RO+CRTH2− T cells were obtained by magnetic selection with an anti-CD45RO biotinylated mAb (StemCell Technologies) and streptavidin-coupled beads (Dynabeads M-280 Streptavidin; Invitrogen). CD45RO was consistently detected on more than 95% of the recovered cells, less than 0.1% of which expressed CRTH2. CRTH2-sorted Th cells were expanded for 3 to 7 days with no substantial changes in the surface phenotype, in media supplemented with anti-CD3/CD28–coated beads and IL-2 as outlined in “Human Th-cell separation and culture.” Where indicated, IL-4–producing cells were enriched from Th2-skewed cultures and stimulated with PMA (20 ng/mL; Sigma-Aldrich, St Louis, MO) and ionomycin (1 μg/mL; Sigma-Aldrich) using the IL-4 Secretion Assay (Miltenyi Biotec) as per manufacturer's specifications.24
Mouse Th-cell separation and culture
C57BL/6 and BALB/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and killed at 5 to 8 weeks of age. Throughout the study mice were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)–endorsed pathogen-free facility and handled in accordance with federal regulations and upon approval by the Johns Hopkins Animal Care and Use Committee (ACUC). CD4+ T cells were isolated from mechanically dispersed splenocytes by negative selection using the StemSep Mouse CD4+ T-Cell Enrichment Cocktail (StemCell Technologies) and LS MACS columns (Miltenyi Biotec). Cells were cultured for 1 week in RPMI supplemented with 5 ng/mL rIL-2 (R&D Systems) and 1 μg/mL each of anti-CD3ϵ (145-2C11; BD Biosciences) and anti-CD28 mAbs (37.51; BD Biosciences) on plates coated with goat anti–armenian hamster IgG (40 μg/mL; Jackson ImmunoResearch Laboratories, West Grove, PA).25 A total of 5 ng/mL rIL-12 (R&D Systems) plus 10 μg/mL anti–IL-4 (11B11; BD Biosciences) and 20 ng/mL rIL-4 (R&D Systems) plus 10 μg/mL anti–IFN-γ (XMG1.2; BD Biosciences) were added to induce Th1 and Th2 skewing, respectively.
Protein expression studies
For surface staining, about 106 cells were incubated 30 minutes at room temperature with the following mAbs: anti-CD4–FITC (S3.5) and anti-CD3–TRI-COLOR (S4.1) from Caltag Laboratories (Invitrogen, Carlsbad, CA); PE-conjugated anti-CCR5 (2D7/CCR5) (BD Biosciences) or anti-CRTH2 (BM16; Miltenyi Biotec); or isotype- and fluorochrome-matched controls (Caltag Laboratories). For intracellular staining, cells were stimulated or not with PMA/ionomycin for 5 hours, the last 4 in the presence of 1 μL/1.5 × 106 cells of a monensin-containing solution (Golgistop; BD Biosciences). Cells were stained with anti–IFN-γ–PE (B27; Caltag Laboratories) and anti–IL-4–APC (11B11; eBioscience) upon fixation in 2% paraformaldehyde and permeabilization with 0.08% Triton X-100. Permeabilized cells were also stained with a GATA-3 mAb (291119; R&D Systems) or the corresponding Ig control and a secondary, FITC-conjugated, F(ab′)2 (Jackson ImmunoResearch Laboratories).26 In all cases cells were loaded on a FACSCalibur flow cytometer (BD Biosciences) and the results analyzed with FCS Express 3.0 (De Novo Software, Thornhill, ON, Canada). Secreted IFN-γ and IL-4 were measured using commercial enzyme-linked immunosorbent assay (ELISA) sets (Ready-SET-Go!; eBioscience) in cell supernatants upon 20-hour stimulation with PMA and ionomycin. Western blot was performed with whole-cell extracts, electrophoresed, and transferred to PVDF membranes on a mini-PROTEAN 3 System (BioRad Laboratories, Hercules, CA). Blots were probed with an anti–GATA-3 mAb (291119; BD Biosciences) and, as protein loading and transfer control, an anti–α-tubulin mAb (DM1A; Lab Vision, Fremont, CA) and then developed with the ECL Western blotting system from GE Healthcare.
RNA studies
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) and its concentration measured on a DU 640 spectrophotometer (Beckman Coulter, Fullerton, CA). cDNA was generated by reverse transcription using the SuperScript First-Strand Synthesis System (Invitrogen) and then amplified using the SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA). Primers were custom synthesized (IDT, Skokie, IL) upon computation using Primer3 27,28 of cDNA template sequences from the UniGene database29 (National Center for Biotechnology Information, National Institutes of Health [NCBI, NIH], Bethesda, MD) aligned onto the corresponding genomic sequences by basic local alignment sequence tool (BLAST) (NCBI). Primer sequences are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Among these, Table S1 lists 2 sets of primers for human GATA-3 yielding nonoverlapping long (L) or short (S) amplicons whose electrophoretic mobilities and melting profiles are shown in Figure S1. Reactions were run for real-time fluorometric quantitation in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The level of transcript expression is presented as percent of the housekeeping RNA for peptidylprolyl isomerase A (PPIA; Table S1),30 calculated as 2−ΔCT, where ΔCT equals the difference of the proband cycle threshold (CT) with PPIA CT. For absolute quantifications, samples were run and analyzed on an Applied Biosystems 7300 Real-Time PCR System in parallel with a duplicate standard curve consisting of serial 2-fold dilutions (0.2 pg to 1 ng) of a cloned cDNA (OriGene Technologies, Rockville, MD).
Results
Failure of polarizing signals to regulate GATA-3 levels in human Th cells in vitro
To understand whether species-specific differences exist in the mechanisms of polarized gene transcription in human and mouse Th cells, we compared the effect of established polarizing conditions on cells isolated from human peripheral blood or mouse spleen tissue.23,25 Th cells were expanded with immobilized anti-CD3 and anti-CD28 Abs and exposed to IL-12 or IL-4. Figure 1A-B shows the frequencies of IFN-γ– and IL-4–producing cells in 7-day cultures restimulated with PMA and ionomycin. Figure 1C compares the concentrations of these cytokines in the supernatants of restimulated cultures. Under these conditions, human Th cells were consistently skewed to a type 1 or 2 cytokine-producing phenotype, IFN-γ–positive cells amounting to 58.1% ± 5.4% and 5.3% ± 1.0% and IL-4+ cells to 0.8% ± 0.2% and 12.2% ± 1.4% in Th1 and Th2 cultures, respectively (P < .001 for IFN-γ or IL-4 in Th1 versus Th2 cells). The frequencies of IL-4– and IL-13–producing cells were in the same range, the latter averaging 1.7% ± 0.7% and 13.5% ± 3.4% in Th1 and Th2 cultures (n = 5), respectively. Besides the expected interindividual variations, these findings—exemplified in the individual fluorescence-activated cell sorter (FACS) profile shown in Figure 1A—confirm that, in both humans and mice, Th1 differentiation is a more efficient process than Th2 differentiation, at least under defined culture conditions in vitro.23,25,31–33 Prolonged culture upon readministration of IL-12 or IL-4 (up to 21 days) did not substantially modify the IFN-γ/IL-4 balance in these cultures (data not shown). A similar profile was consistently observed in polarizing cultures of Th cells isolated from BALB/c or C57BL/6 spleens (data not shown).
In vitro polarization of human Th naive precursors. CD4+CD45RA+ T cells were isolated from peripheral blood by negative selection as specified in “Materials and methods.” Cells were stimulated with anti-CD3/anti-CD8 beads (0.5 beads per cell) and cultured for 7 to 21 days under Th1 or Th2 polarizing conditions (see “Materials and methods”). (A) Shown is a typical experiment in which 7-day Th1 and Th2 cultures were restimulated for 5 hours with PMA (10 ng/mL) and ionomycin (1 μg/mL) in the presence of monensin and then fixed and permeabilized for single-cell detection of IL-4 and IFN-γ. (B) Percentages of IL-4– and IFN-γ–producing cells in similar experiments performed with 7-day Th-cell preparations from 15 donors. Differences were highly significant (P < .001; paired t test). (C) IL-4 and IFN-γ secretion from human in vitro–polarized cultures. IL-4 and IFN-γ were measured by ELISA in the supernatants of cells stimulated (20 hours) with PMA and ionomycin as specified. Means ± SEM of 6 donors. *P < .05 relative to Th1.
In vitro polarization of human Th naive precursors. CD4+CD45RA+ T cells were isolated from peripheral blood by negative selection as specified in “Materials and methods.” Cells were stimulated with anti-CD3/anti-CD8 beads (0.5 beads per cell) and cultured for 7 to 21 days under Th1 or Th2 polarizing conditions (see “Materials and methods”). (A) Shown is a typical experiment in which 7-day Th1 and Th2 cultures were restimulated for 5 hours with PMA (10 ng/mL) and ionomycin (1 μg/mL) in the presence of monensin and then fixed and permeabilized for single-cell detection of IL-4 and IFN-γ. (B) Percentages of IL-4– and IFN-γ–producing cells in similar experiments performed with 7-day Th-cell preparations from 15 donors. Differences were highly significant (P < .001; paired t test). (C) IL-4 and IFN-γ secretion from human in vitro–polarized cultures. IL-4 and IFN-γ were measured by ELISA in the supernatants of cells stimulated (20 hours) with PMA and ionomycin as specified. Means ± SEM of 6 donors. *P < .05 relative to Th1.
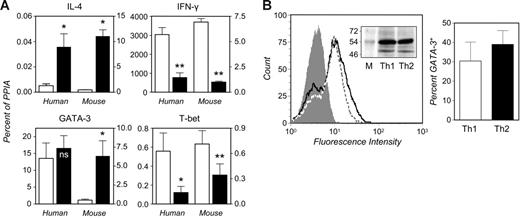
As shown in Figure 2A, comparably significant differences were appreciated in the relative levels of IFN-γ and IL-4 transcripts in human and mouse Th cells, as measured by quantitative, real-time reverse transcriptase–polymerase chain reaction (RT-PCR). Aside from the expected, consistent qualitative differences in cytokine gene expression in human or murine polarized Th cells, it should be noted that human Th cells, irrespective of the polarized state, expressed markedly higher levels of IFN-γ and lower levels of IL-4 mRNA (Figure 2A) and protein (not shown) than mouse cells. It is unlikely that this reflects an increased intrinsic propensity of human versus mouse Th cells toward a type 1 phenotype, because the frequencies of IFN-γ– or IL-4–producing cells in these cultures were comparable (not shown). Human and murine Th cells exhibited substantially similar overall levels of GATA-3 and T-bet transcripts (Figure 2A). However, while T-bet levels were significantly higher in human or murine Th1 than Th2 cells, the reported Th2-selective expression of GATA-3 could be appreciated in mouse but not human Th cells (Figure 2A). Similar results were obtained in experiments in which absolute GATA-3 mRNA levels were calculated against a cDNA standard curve (not shown). A modest 2- to 3-fold increase in GATA-3 levels could be detected in Th2-skewed cultures from 3 of 10 donors, but these interindividual differences did not correlate with overall cytokine production or the frequencies of cytokine-producing cells; nor were they in any apparent connection to an otherwise undocumented atopic background.
The GATA-3 gene is expressed at similar levels in human Th1- and Th2-skewed cultures. (A) Quantitative analysis of gene expression in human and murine in vitro–polarized Th cells. Naive Th cells were isolated from human peripheral blood or BALB/c spleens and cultured under Th1- (□) or Th2-skewing conditions (▪) as specified in “Materials and methods.” Cells were restimulated (5 hours) with PMA and ionomycin to induce cytokine production. RNA was extracted in parallel stimulated and unstimulated cultures for quantitative real-time RT-PCR (see “Materials and methods”). Transcript levels are expressed as percent of the housekeeping control, PPIA, calculated as 2−ΔCT. Values determined in human cells are plotted against the left y axes and those obtained in mouse against the right axes. Means ± SEM of 3 (mouse IL-4 and IFN-γ), 5 (mouse GATA-3 and T-bet; human IL-4 and IFN-γ), or 10 experiments (human GATA-3 and T-bet). *P < .05; **P < .005 relative to Th1; ns indicates nonsignificant. (B) Th1- and Th2-skewed cells were fixed and permeabilized and then stained with a GATA-3 mAb (see “Materials and methods”). The left histogram shows a typical experiment in which the level of staining for GATA-3 is compared in Th1 (dotted line) and Th2 cultures (solid line). The filled histogram is the Th1 population stained with a species- and isotype-matched control (a superimposable histogram was produced upon control staining of Th2 cells). The mean ± SEM frequency of GATA-3+ cells in 4 independent experiments is shown in the bar graph. The inset shows the electrophoretic mobility of the species recognized by the GATA-3 mAb (lower band) in whole-cell extracts from matching numbers of Th1 and Th2 cells. The upper band is generated upon staining for the housekeeping protein, α-tubulin. M indicates molecular weight markers.
The GATA-3 gene is expressed at similar levels in human Th1- and Th2-skewed cultures. (A) Quantitative analysis of gene expression in human and murine in vitro–polarized Th cells. Naive Th cells were isolated from human peripheral blood or BALB/c spleens and cultured under Th1- (□) or Th2-skewing conditions (▪) as specified in “Materials and methods.” Cells were restimulated (5 hours) with PMA and ionomycin to induce cytokine production. RNA was extracted in parallel stimulated and unstimulated cultures for quantitative real-time RT-PCR (see “Materials and methods”). Transcript levels are expressed as percent of the housekeeping control, PPIA, calculated as 2−ΔCT. Values determined in human cells are plotted against the left y axes and those obtained in mouse against the right axes. Means ± SEM of 3 (mouse IL-4 and IFN-γ), 5 (mouse GATA-3 and T-bet; human IL-4 and IFN-γ), or 10 experiments (human GATA-3 and T-bet). *P < .05; **P < .005 relative to Th1; ns indicates nonsignificant. (B) Th1- and Th2-skewed cells were fixed and permeabilized and then stained with a GATA-3 mAb (see “Materials and methods”). The left histogram shows a typical experiment in which the level of staining for GATA-3 is compared in Th1 (dotted line) and Th2 cultures (solid line). The filled histogram is the Th1 population stained with a species- and isotype-matched control (a superimposable histogram was produced upon control staining of Th2 cells). The mean ± SEM frequency of GATA-3+ cells in 4 independent experiments is shown in the bar graph. The inset shows the electrophoretic mobility of the species recognized by the GATA-3 mAb (lower band) in whole-cell extracts from matching numbers of Th1 and Th2 cells. The upper band is generated upon staining for the housekeeping protein, α-tubulin. M indicates molecular weight markers.
Figure 2B shows that, in line with the RNA determinations, no substantial differences could be appreciated in the levels of immunoreactive GATA-3 in human Th1- and Th2-skewed cells fixed and permeabilized for flow cytometry. Likewise, its relative amount and electrophoretic mobility were similar in immunoblots of whole-cell extracts from either culture. This ruled out the possible impact of translational or posttranslational events (eg, ubiquitination34 ) in the regulation of this factor's expression in human Th cells and contributed to validating real-time PCR as a convenient, yet sufficient, tool for the appreciation of quantitative differences in distinct culture conditions. Furthermore, as indicated in Figure S1, the primers used throughout the study consistently yielded a single amplicon (S) of expected electrophoretic mobility and melting profile.
“Enhanced” Th2 features in Th2-skewed cells expressing CRTH2
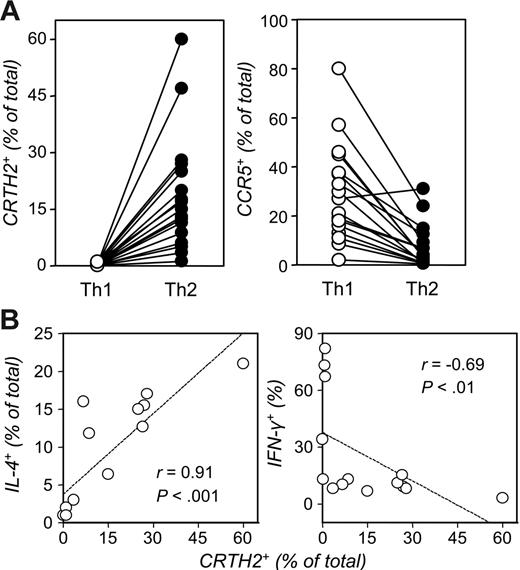
We entertained the possibility that the relatively low proportion of IL-4–producing cells in Th2-skewed cultures could, at least in the human species, hinder the appreciation of differences in GATA-3 expression. The recent characterization of CRTH2 as a faithful Th2 marker prompted us to pursue this line of investigation. CRTH2 has been reported to be selectively expressed on the surface of IL-4–producing T cells.18,33 We consistently detected markedly increased frequencies of cells expressing this surface marker in human Th2-skewed cultures (P < .001; Figure 3A). In contrast, as Figure 3A also shows, the CCL3-5 receptor, CCR5, was expressed on a significantly higher percentage of Th1- than Th2-skewed cells (P < .001). As reported,18,33,35 neither marker was expressed on the surface of unstimulated, naive Th cells (data not shown). Figure 3B shows that the frequencies of CRTH2- and IL-4–expressing Th cells in these cultures were significantly correlated and both inversely correlated with those of IFN-γ–producing cells. Likewise, the frequencies of IL-13–producing cells directly correlated with those of IL-4– and CRTH2-expressing Th cells (not shown). In general, cells that stained for IL-4 also stained for CRTH2 upon fixation-permeabilization (Figure 4A). In fact, while we detected the majority of IL-4–producing cells in the CRTH2+ subset, a variable proportion of CRTH2+ cells did not apparently produce IL-4, partly due to the relatively low sensitivity of intracellular cytokine detection in mildly fixed cells—cells had to be treated with 2% or less paraformaldehyde for CRTH2 and CCR5 detection18 —and, on more conceptual ground, the reported stochastic, quite erratic, monoallelic activation of the IL4 gene in Th2 cells.36 Conversely, even mild fixation decreased by at least 50% the intensity of CRTH2 staining, which, together with the reported down-regulatory effect of cell stimulation on expression of this marker,18,20 might explain the lack of detection of CRTH2 in a relatively low fraction of IL-4–producing cells.
The occurrence of CRTH2+ and CCR5+ cells in human Th-cell–polarizing cultures. (A) Naive CD4+ T cells from 18 donors were polarized as detailed in Figure 1. Seven-day cultures were analyzed by FACS using PE-conjugated Abs for human CRTH2 or CCR5 (see “Materials and methods”). Each dot represents an individual donor (P < .001; paired t test). (B) Polarized Th cells from 14 donors were restimulated with PMA and ionomycin in the presence of monensin and then permeabilized and stained for expression of IL-4 or IFN-γ. Shown is the correlation (Spearman) of the frequencies of IL-4– or IFN-γ–producing cells with baseline expression of CRTH2 in parallel samples of Th2 cultures.
The occurrence of CRTH2+ and CCR5+ cells in human Th-cell–polarizing cultures. (A) Naive CD4+ T cells from 18 donors were polarized as detailed in Figure 1. Seven-day cultures were analyzed by FACS using PE-conjugated Abs for human CRTH2 or CCR5 (see “Materials and methods”). Each dot represents an individual donor (P < .001; paired t test). (B) Polarized Th cells from 14 donors were restimulated with PMA and ionomycin in the presence of monensin and then permeabilized and stained for expression of IL-4 or IFN-γ. Shown is the correlation (Spearman) of the frequencies of IL-4– or IFN-γ–producing cells with baseline expression of CRTH2 in parallel samples of Th2 cultures.
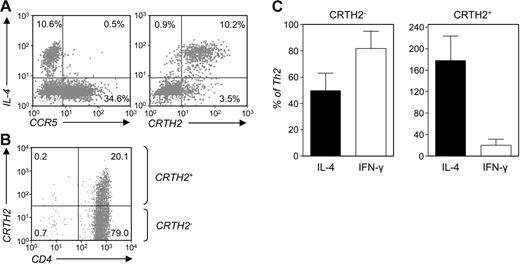
CRTH2+ Th cells exhibit an “enhanced” Th2 phenotype. (A) Two-color staining for IL-4 and CCR5 or CRTH2 in Th2-skewed cells restimulated and monensin-treated as in Figure 2B. (B) Seven-day Th2 cultures were sorted by CRTH2 expression for further phenotypic analysis. The resulting preparations are referred to as “CRTH2+” and “CRTH2−.” (C) IL-4 and IFN-γ levels were measured by ELISA of supernatants collected from either preparation after 3-day subculture and 20-hour stimulation with PMA and ionomycin and expressed as percent of the levels detected in samples of the parental Th2 cultures left unfractionated and stimulated in parallel. Shown are the means ± SEM of 5 independent experiments. Differences in the expression of IL-4 and IFN-γ in CRTH2− versus CRTH2+ cultures were statistically significant (P < .05).
CRTH2+ Th cells exhibit an “enhanced” Th2 phenotype. (A) Two-color staining for IL-4 and CCR5 or CRTH2 in Th2-skewed cells restimulated and monensin-treated as in Figure 2B. (B) Seven-day Th2 cultures were sorted by CRTH2 expression for further phenotypic analysis. The resulting preparations are referred to as “CRTH2+” and “CRTH2−.” (C) IL-4 and IFN-γ levels were measured by ELISA of supernatants collected from either preparation after 3-day subculture and 20-hour stimulation with PMA and ionomycin and expressed as percent of the levels detected in samples of the parental Th2 cultures left unfractionated and stimulated in parallel. Shown are the means ± SEM of 5 independent experiments. Differences in the expression of IL-4 and IFN-γ in CRTH2− versus CRTH2+ cultures were statistically significant (P < .05).
Overall, these results indicated that type 2 cytokine expression is largely restricted to the CRTH2+ subset within human Th2-skewed cultures. To verify whether this corresponded to a consistent pattern of expression of GATA-3 and T-bet, we analyzed the expression of these factors in cells sorted by expression of CRTH2. CRTH2-expressing cells were enriched by positive selection in 7-day Th2-skewing cultures (Figure 4B). The enrichment protocol we adopted for these experiments did not allow for appreciating the degree of enrichment of the CRTH2+ populations before 3 day of subculture under neutral conditions, at which time these cells represented more than 70% of the total. However, in selected parallel separations using PE-labeled anti-CRTH2 and anti-PE–coated beads we could determine an initial purity in the range of 95% to 98%. By contrast, the remaining Th2-skewed cells contained no CRTH2+ cells upon separation and less than 2% at 3 days of subculture. CRTH2+ cells amounted to about 40% and 10% or less in 7-day CRTH2+ and CRTH2− unskewed cultures (U.D.F., M.B., F.M., W.K.L., and V.C., manuscript in preparation). For consistency, these preparations were analyzed at 3 days of subculture. As shown in Figure 4C, CRTH2+ cells produced 3- to 5-fold more IL-4 than CRTH2− cells (P < .05). In addition, CRTH2+ cells produced similarly reduced levels of IFN-γ (P < .05; Figure 4C). These findings are consistent with the notion that CRTH2+ Th cells express a clear-cut Th2 phenotype.17,18
Reverse pattern of T-bet and GATA-3 expression in CRTH2-sorted human Th cells
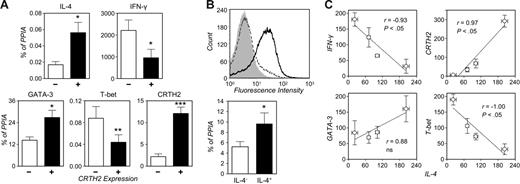
By quantitative RT-PCR we measured the transcript levels for genes undergoing polarized regulation in human CRTH2+ and CRTH2− Th2-skewed cells. As shown in Figure 5A, and consistent with the cytokine secretion profile shown in Figure 4C, CRTH2+ cells expressed significantly higher levels of IL-4 and lower levels of IFN-γ mRNA than CRTH2− cells (P < .05). Not surprisingly, CRTH2 transcripts were markedly more abundant in CRTH2+ than CRTH2− cells (P < .001; Figure 5A). T-bet was consistently expressed at lower levels in CRTH2+ than CRTH2− cells (P < .01), while GATA-3 expression was modestly but significantly higher in the former subset (P < .05). Similar results were obtained in preparations sorted by expression of IL-4 (Figure 5B).24 Thus, expression of both transcriptional regulators seemed to parallel differential cytokine gene expression when CRTH2 and/or IL-4 expression were taken into account. When comparing the normalized transcript levels in the 4 Th-cell preparations studied (ie, Th1-skewed, Th2-skewed, and the CRTH2-sorted fractions of the latter) there was a good overall correlation, either direct or inverse, between IL-4 levels and the abundance of IFN-γ, CRTH2, and T-bet transcripts (Figure 5C). In contrast, GATA-3 expression only seemed to correlate with IL-4 expression within Th2-skewed cultures, with levels of both gene transcripts being lower in CRTH2− and higher in CRTH2+ cells than in the parental Th2 culture (Figure 5C).
Quantitative analysis of gene expression in Th2-skewed cells sorted by CRTH2 or IL-4 expression. (A) Three-day cultures of CRTH2− and CRTH2+ cells were stimulated (5 hours) or not with PMA and ionomycin and RNA extracted for quantitative real-time RT-PCR (see “Materials and methods”). Transcript levels are expressed as percent of the housekeeping control, PPIA. Means ± SEM of 5 experiments. *P < .05; **P < .01; ***P < .001 relative to CRTH2−. (B) IL-4+ cells were enriched from Th2-skewed cultures by positive selection using the MACS IL-4 Secretion Assay as specified in “Materials and methods.” The graph above shows the staining level for IL-4 in the selected cells (solid line), in the residual cells (filled histogram), and in the parental Th2 culture (dotted line) in a typical separation. RNA from IL-4+ and IL-4− cells was extracted for quantitative real-time RT-PCR. GATA-3 transcript levels (expressed as percent of PPIA) are shown below and are the mean ± SEM of 4 experiments. *P = .05 relative to IL-4−. (C) Correlations (Spearman) of IL-4 transcript levels with levels of IFN-γ, CRTH2, GATA-3, and T-bet in Th1 and Th2 cultures and in CRTH2-sorted preparations. Transcript levels, relative to PPIA, were normalized across donors by conversion to percent of the average in parallel preparations. Each dot is the mean ± SEM of 4 donors.
Quantitative analysis of gene expression in Th2-skewed cells sorted by CRTH2 or IL-4 expression. (A) Three-day cultures of CRTH2− and CRTH2+ cells were stimulated (5 hours) or not with PMA and ionomycin and RNA extracted for quantitative real-time RT-PCR (see “Materials and methods”). Transcript levels are expressed as percent of the housekeeping control, PPIA. Means ± SEM of 5 experiments. *P < .05; **P < .01; ***P < .001 relative to CRTH2−. (B) IL-4+ cells were enriched from Th2-skewed cultures by positive selection using the MACS IL-4 Secretion Assay as specified in “Materials and methods.” The graph above shows the staining level for IL-4 in the selected cells (solid line), in the residual cells (filled histogram), and in the parental Th2 culture (dotted line) in a typical separation. RNA from IL-4+ and IL-4− cells was extracted for quantitative real-time RT-PCR. GATA-3 transcript levels (expressed as percent of PPIA) are shown below and are the mean ± SEM of 4 experiments. *P = .05 relative to IL-4−. (C) Correlations (Spearman) of IL-4 transcript levels with levels of IFN-γ, CRTH2, GATA-3, and T-bet in Th1 and Th2 cultures and in CRTH2-sorted preparations. Transcript levels, relative to PPIA, were normalized across donors by conversion to percent of the average in parallel preparations. Each dot is the mean ± SEM of 4 donors.
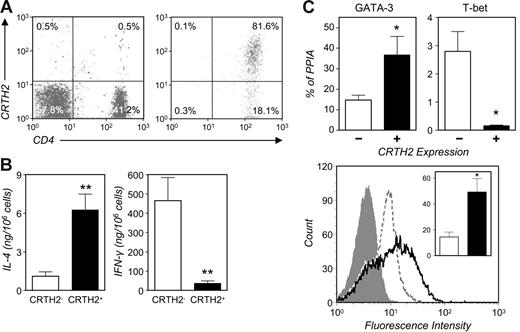
To further substantiate these findings, we studied the expression of these genes in freshly isolated peripheral-blood Th cells. CRTH2+ cells represent about 1% to 2% of the circulating CD3+CD4+ population and almost invariably express the memory marker, CD45RO, and the activation marker, CD25 (data not shown).18 We enriched these cells by positive selection from the CD45RO+ pool. Using a PE-labeled anti-CRTH2 mAb and anti-PE beads as outlined in the previous paragraph allowed us to verify the degree of enrichment of CRTH2+ Th cells immediately after magnetic separation. As in the experiment shown in Figure 6A, a 50- to 200-fold enrichment of these cells was observed in preparations obtained with this protocol. These cells exhibited a remarkably high degree of polarization, as evidenced in experiments comparing IL-4 and IFN-γ production with that in CRTH2−CD45RO+ Th cells (Figure 6B), the latter including a variable percentage of cells expressing the Th1 surface marker, CCR5 (data not shown). As shown in Figure 6C, circulating CRTH2+ Th cells expressed significantly higher RNA levels of GATA-3 and strikingly lower levels of T-bet (P < .05) than CRTH2− cells. Figure 6C (right panel) also shows that these CRTH2+ preparations exhibited a higher frequency of cells expressing high levels of immunoreactive GATA-3, as determined by flow cytometry in fixed/permeabilized cells.
Ex vivo characterization of circulating CD4+CRTH2+ T cells. (A) CRTH2+ cells were enriched to more than 70% by positive selection from peripheral-blood CD4+CD45RO+ T cells (see “Materials and methods”). Shown is their frequency in the parental PBMC preparation and in the enriched fraction in a typical experiment. (B) CRTH2+ cell–enriched preparations consistently produced higher levels of IL-4 and lower levels of IFN-γ than CD4+CD45RO+CRTH2− cells in response to stimulation with PMA and ionomycin. Mean ± SEM cytokine production from 6 (IL-4) or 5 donors (IFN-γ). **P < .005. (C) GATA-3 expression in CRTH2-sorted peripheral-blood CD4+CD45RO+ T cells. Real-time RT-PCR and flow cytometry were used for quantitative analysis of GATA-3 expression at the RNA and protein level. The top panels show the mean (± SEM) GATA-3 and T-bet RNA levels in sorted preparations from 6 donors. The bottom panel shows GATA-3 flow cytometric determination in CRTH2+ (solid line) and CRTH2− cells (dotted line) from an individual donor (filled histogram: isotype control) and, inset, the mean ± SEM percent GATA-3+ cells in CRTH2+ (filled bar) and CRTH2− preparations (empty bar) in 3 donors. *P < .05.
Ex vivo characterization of circulating CD4+CRTH2+ T cells. (A) CRTH2+ cells were enriched to more than 70% by positive selection from peripheral-blood CD4+CD45RO+ T cells (see “Materials and methods”). Shown is their frequency in the parental PBMC preparation and in the enriched fraction in a typical experiment. (B) CRTH2+ cell–enriched preparations consistently produced higher levels of IL-4 and lower levels of IFN-γ than CD4+CD45RO+CRTH2− cells in response to stimulation with PMA and ionomycin. Mean ± SEM cytokine production from 6 (IL-4) or 5 donors (IFN-γ). **P < .005. (C) GATA-3 expression in CRTH2-sorted peripheral-blood CD4+CD45RO+ T cells. Real-time RT-PCR and flow cytometry were used for quantitative analysis of GATA-3 expression at the RNA and protein level. The top panels show the mean (± SEM) GATA-3 and T-bet RNA levels in sorted preparations from 6 donors. The bottom panel shows GATA-3 flow cytometric determination in CRTH2+ (solid line) and CRTH2− cells (dotted line) from an individual donor (filled histogram: isotype control) and, inset, the mean ± SEM percent GATA-3+ cells in CRTH2+ (filled bar) and CRTH2− preparations (empty bar) in 3 donors. *P < .05.
Discussion
Expression of T-bet and GATA-3 follows an opposite pattern in in vitro–generated Th1 and Th2 cells.3–8,25,37 Studies in murine polarized cultures, lines, and clones consistently show a clear-cut bias in the expression of these factors both at the RNA and protein level. This led to the conclusion that transcriptional regulation at the TBX21 and GATA3 loci is a first limiting step in the Th-cell effector response to polarizing signals. A more extreme view of this now-established paradigm postulates that T-bet and/or GATA-3 up-regulation occurs stochastically in naive cells prior to exposure to polarizing signals. These, in this case, would be acting by selecting for expansion those cells that, by exclusive expression of either factor, are predisposed to IL-4 or IFN-γ gene activation.3,38
Studies in human Th cells do not necessarily lend support to this notion. Following earlier attempts using semiquantitative or nonquantitative techniques (eg, end-point RT-PCR or immunofluorescence),39,40 the introduction of real-time RT-PCR has offered the opportunity for more precise quantification of T-bet and GATA-3 transcripts in human Th1 and Th2 cells. Smits et al39 reported less than 3-fold lower T-bet levels and more than 4-fold higher GATA-3 levels in Th cells from 3 donors, which had been cultured for 30 days under Th2-polarizing conditions. In the study by Messi et al33 CRTH2+ memory Th cells from 2 donors expressed negligible levels of T-bet and less than 3-fold higher GATA-3 levels than CCR5+ Th cells from the same donors. In contrast, quite modest changes, if any, in the expression of either factor were appreciated by Kitamura et al41 in a limited panel of Dermatophagoides farinae–reactive human Th1 and Th2 clones. In any of these instances, though, the samples and/or donors studied were too few to extrapolate a general pattern.
In the present study we have examined Th cells from several donors and from 2 mouse strains using a well-established in vitro differentiation protocol. While we observed a clear-cut pattern of IL-4, IFN-γ, CRTH2, and T-bet expression in human and mouse polarizing cultures, we did not appreciate a consistent Th2 bias in the expression of GATA-3 in human Th cells (Figure 2A) until we examined the CRTH2+ fraction of in vitro–skewed cultures or the circulating memory pool (Figures 5A and 6C). Even when factoring in CRTH2 surface expression, however, differences in GATA-3 levels in human Th cells, albeit significant, were in no way comparable with those observed in mouse cultures. For instance, average GATA-3 levels were no more than 2-fold higher in human in vitro–generated, enriched CRTH2+ Th cells than in parallel Th1 cultures from the same donors, while a more than 10-fold difference was consistently appreciated in mouse unfractionated Th2 versus Th1 cultures. Yet, at least in terms of polarized cytokine production, IL-4–driven Th2 polarization is no more efficient in mouse than human Th cells, with 7-day Th2 cultures from either species containing at the most about 25% of IL-4–producing cells by single-cell analysis (data not shown), a figure well in line with earlier published studies of mitogen-primed Th cells.23,25,32 Thus, GATA-3 expression is not ipso facto a molecular determinant of Th2-cell differentiation in the human species.
Our results extend previous work by Nagata and others on the validity of CRTH2 surface expression as a molecular identifier for the Th2 subset in humans.16–18 CRTH2 can mediate recruitment of Th2 cells to the sites of allergic inflammation.16–18,22 However, its role in Th2-cell physiology, differentiation, and overall function is not fully understood. Agonists and antagonists of this receptor can up- and down-regulate IL-4 expression in effector T cells, respectively, and there appears to be a correlation between the levels of CRTH2 and IL-4 expression.18,19 This points to the involvement of a unique pathway of IL-4 gene regulation, downstream of PGD2/CRTH2 signaling, whose definition should prove worthwhile in understanding the physiology and pharmacology of Th2 cells. Our findings would also imply the existence of a causative link between CRTH2 expression and GATA3 up-regulation. GATA-3 overexpression can induce, and T-bet strongly repress, the appearance of CRTH2 on the surface of in vitro–differentiated human Th cells.31 Conversely, our experiments using selective agonists and antagonists (not shown) in differentiating and fully committed Th2 cells seem to exclude the possibility that the very expression of this receptor, through engagement of endogenous ligands, may be functional to certain aspects of Th2-cell development, including GATA3 up-regulation. Whatever the relationship between CRTH2 signaling and GATA-3 gene regulation, the overall significance of this pathway in Th2 responses is to be pursued in a human model, because CRTH2 does not appear to be a marker of mouse Th2 cells (data not shown and Abe et al21 ). Studies in CRTH2-defective lines are therefore unlikely to provide a meaningful response to these functional issues.20
Our findings emphasize the existence of nontrivial differences in the regulation of T-cell effector responses in mice and humans. Aside from CRTH2 and GATA-3 expression, there is a growing appreciation of a substantial degree of divergence in human versus mouse T-cell phenotype and function and overall immune responsiveness (summarized by Mestas and Hughes12 ). Of particular interest to this study, species-specific differences have emerged with respect to the mechanisms that regulate cytokine gene expression in Th2 cells (reviewed by Georas et al42 ). For instance, like GATA-3, the bZip protein c-Maf is not as clearly a Th2-restricted factor in human as it is in mouse Th cells, and the c-Maf binding site within the proximal IL-4 promoter is dispensable for maximal transcriptional activation in human Th cells.40,43,44 We have confirmed this finding in CRTH2-sorted Th cells, in which, out of a panel of genes encoding for nuclear factors known or implied to be differentially expressed in Th1 and Th2 cells, only FOXP3 exhibited a substantial degree of polarization, possibly due to the expansion and/or development of Treg clones within the CRTH2− fraction (Table S2).45
We have shown that mismatches in human and mouse IL-4 promoter sequences can account for differential species-specific regulation of this gene by NFAT and NF-κB molecular species.46 It is conceivable that analogous differences in the cis-trans interactions at the Th2 cytokine gene locus and other loci may account for experimental findings quite unique to human Th cells, such as a low level of phenotype stability and an increased propensity to switch phenotypes or accommodate intermediate phenotypes in response to developmental signals.31,33,35,39
Our study, by highlighting species-specific features in the regulation of GATA-3 expression, further defines the phenotype of human Th2 cells. Our findings do not downplay the importance of this factor in Th-cell physiology. In line with studies of mouse T cells, forced expression of GATA-3 in human T cells leads to the induction of clear Th2 phenotypic traits, such as IL-4 and IL-13 production and CRTH2 surface expression,31 and indeed GATA-3 expression is found up-regulated in Th2-biased conditions like asthma.10,47 Rather, our findings shift the focus from the mechanisms regulating GATA-3 transcription (eg, STAT-6 and NF-κB),37,48 to those that account for this factor's subcellular location and function. For instance, GATA-3 may undergo posttranslational modifications in activated T cells that affect it affinity for certain DNA motifs and/or the interaction with other factors, most notably NFAT species, at proximal or distal Th2 gene regulatory clusters.25,34,49
A recent study highlights the ability of T-bet to regulate GATA-3 function via direct protein-protein interaction.50 In fact, inhibition of GATA-3 expression and/or function, rather than direct interaction with the IFN-γ locus, appears to play a prominent role in T-bet–dependent IFN-γ gene activation in developing Th1 cells.51 While the exact mechanism of GATA-3 inhibition by T-bet is not clarified, its very appreciation modifies the “2-player” paradigm in favor of a more central role for T-bet gene regulation in the genesis of T-cell–polarized responses. In line with this notion, a clear pattern of inheritance has been demonstrated for the Th1 markers, IFN-γ and T-bet, but not for IL-4 or GATA-3,52 hinting at the possibility that mutations within these genes are sufficient to account for Th-cell dysregulation in allergic and autoimmune conditions.
Authorship
Contribution: U.D., N.F.A., and V.C. designed research; U.D., F.M., R.J.K., W.K.L., and M.B. performed research; U.D., F.M., and R.J.K. collected data; U.D. and V.C. analyzed data; V.C. wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vincenzo Casolaro, Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD 21224; e-mail: casolaro@jhmi.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the National Institutes of Health (AI041463) and the Asthma and Allergy Foundation of America (V.C.).
The authors thank Drs Steve N. Georas, Shau-Ku Huang, and Cristiana Stellato for their valuable input and constructive criticisms; Drs Francesco Boin, Sean X. Leng, and George Wang for helpful discussions; Mrs Sherry Hudson for expert technical assistance with flow cytometric data acquisition and analysis; Mrs Nancy D. Bollers and Mrs Deborah Bull for kind help with subject recruitment and characterization; and Mrs Lisa Leventhal for competent advice on regulatory matters.
U.D.F. is on a leave of absence from the Second University of Naples PhD program in Medical and Surgical Oncology and Clinical Immunology.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal