In this issue of Blood, a report is presented, suggesting that the anti-inflammatory effect of prolonged laminar shear stress on the endothelium is mediated by modulation of the amount of nuclear phospho-ATF2 but not by interfering with NF-κB and is therefore restricted to the nonacute inflammatory state.
It has been known for some time that being exposed to laminar shear stress is good when you are a vascular endothelial cell.1,2 It keeps you calm and quiescent and overall relaxed. There seem to be several possible mechano-transduction mechanisms3 mediating flow-activated effects, including up-regulation of Krupple-like factor 24 (KLF2). This transcription factor regulates prominent flow-induced genes such as endothelial nitric oxide synthase (NOS3) and the anticoagulatory endothelial membrane protein thrombomodulin as well as “antiinflammatory” effects.
Atherosclerosis is a vascular disease with a major inflammatory component.5 Sites in the vascular system, predisposing for atherosclerotic vascular lesions are those where laminar flow is disturbed. This suggests that in such areas the “antiinflammatory” effects of laminar flow are absent. How is this flow-dependent anti-inflammatory effect achieved? The major proinflammatory transcription factor is the nuclear factor–κB (NF-κB). This heterodimeric transcription factor is held in the cytosol by its inhibitor (IκB); upon activation by inflammatory cytokines such as TNF-α, IκB is phosphorylated, ubiquitinylated, and degraded in the proteasome. In turn NF-κB is set free, translocates to the nucleus, and drives transcription of inflammatory target genes. For full activation of such genes, binding of additional factors such as activator protein 1 (AP-1) complex and coactivators such as CREB-binding protein CBP/p300 and in turn formation of a transcriptional complex is, however, necessary.
In addition to this cytokine-induced inflammatory response, also a basal, “background” or constitutive inflammatory activity seems to be operative in endothelial cells. This state is characterized by the absence of nuclear translocated NF-κB and low transcription of inflammatory target genes and might be reflected by the slightly increased CRP levels found in atherosclerotic patients.
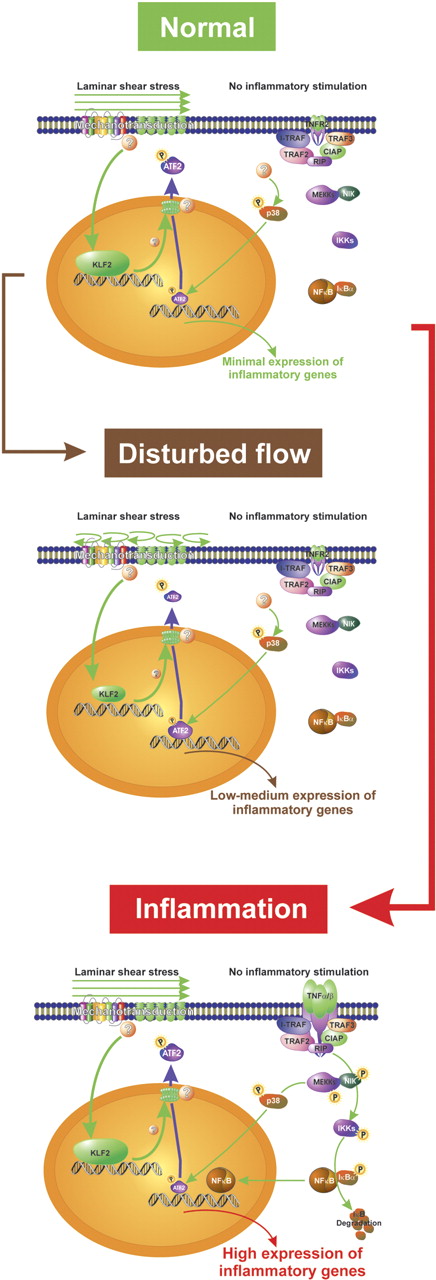
Fledderus and colleagues in this issue analyzed in an elegant experiment the relative abundance of transcription factor binding sites in the promoter of 3 groups of genes regulated differentially in endothelial cells by prolonged laminar shear stress and TNF-α, respectively: in the promoter of genes down-regulated by laminar shear stress the authors found an enrichment of ATF binding sites. ATF (also cyclic AMP response element, CRE) consensus sites bind homodimers or heterodimers of members of the ATF family and c-jun, while the slightly different AP-1 consensus site is occupied by homodimers or heterodimers of the major members of the AP-1 family, c-jun and c-fos. Of importance, among the possible members forming a complex that binds to the ATF consensus site, only ATF2 is constitutively expressed in endothelial cells and regulated by phosphorylation and not by transcription. In fact, the authors found phosphorylated ATF2 (pATF2) only in those endothelial cells overlaying atherosclerotic lesions. Furthermore, prolonged laminar shear stress or lentiviral-mediated overexpression of KLF2 reduced nuclear levels of pATF2, and a reduction of ATF2 led to reduced expression of proinflammatory genes while their TNF-α–dependent up-regulation was preserved. From these data, one can conclude that laminar shear stress suppresses basal but not inducible proinflammatory gene transcription by decreasing the amount of phosphorylated ATF2 in the nucleus in a KLF2-dependent fashion (see figure).
Schematic representation of the effect of flow on the expression of inflammatory genes in endothelial cells. Laminar shear stress induces KLF2 that in turn changes distribution of pATF2 between the nucleus and the cytosol. Less nuclear pATF2 leads to a decrease in the transcription of inflammatory target genes (normal condition). Under disturbed flow conditions, more pATF2 remains in the nucleus and expression of inflammatory genes is increased. Upon inflammatory stimulation, NF-κB is activated and overrules the flow-dependent decrease in nuclear pATF2 (TNF-α/β, TNFR2, I-TRAF, TRAF2, TRAF3, CIAP, RIP, NIK, IKKs; components of NF-κB signaling).
Schematic representation of the effect of flow on the expression of inflammatory genes in endothelial cells. Laminar shear stress induces KLF2 that in turn changes distribution of pATF2 between the nucleus and the cytosol. Less nuclear pATF2 leads to a decrease in the transcription of inflammatory target genes (normal condition). Under disturbed flow conditions, more pATF2 remains in the nucleus and expression of inflammatory genes is increased. Upon inflammatory stimulation, NF-κB is activated and overrules the flow-dependent decrease in nuclear pATF2 (TNF-α/β, TNFR2, I-TRAF, TRAF2, TRAF3, CIAP, RIP, NIK, IKKs; components of NF-κB signaling).
Several questions remain: Most importantly, how shear stress and in turn KLF2 mediates the decrease of nuclear pATF2. It is generally assumed that ATF2 is phosphorylated in the nucleus by p38-MAPKinase. However, it was shown recently that ATF2 can shuttle between the nucleus and the cytosol and nonphosphorylated ATF2 is found in the cytosol. Furthermore, does KLF2 cause this effect via transcription of another (known or hitherto unknown) protein or is this effect transcription independent? In any case, the fact that the basal inflammatory “tone” of the vasculature can be blunted by modulating nuclear pATF2, thereby mimicking normal laminar shear stress, opens new horizons for developing anti-inflammatory drugs or regimens effective in the vasculature while preserving the normal cytokine-induced inflammatory response.
Conflict-of-interest disclosure: the author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal