Abstract
The molecular mechanisms by which mesenchymal stem cells (MSCs) suppress T-cell proliferation are poorly understood, and whether a soluble factor plays a major role remains controversial. Here we demonstrate that the T-cell–receptor complex is not a target for the suppression, suggesting that downstream signals mediate the suppression. We found that Stat5 phosphorylation in T cells is suppressed in the presence of MSCs and that nitric oxide (NO) is involved in the suppression of Stat5 phosphorylation and T-cell proliferation. The induction of inducible NO synthase (NOS) was readily detected in MSCs but not T cells, and a specific inhibitor of NOS reversed the suppression of Stat5 phosphorylation and T-cell proliferation. This production of NO in the presence of MSCs was mediated by CD4 or CD8 T cells but not by CD19 B cells. Furthermore, inhibitors of prostaglandin synthase or NOS restored the proliferation of T cells, whereas an inhibitor of indoleamine 2,3-dioxygenase and a transforming growth factor–β–neutralizing antibody had no effect. Finally, MSCs from inducible NOS−/− mice had a reduced ability to suppress T-cell proliferation. Taken together, these results suggest that NO produced by MSCs is one of the major mediators of T-cell suppression by MSCs.
Introduction
Because mesenchymal stem cells (MSCs) differentiate into osteocytes, chondrocytes, myotubes, and adipocytes,1-3 they are expected to become a source of cells for regenerative therapy. Also, MSCs support hematopoietic stem cell engraftment4-9 and modulate immunologic responses by unknown mechanisms.9-14 Here, we investigated the molecular mechanisms by which MSCs suppress T-cell proliferation.
Transforming growth factor–β (TGF-β), hepatocyte growth factor, indoleamine 2,3-dioxygenase (IDO), and prostaglandin E2 (PGE2) have been reported to mediate T-cell suppression by MSCs.13-15 Specifically, neutralizing antibodies against TGF-β or hepatocyte growth factor,13 an inhibitor of IDO,14 or an inhibitor of prostaglandin production reverse the inhibition of T-cell proliferation by MSCs.15 In addition, some reports have shown that a soluble factor is the major mediator of suppression,13-17 whereas some reports have demonstrated that T-cell–MSC contact is required for this suppression.12-14,16,17 In the current study, we sought to resolve these conflicting results by using a mouse bone marrow–derived MSC system.
One candidate soluble factor for T-cell suppression is nitric oxide (NO) because it is known to inhibit T-cell proliferation.18-25 NO is produced by NO synthases (NOSs), of which there are 3 subtypes: inducible NOS (iNOS), endothelial NOS, and neuronal NOS. Like MSCs, it has been known that macrophages suppress T-cell proliferation. This suppression was reported to be mediated by NO inhibition of Stat5 phosphorylation.18,19 Also, MSCs were reported to produce NO when they differentiate into chondrocytes.26 We therefore investigated whether MSCs can produce NO and whether NO is involved in their ability to suppress T-cell proliferation.
Materials and methods
Materials
N-nitro-l-arginine methyl ester (L-NAME), indomethacin, and concanavalin A (Con A) were purchased from Wako (Osaka, Japan). Con A was used at 5 μg/mL. Indomethacin was used at 5 μM. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were from Sigma (St Louis, MO) and were used at concentrations of 50 ng/mL and 1 μg/mL, respectively. Antimouse CD3/CD28 beads (Dynal Biotech ASA, Oslo, Norway) were used at 10 μL per 106 cells. The transwell system with 1-μm pores for 12-well dishes was from BD Falcon (Franklin Lakes, NJ). Monoclonal antibodies for CD4, CD8, CD11b, CD25, CD29, CD44, CD45, CD69, Sca-1, B220, Gr-1, and interferon-γ (IFN-γ) were from BD Pharmingen (San Diego, CA). An inhibitor of IDO, 1-methyl-DL-tryptophan (1-MT), was purchased from Sigma. An antibody for TGF-β was purchased from Peprotech (Rocky Hill, NJ). Lipopolysaccharide was from Sigma.
MSCs
MSCs were obtained from wild-type or iNOS−/− C57BL/6 mice. Bone marrow cells were harvested from femurs and tibias by a standard flushing method1 and then cultivated in a plastic dish in Iscove modified Dulbecco medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Sigma), 2 mM l-glutamine, 0.1 mg/mL streptomycin, and 100 U/mL penicillin G (Invitrogen) or in MF medium (Toyobo, Tokyo, Japan).
All primary MSCs were characterized at least once by flow cytometry and an in vitro differentiation assay. All MSCs were positive for CD29, CD44, and Sca-1, negative for CD11b, Gr-1, and CD45, and able to differentiate into adipocytes and osteoblasts (Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). We used at least 2 independently isolated batches of MSCs. These cells can be propagated for a long time and retain their surface phenotype and capacity to differentiate for at least 4 months.
Flow cytometric analysis
Cells were incubated with Fc block (BD Pharmingen) to inhibit nonspecific binding of antibodies to Fc receptors. Next, cells were stained in FACS buffer (phosphate-buffered saline [PBS] supplemented with 10% fetal bovine serum) with antibodies for 30 minutes on ice, washed with FACS buffer, and analyzed on a BD LSR flow cytometer (Becton Dickinson, Franklin Lakes, CA). Collected data were analyzed with CELLQUEST software (Becton Dickinson).
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits for mouse IFN-γ and mouse interleukin-2 (IL-2; BD Pharmingen) were used according to the manufacturer's instructions.
Selection of CD4+, CD8+, and CD19+ cells
CD4+, CD8+, and CD19+ cells were selected using mouse CD4, CD8, and CD19 MACS beads and an autoMACS system (Miltenyi Biotech, Auburn, CA). The purity of the cells as determined by flow cytometry with antibodies against CD4, CD8, and B220 was more than 80%.
Thymidine incorporation
Splenocytes were grown in 96-well plates containing RPMI 1640 (Invitrogen) supplemented with 10% fetal calf serum (Sigma), 2 mM l-glutamine (Invitrogen), 50 μM 2-mercaptoethanol (Sigma), 0.1 mg/mL streptomycin, and 100 U/mL penicillin G (Invitrogen). During the cultivation, the cells were pulsed for the last 8 hours of culture with 1 mCi (3.7 × 107 Bq) of [3H]-thymidine (Amersham Biosciences, Piscataway, NJ). After 48 hours of growth, cells were harvested with a Packard FilterMate harvester (Perkin Elmer Life Sciences, Boston, MA), transferred to a UniFilter plate (Perkin Elmer Life Sciences), and analyzed using a TopCount microplate scintillation counter (Perkin Elmer Life Sciences). In the coculture system, MSCs were γ-irradiated (30 Gy) prior to cultivation to prevent thymidine incorporation. When using the transwell system (BD Falcon), one tenth of the cells were harvested and counted because the system includes 12-well culture dishes, which contain 10-fold more cells than the wells of a 96-well plate.
Western blot analysis
Polyclonal antibodies to phosphorylated Stat5 (Cell Signaling Technology, Danvers, MA), Stat5 (Santa Cruz Biotechnology, Santa Cruz, CA), cyclin D2 (Cell Signaling Technology), and Kip1 (Cell Signaling Technology) were used for Western blotting. Cells were lysed on ice for 15 minutes with a buffer consisting of 50 mM Tris (pH 7.5), 150 mM NaCl, 0.5% NP40, Complete proteinase inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), and 1 mM Na3VO4. Lysates were centrifuged at 13 000g for 15 minutes, and supernatants were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred from the gel to a PVDF membrane (Invitrogen), and Western blotting was performed using enhanced chemiluminescence reagents (Pierce, Rockford, IL) to visualize the immunoreactive proteins.
Assay for NO production
NO is quickly converted to NO2 and NO3 in culture medium. Because of the presence of NO3 in RPMI medium, we measured NO2 production using a Griess reagent kit (Wako).
Detection of iNOS expression
Total RNA was prepared using an RNeasy kit (Qiagen, Valencia, CA) and 1 μg was reverse-transcribed using a First Strand Synthesis Kit (Invitrogen), and one tenth of the product was subjected to PCR using the following primers: for iNOS, 5′-GAGATTGGAGTTCGAGACTTC-3′ and 5′-TGGCTAGTGCTTCAGACTTC-3′; and for β-actin, 5′-CCATCATGAAGTGTGACGTTG-3′ and 5′-GTCCGCCTAGAAGCACTTGCG-3′.
Western blotting was performed using a polyclonal antibody for iNOS (BD Transduction Laboratories, Lexington, KY) or a monoclonal antibody for β-actin (Sigma) to confirm equal loading. Immunofluorescence was carried out using the polyclonal iNOS antibody (BD Transduction Laboratories) followed by Alexa Fluor 488 goat anti–rabbit IgG (Molecular Probes, Eugene, OR). To distinguish splenocytes from MSCs, cells were simultaneously stained with phycoerythrin-conjugated anti-CD45 monoclonal antibody (BD Pharmingen). Cells were fixed with ProLong Gold antifade reagent (Molecular Probes) and were visualized by confocal microscopy (Nikon, Tokyo, Japan), and images were analyzed with the accompanying confocal image analysis software (Bio-Rad, Hercules, CA).
Intracellular staining
Intracellular staining was performed using a BD Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions.
Mice
Wild-type mice (C57BL/6) were purchased from Clea Japan (Tokyo). The iNOS−/− mice (C57BL/6 background) were purchased from Jackson Laboratory (Bar Harbor, ME).
Cell lines
RAW264.7 mouse macrophage cells were a generous gift from Dr Matsuura (Jichi Medical University, Tochigi, Japan). HeLa human cervical carcinoma cells were used as negative control for iNOS expression.
Statistical analysis
We used the Student t test for statistical analysis. Differences were considered statistically significant at P values less than .05.
Results
Characteristics of T-cell suppression by MSCs
Although many reports9-17,27 have shown that MSCs suppress T-cell proliferation, the molecular mechanisms and the signaling molecules inhibited by MSCs have not been defined. We therefore investigated the status of activated T cells in the presence of MSCs. The expression of activation markers and the production of IL-2 and IFN-γ were evaluated by flow cytometry, ELISA, and intracellular staining. The expression of the activation markers CD25 and CD69 on CD4 or CD8 T cells was not changed by the presence of MSCs (Figure 1A) In addition, MSCs suppressed the production of IFN-γ but not IL-2 (Figure 1B, upper panel). Also, IFN-γ production was diminished after 24 hours in the presence of MSCs (Figure 1B, lower panel). These findings are in agreement with previous results,27 but they do not explain the strong suppression of T-cell proliferation by MSCs; for example, thymidine incorporation by T cells is reduced more than 10-fold in the presence of MSCs (data not shown).
Status of activated T cells in the presence of MSCs. (A) Expression of the T-cell activation markers CD25 and CD69 on CD4 or CD8 cells 24 hours after stimulation of splenocytes (1 × 106 cells) in a 12-well dish with anti-CD3/CD28 beads (10 μL) in the presence or absence of 1 × 105 MSCs. The numbers in the top right quadrants indicate the percentage ± the standard deviation (SD). (B) Top panel, cytokine production in the same condition as in panel A. Concentrations of IL-2 and IFN-γ were determined at 48 hours by ELISA. The values are the means ± SD from 3 independent experiments. Bottom panel, intracellular staining of IFN-γ at 24 hours. GolgiStop (monensin) was used for the last 8 hours. The values are the mean percentages of CD8/IFN-γ–positive cells ± SD from 3 independent experiments. (C) MSCs suppress the induction of CD4+ and CD8+ T-cell proliferation by PMA and ionomycin. Splenocytes (1 × 105), CD4+ cells (1 × 105), or CD8+ cells (1 × 105) were stimulated in the presence or absence of irradiated MSCs (1 × 104) in the wells of a 96-well plate. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. NS indicates P > .05.
Status of activated T cells in the presence of MSCs. (A) Expression of the T-cell activation markers CD25 and CD69 on CD4 or CD8 cells 24 hours after stimulation of splenocytes (1 × 106 cells) in a 12-well dish with anti-CD3/CD28 beads (10 μL) in the presence or absence of 1 × 105 MSCs. The numbers in the top right quadrants indicate the percentage ± the standard deviation (SD). (B) Top panel, cytokine production in the same condition as in panel A. Concentrations of IL-2 and IFN-γ were determined at 48 hours by ELISA. The values are the means ± SD from 3 independent experiments. Bottom panel, intracellular staining of IFN-γ at 24 hours. GolgiStop (monensin) was used for the last 8 hours. The values are the mean percentages of CD8/IFN-γ–positive cells ± SD from 3 independent experiments. (C) MSCs suppress the induction of CD4+ and CD8+ T-cell proliferation by PMA and ionomycin. Splenocytes (1 × 105), CD4+ cells (1 × 105), or CD8+ cells (1 × 105) were stimulated in the presence or absence of irradiated MSCs (1 × 104) in the wells of a 96-well plate. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. NS indicates P > .05.
We next induced T-cell proliferation using a combination of PMA and ionomycin, which act downstream of the T-cell–receptor complex by activating protein kinase C and inducing Ca2+ influx, respectively. This proliferation was suppressed by MSCs (Figure 1C), suggesting that the T-cell receptor complex is not a target for the suppression and that MSCs influence signals downstream of protein kinase C and Ca2+ influx. As demonstrated in Figure 1C, the proliferation of both purified CD4 and CD8 T cells as well as unfractionated splenocytes was suppressed by MSCs.
Stat5 phosphorylation is inhibited by MSCs
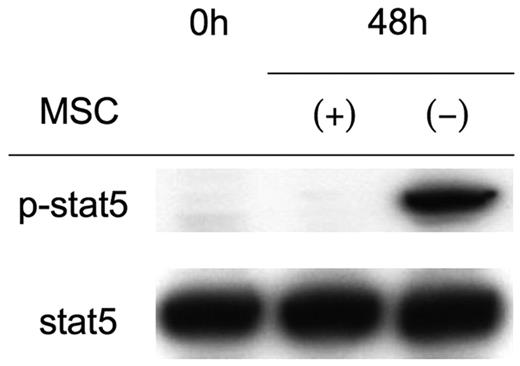
Although T cells from Stat5ab−/− mice do not proliferate upon stimulation with anti-CD3, they up-regulate CD25.28 Because this phenotype is similar to the status of activated T cells in the presence of MSCs (Figure 1A), we hypothesized that they suppress Stat5 phosphorylation. Indeed, as shown in Figure 2, Stat5 phosphorylation was diminished in activated T cells in the presence of MSCs despite equivalent IL-2 production (Figure 1B). The reported changes in cell-cycle–related proteins, including the down-regulation of cyclin D2 and up-regulation of p27 Kip1 in the presence of MSCs,27 were observed less consistently; in most cases, we found that MSCs up-regulated cyclin D2 and down-regulated p27 Kip1 in activated splenocytes compared with freshly isolated splenocytes (data not shown).
Inhibition of Stat5 phosphorylation in the presence of MSCs.
Western blot analysis of Stat5 phosphorylation. Splenocytes (2 × 106) were activated with anti-CD3/CD28 beads in the presence or absence of 1 × 105 MSCs. After 48 hours, splenocytes were collected, lysed, and analyzed by Western blotting. Each lane contains 20 μg protein. Western blotting with anti-Stat5 is shown as a loading control. Shown are representative results from more than 5 experiments.
Inhibition of Stat5 phosphorylation in the presence of MSCs.
Western blot analysis of Stat5 phosphorylation. Splenocytes (2 × 106) were activated with anti-CD3/CD28 beads in the presence or absence of 1 × 105 MSCs. After 48 hours, splenocytes were collected, lysed, and analyzed by Western blotting. Each lane contains 20 μg protein. Western blotting with anti-Stat5 is shown as a loading control. Shown are representative results from more than 5 experiments.
Dose dependency and time course of NO production
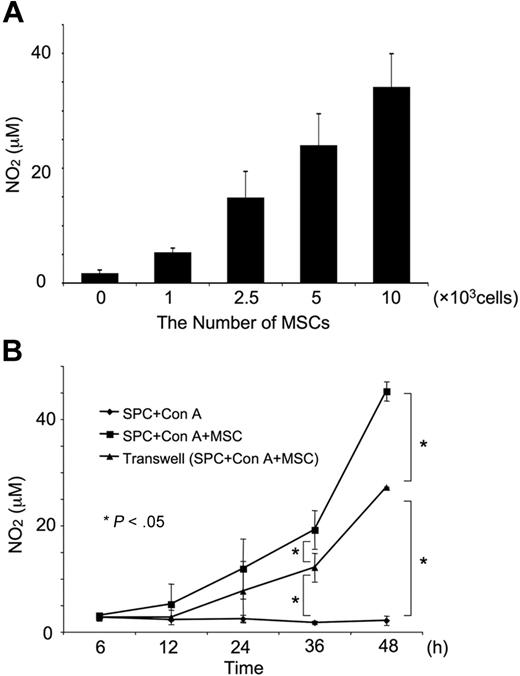
Macrophages have been reported to suppress T-cell proliferation19-23 due to the production of NO and its inhibition of Stat5 phosphorylation.18,19 This prompted us to examine the production of NO in our mouse MSC system. We found that MSCs caused a significant and dose-dependent production of NO (Figure 3A) NO could be first detected approximately 12 hours after the activation of T cells in the presence of MSCs. In the transwell system, in which the T cells were separated from the MSCs by a 1-μm-pore membrane, NO production was initially detected approximately 24 hours after the activation of T cells (Figure 3B).
NO production in the presence of MSCs. (A) Dose-dependent effect of MSCs on NO production. Splenocytes (1 × 106) were activated with Con A (5 μg/mL) in the presence of the indicated number of MSCs for 48 hours in a 12-well dish. The concentrations of NO were determined by Griess assay. (B) Time course of NO production. MSCs (1 × 105/well) were treated as in panel A for the indicated amount of time. “Transwell” indicates experiments performed in 12-well dishes in which the T cells were separated from MSCs by a 1-μm-pore membrane. Values represent the means ± SD from 3 independent experiments. *P < .05.
NO production in the presence of MSCs. (A) Dose-dependent effect of MSCs on NO production. Splenocytes (1 × 106) were activated with Con A (5 μg/mL) in the presence of the indicated number of MSCs for 48 hours in a 12-well dish. The concentrations of NO were determined by Griess assay. (B) Time course of NO production. MSCs (1 × 105/well) were treated as in panel A for the indicated amount of time. “Transwell” indicates experiments performed in 12-well dishes in which the T cells were separated from MSCs by a 1-μm-pore membrane. Values represent the means ± SD from 3 independent experiments. *P < .05.
T-cell suppression and NO
Significant amounts of NO were not produced by MSCs cocultured with T cells in the absence of Con A or by Con A–treated MSCs or T cells (Figure 4A) In the presence of a direct interaction between T cells and MSCs, there was a high level of NO production accompanied by a strong suppression of T-cell proliferation (Figure 4A-B). In contrast, both NO production and T-cell suppression were reduced in a transwell system (Figure 4A-B). We further examined whether such a difference is observed using the RAW264.7 macrophage cell line, a well-characterized producer of NO. As with MSCs, T-cell suppression and NO production were inhibited in the transwell system using the RAW264.7 cells (Figure 4B, right side, and data not shown), suggesting that the difference reflects common aspects of T-cell suppression by NO.
Relationship between NO production and T-cell suppression. (A) Production of NO. Splenocytes (1 × 106) were incubated with or without Con A in the presence or absence of MSCs for 48 hours. “Transwell” indicates experiments performed in 12-well dishes in which the T cells were separated from MSCs by a 1-μm-pore membrane. The values are the means ± SD from 3 independent experiments. *P < .05. (B) T-cell proliferation in the presence or absence of the transwell system. Splenocytes (1 × 106) were activated with Con A in the presence or absence of 1 × 105 MSCs or RAW264.7 cells for 48 hours with or without the transwell. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. (C) T cells but not B cells induce NO production. Purified CD4 or CD8 T cells (1 × 105; ∼80% purity) induce NO in the presence of MSCs (1 × 104), whereas purified CD19 B cells (1 × 105; ∼95% purity) do not induce significant NO production. The mitogen for T cells was Con A, and for B cells, it was lipopolysaccharide (1 μg/mL). The values are the means ± SD from 3 independent experiments. *P < .05.
Relationship between NO production and T-cell suppression. (A) Production of NO. Splenocytes (1 × 106) were incubated with or without Con A in the presence or absence of MSCs for 48 hours. “Transwell” indicates experiments performed in 12-well dishes in which the T cells were separated from MSCs by a 1-μm-pore membrane. The values are the means ± SD from 3 independent experiments. *P < .05. (B) T-cell proliferation in the presence or absence of the transwell system. Splenocytes (1 × 106) were activated with Con A in the presence or absence of 1 × 105 MSCs or RAW264.7 cells for 48 hours with or without the transwell. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. (C) T cells but not B cells induce NO production. Purified CD4 or CD8 T cells (1 × 105; ∼80% purity) induce NO in the presence of MSCs (1 × 104), whereas purified CD19 B cells (1 × 105; ∼95% purity) do not induce significant NO production. The mitogen for T cells was Con A, and for B cells, it was lipopolysaccharide (1 μg/mL). The values are the means ± SD from 3 independent experiments. *P < .05.
T cells but not B cells induce NO
We next asked which cell type causes the NO production. We found that purified CD4+ and CD8+ T cells induce similar degrees of T-cell suppression as unfractionated splenocytes (Figure 1C). Therefore, it is not surprising that they also produce NO in the presence of MSCs (Figure 4C). Although MSCs suppress B-cell proliferation (∼50%; data not shown), purified CD19+ B cells did not appear to induce NO production in the presence of MSCs, suggesting that the mechanisms of B-cell and T-cell suppression are different.
MSC–T-cell interaction and NO production
There are 2 possible explanations for the difference in NO production in the presence and absence of the transwell system. First, it is possible that there is a difference in the time course of NO production in the 2 systems. In the transwell system, a significant level of NO was typically detected after 24 hours, whereas NO production was detected after 12 to 18 hours in the presence of a direct interaction. Thus, the amount of NO produced in the transwell system was always lower than that in the presence of a direct interaction (Figure 3B). These findings suggest that a direct interaction is critical for the early and efficient production of NO as well as for the strong suppression of T-cell proliferation. A second possible explanation for the different results obtained in the transwell and direct interaction systems is that, because NO is highly unstable, in the transwell system it can lose its activity before it influences T cells.
MSCs are a producer of NO
If this second explanation is correct, MSCs should be the main producer of NO. Therefore, we examined whether MSCs can produce NO. It is known that there are 3 NO synthases (iNOS, endothelial NOS, and neuronal NOS), and only one of these, iNOS, can be induced by cell stimulation.29 Therefore, we suspected that iNOS is induced in either T cells or MSCs. Reverse transcriptase–polymerase chain reaction (RT-PCR) (Figure 5A), Western blot analysis (Figure 5B), and immunofluorescence (Figure 5C) detected the induction of iNOS in MSCs cocultured with activated splenocytes but not in MSCs alone, splenocytes alone, or HeLa cells. The immunofluorescence studies showed that iNOS was exclusively expressed by large adherent CD45− cells, which correspond to MSCs (Figure 5C). In addition, iNOS appeared to be expressed throughout the cytoplasm as previously found in Kupffer cells and hepatocytes (Figure 5C).30
Induction of iNOS in MSCs. Total RNA and cell lysates were collected from MSCs alone, splenocytes alone, MSCs cocultured with activated T cells, or activated splenocytes cocultured with MSCs. MSCs were harvested just after washing out activated T cells with PBS. (A) RT-PCR analysis of iNOS mRNA. β-actin is shown as a control. (B) Western blot analysis of iNOS protein. Each lane contains 20 μg protein. β-actin is shown as a loading control. HeLa cells were used as negative control because the antibody also reacts with human iNOS protein. (C) Immunofluorescence of iNOS protein. Left panel, confocal immunofluorescent image of CD45 protein. Middle panel, confocal immunofluorescent image of iNOS protein. Right panel, merged confocal immunofluorescent images of CD45 protein and iNOS protein. Splenocytes (1 × 106) were activated with Con A in the presence of 1 × 105 MSCs for 48 hours. Images were visualized using a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with a 100×/1.40 numerical aperture oil objective lens, Nikon CFI Plan APO (Nikon). Images were acquired using Lasersharp software version 2.1 (Bio-Rad).
Induction of iNOS in MSCs. Total RNA and cell lysates were collected from MSCs alone, splenocytes alone, MSCs cocultured with activated T cells, or activated splenocytes cocultured with MSCs. MSCs were harvested just after washing out activated T cells with PBS. (A) RT-PCR analysis of iNOS mRNA. β-actin is shown as a control. (B) Western blot analysis of iNOS protein. Each lane contains 20 μg protein. β-actin is shown as a loading control. HeLa cells were used as negative control because the antibody also reacts with human iNOS protein. (C) Immunofluorescence of iNOS protein. Left panel, confocal immunofluorescent image of CD45 protein. Middle panel, confocal immunofluorescent image of iNOS protein. Right panel, merged confocal immunofluorescent images of CD45 protein and iNOS protein. Splenocytes (1 × 106) were activated with Con A in the presence of 1 × 105 MSCs for 48 hours. Images were visualized using a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with a 100×/1.40 numerical aperture oil objective lens, Nikon CFI Plan APO (Nikon). Images were acquired using Lasersharp software version 2.1 (Bio-Rad).
Specific inhibitor of NOS restores T-cell proliferation and Stat5 phosphorylation
Next, we investigated the effects of L-NAME, a specific inhibitor of NOS. As expected, L-NAME dose-dependently inhibited the production of NO by MSCs in the presence of activated T cells (Figure S2). Importantly, L-NAME restored T-cell proliferation (Figure 6A,left panel). The effect of L-NAME was dose dependent and more efficient when lower numbers of MSCs were used (Figure 6A, right panel). Using 2.5 × 103 MSCs, 1 mM L-NAME resulted in up to an approximately 80% recovery compared with the positive control (Figure 6A, left panel), suggesting that NO is one of the most important factors for T-cell suppression under the stringent conditions of our assays. On the other hand, even under these conditions, 100% recovery was not achieved, implying that other factors also contribute to the suppression of T-cell proliferation by MSCs.
A specific inhibitor of NOS restores T-cell proliferation and Stat5 phosphorylation. (A) Effect of L-NAME on thymidine incorporation. Top panel, splenocytes (1 × 105) were activated with Con A in the presence or absence of 2.5 × 103 irradiated MSCs and in the presence or absence of 1 mM L-NAME. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. Bottom panel, dose-dependent restoration of T-cell proliferation by L-NAME. Splenocytes (1 × 105) were activated with Con A in the presence of the indicated number of irradiated MSCs for 48 hours. The concentrations of L-NAME are shown. Shown is a typical result of 3 independent experiments. (B) L-NAME restores Stat5 phosphorylation. Splenocytes (2 × 106) were activated with anti-CD3/CD28 beads in the presence or absence of 0.5 × 105 to 1 × 105 MSCs for 48 hours and in the presence or absence of 1 mM L-NAME. Western blotting for phosphorylated and total Stat5 was performed as described in Figure 2. Shown is a representative result from 5 independent experiments.
A specific inhibitor of NOS restores T-cell proliferation and Stat5 phosphorylation. (A) Effect of L-NAME on thymidine incorporation. Top panel, splenocytes (1 × 105) were activated with Con A in the presence or absence of 2.5 × 103 irradiated MSCs and in the presence or absence of 1 mM L-NAME. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. Bottom panel, dose-dependent restoration of T-cell proliferation by L-NAME. Splenocytes (1 × 105) were activated with Con A in the presence of the indicated number of irradiated MSCs for 48 hours. The concentrations of L-NAME are shown. Shown is a typical result of 3 independent experiments. (B) L-NAME restores Stat5 phosphorylation. Splenocytes (2 × 106) were activated with anti-CD3/CD28 beads in the presence or absence of 0.5 × 105 to 1 × 105 MSCs for 48 hours and in the presence or absence of 1 mM L-NAME. Western blotting for phosphorylated and total Stat5 was performed as described in Figure 2. Shown is a representative result from 5 independent experiments.
Other candidates as mediators of suppression by MSCs
Because TGF-β, IDO, and PGE2 were reported as mediators of T-cell suppression by MSCs,13-15 we further compared the effects of L-NAME with inhibitors of each mediator. Indomethacin (inhibitor of PGE2 production) but not 1-MT (inhibitor of IDO) or an anti–TGF-β–neutralizing antibody restored T-cell proliferation as effectively as L-NAME (Figure 7A); however, the effects of L-NAME and indomethacin were not additive, suggesting that the NO and PGE2 share signaling pathways leading to T-cell suppression (Figure 7A).
Effect of inhibitors and T-cell suppression by MSCs from iNOS−/− mice. (A) L-NAME and indomethacin (INDO) restored T-cell proliferation, but TGF-β antibody and 1-MT had no effect. Splenocytes (1 × 105) were activated with Con A in the presence or absence of irradiated MSCs (2.5 × 103), 1 mM L-NAME, 5 μM indomethacin,14 1 μg/mL or 10 μg/mL TGF-β antibody, and 1 mM 1-MT for 48 hours. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. Shown is the mean ± SD of 3 independent experiments. *P < .05. NS indicates P > .05. (B) MSCs from iNOS−/− mice have a reduced ability to inhibit T-cell proliferation. Splenocytes (1 × 105) were activated with Con A in the presence or absence of MSCs (2.5 × 103) from either wild-type or iNOS−/− mice. Left panel, incorporation of [3H]-thymidine relative to that in the absence of MSCs. Right panel, production of NO. The values are the means ± SD from 3 independent experiments. *P < .05.
Effect of inhibitors and T-cell suppression by MSCs from iNOS−/− mice. (A) L-NAME and indomethacin (INDO) restored T-cell proliferation, but TGF-β antibody and 1-MT had no effect. Splenocytes (1 × 105) were activated with Con A in the presence or absence of irradiated MSCs (2.5 × 103), 1 mM L-NAME, 5 μM indomethacin,14 1 μg/mL or 10 μg/mL TGF-β antibody, and 1 mM 1-MT for 48 hours. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. Shown is the mean ± SD of 3 independent experiments. *P < .05. NS indicates P > .05. (B) MSCs from iNOS−/− mice have a reduced ability to inhibit T-cell proliferation. Splenocytes (1 × 105) were activated with Con A in the presence or absence of MSCs (2.5 × 103) from either wild-type or iNOS−/− mice. Left panel, incorporation of [3H]-thymidine relative to that in the absence of MSCs. Right panel, production of NO. The values are the means ± SD from 3 independent experiments. *P < .05.
MSCs from iNOS−/− mice have reduced activity in T-cell suppression
Finally, we used MSCs from iNOS−/− mice to confirm that NO is produced by MSCs and that NO suppresses T-cell proliferation. MSCs from iNOS−/− mice were less effective than MSCs from wild-type mice at suppressing T-cell proliferation, suggesting that NO produced by MSCs is a major mediator of this effect (Figure 7B, left panel). We also confirmed that MSCs from iNOS−/− mice do not produce NO even in the presence of activated T cells (Figure 7B, right panel).
Discussion
Here, we demonstrate for the first time that the production of NO is involved in the suppression of T cells by MSCs. We also showed that NO inhibits Stat5 phosphorylation. Although NO was already known to suppress T-cell proliferation, NO has not been previously reported to mediate T-cell suppression by MSCs.29 Our hypothesis that NO is produced by MSCs and that it suppresses T-cell proliferation in part through Stat5 inhibition was supported by the following facts: (1) NO was readily detected in the medium in the presence of MSCs; (2) L-NAME restored T-cell proliferation as well as Stat5 phosphorylation; and (3) MSCs from iNOS−/− mice had markedly reduced abilities to suppress T-cell proliferation. This hypothesis was further confirmed by the finding that iNOS was detected only in MSCs.
Compared with experiments in which cells were in direct contact, experiments performed in transwells showed a lag in NO production, suggesting that T-cell–MSC contact is critical for the early and efficient production of NO and, thus, T-cell suppression. Whether a soluble factor is a main mediator of T-cell suppression by MSCs has been controversial because results from transwell systems have been inconsistent.12,14,16,17 Our finding that the transwell reduces but does not abolish T-cell suppression (Figure 4B) may help explain these conflicting reports. Although we could not define the mechanism by which NO production is suppressed in the transwell system, the amount of NO production appears to correspond with the extent of T-cell suppression.
Under stringent conditions, in which a lower number of MSCs was used, the restoration of T-cell proliferation by L-NAME reached up to approximately 80%, suggesting that NO is one of the major mediators; however, 100% restoration was never attained, suggesting that other factor(s) contribute to the suppression. Because TGF-β, IDO, and PGE2 have been considered as possible mediators of T-cell suppression by MSCs,13-15 we examined the effect of specific inhibitors of each. We found that indomethacin (inhibitor of PGE2 production) restores T-cell proliferation as previously reported15 but that neither 1-MT (inhibitor of IDO) nor the TGF-β antibody had an effect. Also, the effects of indomethacin were not additive with those of L-NAME. These results, combined with previous reports,31,32 suggest that NO acts upstream of PGE2. Furthermore, our results imply that NO may be the central mediator of T-cell proliferation.
Under our standard conditions (1:10 ratio of MSCs to splenocytes), 1 mM L-NAME restored T-cell proliferation to approximately 25%. Similarly, 5 μM indomethacin also produced an approximately 25% recovery (data not shown). These results suggest that the restoration by L-NAME or indomethacin is not specific to the more stringent conditions (1:40 ratio of MSCs to splenocytes).
Although most of the results from our mouse MSC system are consistent with previous reports,12,15,27 we did not find a clear correlation between T-cell suppression and the up-regulation of Kip1 or the down-regulation of cyclin D2. Instead, our results suggest that the inhibition of Stat5 phosphorylation is more important for T-cell suppression, at least under the conditions of our experiments. In the conditions studied here, after coculture with MSCs, T cells could respond to a second mitogenic stimulation (data not shown), whereas they could not respond to a second stimulation in a previous report,27 suggesting that the status of T cells in our experiments is different than that in the previous report.
Our results provide new insight into how MSCs modulate immune function. Although it is known that the NO-Stat5 pathway is important for T-cell suppression by macrophages, this is the first report demonstrating that the NO-Stat5 pathway is also critical for T-cell suppression by MSCs. The physiologic role of NO produced by MSCs is unknown, and we are currently investigating the possibility that MSCs in bone marrow protect hematopoietic stem cells from T-cell–mediated destruction by inhibiting T-cell proliferation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: K.S. performed the research and analyzed data; K. Ozaki designed the research and wrote the paper; K.H. performed Western blotting; I.O. carried out experiments regarding PMA plus ionomycin; T.N. provided technical advice; A.M. and K.M. provided some reagents and analyzed data; and K. Ozawa organized the research project.
Acknowledgments
We would like to thank Dr Hitoshi Endo (Jichi Medical University) for technical assistance with immunofluorescence microscopy, Dr David Munn (MCG Immunotherapy Center, Medical College of Georgia, Augusta) for technical advice with dissolving 1-MT, and Dr Motohiro Matsuura (Jichi Medical University) for providing the RAW264.7 macrophage cell line.
This work was supported in part by grants from the Ministry of Health, Welfare, and Labor of Japan and Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Technology of Japan.

![Figure 1. Status of activated T cells in the presence of MSCs. (A) Expression of the T-cell activation markers CD25 and CD69 on CD4 or CD8 cells 24 hours after stimulation of splenocytes (1 × 106 cells) in a 12-well dish with anti-CD3/CD28 beads (10 μL) in the presence or absence of 1 × 105 MSCs. The numbers in the top right quadrants indicate the percentage ± the standard deviation (SD). (B) Top panel, cytokine production in the same condition as in panel A. Concentrations of IL-2 and IFN-γ were determined at 48 hours by ELISA. The values are the means ± SD from 3 independent experiments. Bottom panel, intracellular staining of IFN-γ at 24 hours. GolgiStop (monensin) was used for the last 8 hours. The values are the mean percentages of CD8/IFN-γ–positive cells ± SD from 3 independent experiments. (C) MSCs suppress the induction of CD4+ and CD8+ T-cell proliferation by PMA and ionomycin. Splenocytes (1 × 105), CD4+ cells (1 × 105), or CD8+ cells (1 × 105) were stimulated in the presence or absence of irradiated MSCs (1 × 104) in the wells of a 96-well plate. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. NS indicates P > .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-02-002246/4/m_zh80010705930001.jpeg?Expires=1769822255&Signature=c40kV6Zr0Z5EQ4myNFqylFrKCe~lX8nPSru4M54wuEq8zuCXXNrWTpG9LhG4MH~LUZlrIAo5yDt30eeegRfipzBD6Y3NhBBh1O3fBrv-VJs9kio1SXMcOZE7pngoaQrjPEhxn9kAZVqVmbYDcEnDWxfsT~G1JJDNPO9WDscNhAgUH~8mm3~tZ9jWtuhFnQDn5HUbJkbMY9O9JgftBslHsAhv4s1FL~tAlGml3lPBBo~RhBhktuwHbzZgfS9pa2aDyBIuzZRNgJJJhXuZIie0aJbi4TQ3GIGvuqkcwGuGPLjSwydKKoPCH4H7CEzsGTeAlmTME3sCm9yqzKX1snBOJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Relationship between NO production and T-cell suppression. (A) Production of NO. Splenocytes (1 × 106) were incubated with or without Con A in the presence or absence of MSCs for 48 hours. “Transwell” indicates experiments performed in 12-well dishes in which the T cells were separated from MSCs by a 1-μm-pore membrane. The values are the means ± SD from 3 independent experiments. *P < .05. (B) T-cell proliferation in the presence or absence of the transwell system. Splenocytes (1 × 106) were activated with Con A in the presence or absence of 1 × 105 MSCs or RAW264.7 cells for 48 hours with or without the transwell. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. (C) T cells but not B cells induce NO production. Purified CD4 or CD8 T cells (1 × 105; ∼80% purity) induce NO in the presence of MSCs (1 × 104), whereas purified CD19 B cells (1 × 105; ∼95% purity) do not induce significant NO production. The mitogen for T cells was Con A, and for B cells, it was lipopolysaccharide (1 μg/mL). The values are the means ± SD from 3 independent experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-02-002246/4/m_zh80010705930004.jpeg?Expires=1769822255&Signature=DoFR6nO00B-YygPqmLClzrOR~OZWwia2hAy~2aiXdcwnLQgty4XwEv1wtnwU7W~GUoqDCADvzXoIq2yl6HiSgBafVsceLxtfSWZbqcpEcfICyMWuD5ImYzxD67a8E45PFozf1Je17fQyUeQwdbeKazRWlno7DqXdLuaOxzIOsG2hNyWe4IDbdzFu0zUOzbZxmuTrkBpscHo~Vy7NlWP4gMWGPMocGbMy0hcGOon4kJp-Gclp2RWQfNaGI8ACYNPDcwDGgZzXrL6cNtCoGqXo04Zn52CW1kbTTpVer1K4HIaxmaI59davcQTfkBDXyIWhRvmty7i5si7~pLGlKERrYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. A specific inhibitor of NOS restores T-cell proliferation and Stat5 phosphorylation. (A) Effect of L-NAME on thymidine incorporation. Top panel, splenocytes (1 × 105) were activated with Con A in the presence or absence of 2.5 × 103 irradiated MSCs and in the presence or absence of 1 mM L-NAME. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. The values are the means ± SD from 3 independent experiments. *P < .05. Bottom panel, dose-dependent restoration of T-cell proliferation by L-NAME. Splenocytes (1 × 105) were activated with Con A in the presence of the indicated number of irradiated MSCs for 48 hours. The concentrations of L-NAME are shown. Shown is a typical result of 3 independent experiments. (B) L-NAME restores Stat5 phosphorylation. Splenocytes (2 × 106) were activated with anti-CD3/CD28 beads in the presence or absence of 0.5 × 105 to 1 × 105 MSCs for 48 hours and in the presence or absence of 1 mM L-NAME. Western blotting for phosphorylated and total Stat5 was performed as described in Figure 2. Shown is a representative result from 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-02-002246/4/m_zh80010705930006.jpeg?Expires=1769822255&Signature=2HMltEn-JaPkfsye025bT3gg5z7GOeob28lsUa1xc8M9JASD9hfrKR~5vuNsHNDTK7jYfjKk0RjGV5ryPhqBRVrePgZ9TotpKltjy6Khdys9H6fTmu9FnlM2qgiq06K3FokOwIlpSKXn86KdUabq4yAkDy2zP~spmnX7c88OXRZsh2JhIjRJhrdZ0fP9CmlApls5JqCAg~bp~CUu2Em-4hNoN-pXIdJMD9taYcz7unq06uzJzP8pGKQNfB19mxyK-6GmQXySXezsQSJ7v-tKWXbT3D00KMzY68m5vhDeMDPI4DIxfQMpqjzMJsen~xH98aUPgwoTnABdRSrh3faYhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Effect of inhibitors and T-cell suppression by MSCs from iNOS−/− mice. (A) L-NAME and indomethacin (INDO) restored T-cell proliferation, but TGF-β antibody and 1-MT had no effect. Splenocytes (1 × 105) were activated with Con A in the presence or absence of irradiated MSCs (2.5 × 103), 1 mM L-NAME, 5 μM indomethacin,14 1 μg/mL or 10 μg/mL TGF-β antibody, and 1 mM 1-MT for 48 hours. The incorporation of [3H]-thymidine is shown relative to that in the absence of MSCs. Shown is the mean ± SD of 3 independent experiments. *P < .05. NS indicates P > .05. (B) MSCs from iNOS−/− mice have a reduced ability to inhibit T-cell proliferation. Splenocytes (1 × 105) were activated with Con A in the presence or absence of MSCs (2.5 × 103) from either wild-type or iNOS−/− mice. Left panel, incorporation of [3H]-thymidine relative to that in the absence of MSCs. Right panel, production of NO. The values are the means ± SD from 3 independent experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-02-002246/4/m_zh80010705930007.jpeg?Expires=1769822255&Signature=B8I6BMc5y-JrPF2PEamoMzxYjf3qszZrPz-M50J~nDdkG9xBkHedkPKdwgy-t3FgvbRHSduOLHy2ia2Vi4Id~Xb~bexYf4CknYSREAtLx-UVOzrbTWDgxrqDzKW5hW5rCavYAqlyC8p6VlbzaIy3fDP4iPI30aWjFKW17lJv7cc86d4Kp-I8UUN6E4wPG6ztYZ4akgLRYGL7GrK1l4beA0CKpUA5LYS34GuCJp~EHkwsqsKIsZKV28xcZD5AfsbStZcJZlTFN5bTGUvwd3muDXuXkWrrS809qzX~eLlAX9HgNCMYKfh1HkdV8px21tSQCW-ItihfIKI0QJsVGNjMCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal