Abstract
Natural killer (NK) cells play an important role in tumor-cell clearance, particularly against leukemia, as shown by killer cell inhibitory receptor (KIR)–mismatched allogeneic stem cell transplantation. Analysis of in vitro IL-2–expanded NK cells from patients with myelocytic/monocytic acute myeloid leukemia (AML-NK cells) has revealed poor cytolytic functions because of deficient expression of pivotal activation molecules—the natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46. To exclude the possibility that this observation was caused by the in vitro amplification of a small NCRdull population, we analyzed the AML-NK phenotype directly, without any in vitro expansion. We first confirmed that the NCRdull phenotype was not an in vitro artifact. Moreover, analysis of a large population of AML patients allowed us to demonstrate that phenotype was not restricted to a French-American-British (FAB) subtype and was not associated with a particular cytogenetic abnormality. Our longitudinal study of AML patients showed that the NCRdull phenotype was acquired during leukemia development because we observed its complete (for NKp46) or partial (for NKp30) reversibility in patients achieving complete remission (CR). Reversibility of the NCRdull phenotype after CR suggested that leukemia cells might be involved in NCR down-regulation. In agreement with this hypothesis, direct contact between leukemic blasts and NK cells (but not leukemia-cell supernatants) induced loss or decrease in NKp30 and NKp46 expression while impeding NKp44 induction by IL-2. We excluded the major implication of TGF-β in NCR down-regulation. Although the clinical antitumor value of NK cells is clearly demonstrated in allogeneic stem cell transplantation, the role of NK cells in autologous transplantation is not proved. Interestingly, we observed a correlation between the NCRdull phenotype and poor survival in AML patients, suggesting that NK-deficient activation caused by NCR down-regulation could play a role in patient outcome. The prognostic value of NCR expression is discussed, and pathophysiologic implication of the NCR phenotype will be further investigated in a larger study.
Introduction
Natural killer (NK) cells function as sentinels for the recognition of virus infection and cellular transformation by monitoring the expression of class I human leukocyte antigen (HLA) molecules at the cell surface.1 Activation of NK cells is the result of a balance between inhibitory and activating signals.2 Inhibitory signals are provided by interactions with HLA class I molecules. Under physiologic conditions, normal cells are protected from NK-cell lysis because they express a sufficient amount of HLA class I molecules to inhibit NK cytotoxicity.3 In the absence of these inhibitory signals, activating receptors, if engaged by ligands on the target-cell surface, activate NK cytotoxicity. Many activating receptors have been described.4 NKG2D is an important activating receptor expressed by NK cells but also by virtually all cytolytic T lymphocytes.5 NKG2D plays a major role in NK-cell–mediated cytotoxicity against certain target cells. Ligands for NKG2D include the major histocompatibility complex class I chain-related (MIC)–A and –B stress-inducible molecules and the UL16-binding protein (ULBP) major histocompatibility complex class I–related molecules. FcγRIII receptor CD16 is responsible for the antibody-dependent cell cytotoxicity (ADCC) mediated by NK cells, but it also mediates cytotoxicity by yet unidentified ligands.6 Another important activating receptor expressed by NK cells is 2B4/CD244.7 This receptor belongs to the CD2 superfamily and is also expressed by all cytolytic lymphocytes.8 This receptor has an important role in the fight against Epstein-Barr virus (EBV) infection because its ligand, CD48, is up-regulated in EBV-infected cells and thus provides a coactivation signal for NK cells.9,10 Finally, among activating receptors, the natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46 are NK-cell restricted.11 Their engagement by still unidentified cellular ligands, or virus-derived components,12,13 results in increased cytotoxicity and cytokine release by NK cells.14 NCR surface density, as measured by the brightness of immunofluorescence, varies. Thus, NK cells from most donors express a high density of NCR (NCRbright), whereas in less than 20% of donors, NK cells display reduced quantities of NCR (NCRdull). Importantly, a strict correlation exists between NCR density and NK-mediated cytolytic activity.15,16
We focused our attention on NK cells in patients with acute myeloid leukemia (AML) because several clues supported the potent antileukemia role of NK cells. Rapid disease progression and the high incidence of relapse after treatment with high-dose chemotherapy or after transplantation of allogeneic hematopoietic stem cells suggested that leukemic blasts can escape recognition by the immune system. It has been shown in patients with AML that impaired NK-cell function and cytokine production are associated with early relapse.17 Moreover, it as been observed that leukemia cells can express membrane-bound heat shock protein 70 (HSP70), which correlates with sensitivity to lysis by autologous NK cells.18 In addition, NK cells adhere to bone marrow fibroblasts and compete with myeloid leukemia cells for binding to the microenvironment, suggesting that they may inhibit the growth of leukemia cells.19 Finally, an important clue arguing for the major role of NK cells in AML clearance comes from allogeneic stem cell transplantation. When patients and donors were mismatched for HLA-specific inhibitory NK receptors (killer-cell–inhibitory receptors [KIRs]), the risk for relapse was virtually null compared with the 75% rate observed when perfect KIR matching was observed between donors and recipients.20,21 Given that NK cells may have a potent antileukemia effect,22 why do patients experience relapse and thus escape the innate immunity-mediated antitumor response? In agreement with this escape, it has recently been shown that low levels of NCR ligands were expressed by leukemia cells. In addition, the ligands might have been expressed by normal myeloid cells, thus disturbing NK-cell function in AML.23 Another hypothesis is poor NCR expression by NK cells of AML patients (the so-called NCRdull phenotype), a phenotypic abnormality observed in most (more than 80%) in vitro–expanded NK cells of leukemia patients.24 This deficient NCR expression leads to poor antitumor cytotoxicity capacity because of the impossibility of NCR ligand engagement, though the cytotoxicity armamentarium of AML-NK cells is preserved as demonstrated by potent CD16-driven redirected killing.24 Moreover, we have recently shown that this phenotypic defect is also correlated with poor killing of immature dendritic cells (iDCs), which could lead to iDC-mediated tolerance in AML-T cells.25 Because phenotypic and functional analyses were performed after IL-2–driven expansion of AML-NK cells, we could not exclude in vitro–induced proliferation of a minor NCRdull NK-cell subset because, in certain healthy donors, NCRdull and NCRbright subsets can coexist.15
In the present study, we first studied the AML-NK phenotype at diagnosis using 4-color flow cytometry analysis directly on total fresh uncultured peripheral-blood mononuclear cells (PBMCs) from 71 patients with AML. We then tested the in vivo reversibility of the NCRdull phenotype and its dependence on leukemia cells by assessing the AML-NK phenotype after complete remission (CR) of leukemia and compared it with that of patients who did not attain CR or who experienced disease relapse. Then, to decipher mechanisms involved in that process, we verified whether NCR down-regulation could be obtained in culture. Finally, we compared NCR expression and clinical behavior of patients with leukemia.
Materials and methods
Peripheral-blood samples were obtained from patients or healthy blood donors after informed consent was obtained in accordance with the Declaration of Helsinki. Patient samples were obtained before or after specific antileukemia therapy and were part of diagnostic procedures. Umbilical cord blood (UCB) samples from full-term newborns were collected at the Department of Gynaecology, Hôpital de la Conception (Marseille, France) after informed consent of the mothers. The entire research procedure was approved by the local ethics committee (Institut Paoli-Calmettes Marseille, France). To increase the statistical potency of our present analysis, data from 14 additional patients from our previous study24 were analyzed regarding survival curves.
Flow cytometry analysis of NK cells
Cell surface analysis was performed through flow cytometry with the use of a FACScalibur cytometer and CellQuest software (Becton Dickinson, Mountain View, CA). NK cells were immunostained with fluorescein isothiocyanate (FITC)–conjugated anti-CD3, phycocyanin 5 (PC5)–conjugated anti-CD56, and allophycocyanin (APC)–conjugated anti-CD45 (to exclude myeloid blasts) monoclonal antibodies (mAbs) (Immunotech, Marseille, France). Indeed, blasts expressed lower levels of CD45 compared with nonleukemia cells. Thus, this marker is used for gating normal cells.26 Triggering receptor expression (NKp44, NKp30, NKp46, CD16, 2B4/CD244, NKG2D) was measured with phycoerythrin (PE)–conjugated mAb (kind gift of Beckman-Coulter Immunotech, Marseille, France).
Discrimination between NCRdull and NCRbright
Freshly analyzed AML-NK cells were classified as NCRbright or NCRdull, depending on NKp30 and NKp46 MFI levels. Cut-off values for NCRdull and NCRbright were chosen on the basis of previously published studies (Costello et al,24 Sivori et al15 ), in accordance with the subtypes described by Alessandro Moretta (Sivori et al15 ). Indeed, 80% of healthy donors have an NK-expressing NCRbright phenotype.15 In our 23 samples from healthy donors, cut-off points to determine NCRbright phenotypes were chosen with respect to this percentage. When NKp30 and NKp46 were expressed at low levels (MFI, respectively, less than 30 and less than 50), patients were classified with the NCRdull phenotype. When NKp30 and NKp46 were both expressed at high levels (MFI, respectively, greater than 30 and greater than 50), patients were classified with the NCRbright phenotype. Patients with the NKp30bright/NKp46dull phenotype or the NKp30dull/NKp46bright phenotype were further classified as NCRdiscordant.
Generation of NK cells
In all experiments, mature NK cells were derived from the peripheral blood of patients or healthy donors. NK cells were isolated by immunomagnetic negative selection according to the manufacturer's instructions (Miltenyi Biotec, Paris, France). Then NK cells were cultured for 2 to 3 weeks before use or were briefly cultured (1-5 days) with leukemia cells. To amplify NK cells, they were first isolated and then cultured for 3 weeks with 1000 IU/mL interleukin-2 (Proleukin; Chiron, Emeryville, CA), 1.5 ng/mL phytohemagglutinin A (Invitrogen, Cergy-Pontoise, France), and irradiated allogeneic normal PBMCs used as feeder cells in RPMI (Cambrex, Emerainville, France) and 10% fetal calf serum (FCS; Biowest, Paris, France). Then NK cells were tested for cytolytic activity or were harvested and cultured with leukemia cells for another 5 or 10 days.
Generation of NK cells from CD34+ hematopoietic progenitor cells (HSPs)
Experiments were performed using a protocol modified from Sivori et al27 and Carayol et al.28 UCB mononuclear cells were obtained by Ficoll-Hypaque density gradient centrifugation. CD34+ cells were separated from mononuclear cells by use of the MACS system (Miltenyi Biotec, Auburn, CA). Cells obtained were 98% or more pure. CD34+ cord-blood precursors (2 × 104 to 6 × 104 cells) were incubated in 24-well plates (Falcon; Becton Dickinson) in 2 mL α-MEM (Cambrex) containing 10% human serum (ABCYS, Paris, France) and 5% FCS (Biowest). Human recombinant (hr) SCF (hrSCF; 20 ng/mL; ABCYS) and hrIL-15 (20 ng/mL; ABCYS) were added in all cultures. In all experiments, cells were also seeded on a confluent layer of murine MS-5 stromal cells. MS-5 cells were expanded in RPMI (Seromed, Biochrom KG, Berlin) with 10% FCS (Biowest) and were plated in 24-well plates at a concentration of 6 × 104 cells/mL 24 hours before the initiation of cocultures. To test the effect of leukemia cells on differentiating NK cells, we added, on the first day of culture, 200 000 to 600 000 irradiated healthy donor PBMCs (control) or irradiated peripheral-blood cells (containing greater than 98% blasts) from patients with NCRdull or NCRbright AML. All cultures were maintained for 2 to 3 weeks, and half the medium was renewed twice a week.
Cytotoxic assays
Cytotoxic assay was performed with the use of a 4-hour chromium (Cr) 51 release assay. Effector cells were long-term cultured NK cells derived from PBMCs of patients at diagnosis or CR and from healthy donors. Target cells used to investigate the functional recovery of CR-AML-NK cells were the murine mastocytoma FcγRII+ P815 cell line (ATCC; LGC Promochem, Molsheim, France). Cytotoxicity was measured after redirected killing of P815 by NK cells using appropriate mAbs. Concentrations of the various mAbs were 5 μg/mL (KD1 anti–CD16 IgG1 mAb, BAB281 anti-NKp46, Z231 anti-NKp30; from the laboratory of Alessandro Moretta, Genova, Italy). All experiments were performed in triplicate in at least 3 independent experiments.
Coculture of NK cells with leukemia cells
Resting or long-term activated NK cells were cultured in 96-well plates at 50 000 cells/mL, with 50 IU/mL rhIL-2 for 1 to 5 days for resting NK cells or 5 to 10 days for long-term activated NK cells (1000 IU/mL rhIL-2), in the presence of leukemia cells or healthy donors PBMCs (control) at 1 × 106 cells/mL. NK cells were then analyzed for cell-surface–receptor expression by flow cytometry excluding blasts by CD45high gating. We used low concentrations of rhIL-2 (50 IU/mL) to obtain resting NK cells (no up-regulation of cell-activation markers CD69 and NKp44), whereas high concentrations of rhIL-2 (1000 IU/mL) was used to obtain activated NK cells.
Survival analysis
Survival analysis was performed with the use of SPSS software (SPSS, Chicago, IL). To analyze the repartition of age and sex, t tests were performed. To analysis survival we used the Kaplan-Meier method with the log-rank count, and for multivariate analysis we used the Cox model. For more homogeneous sample analyses, data from patients with biphenotypic (n = 2) and AML minimally differentiated (AML0) (n = 2) leukemia were not included. Eighty-one patients were included for survival studies. Results were considered significant when P values were less than or equal to .05.
Results
Patient characteristics
AML-NK cells were analyzed at diagnosis and without previous in vitro amplification in 71 consecutive patients (40 males, 31 females; Table 1)Most (66 of 71) patients had AML, 2 had biphenotypic leukemia, and 3 had refractory anemia with excess blasts (RAEB). The median age was 61 years (range, 17-85 years). We chose to include in our study only patients with white blood cell counts lower than 50 × 109/L (mean, 10.5 ± 22 × 109/L) to facilitate direct analysis of NK cells, which, in all cases, represented a rare population. The absolute number of AML-NK cells was 0.248 × 109/L ± 0.277 and, thus, was similar to values found in healthy donors under the same experimental conditions (ie, 0.264 × 109/L ± 0.183). Seven of 71 (10%) patients had favorable karyotype abnormalities—3 with inv(16), 2 with t(8;21), and 2 with t(15;17).
Characteristics of AML patients
| Characteristic . | Value . |
|---|---|
| Sex, no. patients (%) | |
| Male | 40 (56) |
| Female | 31(44) |
| Age, y | |
| Median | 61 |
| Range | 17-85 |
| FAB classification, no. patients (%) | |
| M0 | 2(2.8) |
| M1 | 9(12.6) |
| M2 | 20(28.1) |
| M3 | 4(5.6) |
| M4 | 22(31) |
| M5 | 8(11.3) |
| M6 | 4(5.6) |
| Biphenotypic | 2(2.8) |
| White blood cell count, ×109 cells/L−1 | 10.5 ± 22* |
| NK-cell count, mm3 ± SD | 248 ± 277 |
| Cytogenetic risk group, no. patients (%) | |
| Favorable | 8(11.5) |
| Adverse/intermediate | 63(88.5) |
| Median follow-up, y | 2.65 |
| Complete remission rate, % | 73 |
| Characteristic . | Value . |
|---|---|
| Sex, no. patients (%) | |
| Male | 40 (56) |
| Female | 31(44) |
| Age, y | |
| Median | 61 |
| Range | 17-85 |
| FAB classification, no. patients (%) | |
| M0 | 2(2.8) |
| M1 | 9(12.6) |
| M2 | 20(28.1) |
| M3 | 4(5.6) |
| M4 | 22(31) |
| M5 | 8(11.3) |
| M6 | 4(5.6) |
| Biphenotypic | 2(2.8) |
| White blood cell count, ×109 cells/L−1 | 10.5 ± 22* |
| NK-cell count, mm3 ± SD | 248 ± 277 |
| Cytogenetic risk group, no. patients (%) | |
| Favorable | 8(11.5) |
| Adverse/intermediate | 63(88.5) |
| Median follow-up, y | 2.65 |
| Complete remission rate, % | 73 |
Value is presented as mean ± SD.
NCRdull phenotype pre-exists to in vitro culture and is not associated with French-American-British subclasses
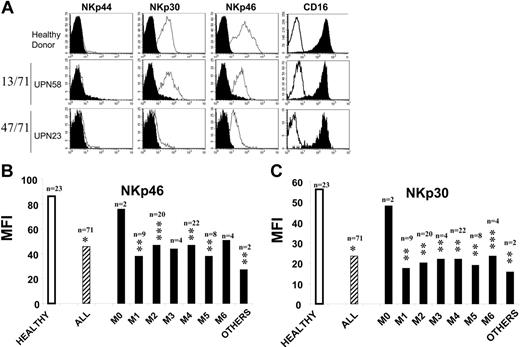
We first analyzed, by flow cytometry, NCR expression on AML-NK cells and compared findings with those of 23 healthy control donors (Figure 1A). Because NKp44 expression is only observed after in vivo or in vitro stimulation, we failed to detect this molecule in healthy donors or unstimulated NK cells in patients with AML. Most (more than 70%) healthy donors had NK cells with an NCRbright phenotype (NKp30 mean MFI, 72 ± 30; NKp46 mean MFI, 102 ± 56; sample of healthy donors in Figure 1A top row). One third of healthy donors had NK cells with the NCRdull phenotype (NKp30 mean MFI, 20 ± 6; NKp46 mean MFI, 30 ± 10; data not shown). No healthy donor had NK cells displaying an NCRdiscordant phenotype. In contrast, we observed that 47 (66.2%) of 71 patients with AML had NK cells with an NCRdull phenotype (NKp30 mean MFI, 12 ± 8; NKp46 mean MFI, 23 ± 15; sample of AML-NK donor in Figure 1A, bottom row). Only 13 (18.3%) of 71 patients with AML had NK cells with a normal NCRbright phenotype (NKp30 mean MFI, 41 ± 8; NKp46 mean MFI, 88 ± 31; sample of healthy donors in Figure 1A, middle row). We observed that 11 (15.5%) patients had an NCRdiscordant phenotype; among them, 7 (10%) patients were NKp30dull/NKp46bright (NKp30 mean MFI, 23 ± 5; NKp46 mean MFI, 72 ± 15; data not shown), and 4 (5.5%) patients were NK30bright/NKp46dull (NKp30 mean MFI, 34 ± 7; NKp46 mean MFI, 46 ± 5; data not shown). In all cases, no difference in CD16 or CD56 expression was observed between normal and AML NK cells (Figure 1A, right column and data not shown, respectively). As shown in Figure 1B, NCR expression was decreased for NKp30 and NKp46 but was not associated with FAB classification subtypes. Patients with AML0 were NCRbright, but this observation has no statistical significance because of the low number of patients with this subtype (n = 2; Figure 1B). In addition, no difference was observed in NCR expression between CD56bright and CD56dull NK subpopulations.
NCRdull phenotype is common and not dependent of AML subclass. NK cells from 71 patients were analyzed by flow cytometry at diagnosis. (A) AML-NK cells were gated on the CD45high CD3−CD56+ fraction of fresh PBMCs. Filled histograms represent isotype-matched mAb staining; open histograms represent staining with specific PE-conjugated mAbs. Represented are 1 healthy donor with NCRbright NK cells (top), 1 of 13 AML patients with NCRbright NK cells (middle), and 1 of 47 AML patients with NCRdull NK cells (bottom). (B) Mean fluorescence intensity (MFI) of surface staining with anti-NKp46 and anti-NKp30 PE-conjugated mAbs of NK cells from healthy donors (Healthy), from AML regardless the FAB subclass (All), and from distinct subclasses (FAB classifications M0-M6). Others are particular forms of AML (Table 1). *P<.001; **P ≤ .01; ***P < .05. n represents number of patients. M0 indicates AML minimally differentiated; M1, AML without maturation; M2, AML with maturation; M3, acute promyelocytic leukemia; M4, acute myelomonocytic leukemia; M5, acute monocytic leukemia; M6, acute erythroid leukemia.
NCRdull phenotype is common and not dependent of AML subclass. NK cells from 71 patients were analyzed by flow cytometry at diagnosis. (A) AML-NK cells were gated on the CD45high CD3−CD56+ fraction of fresh PBMCs. Filled histograms represent isotype-matched mAb staining; open histograms represent staining with specific PE-conjugated mAbs. Represented are 1 healthy donor with NCRbright NK cells (top), 1 of 13 AML patients with NCRbright NK cells (middle), and 1 of 47 AML patients with NCRdull NK cells (bottom). (B) Mean fluorescence intensity (MFI) of surface staining with anti-NKp46 and anti-NKp30 PE-conjugated mAbs of NK cells from healthy donors (Healthy), from AML regardless the FAB subclass (All), and from distinct subclasses (FAB classifications M0-M6). Others are particular forms of AML (Table 1). *P<.001; **P ≤ .01; ***P < .05. n represents number of patients. M0 indicates AML minimally differentiated; M1, AML without maturation; M2, AML with maturation; M3, acute promyelocytic leukemia; M4, acute myelomonocytic leukemia; M5, acute monocytic leukemia; M6, acute erythroid leukemia.
NCRdull phenotype of AML-NK cells is reversible regarding NCR expression and cytolytic function and parallels CR achievement
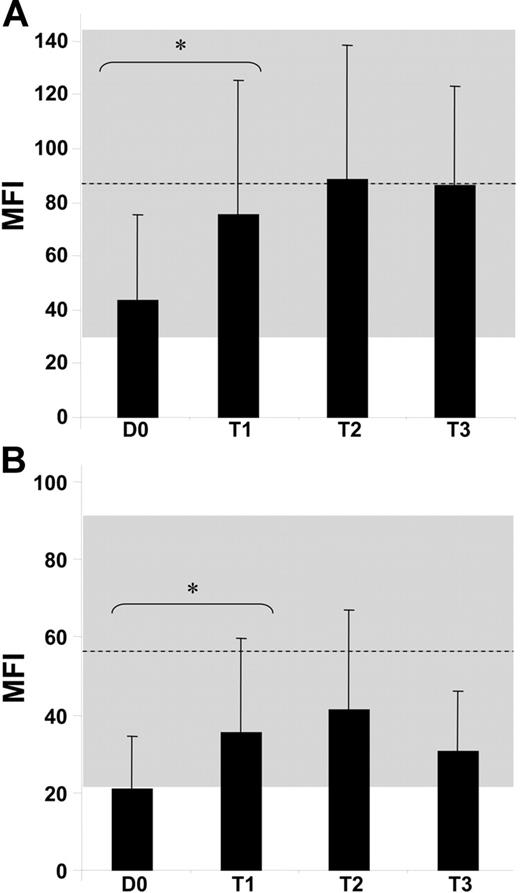
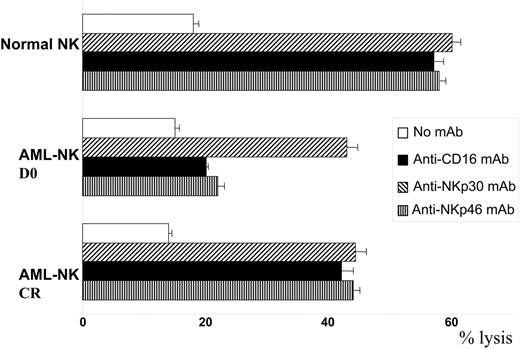
We performed a kinetic study comparing NCR expression and functions on AML-NK cells at diagnosis and after treatment, focusing first on patients who achieved CR. Mean expression of NKp46 was 43 ± 32 at diagnosis and reached 75 ± 50 after CR, corresponding to a statistically significant (P < .05) 1.75-fold increase (Figure 2A). NKp46 expression was further checked at different times after CR and remained stable at T2 (MFI, 88 ± 50) and T3 (MFI, 86 ± 37). Mean expression of NKp30 was 21 ± 14 at diagnosis and reached 35 ± 24 after CR, corresponding to a statistically significant (P < .05) 1.67-fold increase (Figure 2B). Expression of NKp30 was further checked at different times after CR and remained stable at T2 (MFI, 41 ± 26), with a moderate and nonstatistically significant decrease at T3 (MFI, 31 ± 15). As control, we verified that CD16 expression was comparable to that of healthy donor NK cells and did not change expression before (MFI, 242 ± 141) or after CR was achieved (MFI, 218 ± 133; data not shown). We then tested the putative cytolytic functions of AML-NK cells at diagnosis and after achievement of CR. AML-NK cells were tested in redirected killing with anti-CD16, anti-NKp30, and anti-NKp46 mAb (Figure 3). NCRdull AML-NK cells derived from patients at diagnosis tested in redirected killing with anti-CD16 mAb displayed a cytolytic level comparable to that of healthy donor NCRbright NK cells (43% ± 2% vs 60% ± 1%). In contrast, anti-NKp30 mAbs and NKp46 mAbs failed to induce an increased cytotoxicity in NCRdull AML-NK cells compared with nonredirected killing (20% ± 2% and 25% ± 4%, respectively, vs 15% ± 1%). As control, NKp30- and NKp46-redirected killing in healthy donor NK cells was as efficient as CD16-redirected killing (57% ± 2% and 58% ± 1%, respectively, vs 60% ± 1%). The CD16-driven redirected killing of AML-NK cells derived from patients in CR induced cytotoxicity that was comparable to that of healthy donor NK cells (44% ± 2%). AML-NK cells from patients in CR had restored NCR-driven cytotoxicity because NKp30- and NKp46-redirected killing induced a cytotoxicity that was comparable to that of control-redirected killing with anti-CD16 (42% ± 1% and 44% ± 1% respectively, vs 44% ± 2%). Restored NKp46 expression was also followed by restoration of NKp46-redirected killing (data not shown).
Partial or complete restoration of NCRs. MFI of surface staining with anti-NKp46 (A) and anti-NKp30 (B) PE-conjugated mAbs on AML-NK cells at diagnosis when patients achieved CR (30-100 days after diagnosis, T1), at midterm (101-200 days after diagnosis, T2), and at long term (from 200 days after diagnosis, T3) compared with MFI of NK cells from 23 healthy donors (mean represented by the dashed line; SD represented by the gray zone). *P < .001. Error bars indicate SD between donors.
Partial or complete restoration of NCRs. MFI of surface staining with anti-NKp46 (A) and anti-NKp30 (B) PE-conjugated mAbs on AML-NK cells at diagnosis when patients achieved CR (30-100 days after diagnosis, T1), at midterm (101-200 days after diagnosis, T2), and at long term (from 200 days after diagnosis, T3) compared with MFI of NK cells from 23 healthy donors (mean represented by the dashed line; SD represented by the gray zone). *P < .001. Error bars indicate SD between donors.
Functional restoration of AML-NK cells after CR. AML-NK cells from diagnosis (D0, NCRdull) or the same patients at CR (NCRbright) were obtained and amplified as described in “Materials and methods” and assessed for cytolytic activity against the P815 murine cell line. Results are presented as the mean ± SD of maximum lysis percentage of triplicate values at the effector-target ratio of 30:1. Data are from 1 of 3 representative experiments independently performed.
Functional restoration of AML-NK cells after CR. AML-NK cells from diagnosis (D0, NCRdull) or the same patients at CR (NCRbright) were obtained and amplified as described in “Materials and methods” and assessed for cytolytic activity against the P815 murine cell line. Results are presented as the mean ± SD of maximum lysis percentage of triplicate values at the effector-target ratio of 30:1. Data are from 1 of 3 representative experiments independently performed.
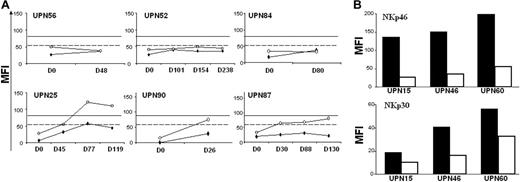
We then performed the same study for patients who failed to attain CR (Figure 4A) or who experienced disease relapse (Figure 4B). For 3 of the 6 patients who did not attain CR (Figure 4A, upper row), we did not observe the restoration of NKp30 or NKp46. The observed pattern was more complex for other patients. For unique patient number 87 (UPN87), we observed that NKp46 increased while NKp30 remained at a “dull” level. Patient UPN90 experienced NKp46 restoration to a normal level (represented by the plain line) whereas NKp30 remained lower than at the mean expression level observed for healthy donor NK cells (dashed line). For UPN25, we observed the complete restoration of NKp46 and the partial restoration of NKp30. In patients who experienced relapse (Figure 4B), we observed a homogeneous pattern regarding NKp46—a drastic decrease in NKp46 expression from CR to relapse (left panel). The same trend was observed regarding NKp30 (right panel). Moreover, for one patient in whom NCR expression was not restored after CR, no modification of NCR expression was observed after relapse (data not shown).
Nonrestoration of NCR surface expression on patients in therapeutic failure or relapse. (A) NK cells from patients in therapeutic failure were analyzed for NKp46 (○) or NKp30 (♦) surface expression. Y-axis represents MFI of PE-conjugated mAbs. X-axis represents number of days after diagnosis (D0). (B) NK cells from patients in relapse. NKp46 (left panel) and NKp30 (right panel) were analyzed on NK cells of AML patients at CR (▪) and after relapse (□). Data represent MFI of PE-conjugated mAb staining for 3 of 4 patients in relapse. Dashed lines correspond to mean of NKp30 expression by NK cells from 23 healthy donors. Solid horizontal lines correspond to mean of NKp46 expression by NK cells from 23 healthy donors.
Nonrestoration of NCR surface expression on patients in therapeutic failure or relapse. (A) NK cells from patients in therapeutic failure were analyzed for NKp46 (○) or NKp30 (♦) surface expression. Y-axis represents MFI of PE-conjugated mAbs. X-axis represents number of days after diagnosis (D0). (B) NK cells from patients in relapse. NKp46 (left panel) and NKp30 (right panel) were analyzed on NK cells of AML patients at CR (▪) and after relapse (□). Data represent MFI of PE-conjugated mAb staining for 3 of 4 patients in relapse. Dashed lines correspond to mean of NKp30 expression by NK cells from 23 healthy donors. Solid horizontal lines correspond to mean of NKp46 expression by NK cells from 23 healthy donors.
NCRdull phenotype induced by leukemia cells
We sought to determine whether the NCRdull phenotype could be induced in vitro by coculture of leukemia cells with developing or mature NK cells (Figure 5). NK cells at different stages of development were tested. These were NK cells undergoing differentiation from CD34 progenitors (Figure 5A), fresh mature NK cells from peripheral blood (Figure 5B), and long-term expanded and activated NK cells (data not shown).
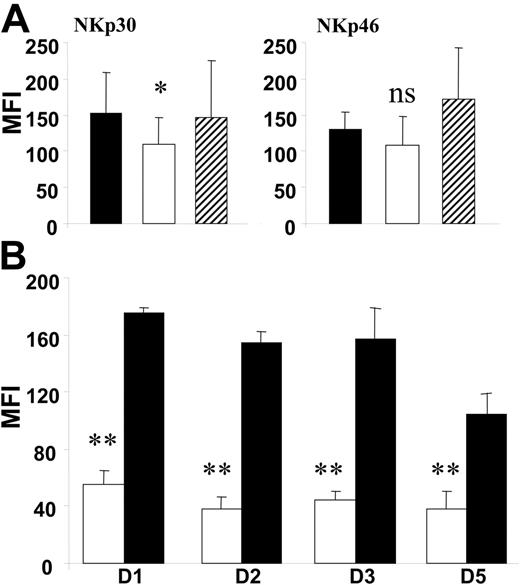
Leukemia cells induce in vitro down-regulation of NCRs. Effect of leukemia-cell coculture with differentiating healthy donor NK cells (A) and resting healthy donor NCRbright NK cells (B). (A) MFI of surface staining with anti-NKp30 and anti-NKp46 PE-conjugated mAbs on CD45high CD3−CD56+ NK cells are represented. ▪ represent NK cells differentiated from CD34+ progenitors in the presence of irradiated allogeneic PBMCs. □ represent NK cells differentiated in the presence of irradiated blasts from patients with NCRdull AML. ▨ represent NK cells differentiated in the presence of irradiated blasts from patients with NCRbright AML. NK cells were analyzed 15 days after the starting culture of CD34+ cells. Data represent the mean ± SD of 6 independent experiments. *P = .05 (NS). (B) MFI of surface staining of anti-NKp30 PE-conjugated mAb on resting NCRbright NK cells from healthy donors. Expression of NKp30 was explored on NK cells cultured for 1 to 5 days in the presence of nonirradiated blasts from patients with NCRdull AML (□) or nonirradiated healthy donor PBMCs (▪). Data present mean MFI ± SD of at least 3 independent experiments.
Leukemia cells induce in vitro down-regulation of NCRs. Effect of leukemia-cell coculture with differentiating healthy donor NK cells (A) and resting healthy donor NCRbright NK cells (B). (A) MFI of surface staining with anti-NKp30 and anti-NKp46 PE-conjugated mAbs on CD45high CD3−CD56+ NK cells are represented. ▪ represent NK cells differentiated from CD34+ progenitors in the presence of irradiated allogeneic PBMCs. □ represent NK cells differentiated in the presence of irradiated blasts from patients with NCRdull AML. ▨ represent NK cells differentiated in the presence of irradiated blasts from patients with NCRbright AML. NK cells were analyzed 15 days after the starting culture of CD34+ cells. Data represent the mean ± SD of 6 independent experiments. *P = .05 (NS). (B) MFI of surface staining of anti-NKp30 PE-conjugated mAb on resting NCRbright NK cells from healthy donors. Expression of NKp30 was explored on NK cells cultured for 1 to 5 days in the presence of nonirradiated blasts from patients with NCRdull AML (□) or nonirradiated healthy donor PBMCs (▪). Data present mean MFI ± SD of at least 3 independent experiments.
To test the leukemic blast effect on NCR expression during NK ontogeny, NK cells were generated from CD34+ progenitors and analyzed after 15 days (greater than 98% CD3−CD56+ cells; data not shown) in the presence of normal allogeneic feeders (filled histograms), irradiated leukemia cells from NCRdull patients (NKp30dull/NKp46dull; open histograms), or irradiated leukemia cells from NCRbright patients (NKp30bright/NKp46bright; striped histograms). NKp30 expression obtained in the presence of leukemia cells from NCRdull patients (MFI, 109 ± 37) was lower than expression in the presence of allogeneic normal feeders (MFI, 152 ± 57) or leukemia cells from NCRbright patients (MFI, 147 ± 78). NKp46 expression obtained in the presence of leukemia cells from NCRdull patients (MFI, 108 ± 40) was lower than expression in the presence of allogeneic normal feeders (MFI, 130 ± 23) or leukemia cells from NCRbright patients (MFI, 171 ± 71). No significant difference in the activation-induced NKp44 expression and in NKG2D or 2B4/CD244 expression was observed (data not shown). As control, no significant difference in CD16 expression was observed between the different culture conditions (data not shown).
We then tested the effect of leukemia cells on mature NK cells. NCRbright NK cells were isolated from healthy donors and cultured for 1 to 5 days in IL-2–supplemented medium and leukemia cells (Figure 5B). Rapidly, and at all times of culture, we observed a significant decrease (P < .05) in NKp30 expression when leukemia cells from patients with NCRdull leukemia were used as feeders (open bars), in contrast to feeders from healthy donors (filled bars). In contrast, leukemia cells from patients with NCRbright did not induce significant modification in NCR expression (data not shown). In addition, no difference in NKp46 expression was observed (data not shown). As control, CD16, 2B4/CD244, and NKG2D expression was checked but did not change when leukemia or normal feeder cells were used (data not shown).
NCRdull phenotype is associated with a decreased survival
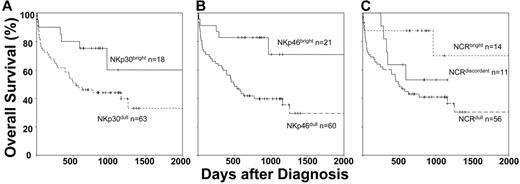
We measured patient overall survival using the Kaplan-Meier model, determining different subgroups based on NKp30 and NKp46 expression. To obtain a sufficient number for potent statistical analysis, we included in our survival analyses patients phenotypically and functionally characterized in our previous study.24 Among the 14 additional AML patients, 11 had NCRdull and 3 had NCRbright leukemia. Resultant duration of follow-up was 3.8 years. We first analyzed patient survival according to NKp30 expression (Figure 6A). Probability of survival at 5 years was 60% for patients with NKp30bright AML and 33% in patients with NKp30dull AML (P = .04). We then analyzed patient survival according to NKp46 expression (Figure 6B). We observed higher survival rates for patients with NKp46bright than for those with NKp46dull (72% vs 28% respectively; P = .003). We then compared the 3 following subgroups of patients: NCRbright (NKp30bright/NKp46bright), NCRdull (NKp30dull/NKp46dull), and NCRdiscordant (Figure 6C). Probability of survival at 5 years was 64% for NCRbright patients, 43% for NCRdiscordant patients, and 31% for NCRdull patients. These observations strongly suggest a correlation between poor survival and deficiency of NK-cell–triggering receptors. To analyze crucial variables that could affect overall survival, we performed multivariate analyses (Cox model; data not shown). We failed to reveal a statistical association between NCR phenotype and age, sex, white blood count, NK-cell count, karyotypic abnormality, relapse, and cause of death (equally represented by leukemia evolution and lethal infectious events). Along with karyotypic abnormalities, NCR expression was the only parameter that showed a significant association with survival.
NCRdull is associated with poor survival. Kaplan-Meier test was performed on data from the 71 patients analyzed in this study and 14 additional patients from our previous study. We compared NCR phenotypes and overall survival of those patients. Statistical significance was calculated according to the log-rank method. (A) Patients were compared on NKp30 expression. (B) Patients were compared on NKp46 expression. (C) Patients were compared on NCRbright (NKp30bright/NKp46bright) versus NCRdull (NKp30dull/NKp46dull) versus NCRdiscordant (NKp30bright/NKp46dull and NKp30dull/NKp46bright) phenotypes. n indicates the number of corresponding patients in each arm.
NCRdull is associated with poor survival. Kaplan-Meier test was performed on data from the 71 patients analyzed in this study and 14 additional patients from our previous study. We compared NCR phenotypes and overall survival of those patients. Statistical significance was calculated according to the log-rank method. (A) Patients were compared on NKp30 expression. (B) Patients were compared on NKp46 expression. (C) Patients were compared on NCRbright (NKp30bright/NKp46bright) versus NCRdull (NKp30dull/NKp46dull) versus NCRdiscordant (NKp30bright/NKp46dull and NKp30dull/NKp46bright) phenotypes. n indicates the number of corresponding patients in each arm.
Discussion
Reduced NCR expression was observed in patients with long-term IL-2–expanded AML-NK cells24 that could have been a consequence of the in vitro selection of NCRdull NK cells. Against this hypothesis, we observed that the in vitro growth rate of NCRdull AML-NK cells was comparable to that of their NCRbright counterparts (data not shown). We then directly analyzed, without in vitro culture, AML-NK cells from 71 AML patients at diagnosis and observed an NCRdull phenotype in most patients. Interestingly, we observed discordance between NKp30 and NKp46 expression—that is, cells were either NKp30dull/NKp46bright or NKp30bright/NKp46dull—in a significant fraction (15%) of patients. This phenotype has never been observed, to our knowledge, in healthy donors with parallel levels of expression for NKp30 and NKp46, with only NKp30dull/NKp46dull (NCRdull) or NKp30bright/NKp46bright (NCRbright) NK.15 This unusual differential expression of NKp30 and NKp46 sustains the hypothesis that the down-regulation of NKp30 and NKp46 observed in AML patients could rely on distinct mechanisms. An important issue raised by the abnormal NCR phenotype observed in AML patients was congenital origin (perhaps favoring leukemia occurrence) compared with acquired origin.24 Because of the rarity of AML (incidence rate less than 1/100 000 per year), the possibility of a prospective study implicating extensive flow cytometry analysis of healthy subjects to determine NCR status before leukemia was excluded. As a surrogate, we initiated a kinetic study of NCR expression in AML patients. In most patients, we observed partial (NKp30) or complete (NKp46) recovery of NCR expression. Thus, NCRdull was probably acquired as leukemia developed. This observation was completed by the detailed analysis of NCR recovery, depending on leukemia evolution during and after treatment. Interestingly, we observed that NCR recovery was mainly restricted to patients achieving CR. In addition, we observed a down-regulation of NCR expression in patients experiencing leukemia relapse. These data at least suggest a positive correlation between the NCRdull phenotype and the presence of leukemia blasts. Given that few data are available regarding the regulation mechanism of NCR expression, we explored the unique clue available from the literature, which is the involvement of TGF-β1. TGF-β1 induces a drastic down-modulation of NKp30.29 We thus measured TGF-β1 concentrations in peripheral-blood and bone marrow aspirate of AML patients (data not shown). As previously observed by another group, detected levels of TGF-β1 were lower in patients with AML than in healthy subjects.30 Moreover, we failed to detect significant TGF-β1 concentrations in leukemia blast supernatants (data not shown). These experiments strongly suggest that TGF-β1 is probably not the mediator of NCR down-regulation in AML patients. In addition, TGF-β1 overexpression could explain NKp30, but not NKp46, down-regulation because the expression of the latter was not affected by TGF-β1,29 suggesting at least the existence of another mechanism leading to NKp46 down-regulation. Nevertheless, we cannot exclude a paracrine effect of leukemia-secreted TGF-β1 undetectable in the sera of patients and not in our culture conditions. Because clinical data suggest that leukemia blasts induce the NCRdull phenotype, we performed an in vitro analysis of the effects of neoplastic cells on NCR expression. Under NK-differentiating conditions, the coculture of CD34+ hematopoietic stem cells with leukemia cells of patients with an NCRdull phenotype induced a decreased expression of NKp30 and, to a lesser degree, of NKp46. In those culture conditions, we did not observe the NCRdull phenotype, as expected. This inability for blasts cells to induce the NCRdull phenotype in differentiating NK cells might have had several origins: the process took place in mature NK cells, not during NK differentiation, or culture conditions were not favorable for AML blast effect (high cytokine concentration, leukemia cells, and CD34+ progenitor containment). Nevertheless, these data suggest leukemia cells could interfere with the initial steps of NK-cell differentiation. Moreover, culture of mature peripheral-blood normal NCRbright NK cells with leukemia cells induced a significant down-regulation of NKp30, but not of NKp46. Because NKp30 and NKp46 are both down-regulated in AML-NK cells, our data suggest that leukemia cells may act during NK ontogeny and on mature NK cells to down-regulate NCR. We further completed our analysis by study of the in vitro reversibility of the NCRdull phenotype. In most cases (there were some exceptions), the expansion of NCRdull AML-NK cells with normal feeders, after the depletion of leukemia cells, failed to restore a normal NCR phenotype (data not shown). Thus, in vitro restoration of a normal NCR phenotype is likely to have been caused by novel NK populations and not by the restoration of normal NCR expression by primarily NCRdull NK cells. We also performed experiments using leukemia blast culture supernatants (in resting or GM-CSF–stimulated conditions), but we failed to detect significant modification in NCR expression (data not shown). Altogether, these data argue for a mechanism of NCR down-regulation involving cell-to-cell contact between leukemia blasts and NK. Leukemia cells are able to down-regulate NCR expression. Therefore, what could have been the physiopathologic meaning of such a phenomenon? We can speculate that NCR down-regulation is another mechanism of tumor escape from innate immunity. Nonetheless, it could be argued that NCR expression, in most cases, was still present, though at reduced levels. We have shown that NCR down-regulation is sufficient to drastically reduce in vitro NK-cell cytotoxicity. Moreover, a recent study has shown that leukemia blasts express low amounts of the still unidentified NCR ligands.23 The convergent effect of low NCR expression by AML-NK cells and low ligand expression by leukemia cells may suggest an evasion of leukemia from NK immune surveillance. From all these in vitro data, it could be hypothesized that the NCRdull phenotype could have a consequence on leukemia clinical evolution. Kaplan-Meier survival curves markedly showed that patients with NKp30bright or NKp46bright leukemia survive longer than patients with NKp30dull or NKp46dull leukemia and that patients with NCRbright leukemia survive longer than those with NCRdiscordant leukemia, who in turn survive longer than those with NCRdull leukemia. The NCR phenotype may influence patient outcome through an antileukemia effect or through anti-infection control because leukemia evolution and infectious complications equally contribute to patient outcomes. We can hypothesize that higher NCR expression at diagnosis and better recovery after CR enable better antiopportunistic infection and better antileukemia responses to AML-NK. Finally, our results showed that NCR expression can be considered a new prognostic marker for overall survival in AML patients. Aside from immunotherapy protocols with autologously restored NK cells, this new karyotype-independent prognostic marker deserves consideration in chemotherapy protocol design and analysis. Our findings represent a rationale for clinical immunotherapy studies aimed at restoring NK cytotoxic function through the restoration of NCR expression.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: C.F. was involved in the design of the study, in the management of patient data, and in the management of biological data; provided the major technical contribution to the study; and provided a major contribution to the writing of the article. S.J.-L. and F.M. provided technical help both for in vitro experiments and for management of patient data; they also participated in the design of some experiments. C.A. and D.S. provided a major contribution to patient diagnosis, provided technical help for cytometry experiments, and participated in all study design. D.O. was involved in the study design and contributed to the writing of the article. R.C. was involved in study design, in clinical and data management of patients, and in the management of technical biological help, and provided a major contribution to the writing of the article.
Acknowledgments
This work was supported by Groupement Entreprise Français Lutte Cancer, Fédération Nationale des Centres de Lutte Contre le Cancer, Fondation pour la Recherche Médicale, and Ligue Nationale contre le Cancer.
We thank Professor Alessandro Moretta for his valuable advice and commentary from the beginning of the study. We thank Florence Baratier and Dany Jayme for flow cytometry analysis. We thank Christine Fornelli of Beckman-Coulter-Immunotech for her help in mAb settings and for critical discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal