Abstract
Regulatory T cells play an essential role in the control of self-tolerance and processes of adaptive immunity. Tolerogenic IL-10–modulated human dendritic cells (IL-10DCs) induce anergic T cells with strong suppressive properties (iTregs) that inhibit the activation of effector T cells. In this study, we evaluated the interaction between cell-cycle regulation and intracellular signaling in these iTregs. Analysis of signal transduction events revealed a down-regulation of the mitogen-activated protein kinases (MAPKs) Jun N-terminal kinase (JNK) and a nonactivation of extracellular-signal–regulated kinase (ERK) in contrast to a marked activation of p38 MAPK and the p38 effector MAPK-activated protein kinases 2/3 (MAPKAP2/3). The elevated activation of p38 is critical for the induction and maintenance of anergy controlled by an increased expression of the cell-cycle inhibitor p27Kip1. Moreover, blocking experiments with the specific inhibitor SB203580 demonstrated that the regulatory function of iTregs is associated with an enhanced p38 MAPK activity. In contrast to other Treg populations, the suppressor function of iTregs is independent of IL-10. In conclusion, our data indicate that a cross-talk of cell-cycle regulation and p38-dependent signal transduction is required for the suppressor function of iTregs.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) of the immune system and are uniquely capable of stimulating clonal expansion of naive T cells, providing a plethora of costimulating molecules and cytokines.1,2 However, there is also experimental evidence for a function of DCs in the induction of tolerance.3,4 Defining the mechanisms responsible for this phenomenon has become one of the most intriguing challenges in immunology. It is widely assumed that various subpopulations of DCs are involved in the initiation and maintenance of peripheral tolerance. In addition, the generation of regulatory T cells (Tregs) induced by DCs plays a critical role for maintenance of peripheral tolerance. Currently, various T-cell populations are considered to be regulatory T cells, including CD4+CD25+ Tregs TR1 and TH3 cells.5–9 These heterogeneous cell populations are capable of suppressing effector T-cell functions through diverse contact-dependent and/or -independent mechanisms, specific for the respective regulatory cell type.5–9
Previously, we have demonstrated that IL-10–modulated tolerogenic DCs (IL-10DCs) induce anergic CD4+ and CD8+ T cells with antigen-specific regulatory properties (iTregs) inhibiting activated CD8+ and CD4+ effector T cells.10–12 These iTregs are characterized by an G1 arrest of the cell cycle mediated via high expression of the cyclin-dependent kinase (cdk) inhibitor p27Kip1.13 Addition of IL-2 and blocking of the CTLA-4 pathway abolished the state of anergy and also resulted in a loss of suppressive properties of iTregs, indicating an interaction between cell-cycle regulation, mechanisms of signal transduction, and suppressor function of iTregs induced by tolerogenic IL-10DCs.13
Mitogen-activated protein kinases (MAPKs) play crucial roles in mediating cellular responses to various extracellular signals. Currently, 4 major subgroups of the MAPK family have been identified: stress-activated protein kinases (SAPKs)/Jun N-terminal kinases (JNK1/2), p38 kinases, extracellular signal–regulated kinases 1 and 2 (ERK1/2), and, more recently, ERK5.13–15 It was demonstrated that activation of the MAPKs ERK16 and JNK17 is reduced in anergic T cells induced in the absence of costimulation due to a blocked activity of p21RAS in the case of ERK. Some reports described a reduced activity of p38 in anergy but so far the function of these signal transduction events in Tregs has not been defined.
In this study, we show that the activity of the MAPK p38 and its downstream effectors MAPK-activated protein kinases 2/3 (MAPKAP-K2/3) is markedly enhanced in iTregs induced by IL-10DCs, whereas the activation of the MAPKs JNK and ERK is significantly reduced. Inhibition of p38 in iTregs induces a markedly reduced expression of the cdk inhibitor p27Kip1, resulting in cell-cycle progression and complete loss of regulatory function. Taken together, these data suggest a critical role of altered MAPK signaling in iTregs, affecting the cross-talk between signal transduction and cell-cycle progression, thereby modulating the suppressor activity.
Materials and methods
Culture medium
X-VIVO15 supplemented with 0.5% autologous plasma was used for generation of DCs. T cells were cultured and stimulated in X-VIVO20 (both from Cambrex, Verviers, Belgium).
Antibodies
For fluorescence-activated cell-sorting (FACS) analysis, antibodies against CD2 (6F10.3), CD14 (RM052), CD19 (J4.119), CD80 (MAB104), CD83 (HB15A) (all from Beckmann Coulter, Kretfold, Germany), CD86, (BU63; Serotec, Raleigh, NC), HLA-DR (Serotec), and mouse (MOPC-31-C, MOPC-173, and 27-35) and rat (A95-1, R35-95) subclass–specific isotypes (all from Beckmann Coulter) were used. For conjugated secondary reagents, DTAF-conjugated goat anti–mouse IgG and PE-conjugated donkey anti–mouse IgG (Jackson Immunoresearch, Bar Harbor, ME) were used. For staining of magnetic-activated cell-sorter (MACS)–sorted T cells, FITC-conjugated CD4 (13B8.2) or CD8 (B9.11) and FITC-conjugated mouse IgG (697.1Mc7) (all from Beckmann Coulter) were used.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats using Ficoll gradients and suspended in culture dishes for 45 minutes. Nonadherent cells were rinsed off the plates, and remaining cells were cultured in 3 mL X-VIVO15 supplemented with 400 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine; Berlex Laboratories, Seattle, WA), 1000 U/mL IL-4 (Strathmann, Hamburg, Germany) and 0.5% autologous plasma. At day 6, nonadherent cells were collected, resuspended in X-VIVO15 supplemented with 0.5% autologous plasma, 400 U/mL GM-CSF and 1000 U/mL IL-4, and additionally stimulated with 2.5 ng/mL IL-1β, 2.5 ng/mL TNF-α, 250 U/mL IL-6 (Strathmann), and 0.25 μg/mL PGE2 (Minprostin; Pharmacia-Upjohn, Erlangen, Germany) to induce mature DCs (mDCS). Tolerogenic IL-10DCs were induced by addition of 20 ng/mL IL-10 (DNAX, Bergisch-Gladbach, Germany) to the culture.
Isolation of CD4+ T cells
CD4+ T cells were prepared from buffy coats using CD4-MACS beads (MACS systems; Miltenyi Biotec, Auburn, CA) according to standard protocols (purity > 95%).
Induction and restimulation of iTregs and activated CD4+ effector T cells
mDCS, IL-10DCs, and CD4+ T cells were prepared as described above, and 5 × 105 mDCs or IL-10DCs were cocultured with 5 × 106 CD4+ T cells per well in 3 mL X-VIVO20 supplemented with 0.5% autologous plasma and 2 U/mL IL-2 in 6-well plates for 5 days during primary culture to induce effector T cells (Teffs) and iTregs. After 5 days, T cells (1 × 106/well) were suspended in X-VIVO20 supplemented with 2.5% autologous plasma and 2 U/mL IL-2 and restimulated in 6-well plates coated with 0.1 μg/mL anti-CD3 mAb (OKT3, CRL 8001; ATCC, Manassas, VA) or mDCs (1 × 105/well) generated from the same donor used for the primary culture or left unstimulated.
Suppressor assays of iTregs and Teffs
iTregs and Teffs were generated as described above using the same donor (syngeneic T cells). To assess the suppressor activity, anergic iTregs (1 × 105) and Teffs (1 × 105) suspended in X-VIVO20 supplemented with 2.5% autologous plasma of T-cell donor and 2 U/mL IL-2 were mixed and restimulated with immobilized anti-CD3 mAb (0.1 μg/mL) or mDCs (1-2 × 105) generated from the same donor used for the primary culture in 96-well plates (Costar Corning, Bodenheim, Germany). Proliferation was measured 48 to 72 hours later by [3H]-thymidine incorporation. In some experiments, anti–IL-10, anti-IL10R, and anti–TGF-β antibodies (10 μg/mL) were used (R&D Systems, Minneapolis, MN).
Inhibition of p38 MAPK
Inhibition of p38 was performed by adding SB203580 (Calbiochem/Merck Biosciences, San Diego, CA) to a final concentration of 10 μM to either primary culture, coculture, or restimulation experiments.
Flow cytometric analysis
Intracellular FACS analyses were performed using Cytofix/Cytoperm (BD Biosciences, Palo Alto, CA) according to the manufacturer's instructions and analyzed by flow cytometry (FACScalibur and Cellquest software; Becton Dickinson, San Jose, CA). For IL-2 analysis, APC-labeled monoclonal rat anti–IL-2 (clone MQ1-17H12, 10 μg/mL; BD Biosciences) and control APC-labeled rat IgG2a κ (clone R35-95, 10 μg/mL; BD Biosciences) were used. Intracellular staining was performed 24 hours after restimulation, and IL-2 expression was boosted with PHA (2.4 μg/mL) and PMA (1 ng/mL) while cytokine secretion was blocked using Golgi Stop (BD Biosciences, Palo Alto, CA) for 4 hours. For CFDA labeling of T cells, the Vybrant CFDA SE Cell Tracer Kit (Molecular Probes, Eugene, OR) was used according to the manufacturer's instructions. The proliferative response of the CFDA-labeled population was assessed.
Cell-cycle analysis
Cell-cycle analysis were performed by DNA staining with propidium iodide (Sigma, St Louis, MO) as described previously.13
Cytokine analysis
For assessment of cytokine production, supernatants were collected 48 hours after restimulation of iTregs, Teffs, or coculture experiments, and stored at −70°C. The amounts of IL-2 and IFN-γ were assessed by enzyme-linked immunosorbent assay (ELISA) using commercially available antibodies and standards according to the manufacturer's protocols (Pharmingen, San Diego, CA). For assessment of IL-10 production, cell culture supernatants were collected 0, 24, 48, or 72 hours after restimulation. The amounts of IL-10 were measured by ELISA using the BD OptEIA Human IL-10 ELISA Kit II (Becton Dickinson).
qRT-PCR of IL-10 mRNA
For assessment of IL-10 mRNA, RNA samples of iTregs or Teffs (5 × 106/3 mL) were collected 0, 24, 48, or 72 hours after restimulation. The amount of IL-10 mRNA was determined by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) using a Light Cycler (Roche, Indianapolis, IN). Commercially available primers and specific probes for IL-10 (Human Interleukin-10 Real-Time PCR Primer Set, FRET Probe and standard; Biosource, Heidelberg, Germany) and for GAPDH (QuantiTect Gene Expression Assay; Qiagen, Valencia, CA) were used.
Immunoprecipitation and kinase assay
Total protein (500 μg) dissolved in Triton-lysis buffer (TLB; 20 mM Tris (pH 7.4), 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 50 mM b-glycerophosphate, 20 mM sodium-pyrophosphate, 0.2 mM pefablock, 5 μg/mL aprotinin, 1 mM Na3VO4, and 5 mM benzamidine) was used to immunoprecipitate the MAPKs ERK2, JNK1, and p38 as well as MAPKAP-K2/3 with the respective antibodies (1 μg) anti-JNK1, anti-ERK2, anti-p38 (Santa Cruz Biotechnology, Santa Cruz, CA), and MAPKAP-K2/318 (Upstate Biotechnology, Lake Placid, NY), and 25 μL protein A agarose (Amersham Pharmacia, Freiburg, Germany) by incubation head-over-end for 2 hours at 4°C, followed by washing twice with kinase buffer (10 mM MgCl2, 25 mM HEPES [pH 7.5], 25 mM b-glycerophosphate, 5 mM benzamidine, 0.5 mM DTT, and 1 mM Na3VO4). MBP (1 μg; Sigma,), GST–c-Jun (S. Ludwig, Mülnster, Germany), ATF-2, or HSP25 (Cell Signaling Technologies, Beverly, MA)–specific substrate for ERK2, JNK1, p38, and MAPKAP-K2/3, respectively, 200 mM ATP, and 5 μCi (0.185 MBq) γ-[32P]-ATP (Amersham Pharmacia) were added to kinase buffer to a total volume of 20 μL kinase buffer. The kinase reaction was performed for 15 minutes at 30°C, separated in SDS-PAGE (12.5%), and analyzed with a phophoimager (Ray Test; Fuji Systems, Düsseldorf, Germany). Analysis of the kinase activities in control lysates of mDCs and IL-10DCs at concentrations reflecting the percentage of APCs (< 5%) in the coculture experiments did not show any phosphorylation of the substrates, excluding influences of DC-derived kinases on the results (data not shown).
Immunoblotting
Immunoblotting was performed as described previously.12 Membranes were probed with anti-JNK1, anti-ERK2, anti-p38 (all from Santa Cruz Biotechnology), anti-p27 (Kip1; Signal Transduction Laboratories, Heidelberg, Germany), anti-MAPKAP-K2/318 or antiactin (Santa Cruz Biotechnology) overnight at 4°C. Detection was performed by incubation with horseradish peroxidase–conjugated antibodies (goat antimouse from Dianova [Hamburg, Germany]; and goat antirabbit from New England Biolabs, Beverly, MA). Proteins were visualized by ECL Plus using Hyperfilm ECL (Amersham Pharmacia).
Statistical analyses
Statistical significances of differences between experimental groups were evaluated using the unpaired Student t test and the Statview 5.0 software package (SAS Institute, Cary, NC). Differences of P values of .05 or less were considered significant.
Results
Activity of MAPKs ERK and JNK is down-regulated in iTregs
To analyze the role of MAPKs in iTregs induced by IL-10DCs, we first assessed the activation status of the MAPKs ERK and JNK by in vitro kinase assays after restimulation. We compared iTregs with T cells cocultured with mDCs, resulting in the generation of fully activated Teffs.10–13 Briefly, CD4+ T cells were cultured with allogeneic mDCs (Teffs) or IL-10DCs (iTregs; primary culture) for 5 days and then restimulated. Subsequently, immunoprecipitations of cell lysates were performed and used for in vitro kinase assays.
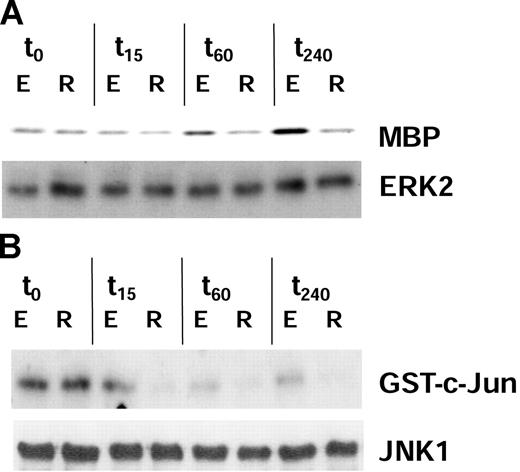
After restimulation, the activity of ERK2 in iTregs (as detected by MBP phosphorylation) was not up-regulated throughout the time period of examination compared with that in Teffs, displaying a maximum of activation after 240 minutes (Figure 1A; top panel). Similar results were observed when the activity of JNK1 was monitored. The activity of MAPK JNK dropped significantly in iTregs 15 minutes after restimulation, whereas the activation level in Teffs was maintained up to 15 minutes and thereafter successively declined, but remained on a significantly higher level compared with that of iTregs (Figure 1B; bottom panel).
The activation of ERK2 and JNK1 is reduced in anergic iTregs induced by coculture with IL-10–modulated DCs. CD4+ T cells were cocultured with allogeneic IL-10DCs or mDCs for 5 days to induce iTregs (R) and Teffs (E). Subsequently, T cells were restimulated by anti-CD3 mAb. Lysates of T-cell populations were prepared at 0, 15, 60, and 240 minutes after restimulation. ERK2 and JNK1 were immunoprecipitated with anti-ERK2 and anti-JNK1 antisera. MBP and GST–c-Jun served as specific substrates for ERK2 (panel A; top) and JNK1 (panel B; top) in in vitro kinase assays to analyze their activation. Western blots using antibodies against ERK2 and JNK1, respectively, demonstrated equivalent protein loading of both T-cell populations (panels A-B; bottom). One representative of 3 independent experiments with similar results is shown.
The activation of ERK2 and JNK1 is reduced in anergic iTregs induced by coculture with IL-10–modulated DCs. CD4+ T cells were cocultured with allogeneic IL-10DCs or mDCs for 5 days to induce iTregs (R) and Teffs (E). Subsequently, T cells were restimulated by anti-CD3 mAb. Lysates of T-cell populations were prepared at 0, 15, 60, and 240 minutes after restimulation. ERK2 and JNK1 were immunoprecipitated with anti-ERK2 and anti-JNK1 antisera. MBP and GST–c-Jun served as specific substrates for ERK2 (panel A; top) and JNK1 (panel B; top) in in vitro kinase assays to analyze their activation. Western blots using antibodies against ERK2 and JNK1, respectively, demonstrated equivalent protein loading of both T-cell populations (panels A-B; bottom). One representative of 3 independent experiments with similar results is shown.
MAPK p38 and MAPKAP-K2/3 are markedly activated in iTregs
In other models of anergy, the activity of a third MAPK, p38, was unaffected or down-regulated.19,20 However, the functional role of p38 MAPK in iTregs remains to be defined. Kinase assays of p38 were performed at various time points after restimulation to analyze the activation state of p38 of iTregs.
After induction of iTregs and Teffs, a strong activation of p38 was observed in both T-cell populations (Figure 2A; top panel; t0). In Teffs, the activity of p38 dropped dramatically after restimulation and decreased almost below detection levels after 60 minutes. In contrast, enhanced activation levels of p38 was observed in iTregs up to 60 minutes after activation. Subsequently, p38 phophorylation was more slowly down-regulated compared with that of activated Teffs.
iTregs display a high activity of the p38 MAPK and MAPKAP-K2/3. Teffs (E) and iTregs (R) were induced and restimulated at day 5 as described in Figure 1. Immunoprecipitation of p38 and MAPKAP-K2/3 was performed with lysates prepared 0, 15, 60, and 240 minutes after restimulation. ATF-2 and HSP 25 served as specific substrates for p38 (panel A; top) and MAPKAP-K2/3 (panel B; top) in in vitro kinase assays. As a control, equivalent levels of the MAPK p38 and MAPKAP-K2/3 were measured in both iTregs and Teffs by Western blot (panels A and B; bottom). Similar results were obtained in 4 independent experiments.
iTregs display a high activity of the p38 MAPK and MAPKAP-K2/3. Teffs (E) and iTregs (R) were induced and restimulated at day 5 as described in Figure 1. Immunoprecipitation of p38 and MAPKAP-K2/3 was performed with lysates prepared 0, 15, 60, and 240 minutes after restimulation. ATF-2 and HSP 25 served as specific substrates for p38 (panel A; top) and MAPKAP-K2/3 (panel B; top) in in vitro kinase assays. As a control, equivalent levels of the MAPK p38 and MAPKAP-K2/3 were measured in both iTregs and Teffs by Western blot (panels A and B; bottom). Similar results were obtained in 4 independent experiments.
To verify the findings concerning the activation state of p38, we examined the activity of downstream substrates of p38, MAPKAP-K2/3, with HSP25 (small heat-shock protein 25) as a specific substrate. MAPKAP-K2/3 is involved in p38 activation via regulation of shuttling of p38 MAPK between cytosol and the nucleus, in the regulation of gene transcription or RNA stabilization, and in the phosphorylation of HSP27.21–23 In contrast to Teffs, which showed a constant weak expression of the kinases, resting and activated iTregs exhibited a markedly higher level of activation of the MAPKAP-K2/3 (Figure 2B; top panel).
Enhanced activation of the p38/MAPKAP-K2/3 module during the induction of iTregs
Next, we assessed the activation of MAPKs during the induction phase of iTregs to analyze the role of p38 MAPK and its downstream substrates MAPKAP-K2/3 for the induction of iTregs. p38 activity was up-regulated in iTregs, reaching a maximum at day 5 in contrast to that of Teffs, (Figure 3A; top panel).
Activation of p38 and MAPKAP-K2/3 during the induction of iTregs. CD4+ T cells were cultured with allogeneic IL-10DCs or mDCs to induce the generation of iTregs (R) and Teffs (E). At days 0, 2, and 5 of culture, lysates of both T-cell populations were used for immunoprecipitation of p38 and MAPKAP-K2/3. Subsequently, in vitro kinase assays were performed using ATF-2 and HSP25 as specific substrates for p38 (panel A; top) and MAPKAP-K2/3 (panel B; top) to test the activation of the kinases. The incorporation of [32P] was visualized after SDS-PAGE. Western blot analysis revealed equal expression of MAPK p38 and MAPKAP-K2/3 during primary culture in both iTregs and effector T cells (panels A-B; bottom). One of 5 representative experiments is shown.
Activation of p38 and MAPKAP-K2/3 during the induction of iTregs. CD4+ T cells were cultured with allogeneic IL-10DCs or mDCs to induce the generation of iTregs (R) and Teffs (E). At days 0, 2, and 5 of culture, lysates of both T-cell populations were used for immunoprecipitation of p38 and MAPKAP-K2/3. Subsequently, in vitro kinase assays were performed using ATF-2 and HSP25 as specific substrates for p38 (panel A; top) and MAPKAP-K2/3 (panel B; top) to test the activation of the kinases. The incorporation of [32P] was visualized after SDS-PAGE. Western blot analysis revealed equal expression of MAPK p38 and MAPKAP-K2/3 during primary culture in both iTregs and effector T cells (panels A-B; bottom). One of 5 representative experiments is shown.
Consistent with the activation of p38, the activity of MAPKAP-K2/3 was also enhanced during induction of iTregs in contrast to that of Teffs (Figure 3B; top panel).
Surprisingly, these results show a distinct pattern of activation of MAPKs in iTregs. Compared with activated Teffs, ERK and JNK activity is reduced, and the activation level of p38 and MAPKAP-K2/3 is markedly enhanced during induction and after restimulation of iTregs. This finding provoked two questions. First, is this strong activation of p38 functionally involved in the induction and/or maintenance of anergy? Second, is this activity essential for the suppressor activity of iTregs?
Activity of p38 is essential for induction and maintenance of anergy in iTregs
To analyze the functional relevance of activated p38 in induction and maintenance of anergy, we performed experiments with the p38-specific inhibitor SB203580.24 First, efficacy and specificity of the inhibitor was tested by adding SB203580 during primary cultures of CD4+ T cells with mDCs or IL-10DCs and subsequent analysis of p38 activity. Significantly reduced activation of p38 in both T-cell populations, Teffs and iTregs, demonstrated the proper function of the inhibitor (data not shown). In contrast, activation levels of the other MAPKs ERK and JNK and of MKK3/6, the latter kinases being upstream activators of p38, were not affected in T cells treated with the specific inhibitor (data not shown).
In addition, we formally proved the functional relevance of p38 activation for the induction of iTregs. In these experiments, SB203580 was added during primary cultures of CD4+ T cells with tolerogenic IL-10DCs or mDCs. Thereafter, T cells were restimulated, and proliferation assays were perfomed. As reported previously, iTregs displayed a significantly reduced proliferation compared with that of Teffs, confirming the induction of an anergic state (Figure 4A; top panel). Inhibition of p38 during primary culture abrogated this effect (Figure 4A; middle panel).
p38 MAPK is crucial for the induction and maintenance of T-cell anergy. IL-10DCs or mature DCs were cocultured with allogeneic CD4+ T cells to stimulate iTregs (R, □) and Teffs (E, ▪), and reactivated. The specific inhibitor SB203580 of MAPK p38 was added during the induction of the T-cell populations (middle panel) or during restimulation (bottom panel). Proliferation was detected 96 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). After 48 hours of culture, supernatants were collected and IL-2 production was measured by ELISA as described in “Materials and methods” (B). One of 5 independent experiments with similar results was shown. *Statistical significance according to the Student t test; P ≤ .05.
p38 MAPK is crucial for the induction and maintenance of T-cell anergy. IL-10DCs or mature DCs were cocultured with allogeneic CD4+ T cells to stimulate iTregs (R, □) and Teffs (E, ▪), and reactivated. The specific inhibitor SB203580 of MAPK p38 was added during the induction of the T-cell populations (middle panel) or during restimulation (bottom panel). Proliferation was detected 96 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). After 48 hours of culture, supernatants were collected and IL-2 production was measured by ELISA as described in “Materials and methods” (B). One of 5 independent experiments with similar results was shown. *Statistical significance according to the Student t test; P ≤ .05.
Alternatively, the inhibitor SB203580 was added later during restimulation of iTregs and Teffs to assess the role of p38 for the maintenance of anergy in iTregs. Blocking of p38 during restimulation also induced a dramatically enhanced proliferation of iTregs to levels similar to that of Teffs (Figure 4A; bottom panel), demonstrating the functional relevance of p38 also for the maintenance of anergy.
In addition, the release of IL-2 was assessed in the presence or absence of the p38 inhibitor. As described previously, iTregs produced significantly lower amounts of IL-2 compared with that of Teffs (Figure 4B). More important, addition of the inhibitor during induction or restimulation of iTregs restored the IL-2 production (Figure 4B). These results likewise support the observation that p38 is critical for both the induction and maintenance of anergy in iTregs.
p38 is involved in G1 cell-cycle arrest of iTregs controlled by cdk inhbitor p27Kip1
The state of anergy in iTregs is the result of a G1 cell-cycle arrest that is mediated by an up-regulation of the cdk inhibitor p27Kip1.13 To address the question of whether there is a cross-talk between cell-cycle arrest and altered signal transduction in iTregs, we performed cell-cycle experiments by analyzing the DNA content in iTregs and Teffs after modulation of the MAPK p38 pathway.
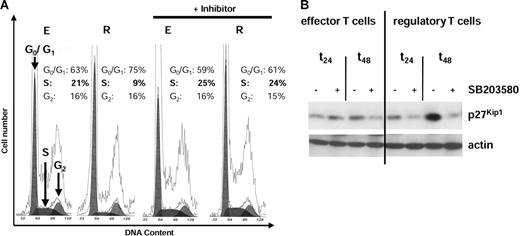
As reported previously, iTregs are characterized by G1 cell-cycle arrest with reduced percentages of cells in the S (synthesis) phase of the cell cycle (S phase, 9%) in contrast to strongly proliferating Teffs (S phase, 21%) (Figure 5A). More important, after addition of the inhibitor SB203580, cell-cycle analysis revealed an abrogation of G0/G1arrest and start of DNA synthesis in restimulated iTregs (S phase, 24%) to levels similar to that of Teffs (S phase, 25%) (Figure 5A).
MAPK p38 induces G1 cell-cycle arrest via high expression of the cdk inhibitor p27Kip1. iTregs (R) and Teffs (E) CD4+ T cells were generated by coculture of IL-10DCs or mature DCs with CD4+ T cells. The p38-specific inhibitor SB203580 was added during priming of the T-cell population as indicated. Distribution of DNA content was measured 24 hours after restimulation in iTregs and Teffs. The percentage of T cells in G0/G1, S, and G2 phases is indicated (A). Results represent 2 independent experiments. After restimulation (24 and 48 hours), lysates of T-cell populations were prepared and proteins were separated on SDS-PAGE, blotted, and probed for the cdk inhibitor p27Kip1 (B). The blot was stripped and reprobed for actin as a control for equal loading. Similar results were obtained in 4 independent experiments.
MAPK p38 induces G1 cell-cycle arrest via high expression of the cdk inhibitor p27Kip1. iTregs (R) and Teffs (E) CD4+ T cells were generated by coculture of IL-10DCs or mature DCs with CD4+ T cells. The p38-specific inhibitor SB203580 was added during priming of the T-cell population as indicated. Distribution of DNA content was measured 24 hours after restimulation in iTregs and Teffs. The percentage of T cells in G0/G1, S, and G2 phases is indicated (A). Results represent 2 independent experiments. After restimulation (24 and 48 hours), lysates of T-cell populations were prepared and proteins were separated on SDS-PAGE, blotted, and probed for the cdk inhibitor p27Kip1 (B). The blot was stripped and reprobed for actin as a control for equal loading. Similar results were obtained in 4 independent experiments.
As described above, inhibition of p38 during induction and activation of iTregs is followed by strong proliferative response, indicating an abrogation of anergy and cell-cycle arrest. These findings prompted the question of whether this alteration of the T-cell response is also reflected by a different regulation of the expression of the cdk inhibitor p27Kip1, the high expression of which is critical for cell-cycle arrest in the iTregs.12 In contrast to Teffs, iTreg cells exhibited enhanced expression of the cdk inhibitor p27Kip1, as demonstrated in Figure 5B. However, in the presence of the p38 inhibitor SB203580, a significant down-regulation of p27Kip1 24 to 48 hours after restimulation was observed (Figure 5B), indicating an important function of p38 in cell-cycle regulation and anergy induction.
Inhibition of p38 activity correlates with loss of suppressor activity of iTregs
Activation of p38 in association with down-regulation of ERK and JNK is essential for induction and maintenance of anergy, as shown in our study. Next, we addressed the question of whether highly activated p38 is also critical for the generation of anergic iTregs with suppressive properties. For these experiments, T cells were stimulated with tolerogenic DCs in the presence or absence of the specific p38 inhibitor. Subsequently, the suppressor activity of these T cells was assessed via analysis of the proliferation of cocultured Teffs. As shown in Figure 6A and previously, iTregs inhibited the proliferation of cocultured Teffs. In contrast, in the presence of SB203580, the regulatory function of iTregs was completely abrogated, as shown by an unrestricted proliferative T-cell response in coculture experiments with Teffs (Figure 6A). In addition, we assessed T-cell proliferation by FACS analysis with CFDA-labeled T cells to distinguish between effector and regulatory T cells in these suppressor coculture assays by gating on CFDA-labeled cells (Figure 6B). The experiments were performed in duplicate to label effector as well as suppressor T cells in all experimental settings. Compared with untreated iTregs, T cells cocultured with tolerogenic DCs in the presence of SB203580 showed a high proliferation as a result of anergy loss. Notably, Figure 6B also depicts an unaffected proliferation of Teffs cocultured with these SB203580-treated T cells, supporting the pivotal role of p38 MAPK for the induction of the anergic state as a prerequisite for the regulatory function of iTregs.
Activity of p38 is essential for the induction of iTregs with suppressive properties. iTregs (R) and Teffs (E) were induced as described in “Materials and methods.” In some experiments, during the primary culture, the specific p38 inhibitor SB203580 was added. After primary culture, Teffs and iTregs were cocultured (ratio, 1:1) and activated by anti-CD3 mAb in suppressor assays. Coculture of Teffs or single T-cell populations served as controls. Proliferation was detected 72 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). (B) iTregs (R), SB203580-pretreated iTregs (RI) and Teffs (E) were CFDA labeled or left untreated to determine the proliferation of the T-cell populations after restimulation and in coculture experiments. The proliferative response of the CFDA-labeled population was assessed. FACS analysis was performed after 96 hours. One representative of 3 experiments is shown. IL-2 (C) and IFN-γ (D) production were measured by ELISA in supernatants harvested after 48 hours of culture. Similar results were obtained in 5 independent experiments. To discriminate Teffs, iTregs, and inhibitor-treated iTregs in coculture, the respective populations were CFDA labeled prior to the setup of the experiment (E-F). After 24 hours, intracellular IL-2 production was determined by FACS analysis in the unlabeled population. One representative is shown in panel E. (F) The IL-2 expression is normalized against cytokine expression in Teffs. A total of 5 independent experiments are demonstrated. Asterisks indicate statistical significance according to the Student t test (*P ≤ .05; **P ≤ .005; ***P < .001).
Activity of p38 is essential for the induction of iTregs with suppressive properties. iTregs (R) and Teffs (E) were induced as described in “Materials and methods.” In some experiments, during the primary culture, the specific p38 inhibitor SB203580 was added. After primary culture, Teffs and iTregs were cocultured (ratio, 1:1) and activated by anti-CD3 mAb in suppressor assays. Coculture of Teffs or single T-cell populations served as controls. Proliferation was detected 72 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). (B) iTregs (R), SB203580-pretreated iTregs (RI) and Teffs (E) were CFDA labeled or left untreated to determine the proliferation of the T-cell populations after restimulation and in coculture experiments. The proliferative response of the CFDA-labeled population was assessed. FACS analysis was performed after 96 hours. One representative of 3 experiments is shown. IL-2 (C) and IFN-γ (D) production were measured by ELISA in supernatants harvested after 48 hours of culture. Similar results were obtained in 5 independent experiments. To discriminate Teffs, iTregs, and inhibitor-treated iTregs in coculture, the respective populations were CFDA labeled prior to the setup of the experiment (E-F). After 24 hours, intracellular IL-2 production was determined by FACS analysis in the unlabeled population. One representative is shown in panel E. (F) The IL-2 expression is normalized against cytokine expression in Teffs. A total of 5 independent experiments are demonstrated. Asterisks indicate statistical significance according to the Student t test (*P ≤ .05; **P ≤ .005; ***P < .001).
Activated Th1 effector T cells are characterized by high proliferation, enhanced release of IL-2, and high production of the characteristic Th1 cytokine IFN-γ. To analyze the inhibition of the Th1 T-cell response in the presence of iTregs in more detail, we next assessed the production of IL-2 and IFN-γ. The secretion of IL-2 and IFN-γ is markedly decreased in iTregs compared with Teffs characterized as activated Th1 cells as shown above and in Figure 6C and 6D. When Teffs are cultured in the presence of iTregs, the release of the 2 cytokines is significantly reduced as demonstrated in Figure 6C and 6D, demonstrating the inhibition of a Th1 T-cell response by iTregs. In contrast, T cells generated by tolerogenic IL-10DCs in the presence of SB203580 did not abolish the secretion of IL-2 and IFN-γ (Figure 6C-D). Moreover, we performed FACS analyses with CFDA-labeled Teffs or iTregs to investigate the regulation and suppression of IL-2 expression in both particular T-cell populations (Figure 6E-F). Experiments revealed that the reduced IL-2 production in coculture assays was due to a decreased expression of IL-2 in Teffs inhibited by iTregs. In the presence of the p38 inhibitor during the induction phase of iTregs, the development of these anergic T cells was abolished as demonstrated by an enhanced IL-2 production compared with untreated iTregs (Figure 6E-F). In addition, an unaffected IL-2 production of Teffs in suppressor assays comparable with control Teffs was observed, indicating that the induction of anergic iTregs with strong suppressor properties was abrogated (Figure 6E-F). The data of 5 independent experiments are summarized in Figure 6F to demonstrate the significance of the results.
Suppressor function of iTregs is not mediated by IL-10
The important role of IL-10 and TGF-β for the regulatory function of Tregs was described for TR1 and TH3 cells, and discussed for naturally occurring CD4+/CD25+ T cells in vivo.6–8 Moreover, the MAPK p38 is known to be involved in signal transduction pathways, inducing the expression of IL-10 in T cells after TCR/CD28- or mitogen-induced activation.25 In order to analyze the function of IL-10 for the suppressor activity of iTregs and the role of p38 for IL-10 production in iTregs, we assessed the expression of IL-10 on the mRNA and protein level in iTregs generated in the presence and absence of the p38 inhibitor in primary and restimulation experiments at several time points. As demonstrated in Figure 7A, after incubation with SB203580 a reduction of IL-10 protein in iTregs was only observed at day 5 of primary culture. At earlier time points or on mRNA levels, no differences were found (Figure 7A; data not shown). During restimulation experiments of iTregs, a significant inhibition of IL-10 expression was not detected at any time point (Figure 7A; data not shown). However, in coculture assays of iTregs and Teffs, slightly reduced amounts of IL-10 were detected after pretreatment of Tregs with the p38 inhibitor (Figure 7B). As previously shown, under certain conditions, TGF-β plays a role for the function of Tregs. However, we did not detect significant amounts of TGF-β in the supernatants of iTregs or coculture experiments with Teffs (data not shown).6–8
IL-10 is not critical for regulatory activity of iTregs. Expression of IL-10 mRNA and protein levels of iTregs (R), Teffs (E), and SB203580-pretreated iTregs (RI) and Teffs (EI) was quantified at day 5 of primary culture and day 4 after restimulation by ELISA and qRT-PCR, respectively (A). Relative IL-10 mRNA expression was normalized to GAPDH. Secretion of IL-10 in cocultures of iTregs and Teffs (E+R) or SB203580-pretreated iTregs and Teffs (E+RI) was determined by ELISA (n = 4) (B). In cocultures of iTregs and Teffs, IL-10 and TGF-β bioactivity was neutralized by anti–IL-10, anti–IL-10R, or anti–TGF-β antibodies (C). Proliferation was measured in triplicate 72 h after restimulation as incorporation of [3H]-thymidine. *Statistical significance according to the Student t test (P ≤ .05). n.s. indicates not significant. One representive experiment of 3 is shown.
IL-10 is not critical for regulatory activity of iTregs. Expression of IL-10 mRNA and protein levels of iTregs (R), Teffs (E), and SB203580-pretreated iTregs (RI) and Teffs (EI) was quantified at day 5 of primary culture and day 4 after restimulation by ELISA and qRT-PCR, respectively (A). Relative IL-10 mRNA expression was normalized to GAPDH. Secretion of IL-10 in cocultures of iTregs and Teffs (E+R) or SB203580-pretreated iTregs and Teffs (E+RI) was determined by ELISA (n = 4) (B). In cocultures of iTregs and Teffs, IL-10 and TGF-β bioactivity was neutralized by anti–IL-10, anti–IL-10R, or anti–TGF-β antibodies (C). Proliferation was measured in triplicate 72 h after restimulation as incorporation of [3H]-thymidine. *Statistical significance according to the Student t test (P ≤ .05). n.s. indicates not significant. One representive experiment of 3 is shown.
In order to address the question of the function of the immunosuppressive cytokines IL-10 or TGF-β for the regulatory activity of iTregs, we performed inhibition experiments with neutralizing or blocking antibodies raised against IL-10 or TGF-β, or the IL-10 receptor, respectively. As demonstrated in Figure 7C, the regulatory capacity of iTregs was not affected in these assays, indicating that IL-10 and TGF-β are not critical for the suppressor function of iTregs.
Discussion
In this study, we describe a novel mechanism of altered signal transduction via MAPKs in human regulatory T cells. Strong activation of MAPK p38 and its downstream substrates MAPKAP-K2/3 accompanied by an inhibited activity of MAPK ERK and JNK is critical for the induction of anergy and for the suppressor function of iTregs induced by tolerogenic IL-10–modulated DCs.
These iTregs share some distinct characteristics with anergic T cells. They are characterized by low proliferative capacities due to G1 cell-cycle arrest and impaired IL-2 production.10 However, in contrast to conventional anergic T cells, these iTregs additionally display suppressive function as they have the capacity to inhibit the activation of resting and effector T cells.11,12 Previous reports have shown that these anergic iTregs differ from “classical” regulatory T cells (ie, TR1 cells; characterized by IL-10–dependent suppression of effector T-cell function), TH3 cells (effecting suppression by secretion of TGF-β), and naturally occurring regulatory CD4+CD25+ T cells.6–8,10–13,26,27 Although early experiments in vitro suggested CD4+CD25+ T cells to be anergic,27,28 recent experiments in vitro and in vivo have questioned this feature to be essential for the regulatory function of CD4+CD25+ Tregs.6,25,28 iTregs induced by IL-10DCs mediate their suppressive function similar to CD4+CD25+ Tregs via cell-to-cell contact-dependent mechanisms.11,12 In addition, the iTregs described in this paper hinder activation of the immune response independent of IL-10, a clear distinction from TR1 cells. Thus, iTregs induced by tolerogenic IL-10DCs represent a unique population of secondary induced regulatory T cells that can be distinguished from all described groups of regulatory T cells to date.
A first indication for impaired pathways of signal transduction in anergic T cells has been given by the finding that the activation of p21RAS, a GTP-binding protein upstream of the ERK MAPK pathway, is blocked, leading to reduced ERK activity in anergic T cells.16 Inhibition of ERK, JNK, and p38 activation was observed in various models of anergy in vitro and in vivo, suggesting a complete block of the MAPK pathway.17,19,20,30 JNK and ERK are known to be involved in the regulation of IL-2 expression in activated T cells by control of downstream transcription factors and/or mRNA stability.31–34 A reduced activation of JNK and ERK and perturbed transcription factors resulting in a diminished IL-2 production is well documented in anergic T cells.16,17,19,35–38 Notably, in our model of iTregs, we also observed an impaired activity of ERK and JNK after anergy induction. Therefore, the decreased production of IL-2 by iTregs might be the result of this reduced activation of MAPKs ERK and JNK, which are important upstream regulators of IL-2 transcription.
In striking contrast to previously described models of T-cell anergy,17,19,30 iTregs exhibit a markedly enhanced activity of the MAPK p38 compared with activated effector T cells stimulated with mDCs. An increased activation of p38 was observed during the induction of iTregs and in restimulated iTregs, as measured in kinase assays. The functional relevance of the distinct pattern of this signal transduction pathway is also reflected by the enhanced activity of the p38 effector kinase MAPKAP-K2/3. More important, activation of p38 is essential for the induction and maintenance of anergy in iTregs as formally proven by experiments using a specific inhibitor. A blockade of p38 activity induced a complete abrogation of T-cell anergy as demonstrated by an enhanced cell-cycle progression associated with the down-regulation of the cdk inhibitor p27Kip1. This resulted in an unrestricted proliferation and high IL-2 production of iTregs.
Several studies provide evidence for a restrictive role of p38 in cell-cycle control in various cell systems as conditional or constitutive experimental activation of p38 resulted in inhibition of the cell cycle at the G1/S transition.39–41 However, in immature thymocytes, activation of p38 results in cell-cycle arrest and blockade of differentiation in vivo,42 demonstrating that the p38 MAPK pathway is a critical regulatory element during thymocyte proliferation and maturation. Moreover, in line with our data, it was demonstrated in a mouse model of anergy that an enhanced activity of the p38 MAPK was required for cell-cycle arrest of the anergic T cells.43 This data support our results indicating a critical role of p38 for cell-cycle control in iTregs.
In addition to the critical role of p38 for cell-cycle control, p38 activation is also associated with the suppressive properties of iTregs. Our experiments demonstrated that T cells primed by tolerogenic DCs in the presence of the p38 inhibitor did not develop suppressor activity in contrast to fully differentiated iTregs.11,12 This phenomenon may be due to the prevention or reversal of the anergic state in iTregs induced by blocking of p38 activity as anergy may be a requirement for iTregs induced by tolerogenic DCs to develop suppressor function. As demonstrated previously, an anergic state characterized by low proliferation and IL-2 production is typical for various populations of regulatory T cells like CD4+/CD25+ T cells or TR1 cells.6–8 In some cases, the reversal of anergy (eg, by addition of IL-2) induced the loss of the suppressor function of the regulatory T cells.6–8 In contrast, other studies revealed that anergy and suppressor activity are regulated by different processes (eg, the stimulation of murine CD4+/CD25+ with DCs reverses their anergic state but only partially inhibits their suppressor activity).44,45 In addition, it was demonstrated that natural occurring CD4+/CD25+ T cells characterized as anergic T cells can be expanded by stimulation with DCs or by polyclonal activation in the presence of high amounts of IL-2 without losing their suppressive capacity after a resting period.45–47
The MAPK p38 in T cells is primarily known for its function in regulating cytokine expression (eg, IFN-γ, TNF-α, and IL-10), in TCR/costimulation mediated immune responses and in Th1 and Th2 effector function, demonstrating an important role for this MAPK for T-cell activation.22,25,32,48,49 However, the biochemical signaling that determines the regulatory activity of T cells is poorly understood. Recently, it was described that the transcription factor FOXP3 is important for the development and activation of CD4+CD25+ Tregs.50–52 Nevertheless, in iTregs induced by tolerogenic DCs the expression of FOXP3 was unaffected compared with that of activated effector T cells (H.S.A., unpublished results, June 2006).
Former studies demonstrate that under certain circumstances p38 may be involved in the repression of the transcription of IL-2 by regulation of transcription factors like NFAT and NFκB or repressor elements binding to silencer regions of the IL-2 gene.36,38
Intracellular kinases regulating the induction of anergic regulatory T cells may serve as new targets for a specific modulation of a variety of harmful immune responses. Our data provide evidence for an essential biochemical feature of a population of iTregs induced by tolerogenic IL-10DCs. A distinct regulation of the MAPK signaling network in iTregs with an inhibited activity of ERK and JNK and an elevated activation of p38 is critical for cell-cycle–controlled induction of anergic T cells with suppressor properties.
Authorship
Contribution: H.A. designed and performed research, analyzed and collected data, and wrote the paper; S.K. designed research, performed research and collected data; E.G. performed research and collected data; S.L. performed research and contributed analytical tools; J.K. supervised the project, provided economic support, and contributed to analysis of the data; and K.S. designed research, analyzed and collected data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kerstin Steinbrink, Department of Dermatology, University of Mainz, Langenbeckstrasse 1, 55131 Mainz, Germany; e-mail: steinbrink@hautklinik.klinik.uni-mainz.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Esther von Stebut, Helmut Jonuleit, Marcus Maurer, and Dennis Strand for critically reading the manuscript.
This work was supported by grants from the German Research Foundation (SFB548/B6) (K.S.) and the University of Mainz, Germany (MAIFOR) (K.S.).

![Figure 3. Activation of p38 and MAPKAP-K2/3 during the induction of iTregs. CD4+ T cells were cultured with allogeneic IL-10DCs or mDCs to induce the generation of iTregs (R) and Teffs (E). At days 0, 2, and 5 of culture, lysates of both T-cell populations were used for immunoprecipitation of p38 and MAPKAP-K2/3. Subsequently, in vitro kinase assays were performed using ATF-2 and HSP25 as specific substrates for p38 (panel A; top) and MAPKAP-K2/3 (panel B; top) to test the activation of the kinases. The incorporation of [32P] was visualized after SDS-PAGE. Western blot analysis revealed equal expression of MAPK p38 and MAPKAP-K2/3 during primary culture in both iTregs and effector T cells (panels A-B; bottom). One of 5 representative experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047563/4/m_zh80100701570003.jpeg?Expires=1765883506&Signature=UmR3w3qSVCAsvKku-nsHC~N-sQpz80h1AirhaqU59emh70d4EsnlQQDLHastelpS86urUoJq2sMLC3GccF-hjBIjy1W49N3z6jmxd1ydceD9cwGRYREFcFlc09PcPIX~2-UZtkodvNgL42nMgixJzHonmcO8gcJGeSXXLV84YcZQx80hjKMfbHXb7na1y9zQULICUsyILUAueVrCPWJ37xbylSvap5uKcxLWDhBOfK3hSoGw3L3b7kZRmUIR44MXcGl0RDA6vOS6xbpTTocr9ZJ5~r8BnFjPJtp6TOXp1QoGZ9DUdG0EjejStzojdLRe5VNtud4FKZ75AtS-JHg4aw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. p38 MAPK is crucial for the induction and maintenance of T-cell anergy. IL-10DCs or mature DCs were cocultured with allogeneic CD4+ T cells to stimulate iTregs (R, □) and Teffs (E, ▪), and reactivated. The specific inhibitor SB203580 of MAPK p38 was added during the induction of the T-cell populations (middle panel) or during restimulation (bottom panel). Proliferation was detected 96 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). After 48 hours of culture, supernatants were collected and IL-2 production was measured by ELISA as described in “Materials and methods” (B). One of 5 independent experiments with similar results was shown. *Statistical significance according to the Student t test; P ≤ .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047563/4/m_zh80100701570004.jpeg?Expires=1765883506&Signature=1vv3j9KsMGpDn6jQ9t52ZWwEKWDygYwJyYjPIo1GY82K5I3DuRhqDEo5Y0c9Jy5Qaih0R49e~v3g~SLstP56-I8-1ZHVuaAlJjOeRPAoKrcYFJS7OVb3UL8Xet671FUcM7rHKMT1kcFLvtEs2Tc6B0Kf5ZwaWpMzIPyb91e86hoWob5az~L2K1m1u-X6Z6FJzYYCQkOuTne8NnqVNbNC~N3ACxvDinsk5naGQq7Zd7pPSGSYCq~glIfMQKMrtt5-UVZeF-HfxXG1fQneSanw1r5T1wpF0F1KUtRsI~Q4kWqOYYHkcqHXRu52rw8pUCTD-FeO2Y~6XjS9UMcyX6e~Vg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Activity of p38 is essential for the induction of iTregs with suppressive properties. iTregs (R) and Teffs (E) were induced as described in “Materials and methods.” In some experiments, during the primary culture, the specific p38 inhibitor SB203580 was added. After primary culture, Teffs and iTregs were cocultured (ratio, 1:1) and activated by anti-CD3 mAb in suppressor assays. Coculture of Teffs or single T-cell populations served as controls. Proliferation was detected 72 hours after restimulation as incorporation of [3H]-thymidine and demonstrated as cpm ± SEM (A). (B) iTregs (R), SB203580-pretreated iTregs (RI) and Teffs (E) were CFDA labeled or left untreated to determine the proliferation of the T-cell populations after restimulation and in coculture experiments. The proliferative response of the CFDA-labeled population was assessed. FACS analysis was performed after 96 hours. One representative of 3 experiments is shown. IL-2 (C) and IFN-γ (D) production were measured by ELISA in supernatants harvested after 48 hours of culture. Similar results were obtained in 5 independent experiments. To discriminate Teffs, iTregs, and inhibitor-treated iTregs in coculture, the respective populations were CFDA labeled prior to the setup of the experiment (E-F). After 24 hours, intracellular IL-2 production was determined by FACS analysis in the unlabeled population. One representative is shown in panel E. (F) The IL-2 expression is normalized against cytokine expression in Teffs. A total of 5 independent experiments are demonstrated. Asterisks indicate statistical significance according to the Student t test (*P ≤ .05; **P ≤ .005; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047563/4/m_zh80100701570006.jpeg?Expires=1765883506&Signature=cHpW9pMc3FNtP7m2xWvwbIxcDZfdcGG4inbl9~pwJuN0Xo3cOS4O7-BwytcsHBoXQ1eZWouMWcbVFyNvmtAcBkgike-~5s5ww8T1OmVNkCxfm1jjjvcdx~LH0kZovZboXciEEOFjVduQDxmo4uDm7dScjd~Ybu-muTCPxw8O7bKxF0JA63j97z5yQgJ4iLPyyIYiMcPMfM3knXbdztiBwQhBTdpfRHE52zh05DJqQyglerzD-0STUD5q~uAf6DEksy-JMRUjjK2QwPxaj~HA1YElb0n~jIjvxNY3wW~fObMA0ibVVUnrRASOI0yrCZgGCYvll~YWSW9ql9fGTGNK4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. IL-10 is not critical for regulatory activity of iTregs. Expression of IL-10 mRNA and protein levels of iTregs (R), Teffs (E), and SB203580-pretreated iTregs (RI) and Teffs (EI) was quantified at day 5 of primary culture and day 4 after restimulation by ELISA and qRT-PCR, respectively (A). Relative IL-10 mRNA expression was normalized to GAPDH. Secretion of IL-10 in cocultures of iTregs and Teffs (E+R) or SB203580-pretreated iTregs and Teffs (E+RI) was determined by ELISA (n = 4) (B). In cocultures of iTregs and Teffs, IL-10 and TGF-β bioactivity was neutralized by anti–IL-10, anti–IL-10R, or anti–TGF-β antibodies (C). Proliferation was measured in triplicate 72 h after restimulation as incorporation of [3H]-thymidine. *Statistical significance according to the Student t test (P ≤ .05). n.s. indicates not significant. One representive experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047563/4/m_zh80100701570007.jpeg?Expires=1765883506&Signature=NgX79NWMKf34-VJAQMVU65Ll-kBZIrjJV4cfrb4yfpBZ66JI-c~5mTKsFHbI5nzm~JbUFAH0n1ctYhvmRG7pjVEZcwrgvDh1RZKHnYllQ91~1uiDaCH-dcUtF82j8SFGQQLoIuROCaNBwKMA6lqfH~DdWcqoIcpb8bMsujfIJCpxpGHsT9S~kpV9iyBpRn7pLj~wEOJpwhoTsq4~ce6k3lasfkmf8wod4oulsiuFEos-Cd2FUMMpOZfPmM1~ZiXS4vpQ8fOvNfcvU2WvaZdcnyJH8o~4NdUhg-PdO3Yzuvk8vIMWG5F0jWdlylORa8ovN4o6mbITHOmj6k1vHi0eHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal