Abstract
Epigenetic therapy with hypomethylating drugs is now the standard of care in myelodysplastic syndrome (MDS). Response rates remain low, and mechanism-based dose optimization has not been reported. We investigated the clinical and pharmacodynamic results of different dose schedules of decitabine. Adults with advanced MDS or chronic myelomonocytic leukemia (CMML) were randomized to 1 of 3 decitabine schedules: (1) 20 mg/m2 intravenously daily for 5 days; (2) 20 mg/m2 subcutaneously daily for 5 days; and (3) 10 mg/m2 intravenously daily for 10 days. Randomization followed a Bayesian adaptive design. Ninety-five patients were treated (77 with MDS, and 18 with CMML). Overall, 32 patients (34%) achieved a complete response (CR), and 69 (73%) had an objective response by the new modified International Working Group criteria. The 5-day intravenous schedule, which had the highest dose-intensity, was selected as optimal; the CR rate in that arm was 39%, compared with 21% in the 5-day subcutaneous arm and 24% in the 10-day intravenous arm (P < .05). The high dose-intensity arm was also superior at inducing hypomethylation at day 5 and at activating P15 expression at days 12 or 28 after therapy. We conclude that a low-dose, dose-intensity schedule of decitabine optimizes epigenetic modulation and clinical responses in MDS.
Introduction
Aberrant DNA methylation has been implicated in the pathogenesis of many tumors including myelodysplastic syndrome (MDS).1-3 Hypomethylating agents like azacitidine and decitabine have demonstrated anti-MDS activity.4-6 Both decitabine and azacitidine are now approved for the treatment of MDS and chronic myelomonocytic leukemia (CMML).6 However, response rates to these agents remain low, with complete responses (CRs) observed in fewer than 10% of patients in randomized phase 3 studies.4,5 Both azacitidine and decitabine were tested clinically before a good understanding of their mechanism of action, and there are very few studies that attempted mechanism-based optimization of these agents.7 In one study inspired by laboratory observations suggesting loss of the hypomethylation effect at high doses,8 decitabine appeared significantly more active when given daily at low doses compared with higher doses.9 Other strategies suggested to improve the results with these agents include the use of multiple cycles before first clinical evaluation4,-6 and avoiding dose delays (ie, higher dose-intensity). These last 2 approaches have been hypothesized to maximize hypomethylation induction.
In this study, we sought to formally test mechanism-based approaches to optimize therapy with decitabine in MDS and CMML. First, we reduced the dose of decitabine per course from 135 mg/m2 to 100 mg/m2 and investigated, in an adaptive randomization design, which of 3 schedules was superior: (1) 10 mg/m2 intravenously over 1 hour daily for 10 days; (2) 20 mg/m2 intravenously over 1 hour daily for 5 days (exactly double dose-intensity); and (3) 20 mg/m2 subcutaneously daily for 5 days. Second, we implemented a strategy of delivering courses of decitabine every 4 weeks (rather than 6 to 8 weeks) regardless of counts, as long as there was persistent disease and no significant myelosuppression-associated complications; and therapy was continued for at least 3 courses before evaluating response or failure on therapy. These latter approaches also maximize dose intensity. This report summarizes the results of our clinical trial.
Patients, materials, and methods
Study group
Adults with a diagnosis of MDS and CMML who were referred to MD Anderson Cancer Center were enrolled in the study after informed consent was obtained according to institutional guidelines and in accordance with the Declaration of Helsinki. The studies reported in the manuscript have been fully approved by The University of Texas MD Anderson Cancer Center Surveillence Committee. The French-American-British (FAB) morphologic classification was used for MDS and CMML diagnosis. Eligibility criteria included (1) age 16 years or older; (2) diagnosis of MDS with International Prognostic Scoring System (IPSS) intermediate or high risk, or diagnosis of CMML; (3) normal organ functions including creatinine 176.8 μM (2 mg/dL) or less, and bilirubin 34.2 μM (2 mg/dL) or less.10,11 Patients with prior intensive chemotherapy with cytarabine 1 g/m2 or more were not eligible. Diagnosis of CMML was based on the typical morphologic picture, unexplained leukocytosis greater than 12 × 109/L lasting for at least 3 months, exclusion of other myeloproliferative disorders, and presence of at least 1 × 109/L monocytes.10
Therapy
Patients were randomized to receive decitabine in 1 of 3 schedules: (1) 20 mg/m2 intravenously over 1 hour daily for 5 days; (2) 20 mg/m2 daily for 5 days, given in 2 subcutaneous doses daily for 5 days; or (3) 10 mg/m2 intravenously over 1 hour daily for 10 days. All patients received the same decitabine total dose per course, 100 mg/m2. Preferential randomization to the arm demonstrating a higher CR rate started after the 45th patient. Courses of decitabine were given every 4 weeks, at least in the first 3 courses, regardless of the counts, as long as (1) there were no significant myelosuppressive, life-threatening complications with a particular course, such as pneumonia severe infection or bleeding, or severe organ damage, and (2) there was evidence of persistent disease. Therefore, bone marrow aspirations and biopsies (including cytogenetics if abnormal before therapy) were performed before every course to decide on delivering the courses every 4 weeks, until CR or for the first 3 courses (whichever was first), then every 2 to 3 courses. Patients were considered to have not responded only after having received at least 3 courses of therapy, unless prohibitive toxicities, clear progression to acute myeloid leukemia (AML), or patient request did not allow delivery of such therapy. No dose escalations were considered. Dose reductions by 25% to 30%, rounded to 15, 10, 7.5, and 5 mg/m2, were allowed for grade 3 or 4 nonmyelosuppressive toxicities, for severe myelosuppression-associated complications (infections, bleeding), or for prolonged myelosuppression defined as a hypocellular marrow (5% or less cellularity) without evidence of disease for 6 weeks or more after the start of a course of therapy. Other dose modifications (eg, 50% dose reductions) were occasionally considered for severe complications, if judged in the best safety interest of the patient. Use of erythropoietin and granulocyte–colony-stimulating factor (G-CSF) was allowed as indicated by the clinical condition. However, coding of responses as detailed later, followed the modified International Working Group (IWG) criteria,11-13 whereby response is recorded when patients were off growth factor support. Antibiotic prophylaxis and therapy for fever of infections was according to institutional guidelines. In general, erythropoietin 40 000 U subcutaneously weekly was allowed for red cell transfusion dependence or for a hemoglobin level below 100 g/L (10 g/dL). G-CSF 300 to 480 μg subcutaneously was given if the granulocyte count was less than 1 × 109/L in the setting of a febrile episode or documented infection, or in a patient in CR but with granulocyte counts less than 1 × 109/L prior to initiation of the next course of decitabine.
Response criteria and statistical considerations
At the time of the study design, the criteria for CR, partial response (PR), and hematologic improvements were as published11,12 except not requiring durability of response for at least 4 weeks. Thus, the adaptive randomization was based on the incidence of CR in the 3 schedules as soon as they were documented. As the IWG criteria gained acceptance and were modified, it was judged more appropriate to recode the responses according to the modified IWG criteria13 to allow comparisons of the decitabine results with other agents and regimens. Four authors (H.K., J.S., J.D., and C.B.R.) reviewed all responses and agreed on the final response assigned to each patient. This response reclassification did not alter (as shown later) the selection of the decitabine 5-day intravenous schedule as the optimal one from this study. The response criteria used to report the final results from this study are detailed below (ie, the modified IWG criteria).
Response criteria for CR and PR were identical to the ones used for AML,11 but required response durability for at least 4 weeks. A CR required normalization of the bone marrow and peripheral counts with 5% or less marrow blasts, a granulocyte count of 1 × 109/L or more, and a platelet count of 100 × 109/L or more, lasting for at least 4 weeks. A PR was similar to CR except for persistent marrow blasts above 5%, but which were reduced by 50% or more.11 A marrow CR referred to reduction of marrow blasts to 5% or less without normalization of peripheral counts.
Hematologic improvements (HIs) were coded as the modified IWG criteria: HI-E referred to a hemoglobin increase by at least 15 g/L (1.5 g/dL) or transfusion independence; HI-P referred to an absolute increase of platelet counts from less than 20 to more than 20 × 109/L and by at least 100%, or if more than 20 × 109/L, by an absolute increase of at least 30 × 109/L; HI-N referred to a granulocyte increase by at least 100% and by an absolute increase of at least 0.5 × 109/L. HIs were required to last for at least 2 months.12,13 Persistence of dysplastic changes in CR were allowed as proposed.13 Abnormal pretreatment counts were the averages of at least 2 measurements obtained over at least 1 week before treatment and not influenced by transfusions.13 Transient myelosuppression between courses due to chemotherapy did not interrupt the coding of response durability. The above response criteria incorporated the original IWG criteria with the proposed modifications.12,13
Cytogenetic responses were as defined: complete cytogenetic response referred to disappearance of the cytogenetic abnormality; partial cytogenetic response referred to 50% or more reduction of the cytogenetic abnormality.12,13 Response duration was dated from first evidence of response until disease progression as defined by IWG. Survival was dated from start of therapy. Time to transformation was dated from start of therapy until increased blasts to 30% or more blasts.
The statistical design was an adaptive randomization Bayesian method of Berry.14 Initially, the randomization was balanced with probability of one third to each of the 3 schedules. Beginning with the 46th patient, and for each subsequent patient, the probability that the current patient was assigned to a particular treatment schedule was proportional to current estimates of the probability that the CR rate of this schedule was superior to the other 2 schedules. If the assignment probability to a schedule was below 0.05, assignment to this schedule would be temporarily suspended, and would be resumed once it was above 0.05. A schedule would be selected as the optimal one if its assignment probability was above 0.95. Prior CR rate distributions for all 3 treatment schedules were assumed as beta (0.2, 0.8), which was equivalent to a CR rate of 20%. The amount of information in this prior distribution was equivalent.
A total of up to 95 patients were to be treated. If an arm was selected before accrual of the 95 patients, the remaining patients would be treated with the selected best treatment schedule. Toxicities were graded according to the National Cancer Institute (NCI)/Common Toxicity Criteria (CTC) guidelines, version 3.
Methylation and expression studies
Peripheral blood samples were collected from consenting patients. Whenever possible, blood was obtained before or on the first day of treatment (day 0 or day 1), at the end of the first week (days 5 to 7), at the end of the second week (day 12), and at recovery of counts. DNA and RNA were isolated from peripheral blood samples after ficoll separation of mononuclear cells using standard phenol-chloroform extraction methods. Global DNA methylation was measured by the LINE1 bisulfite pyrosequencing assay, as previously described.15 P15INK4B methylation was also measured by pyrosequencing assay, using primers 5′-GTTTTTTTTTAGAAGTAATTTAGG-3′ and biotinylated-5′-TCCTTCTACRACTTAAAACC-3′, and sequencing primer 5′-TTTTTAGAAGTAATTTAGG-3′. P15INK4B expression was measured by real-time PCR (qPCR) using a commercially available assay (TaqMan Gene Expression Assay Hs00 365 249_m1; Applied Biosystems, Foster City, CA), and the level of expression was marked by delta CT, using GAPDH as an internal control (primer sets, 5′-ATGGAAATCCCATCACCATCTT-3′ and 5′-CGCCCCACTTGATTTTGG-3′; probe, FAM-5′-CAGGAGCGAGATCC-3′-MGBNFQ [minor groove binder, nonfluorescent quencher]). Fold changes between 2 sample groups were calculated by 2 to the power of delta-delta CT.15,16 The Fisher exact test and Mann-Whitney test were used for analysis of categorical and continuous data, respectively, and a 2-sided P less than .05 was considered significant. Methylation or expression in each group is described by mean value plus or minus standard error of mean.
Results
Study group
A total of 95 patients were treated. Their characteristics are detailed in Table 1. Seventy-seven patients had MDS, and 18 had CMML. These patients had relatively advanced MDS, with 66% of patients eligible for IPSS classification (ie, not secondary MDS and CMML with white blood cell [WBC] count > 12 × 109/L) being intermediate-2 or high risk. After the 45th patient was enrolled, more patients were increasingly treated with the decitabine schedule of 20 mg/m2 daily for 5 days since it showed a higher CR rate than the other 2 arms. At the 65th patient, this schedule was chosen according to the statistical design of the trial as the one most likely to be associated with the highest CR rate. Therefore, the remaining 30 patients were all treated on this selected dose schedule.
Characteristics of the study group (95 patients)
| Characteristic . | Value . |
|---|---|
| Age at least 60 y, no. (%) | 67 (71) |
| Median age, y | 65 |
| No. female (%) | 29 (31) |
| Hemoglobin level less than 110 g/L, no. (%) | 81 (85) |
| Granulocyte count less than 1.0 × 109/L, no. (%) | 42 (44) |
| Platelet count less than 100 × 109/L, no. (%) | 68 (72) |
| Chromosomal abnormalities present, no. (%) | 53 (56) |
| Bone marrow blasts, no. (%) | |
| Less than 5% | 23 (24) |
| 5%–9% | 29 (31) |
| 10%–19% | 37 (39) |
| 20%–29% | 6 (6) |
| Duration of MDS | |
| 3–6 mo, no. (%) | 13 (14) |
| More than 6 mo, no. (%) | 36 (38) |
| Median, mo | 3.2 |
| Prior therapy, no. (%) | 58 (61) |
| Growth factors | 35 (37) |
| Others | 23 (24) |
| Secondary MDS, no. (%) | 30 (32) |
| Prior therapy for primary malignancy, no. | |
| Chemotherapy | 7 |
| Irradiation | 5 |
| Both/other | 10 |
| Surgery only | 8 |
| Morphology, no. (%) | |
| MDS | 77 (81) |
| CMML | 18 (19) |
| IPSS risk,* no. (%) | |
| Intermediate 1 | 19 (34) |
| Intermediate 2 | 26 (46) |
| High | 11 (20) |
| Not applicable† | 39 |
| Decitabine schedule, no. (%) | |
| 20 mg/m2 intravenously for 5 d | 64 (67) |
| 20 mg/m2 subcutaneously for 5 d | 14 (15) |
| 10 mg/m2 intravenously for 10 d | 17 (18) |
| Characteristic . | Value . |
|---|---|
| Age at least 60 y, no. (%) | 67 (71) |
| Median age, y | 65 |
| No. female (%) | 29 (31) |
| Hemoglobin level less than 110 g/L, no. (%) | 81 (85) |
| Granulocyte count less than 1.0 × 109/L, no. (%) | 42 (44) |
| Platelet count less than 100 × 109/L, no. (%) | 68 (72) |
| Chromosomal abnormalities present, no. (%) | 53 (56) |
| Bone marrow blasts, no. (%) | |
| Less than 5% | 23 (24) |
| 5%–9% | 29 (31) |
| 10%–19% | 37 (39) |
| 20%–29% | 6 (6) |
| Duration of MDS | |
| 3–6 mo, no. (%) | 13 (14) |
| More than 6 mo, no. (%) | 36 (38) |
| Median, mo | 3.2 |
| Prior therapy, no. (%) | 58 (61) |
| Growth factors | 35 (37) |
| Others | 23 (24) |
| Secondary MDS, no. (%) | 30 (32) |
| Prior therapy for primary malignancy, no. | |
| Chemotherapy | 7 |
| Irradiation | 5 |
| Both/other | 10 |
| Surgery only | 8 |
| Morphology, no. (%) | |
| MDS | 77 (81) |
| CMML | 18 (19) |
| IPSS risk,* no. (%) | |
| Intermediate 1 | 19 (34) |
| Intermediate 2 | 26 (46) |
| High | 11 (20) |
| Not applicable† | 39 |
| Decitabine schedule, no. (%) | |
| 20 mg/m2 intravenously for 5 d | 64 (67) |
| 20 mg/m2 subcutaneously for 5 d | 14 (15) |
| 10 mg/m2 intravenously for 10 d | 17 (18) |
For IPSS risk data, n = 56 patients.
Not applicable because of either secondary MDS or CMML with WBC count above 12 × 109/L.
Response and outcome
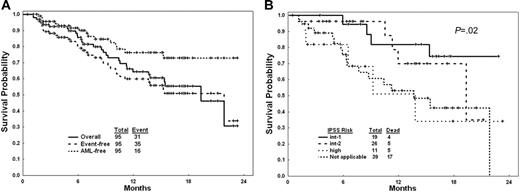
Overall, 32 patients (34%) achieved CR, 1 (1%) had PR, 23 (24%) had marrow CR without (n = 10, 11%) or with other HI responses (n = 13, 14%), and 13 (14%) had HIs including 2 or more HIs in 5 (5%). Overall, 69 of 95 patients (73%) demonstrated objective responses (Table 2). The CR rate was higher with the 5-day intravenous schedule (39%) compared with the other 2 schedules (21% for the 5-day subcutaneous and 24% for the 10-day intravenous; Table 3). The IWG modified CR rates by IPSS risk categories were intermediate-1, 9 of 19 (47%); intermediate-2, 7 of 26 (27%); high, 4 of 11 (36%); nonapplicable, 12 of 39 (31%). With a median follow-up of 10 months, 16 (17%) patients have progressed to AML (10 of them while on decitabine) and 31 patients have died. Causes of death were AML progression complications in 15, MDS complications in 12, and myelosuppression-associated complications in 4. The estimated 18-month survival rate was 56%; the estimated 18-month event (AML or death)–free survival rate was 51%. The median overall survival was 19 months. The estimated 18-month AML transformation rate was 27% (Figure 1A). The survival in different IPSS risk groups and in CMML are shown in Figure 1B. Among 18 patients with CMML, 9 (50%) achieved CR and overall 12 (67%) had an objective response; their estimated 18-month survival rate was 57%.
Response data (95 patients) by the Modified IWG Criteria
| Response . | No. of patients (%) . |
|---|---|
| Complete response | 32 (34) |
| Partial response | 1 (1) |
| Marrow CR | 10 (11) |
| Marrow CR + other HI | 13 (14) |
| Hematologic improvement | |
| Single lineage | 9 (9) |
| 2 or 3 lineages | 4 (4) |
| Response . | No. of patients (%) . |
|---|---|
| Complete response | 32 (34) |
| Partial response | 1 (1) |
| Marrow CR | 10 (11) |
| Marrow CR + other HI | 13 (14) |
| Hematologic improvement | |
| Single lineage | 9 (9) |
| 2 or 3 lineages | 4 (4) |
Comparison of outcome and side effects by dose schedule
| Parameter . | 5-day IV dose . | 5-day SQ dose . | 10-day IV dose . |
|---|---|---|---|
| No. of patients | 64 | 14 | 17 |
| No. of patients achieving complete response (%) | 25 (39) | 3 (21) | 4 (24) |
| Median number of courses (range) | 5+ (1-18) | 8 (1-17) | 9 (1-15) |
| Median duration of therapy, mo (range) | 5.4+ (1.0-20.4+) | 9.7 (0.5-22.9+) | 10.8 (1.9-17.7+) |
| Median follow-up time, mo | 6.5 | 15 | 15 |
| No. of patients still on therapy (%) | 39 (61) | 3 (21) | 6 (35) |
| Median days to granulocyte recovery to 1 × 109/L or more | 24 | 14 | 27 |
| Median days to platelet recovery to 50 × 109/L or more | 20 | 31 | 27 |
| Median days to delivery of subsequent courses | 35 | 35 | 40 |
| No. of courses requiring hospitalization (%) | 50 (12) | 14 (14) | 23 (23) |
| Parameter . | 5-day IV dose . | 5-day SQ dose . | 10-day IV dose . |
|---|---|---|---|
| No. of patients | 64 | 14 | 17 |
| No. of patients achieving complete response (%) | 25 (39) | 3 (21) | 4 (24) |
| Median number of courses (range) | 5+ (1-18) | 8 (1-17) | 9 (1-15) |
| Median duration of therapy, mo (range) | 5.4+ (1.0-20.4+) | 9.7 (0.5-22.9+) | 10.8 (1.9-17.7+) |
| Median follow-up time, mo | 6.5 | 15 | 15 |
| No. of patients still on therapy (%) | 39 (61) | 3 (21) | 6 (35) |
| Median days to granulocyte recovery to 1 × 109/L or more | 24 | 14 | 27 |
| Median days to platelet recovery to 50 × 109/L or more | 20 | 31 | 27 |
| Median days to delivery of subsequent courses | 35 | 35 | 40 |
| No. of courses requiring hospitalization (%) | 50 (12) | 14 (14) | 23 (23) |
IV indicates intravenous; SQ, subcutaneous.
Outcome of study group with decitabine. (A) Survival (solid line); event-free survival (event = AML or death; dashed line); and duration of freedom from AML (dotted line). (B) Survival in IPSS risk groups and in CMML.
Outcome of study group with decitabine. (A) Survival (solid line); event-free survival (event = AML or death; dashed line); and duration of freedom from AML (dotted line). (B) Survival in IPSS risk groups and in CMML.
Treatment delivery
The median number of courses delivered so far was 6+ (range, 1-18). The study is ongoing and 48 of the 95 patients are still receiving decitabine. Among 62 patients with a follow-up time of at least 6 months, the median number of courses was 9 (range, 1-18). Only 7 of these 62 patients received fewer than 3 courses of therapy for the following reasons: progression to a worse MDS category or to AML (2 patients); prolonged myelosuppression (2 patients); severe toxicities (0 patients); other causes (3 patients: alternative therapies in 2, patient request in 1). The median number of courses to achieve CR was 3 (range, 1-7); the median number to achieve PR was 5.
Side effects
Severe (grade 3-4) drug-related extramedullary toxicities were uncommon, and included transient liver toxicities (mostly elevations of liver enzymes) in 4 patients (4%) and other in 1 patient (1%). None required treatment interruptions or recurred with dose reductions. Myelosuppression-associated toxicities among 622 courses delivered are described in Table 4. Despite the repeated courses of therapy and median number of 6+ courses delivered so far, 32 (34%) of the 95 patients never required any hospitalization due to decitabine therapy. Mortality directly attributable to decitabine therapy did not occur.
Side effects
| Toxicities . | No. of patients (%) . | |
|---|---|---|
| Grade 1-2 . | Grade 3-4 . | |
| Extramedullary toxicities* | ||
| Fatigue | 6 (6) | — |
| Bone aches | 4 (4) | 5 (5) |
| Liver dysfunction | 10 (11) | 4 (4) |
| Skin rashes | 1 (1) | — |
| Nausea, vomiting | 17 (17) | — |
| Diarrhea | 2 (2) | — |
| Other | 9 (9) | — |
| Myelosuppression-related toxicities† | ||
| Fever of unknown origin | — | 23 (4) |
| Documented bacterial infections | ||
| Sepsis alone | — | 7 (1) |
| Minor infections | — | 24 (4) |
| Pneumonias with or without other infections | — | 20 (3.5) |
| Fungal infections | ||
| Confirmed | — | 5 (0.8) |
| Suspected | — | 1 (0.2) |
| With other infections | — | 1 (0.2) |
| Bleeding | 46 (7) | 7 (1) |
| Hospitalization | — | 110 (18) |
| Toxicities . | No. of patients (%) . | |
|---|---|---|
| Grade 1-2 . | Grade 3-4 . | |
| Extramedullary toxicities* | ||
| Fatigue | 6 (6) | — |
| Bone aches | 4 (4) | 5 (5) |
| Liver dysfunction | 10 (11) | 4 (4) |
| Skin rashes | 1 (1) | — |
| Nausea, vomiting | 17 (17) | — |
| Diarrhea | 2 (2) | — |
| Other | 9 (9) | — |
| Myelosuppression-related toxicities† | ||
| Fever of unknown origin | — | 23 (4) |
| Documented bacterial infections | ||
| Sepsis alone | — | 7 (1) |
| Minor infections | — | 24 (4) |
| Pneumonias with or without other infections | — | 20 (3.5) |
| Fungal infections | ||
| Confirmed | — | 5 (0.8) |
| Suspected | — | 1 (0.2) |
| With other infections | — | 1 (0.2) |
| Bleeding | 46 (7) | 7 (1) |
| Hospitalization | — | 110 (18) |
Of 95 patients, 32 (34%) were never hospitalized.
— indicates not applicable.
N = 95 patients.
N = 622 courses.
The incidences of myelosuppression-associated complications and of prolonged myelosuppression tended to be worse with the 10-day intravenous schedule, as was the incidence of hospitalization. The median days to subsequent courses were 40 with the 10-day intravenous schedule versus 35 with the 5-day schedules (Table 3).
Dose reductions of decitabine were required in 14 (14%) of 95 patients and in 88 (14%) of 622 courses. Thus, most patients received the full decitabine dose schedule.
Epigenetic modulation
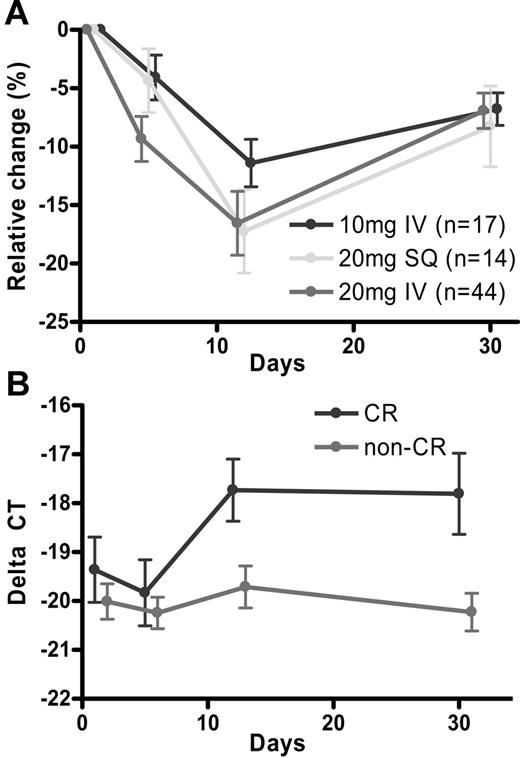
We used a LINE1 assay as a surrogate for global methylation. Relative LINE hypomethylation percentage in patients receiving 20 mg/m2 intravenously for 5 days, 20 mg/m2 subcutaneously for 5 days, and 10 mg/m2 intravenously for 10 days was −11.0% ± 2.1%, −5.6% ± 2.7%, and −3.4% ± 1.9%, on day 5; −18.4% ± 2.5%, −19.0% ± 3.6%, and −10.9% ± 2.5% on day 12; and −9.7% ± 1.9%, −8.2% ± 3.5%, and −6.1% ± 3.2% at recovery, respectively (Figure 2A). On day 5, hypomethylation was more prominent on the 20 mg/m2 intravenous arm compared with the 20 mg/m2 subcutaneous arm (P = .09) or the 10 mg/m2 intravenous arm (P = .02). At the time of peripheral blood recovery, LINE remethylation was observed but the level was not fully back to baseline. The nadir of methylation after repeated cycles of decitabine was similar to cycle 1 (data not shown), and LINE methylation recovered to the same degree after each course of decitabine therapy. To determine the association between clinical response and the degree of hypomethylation, we then focused on the 20 mg/m2 intravenous group and divided patients into 3 groups (CR [n = 16], benefit other than CR [n = 16], and no clinical response [n = 12]). Relative hypomethylation was not different in these 3 groups on day 5 (−4.3% ± 2.7%, −15.5% ± 2.4%, and 9.4% ± 4.7%), on day 12 (13.7% ± 2.6%, 21.9% ± 7.6%, and −16.4% ± 5.5%), and at peripheral blood recovery (−6.6% ± 2.2%, −7.6% ± 2.6%, and −6.3% ± 3.5%).
Epigenetic modulation by decitabine. (A) Relative LINE hypomethylation percentage by schedule. (B) p15 expression levels by response.
Epigenetic modulation by decitabine. (A) Relative LINE hypomethylation percentage by schedule. (B) p15 expression levels by response.
Promoter methylation of the p15INK4B gene was observed in 11 of 80 patients analyzed (14%). The incidence of promoter methylation was similar in patients achieving CR (4/26, 15%), in patients having benefit other than CR (4/32, 13%), and in patients with resistant disease (3/20, 15%). Two patients with no baseline methylation were not evaluable for clinical response. Among those who had baseline p15INK4B methylation, all except one (who had benefit other than CR) showed at least temporary hypomethylation (data not shown). All 4 patients who achieved CR had maintained low methylation at peripheral blood recovery, while all 3 patients who had benefit other than CR, and 2 of 3 patients who had resistant disease, showed significant remethylation of p15INK4B promoter at recovery.
We then assessed if p15 expression changes after decitabine in all patients analyzed. Overall, the expression levels changed from baseline ( = 1 fold) to 0.81-fold on day 5 (P = .67), 1.6-fold on day 12 (P = .15), and 1.3-fold at recovery (P = .51). Next we assessed if expression levels were different in 3 dosing schedule groups. Expression levels at baseline and on day 5 were similar in all 3 groups. On day 12 and at recovery, the 20 mg/m2 intravenous group tended to have higher p15 expression compared with the 20 mg/m2 subcutaneous group (2.8-fold [P = .32] on day 12, and 2.7-fold [P = .36] at recovery) or the 10 mg/m2 intravenous group (3.9-fold [P = .039], and 3.0-fold [P = .25], respectively). We then assessed the impact of p15INK4B activation on response. p15INK4B expression levels were not different in the CR group and non-CR groups at baseline and on day 5. The expression levels became significantly higher in the CR group than in the non-CR group on day 12 (3.9-fold difference; P = .012) and this difference was maintained at peripheral blood recovery (5.4-fold difference; P = .011) (Figure 2B).
Response and outcome of patients with thrombocytopenia
Response of thrombocytopenia was particularly relevant. Among 68 patients with pretreatment platelet counts less than 100 × 109/L, 33 (49%) ultimately achieved platelet counts of at least 100 × 109/L. Such responses were noted in 4 of 15 (27%) with pretreatment platelet counts of less than 20 × 109/L, in 14 of 31 (45%) with counts 20 to 50 × 109/L, and in 15 of 22 (68%) with counts 51 to 99 × 109/L. The estimated 18-month survival rates in the 3 groups were 28%, 63%, and 55%, respectively. One-year survival dated from 3 months into therapy by platelet responses (more than 100 × 109/L) were 86% for responders and 54% for nonresponders (P = .03). By IWG response criteria, 39 of 68 patients (57%) with pretreatment platelet counts less than 100 × 109/L had an HI-P response, either an increase from less than 20 to more than 20 × 109/L and at least a 100% increase (8/15; 53%) or an absolute increase of platelet counts of at least 30 × 109/L (31/53 patients; 58%), lasting for at least 2 months. Similar results were reported by van den Bosch et al.17
Cytogenetic responses
Cytogenetic abnormalities were present before treatment in 53 patients, 51 of whom were evaluable for cytogenetic response. A complete cytogenetic response after therapy was observed in 17 (33%). These included 12 of 16 patients achieving morphologic CR, 4 of 22 patients with other responses, and 1 of 13 patients without morphologic response. A partial cytogenetic response (decrease by at least 50%) was noted in 12 patients, for a total cytogenetic response rate of 57%. Recurrence of the pretreatment cytogenetic abnormalities, but no new chromosomal abnormalities, was noted in 3 patients who had a cytogenetic CR. Survival from start of therapy was longer among the 38 of 53 patients achieving response by whether or not they had a persistence of the cytogenetic abnormality or partial cytogenetic response or not (P = .14). The median time to cytogenetic response was 2.1 months. The estimated 18-month survival rates by a landmark analysis at 3 months for the 38 patients achieving a response by modified IWG criteria were 87% with complete cytogenetic response, 54% with partial cytogenetic response, and 37% without a cytogenetic response (P = .017).
Discussion
In this study, we show that a low dose but high dose-intensity schedule of decitabine optimizes epigenetic modulation (hypomethylation induction, activation of p15INK4B) as well as clinical results based on IWG criteria. The optimal dose in this study, 20 mg/m2 intravenously daily over 1 hour for 5 consecutive days, yields a remarkable high response rate (39% CR) in a poor-prognosis group of patients. This treatment is well tolerated with few nonmyelosuppressive complications, and represents an excellent therapeutic option in a disease where the standard was supportive care a few years ago.
An important question is how the results of this study compare with those of the randomized studies of decitabine and azacitidine versus supportive care.4-6 Key components of this regimen were: (1) timely delivery of decitabine courses every 4 weeks (rather than 6 to 8 weeks) regardless of myelosuppression, as long as there were no myelosuppression-related prohibitive complications (eg, pneumonia, severe infections or bleeding, severe organ dysfunction) or prolonged myelosuppression (no evidence of MDS in a hypocellular marrow with 5% or less cellularity); (2) delivery of at least 3 courses of decitabine before judging response; and (3) reduced decitabine dose from 135 mg/m2 to 100 mg/m2 hoping to alleviate further myelosuppressive complications and optimize hypomethylation induction. The CR rate in the best arm of this study was 39% versus only 9% in the decitabine randomized study. While the 2 study groups appear comparable, a major difference is the dose-intensity delivery of decitabine and the fact that the median number of decitabine courses in this study is 6+ courses versus 3 courses in the decitabine randomized trial. Comparing the current results to the azacitidine randomized study, it appears that a more advanced group of patients with MDS were treated in our study and a comparable median number of cycles was given. Despite that, the CR rate was 39% with decitabine but only 6% with azacitidine. Nevertheless, there are risks associated with comparing single institution studies to multi-institution trials, and the suggestion of a superior response rate with decitabine needs to be confirmed in a randomized study.
The randomized design of the study allowed defining the contribution of pharmacodynamic considerations and epigenetic effects on responses after decitabine. One caveat to this study is that the analyzed cells were not purified MDS cells and therefore represent a mixture of normal and neoplastic cells. A comparison of aberrant DNA methylation in peripheral blood– and bone marrow–derived cells from MDS generally showed identical patterns, suggesting that most of the analyzed cells were in fact part of the neoplastic clone,18 as also confirmed by clonality studies.19 Thus, the observation that the best clinical arm is associated with the most rapid and profound induction of hypomethylation as well as induction of p15INK4B, lends support to the notion that decitabine works in vivo as a hypomethylating agent. However, the fact that within the optimal dose, the degree of hypomethylation did not correlate with responses, suggests that downstream effects are also key to decitabine activity. Indeed, the correlation between p15INK4B activation and response is consistent with this hypothesis. The fact that responses tend to occur slowly suggests that these early changes in DNA methylation and gene expression cannot be explained simply by clonal changes in cell composition. Interestingly, decitabine responses were generally accompanied by cytogenetic responses, indicating that the epigenetic effects lead to eventual clearance of the MDS clone. However, a few hematologic responders did not show cytogenetic responses, which could be explained by clonal heterogeneity and elimination of a more advanced clone, or by the possibility that differentiation is part of the response process with decitabine. How responses are occurring after hypomethylation induction is still unclear. The delayed nature of the responses suggests a noncytotoxic, nonapoptotic mechanism in MDS. Intriguing possibilities to consider are an immune process activated by changes in tumor antigen expression, an effect on the MDS stem cell self-renewal, which may not manifest as a response until weeks later, or an effect on normal stem cells suppressed by the MDS clone and reawakened by epigenetic therapy. All of the 3 latter hypotheses could also account for the intriguing observation that decitabine responses are lost when the dose of the agent is increased.9
This study used a Bayesian design, which allowed more patients to be treated on the schedules suggested to be more effective, and required a smaller number of patients to evaluate the study objectives. Within the operating characteristics of the design, the 5-day intravenous arm was selected over the 5-day subcutaneous arm (CR rates 39% versus 21%). However, only 14 patients were treated on the latter schedule before it was selected out, and a CR rate of 21% is encouraging, even when compared with previous schedules of azacitidine (CR rate 6%) or decitabine (CR rate 10%).4,5 Recognizing the importance of a subcutaneous schedule for ease-of-delivery and for other maintenance strategies, additional studies comparing the 5-day intravenous versus the 5-day subcutaneous schedules in larger numbers of patients may be indicated.
In summary, decitabine provides significant anti-MDS and anti-CMML activities with a safe toxicity profile. Increasing dose intensity to a dose schedule of 20 mg/m2 intravenously over 1 hour daily for 5 days resulted in improved epigenetic reactivation and was selected as producing the best CR rate. Capitalizing on these results, optimizing epigenetic reactivation by combination epigenetic therapy,16 and understanding the key determinants of response could lead to improved management of MDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: H.K. and J.-P.J.I. designed the study, analyzed the results, and wrote the paper. Y.O. and J.-P.J.I. conducted the laboratory studies. X.H. and J.S. designed the studies and analyzed the data. All other authors treated patients and contributed to the final analysis and writing of the paper.
Acknowledgments
This work is supported in part by Leukemia SPORE (Specialized Program of Research Excellence) grant 1P50CA 10063204 and by MDS P01 Grant CA 108631, both from the National Institutes of Health awarded to H.K., J.P.I., and G.G.-M.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal