Abstract
Critical to the development of an effective HIV/AIDS model is the production of an animal model that reproduces long-lasting active replication of HIV-1 followed by elicitation of virus-specific immune responses. In this study, we constructed humanized nonobese diabetic/severe combined immunodeficiency (NOD/SCID)/interleukin-2 receptor γ-chain knockout (IL2Rγnull) (hNOG) mice by transplanting human cord blood–derived hematopoietic stem cells that eventually developed into human B cells, T cells, and other monocytes/macrophages and 4 dendritic cells associated with the generation of lymphoid follicle–like structures in lymphoid tissues. Expressions of CXCR4 and CCR5 antigens were recognized on CD4+ cells in peripheral blood, the spleen, and bone marrow, while CCR5 was not detected on thymic CD4+ T cells. The hNOG mice showed marked, long-lasting viremia after infection with both CCR5- and CXCR4-tropic HIV-1 isolates for more than the 40 days examined, with R5 virus–infected animals showing high levels of HIV-DNA copies in the spleen and bone marrow, and X4 virus–infected animals showing high levels of HIV-DNA copies in the thymus and spleen. Furthermore, we detected both anti–HIV-1 Env gp120– and Gag p24–specific antibodies in animals showing a high rate of viral infection. Thus, the hNOG mice mirror human systemic HIV infection by developing specific antibodies, suggesting that they may have potential as an HIV/AIDS animal model for the study of HIV pathogenesis and immune responses.
Introduction
Current animal models for either human immunodeficiency virus type 1 (HIV-1) or simian immunodeficiency virus (SIV) suffer from the lack of a system precisely mirroring human HIV infection and the progression to disease state.1 In current animal models with HIV infection, such as chimpanzees, animals do not develop AIDS.1 Past animal models for HIV infection have relied on humanized severe combined immunodeficiency (hSCID) mice models to study prospective anti-HIV drugs and vaccines. SCID-hu (Thy/Liv) mice, engrafted with human fetal thymus and liver tissue in the renal subcapsular region, were first reported as the small-animal model.2 Because human T cells are generated within the engrafted thymus, this model has been used for the study of thymopoiesis3-6 and hematopoiesis7,8 under the burden of HIV-1 infection. However, this model allows for a limited systemic HIV-1 infection, which is restricted mainly to the engrafted thymus. Another HIV mouse model, hu-PBL–SCID mice engrafted with human peripheral blood mononuclear cells (PBMCs),9 has been actively used as a tool in developing antiretroviral therapy.9-11 However, the infection persists for only a short time in association with rapid loss of CD4+ T cells because there is no active hematopoiesis or thymopoiesis.9,12,13 Furthermore, these mouse models fail to mirror certain key aspects of the human immune response, lacking normal lymphoid tissue and functional human antigen-presenting cells such as dendritic cells (DCs).14 Thus, although these mouse models are valuable as animal models for HIV infection, the development of a mouse model more analogous to human HIV infection is needed if we are to better understand HIV pathogenesis and develop successful anti-HIV therapies and preventive vaccines.
To solve the difficult issue about the development of an ideal HIV mouse model, we initially selected a humanized nonobese diabetic (NOD)/SCID interleukin-2 receptor (IL-2R) γ-chain knockout (NOG) mouse15 as a model animal because it has been suggested that multilineage cells, including human T, B, and natural killer (NK) cells, differentiate in these mice when given transplants of human CD34+ hematopoietic stem cells.16-18 In the current study, we further reveal the kinetics of differentiation of human B and T cells, monocytes/macrophages, and DCs in the mice that received transplants, and we characterize the animals by infection with both CCR5 (R5)– and CXCR4 (X4)-tropic HIV strains. Since our hNOG mice show stable and systemic infection of both R5- and X4-tropic HIV for more than the 40 days studied, and HIV-specific antibodies are detectable in the animals with high plasma viral loads and HIV-DNA copy numbers, we also discuss the suitability of HIV-hNOG mice as an animal model for HIV-1 infection.
Materials and methods
Transplantation of human CB-derived hematopoietic stem cells in NOG mice
Human cord blood (CB) was obtained from Saiseikai Central hospital (Minato-ku, Tokyo, Japan) and Tokyo Cord Blood Bank (Katsushika-ku, Tokyo, Japan) after obtaining informed consent. All research on human subjects was approved by the Institutional Review Board of each institution participating in the project. CB mononuclear cells were separated by Ficoll-Hypaque density gradient. CD34+ hematopoietic stem cells were isolated using a magnetic-activated cell sorting (MACS) Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. More than 95% of CD34+ cells were positively selected after 2 time-enrichment manipulations. Cells were either immediately used for the transplantation or frozen until use. NOG mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan) and maintained under specific pathogen–free (SPF) conditions in the animal facility of the National Institute of Infectious Diseases (NIID; Tokyo, Japan). Mice used in these studies were free of known pathogenic viruses, herpes viruses, bacteria, and parasites. They were housed in accordance with the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science (1987) under the Japanese Law Concerning the Protection and Management of Animals, and were maintained in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of NIID, Japan. Once approved by the Institutional Committee for Biosafety Level 3 experiments, these studies were conducted at the Animal Center, NIID, Japan, in accordance with the requirements specifically stated in the laboratory biosafety manual of the World Health Organization. Female mice (6 to 10 weeks old) were irradiated (300 cGy) and 1 × 104 to 1.2 × 105 CD34+ cells were intravenously injected within 12 hours.
Flow cytometry
The purity of CB-derived CD34+ cells after separation was evaluated by double staining with FITC-conjugated anti–human CD45 (J.33) and PE-conjugated anti–human CD34 (Class III 581) (all from Beckman Coulter, Fullerton, CA). After transplantation (1-7 months), peripheral blood, spleens, bone marrow (BM), and thymi were collected for flow cytometric analysis following staining with the following monoclonal antibodies (mAbs): FITC-conjugated anti–human CD45 (J.33), CD3 (UCHT1), CD4 (13B8.2), CD19 (J4.119), CD45RO (UCHL1) (all from Beckman Coulter), and CCR5 (2D7; BD Pharmingen, San Diego, CA); PE-conjugated anti–human CD4 (13B8.2), CD8 (B9.11), CD19 (J4.119), CD45RA (ALB11) (all from Beckman Coulter), and CXCR4 (44717; R&D Systems, Minneapolis, MN); anti–mouse CD45 (YW62.3; Beckman Coulter); ECD-conjugated anti–human CD45 (J.33; Beckman Coulter); and PC5-conjugated anti–human CD8 (T8) and CD14 (Rm052) (all from Beckman Coulter). Flow cytometric analysis was conducted by 2- or 4-color staining using an EpicsXL (Beckman Coulter).
Immunohistochemistry
Organs were snap-frozen following embedding in OCT compound (Sakura Finetechnical, Tokyo, Japan). Frozen sections were air-dried and fixed in acetone. HIV-1–infected organs were fixed in 4% paraformaldehyde and embedded in OCT compound following immersion in gradient sucrose (5%-30%). Fixed samples were stained with the following mAbs: anti–human CD45 (1.22/4014; Nichirei, Tokyo, Japan), CD3 (UCHT1; DAKO, Glostrup, Denmark), CD20 (L26; DAKO), CD68 (KP1; DAKO), CD205 (MG38; eBioscience, San Diego, CA), and DRC-1 (R4/23; DAKO) for follicular dendritic cells (FDCs); anti–mouse FDC-M1 (BD Pharmingen) for murine FDCs; and HIV-1 Gag p24 (DAKO) for detection of infected cells. Biotin-labeled goat F(ab′)2 anti–mouse immunoglobulin (Ig; ICN Biomedicals, Aurora, OH)– or biotin-labeled mouse F(ab′)2 anti–rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as the secondary antibody. Samples were treated with alkaline phosphatase (AP) or horseradish peroxidase (HRP)–streptavidin conjugate (ZYMED Laboratories Inc, San Francisco, CA). BCIP/NBT, DAB, or AEC (all from DAKO) was used for the visualization. Photographs were taken by light microscopy (Leica DMRA; Leica Microsystems Wetzlar, Wetzlar, Germany) using Leica HC PLAN APO lenses (10×/0.40 NA PH1). Leica Q550 was used for image processing.
Measurement of human Igs in mice plasma
Plasma concentrations of human IgM, IgG, and IgA in NOG mice that received transplants of human stem cells were determined by conventional human Ig quantitation assay at BML Inc (Tokyo, Japan).
Cells and viruses
Human embryonic kidney 293T cells and monkey kidney COS7 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics. The 293T cells and COS7 cells were used for transfection of DNA plasmids containing HIV-1JRCSF and simian/human immunodeficiency virus (SHIV)–C2/1, respectively. The SHIV-C2/1 strain contains the env gene of pathogenic HIV-1 strain 89.6.19 Cell-free supernatant was collected and stored at −80°C before use. A primary clinical isolate, HIV-1MNp, was kindly provided by Dr J. Sullivan of the University of Massachusetts Medical School (Worcester, MA). PBMCs isolated from HIV-1–seronegative individuals were cultured in RPMI 1640 supplemented with 10% FBS and antibiotics with 5 μg of phytohemagglutinin (PHA)/mL for 3 or 7 days (PHA-PBMCs). HIV-1MNp was propagated in PHA-PBMCs, and cell-free virus stocks were stored at −80°C.
The 50% tissue-culture infectious dose (TCID50) was determined using PHA-PBMCs and the endpoint dilution method. A 4-fold series of dilution was prepared from the virus stock, and then cells were mixed and cultured for 7 days for X4–HIV-1 and 14 days for R5–HIV-1 in RPMI 1640 supplemented with 20% FBS and antibiotics. The endpoints were determined by screening for the p24 antigen using Lumipulse (Fujirevio, Tokyo, Japan).
HIV-1 infection
All procedures for the infection and maintenance of NOG mice were performed in Biosafety Level 3 facilities at NIID under standard caging conditions. On days 102 to 132 after stem cell transplantation, 16 mice were inoculated intravenously with R5-tropic HIV-1JRCSF (65 000 TCID50) or X4-tropic SHIV-C2/1 (50 000 TCID50). On days 18 to 43 after inoculation, plasma was collected to determine HIV-RNA copy numbers, and spleen cells were prepared as single-cell suspensions to analyze the CD4/CD8 ratio using flow cytometry. A number (14) of other mice were inoculated intravenously with R5-tropic HIV-1JRCSF (200 or 65 000 TCID50) or X4-tropic HIV-1MNp (180 or 20 000 TCID50) on days 126 to 146 after transplantation. On days 18 to 40 after inoculation, plasma was collected for the determination of HIV-RNA copy numbers, and single-cell suspensions of the spleen, BM, and thymus were prepared for HIV-DNA measurement. The CD4/CD8 ratio in the spleen and percentages of human CD45+ cells in organs were analyzed using flow cytometry.
Virologic analysis
Plasma viral RNA copy numbers were measured using a real-time quantification assay based on the TaqMan system (Applied Biosystems, Foster City, CA). Plasma viral RNA was extracted and purified using a QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). The RNA was subjected to reverse transcription (RT) and amplification using a TaqMan One-Step RT–polymerase chain reaction (PCR) Master Mix Reagents Kit (PE Biosystems, Foster City, CA) with HIV-1 gag consensus primers (forward, 5′-GGACATCAAGCAGCCATGCAA-3′; and reverse, 5′-TGCTATGTCACTTCCCCTTGG-3′) and an HIV-1 gag consensus TaqMan probe (FAM-5′-ACCATCAATGAGGAAGCTGCAGAA-3′-TAMRA). For SHIV-C2/1 analysis, primers (forward, 5′-AATGCAGAGCCCCAAGAAGAC-3′; and reverse, 5′-GGACCAAGGCCTAAAAAACCC-3′) and a TaqMan probe (FAM-5′-ACCATGTTATGGCCAAATGCCCAGAC-3′-TAMRA) were designed for targeting the SIVmac239 gag region.20 Probed products were quantitatively monitored by their fluorescence intensity with the ABI7300 Real-Time PCR system (PE Biosystems). To obtain control RNA for quantification, HIV-1 gag RNA and SIVmac239 gag RNA were synthesized using T7 RNA polymerase and pKS460. Viral DNA was extracted and purified using a QIAamp DNA Mini Kit (Qiagen). Determination of HIV-1 DNA copy numbers was performed by real-time PCR assay with TaqMan Master mixture (PE Biosystems). Primers (forward, 5′-GGCTAACTAGGGAACCCACTG-3′; and reverse, 5′-CTGCTAGAGATTTTCCACACT-3′) and probes (FAM-5′-TAGTGTGTGCCCGTCTGTTGTGTGAC-3′-TAMRA) were designed for targeting the HIV-1 long terminal repeat region, R/U5. The viral DNA was quantified using LightCycler (Roche Diagnostics, Almere, The Netherlands). Viral RNA and DNA were calculated based on the standard curve of control RNA and DNA. All assays were carried out in duplicate.
HIV-antigen ELISA
Levels of anti–HIV-1 Igs against recombinant HIV-1IIIB Env gp120, recombinant HIV-1MN Env gp120, and recombinant HIV-1IIIB Gag p24 (all from ImmunoDiagnostics Inc, Woburn, MA) in plasma from HIV-1–infected and –uninfected control mice were determined using a standard enzyme-linked immunosorbent assay (ELISA). Microplates (96-well) were coated overnight with 200 ng/well antigens, and plasma diluted 1:20, 1:60, and 1:180 with PBS were incubated for 1 hour. AP-labeled anti–human Igs (γ, α, and μ; Sigma-Aldrich, St Louis, MO) were used as secondary antibodies. P-nitrophenylphosphate (pNPP) Solution (WAKO Chemical USA, Richmond, VA) was used for the visualization. The enzyme reaction was stopped by addition of 0.1 M NaOH and read at 405 nm. All assays were carried out in triplicate.
Statistical analysis
Data were expressed as the mean value ± standard deviation (SD). Significant differences between data groups were determined by 2-sample Student t test analysis. A P value less than .05 was considered significant.
Results
Reconstitution of human lymphoid systems in hNOG mice
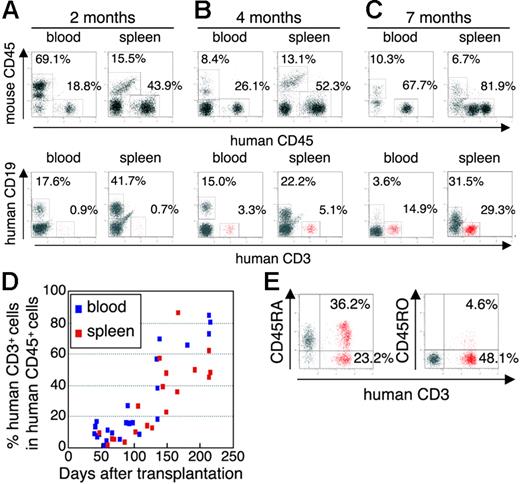
The initial studies describing the construction of humanized SCID mice used the human PBMC for infection of immunodeficiency viruses.9,12,21 However, these hu-PBL-SCID mice showed a partial infection to the R5 virus and a relatively limited period of viral replication. To construct a more suitable mouse model mimicking HIV-1 infection in humans, we selected human CB stem cells as a transplant for NOG mice. NOG mice were inoculated intravenously with human CD34+ hematopoietic stem cells, and their development of human lymphoid systems were then monitored. After transplantation (2 months), human CD45+ leukocytes were recognized in both PB and the spleen, but most of the cells were human B cells (Figure 1A) Human T cells began to be recognized clearly in PB and the spleen 4 months after transplantation (Figure 1B) and gradually increased in level, as did human B cells (Figure 1C).
Flow cytometric analysis of human T cells in the peripheral blood and spleen in NOG mice given intravenous transplants of human CB-derived CD34+ cells. (A-C) Representative profiles of the mice 2 months (A), 4 months (B), and 7 months (C) after transplantation. The ratio of human to murine CD45+ cells and that of human CD3+ to CD19+ cells show an incremental increase in human CD45+ cells and human CD3+ cells from 2 to 7 months. (D) Change of net percentages of human CD3+ T cells among human CD45+ cells in peripheral blood and the spleen from 38 mice 39 to 213 days after transplantation. (E) CD45RA is more efficiently expressed than CD45RO on human CD3+ T cells in spleen. A gate was set on the human CD45+ population. The fluorescence-activated cell sorting (FACS) profile is representative of 1 in a group of 5 mice.
Flow cytometric analysis of human T cells in the peripheral blood and spleen in NOG mice given intravenous transplants of human CB-derived CD34+ cells. (A-C) Representative profiles of the mice 2 months (A), 4 months (B), and 7 months (C) after transplantation. The ratio of human to murine CD45+ cells and that of human CD3+ to CD19+ cells show an incremental increase in human CD45+ cells and human CD3+ cells from 2 to 7 months. (D) Change of net percentages of human CD3+ T cells among human CD45+ cells in peripheral blood and the spleen from 38 mice 39 to 213 days after transplantation. (E) CD45RA is more efficiently expressed than CD45RO on human CD3+ T cells in spleen. A gate was set on the human CD45+ population. The fluorescence-activated cell sorting (FACS) profile is representative of 1 in a group of 5 mice.
In Figure 1D, we summarized percentages of human CD3+ T cells in human CD45+ cells from 38 mice from 39 to 213 days after transplantation. Human CD3+ T cells clearly increased 100 days after transplantation in both PB and the spleen. After transplantation (4 months), human CD3+ T cells in the spleen preferably expressed CD45RA rather than CD45RO (70.8% ± 13.4% and 27.3% ± 38.8% in CD3+ T cells, respectively; n = 5; Figure 1E), demonstrating that most of the T cells were in a naive state. In addition, plasma taken from 5 mice 113 to 143 days after transplantation showed that all mice produced human IgM, with concentrations ranging from 0.025 to 0.5 g/L, and that human IgG and IgA was also detected in some of the mice (ranges, 0.015-0.18 g/L and 0.003-0.012 g/L, respectively) (data not shown).
By 7 months after transplantation, human CD45+ leukocytes comprised more than 80% to 90% of mononuclear cells in the spleen (Figure 1C), and most of the mice showed symptoms of a wasting condition and a hunched back. Based upon these results, we determined that the suitable period for HIV inoculation would be 4 to 5 months after transplantation.
Formation of lymphoid structures, including monocytes/macrophages, DCs, and FDCs
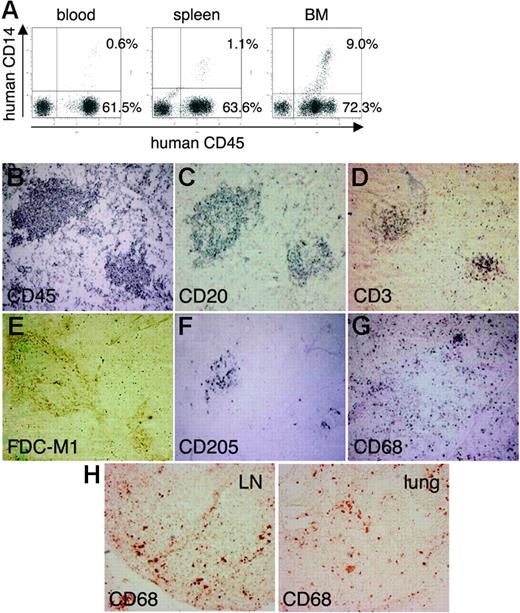
Next, using the hNOG mice at 4 months after transplantation, we investigated lymphoid structure formation and the development of human monocytes, macrophages, DCs, and FDCs, which are very important factors not only for elicitation of immune responses against foreign antigens, but also for the spread of HIV-1 infection in a body.22-24 Human CD14+ monocytes were detected in PB, the spleen, and BM using flow cytometry (Figure 2A) During immunohistochemical analysis, human CD45+ leukocytes gathered in a form of follicle-like structures (FLSs) at the end of the central artery in the spleen (Figure 2B). From a serial section of the same region (Figure 2B-G), these structures consisted mainly of human CD20+ B cells (Figure 2C) admixed with a small number of human CD3+ T cells (Figure 2D). Hardly any human FDCs positive for DRC-1 were detected (data not shown), whereas a loose network of murine FDCs positive for FDC-M1 was recognized in the distal portion of the FLSs (Figure 2E). Human CD205+ DCs were predominantly detected in a cluster form within the FLSs (Figure 2F), while human CD68+ macrophages were scattered throughout the spleen (Figure 2G). Many human CD68+ macrophages were also observed in various other organs, including the lymph nodes (LNs) and the lungs (Figure 2H).
Flow cytometric analysis and immunohistochemical analysis of the expression of myelomonocytic markers in hNOG mice 4 months after transplantation. (A) Human CD14+ monocytes/macrophages are recognized in peripheral blood, the spleen, and BM. (B-G) Immunohistochemical findings from serially sectioned spleen for the expressions of human CD45 (B), human CD20 (C), human CD3 (D), murine FDC (E), human CD205 (F), and human CD68 (G). (H) Human CD68+ macrophages are also detected in the medulla of the LN and lung. Visualization was performed with BCIP (B-D, F-G), DAB (E), and AEC (H). Original magnification, ×100.
Flow cytometric analysis and immunohistochemical analysis of the expression of myelomonocytic markers in hNOG mice 4 months after transplantation. (A) Human CD14+ monocytes/macrophages are recognized in peripheral blood, the spleen, and BM. (B-G) Immunohistochemical findings from serially sectioned spleen for the expressions of human CD45 (B), human CD20 (C), human CD3 (D), murine FDC (E), human CD205 (F), and human CD68 (G). (H) Human CD68+ macrophages are also detected in the medulla of the LN and lung. Visualization was performed with BCIP (B-D, F-G), DAB (E), and AEC (H). Original magnification, ×100.
Expression of HIV-1 coreceptors on CD4+ cells in various tissues
Since the development of lymphoid tissues was recognized in hNOG mice, we focused on the expressions of HIV-1 coreceptors CXCR4 and CCR5 on human CD4+ cells in these tissues. CXCR4 antigen was expressed in 36.5% ± 4.2% (n = 4) of the CD4+ cells in PB (Figure 3A) and 78.1% ± 17.1% (n = 5) in the spleen (Figure 3B). CCR5+ cells were detected in 15.5% ± 1.8% (n = 4) of CD4+ cells in PB and 28.6% ± 12.6% (n = 5) in the spleen (Figure 3A-B). In the thymus, CD4+CD8+ thymocytes existed in 82.9% ± 4.4% (n = 5) as well as small numbers of CD4+CD8− cells (6.4% ± 2.4%; n = 5) and CD4−CD8+ cells (7.7% ± 3.0%; n = 5), with the CXCR4 antigen expressed in 50.1% ± 4.5% (n = 5) of CD4+ cells, while, as with normal human thymocytes,25 CCR5+ cells were almost undetectable, with less than 1% (0.6% ± 0.1%; n = 5) (Figure 3C). Human CD3+ T cells and CD14+ monocytes in BM were detected only in 3.2% ± 2.1% and 5.8% ± 3.8%, respectively, while CD4+ cells were recognized in 18.1% ± 6.5%, with many expressing both CXCR4 (75.0% ± 23.1%) and CCR5 (81.3% ± 6.6%; n = 5; Figure 3D). Thus, distributions of HIV-1 coreceptor–positive cells in these lymphoid tissues suggest that the hNOG mice allow for sufficient development of human cells to make the study of HIV-1 pathogenesis possible.
Surface expression of HIV-1 coreceptors on CD4+ cells in various organs of mice 4 months after transplantation. A representative FACS profile of human CXCR4 and CCR5 on CD4+ cells shows the existence of CXCR4+CD4+ and CCR5+CD4+ cells in blood (A), spleen (B), and BM (D), but no CCR5+CD4+ cells in the thymus (C). BM results show that many CD4+ cells are neither CD3+ T cells nor CD14+ monocytes. A gate was set on the human CD45+ population.
Surface expression of HIV-1 coreceptors on CD4+ cells in various organs of mice 4 months after transplantation. A representative FACS profile of human CXCR4 and CCR5 on CD4+ cells shows the existence of CXCR4+CD4+ and CCR5+CD4+ cells in blood (A), spleen (B), and BM (D), but no CCR5+CD4+ cells in the thymus (C). BM results show that many CD4+ cells are neither CD3+ T cells nor CD14+ monocytes. A gate was set on the human CD45+ population.
Both R5- and X4-tropic HIVs efficiently infect and replicate in hNOG mice
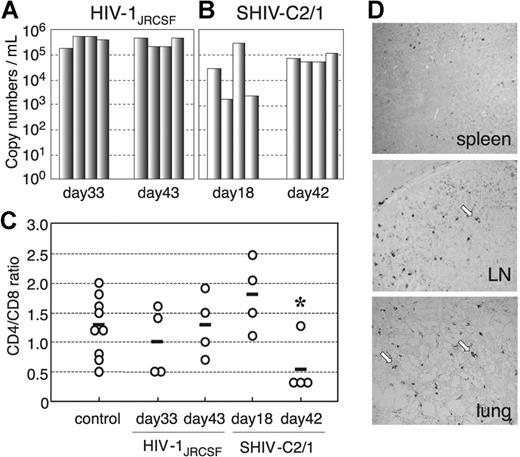
In our preliminary study, using low and high doses of challenge virus, no viral infection was detected in any of the virus-inoculated hNOG mice at 7 days after infection, while some showed detectable plasma viral loads at 14 days (data not shown). Then, we prepared 16 hNOG mice that received transplants of stem cells and inoculated them with a high dose of R5-tropic HIV-1JRCSF (65 000 TCID50) and X4-tropic SHIV-C2/1 (50 000 TCID50) intravenously through the tail vein at 102 to 132 days after transplantation. Upon HIV-1JRCSF infection, viral copy numbers in plasma rose to a level of 1.6 × 105 to 5.8 × 105 copies/mL (n = 4) on day 33 and 2.0 × 105 to 4.7 × 105 copies/mL on day 43 (n = 4) (Figure 4A) Moreover, for SHIV-C2/1 infection, viral copy numbers in plasma were 1.6 × 103 to 3.2 × 105 copies/mL on day 18 (n = 4) and reached 5.4 × 104 to 1.1 × 105 copies/mL on day 42 (n = 4; Figure 4B). In these mice, no significant decline in the CD4/CD8 ratio was observed throughout entire period of follow-up for the R5-tropic virus infection, while CD4+ cell decline was detected for the X4-tropic virus infection on day 42 after infection (P = .044) but not on day 18 after infection (Figure 4C). Four mice that did not receive transplants of human stem cells showed no detectable levels of plasma viral load (less than 500 copies/mL) following HIV/SHIV inoculation (data not shown).
The numbers of RNA viral copies in plasma, CD4+/CD8+ T-cell ratios in the spleen, and p24 detection in the immunohistochemistry of HIV/SHIV-infected mice. (A) Viral copy numbers of 8 mice inoculated with a high infectious dose of HIV-1JRCSF (65 000 TCID50) and killed on days 33 and 43 after inoculation. (B) Viral copy numbers of 8 mice inoculated with a high infectious dose of SHIV-C2/1 (50 000 TCID50) and killed on days 18 and 42 after inoculation. Note that all the mice showed high levels of viremia that lasted more than 40 days after inoculation. (C) CD4/CD8 cell ratios in the spleens of 16 infected mice and 9 uninfected control mice. Control mice were not inoculated with HIV/SHIV and were killed on days 105 to 166 after stem cell transplantation. There was no significant rapid loss of CD4+ cells in HIV-1JRCSF–infected mice, while a decline of the CD4/CD8 ratio was detected in SHIV-C2/1–infected mice on day 42 after infection compared with uninfected control mice (*P < .05). The short bars indicate the means of each group. (D) P24+ cells are clearly observed in the spleen, LNs, and lungs. Arrow indicates p24 positive for macrophage-like cells. Original magnification, ×100.
The numbers of RNA viral copies in plasma, CD4+/CD8+ T-cell ratios in the spleen, and p24 detection in the immunohistochemistry of HIV/SHIV-infected mice. (A) Viral copy numbers of 8 mice inoculated with a high infectious dose of HIV-1JRCSF (65 000 TCID50) and killed on days 33 and 43 after inoculation. (B) Viral copy numbers of 8 mice inoculated with a high infectious dose of SHIV-C2/1 (50 000 TCID50) and killed on days 18 and 42 after inoculation. Note that all the mice showed high levels of viremia that lasted more than 40 days after inoculation. (C) CD4/CD8 cell ratios in the spleens of 16 infected mice and 9 uninfected control mice. Control mice were not inoculated with HIV/SHIV and were killed on days 105 to 166 after stem cell transplantation. There was no significant rapid loss of CD4+ cells in HIV-1JRCSF–infected mice, while a decline of the CD4/CD8 ratio was detected in SHIV-C2/1–infected mice on day 42 after infection compared with uninfected control mice (*P < .05). The short bars indicate the means of each group. (D) P24+ cells are clearly observed in the spleen, LNs, and lungs. Arrow indicates p24 positive for macrophage-like cells. Original magnification, ×100.
To confirm HIV infection, we used immunohistochemistry to detect the presence of the p24 antigen of the HIV-1 Gag protein in various tissues of mice showing viremia. p24+ cells were clearly identified in the spleen, LN, and lungs (Figure 4D), which include macrophage-like cells.
Different distributions of R5- and X4-tropic viruses in lymphoid tissues
A number of mice (14) were further analyzed for HIV-1 infection on days 126 to 146 after transplantation with a low dose (200 TCID50) or a high dose (65 000 TCID50) of R5-tropic HIV-1JRCSF and a low dose (180 TCID50) or a high dose (20 000 TCID50) of X4-tropic HIV-1MNp. Consequently, 2 of the 4 mice given a low dose of HIV-1JRCSF and 2 of the 3 mice given a low dose of HIV-1MNp were successfully infected (Table 1) suggesting that each dose represents an approximately 50% infectious dose of HIV for hNOG mice. High HIV-DNA copy numbers were mainly detected in the spleen and BM of the HIV-1JRCSF –infected mice, and in the thymus and spleen of the HIV-1MNp–infected mice, while their BM showed lower copy numbers (Table 1).
Comparison of viral RNA copies in plasma and HIV-DNA copies in the spleen, BM, and thymus from hNOG mice receiving low- and high-dose viral inoculations
| Mouse ID no. . | HIV strain . | TCID50 . | Time after inoculation, d . | RNA viral copies/mL . | CD4/CD8 ratio . | HIV-DNA copies/106 human cells . | ||
|---|---|---|---|---|---|---|---|---|
| Splee . | BM . | Thymus . | ||||||
| Low-dose viral inoculation group | ||||||||
| 113-1 | HIV-1JRCSF | 200 | 18 | 6 240 | 1.8 | 34 177 | 11 785 | 3 495 |
| 112-2 | HIV-1JRCSF | 200 | 18 | <500 | 1.2 | < 100 | < 100 | < 100 |
| 113-2 | HIV-1JRCSF | 200 | 40 | 6 177 | 1.6 | 25 855 | 27 920 | 3 473 |
| 112-3 | HIV-1JRCSF | 200 | 40 | <500 | 0.9 | < 100 | < 100 | <100 |
| 112-4 | HIV-1MNp | 180 | 18 | 72 477 | 1.3 | 18 873 | 100 | ND |
| 113-4 | HIV-1MNp | 180 | 40 | 70 667 | 0.3 | 4 947 | 653 | 32 163 |
| 112-1 | HIV-1MNp | 180 | 40 | <500 | 0.9 | < 100 | < 100 | < 100 |
| High-dose viral inoculation group | ||||||||
| 136-3 | HIV-1JRCSF | 65 000 | 25 | 252 381 | 0.8 | 958 871 | 1 797 600 | 232 155 |
| 136-2 | HIV-1JRCSF | 65 000 | 29 | 50 167 | 0.7 | 41 172 | 54 521 | 8 600 |
| 141-1 | HIV-1JRCSF | 65 000 | 30 | 67 667 | 2.2 | 27 735 | 52 430 | 429 |
| 161-3 | HIV-1JRCSF | 65 000 | 30 | 13 847 | 0.9 | 104 466 | 14 653 | 111 080 |
| 157-3 | HIV-1MNp | 20 000 | 31 | 1 253 925 | 0.5 | 41 053 | 56 802 | 976 556 |
| 157-4 | HIV-1MNp | 20 000 | 31 | 147 973 | 0.6 | 3 634 | 262 | 40 796 |
| 161-6 | HIV-1MNp | 20 000 | 31 | 108 073 | 1.7 | 4 991 | < 100 | 3 673 |
| Mouse ID no. . | HIV strain . | TCID50 . | Time after inoculation, d . | RNA viral copies/mL . | CD4/CD8 ratio . | HIV-DNA copies/106 human cells . | ||
|---|---|---|---|---|---|---|---|---|
| Splee . | BM . | Thymus . | ||||||
| Low-dose viral inoculation group | ||||||||
| 113-1 | HIV-1JRCSF | 200 | 18 | 6 240 | 1.8 | 34 177 | 11 785 | 3 495 |
| 112-2 | HIV-1JRCSF | 200 | 18 | <500 | 1.2 | < 100 | < 100 | < 100 |
| 113-2 | HIV-1JRCSF | 200 | 40 | 6 177 | 1.6 | 25 855 | 27 920 | 3 473 |
| 112-3 | HIV-1JRCSF | 200 | 40 | <500 | 0.9 | < 100 | < 100 | <100 |
| 112-4 | HIV-1MNp | 180 | 18 | 72 477 | 1.3 | 18 873 | 100 | ND |
| 113-4 | HIV-1MNp | 180 | 40 | 70 667 | 0.3 | 4 947 | 653 | 32 163 |
| 112-1 | HIV-1MNp | 180 | 40 | <500 | 0.9 | < 100 | < 100 | < 100 |
| High-dose viral inoculation group | ||||||||
| 136-3 | HIV-1JRCSF | 65 000 | 25 | 252 381 | 0.8 | 958 871 | 1 797 600 | 232 155 |
| 136-2 | HIV-1JRCSF | 65 000 | 29 | 50 167 | 0.7 | 41 172 | 54 521 | 8 600 |
| 141-1 | HIV-1JRCSF | 65 000 | 30 | 67 667 | 2.2 | 27 735 | 52 430 | 429 |
| 161-3 | HIV-1JRCSF | 65 000 | 30 | 13 847 | 0.9 | 104 466 | 14 653 | 111 080 |
| 157-3 | HIV-1MNp | 20 000 | 31 | 1 253 925 | 0.5 | 41 053 | 56 802 | 976 556 |
| 157-4 | HIV-1MNp | 20 000 | 31 | 147 973 | 0.6 | 3 634 | 262 | 40 796 |
| 161-6 | HIV-1MNp | 20 000 | 31 | 108 073 | 1.7 | 4 991 | < 100 | 3 673 |
Seven mice inoculated with a low infectious dose of HIV-1JRCSF (200 TCID50) or HIV-1MNp (180 TCID50), and 7 mice receiving a high infectious dose of HIV-1JRCSF (65 000 TCID50) or HIV-1MNp (20 000 TCID50) were listed.
ND indicates not done.
Generation of HIV-specific antibodies in hNOG mice at a high multiplicity of infection
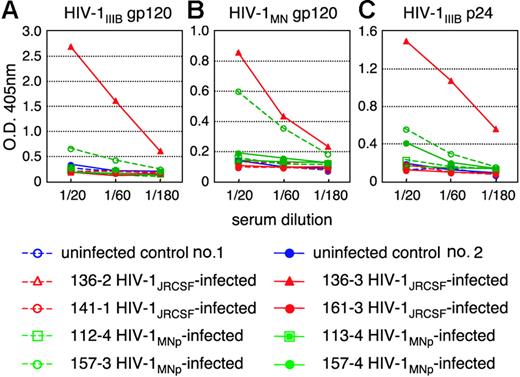
We then tested for generation of human antibodies against HIV-1 from these 14 mice by HIV antigen-specific ELISA. The sera of mice no. 136-3 and no. 157-3 infected with HIV-1JRCSF and HIV-1MNp, respectively, showed significant levels of human antibodies specific for HIV-1IIIB-Env gp120 (Figure 5A), HIV-1MN-Env gp120 (Figure 5B), and HIV-1IIIB-Gag p24 (Figure 5C). In addition, no. 157-4 sera from an HIV-1MNp–infected animal was also weakly positive for their Env and Gag antigens. These animals showed intense plasma viral loads and enhanced proviral DNA copies in the spleen, BM, and thymus (Table 1), suggesting that hNOG mice inoculated with high doses of HIV and showing high rates of viral infection develop HIV-1–specific humoral immune responses that are analogous to those seen in human anti-HIV B-cell responses.
Detection of anti–HIV-1 antibodies from the plasma of HIV-1–infected mice. An ELISA assay was conducted by using plasma from 14 mice inoculated with either HIV-1JRCSF or HIV-1MNp, and from 2 uninfected control mice. Representatives (n = 8) of the 14 HIV-1–inoculated mice, and the 2 uninfected mice, are shown in the panels. Measurements of specific human antibodies for HIV-1IIIB gp120 (A), HIV-1MN gp120 (B), and HIV-1IIIB p24 antigens (C) were shown. Results are expressed as the means from triplicate assays in 3 different experiments.
Detection of anti–HIV-1 antibodies from the plasma of HIV-1–infected mice. An ELISA assay was conducted by using plasma from 14 mice inoculated with either HIV-1JRCSF or HIV-1MNp, and from 2 uninfected control mice. Representatives (n = 8) of the 14 HIV-1–inoculated mice, and the 2 uninfected mice, are shown in the panels. Measurements of specific human antibodies for HIV-1IIIB gp120 (A), HIV-1MN gp120 (B), and HIV-1IIIB p24 antigens (C) were shown. Results are expressed as the means from triplicate assays in 3 different experiments.
Discussion
Current small-animal models fall short of accurately mirroring human HIV-1 infection and thus have limited usefulness in analyzing the natural course of its progression to the disease state and in developing antiviral countermeasures. Although successful HIV-1 infections in immunodeficiency mice humanized with PBMCs have been reported,12,13,21 transplanted human cells are soon depleted and do not elicit virus-specific immune responses, shedding little light on pathogenesis and vaccine development. By using NOG mice that received hematopoietic stem cell transplants showing high rates of viral infection, we demonstrated HIV-specific antibody responses and viral infection parameters, including the following: (1) similar levels of susceptibility to both R5- and X4-tropic HIV-1; (2) high levels of viremia stably observed over 40 days; (3) immunohistochemical detection of infected cells in various organs; and (4) a distinct tissue distribution for R5- versus X4-tropic HIV-1s.
Among CD4+ T cells, CXCR4 antigen is primarily expressed on naive and CCR5 on activated or memory cells.26 hu-PBL-SCID mice become susceptible to R5-tropic HIV-1 strains,27 since T cells are initially activated in the xenogenic environment and then become anergic.14 In contrast, SCID-hu (Thy/Liv) mice are more susceptible to X4 than to R5 strains6 because HIV-1 infection is restricted mainly to the engrafted thymus that is primarily comprised of immature T cells, suggesting that this model may not be practical overt HIV infection. Our study represents the first attempt to infect NOG mice that received transplants of human hematopoietic stem cells with HIV-1. Very similar infection rates were seen for both R5 and X4 strains in the mouse model. Flow cytometry revealed both CXCR4+CD4+ and CCR5+CD4+ cells in PB, the spleen, and BM, but only CXCR4 on thymic CD4+ T cells. It also showed the scattering of human macrophages, known to be susceptible to R5-tropic HIV-1 strains28,29 and the source of HIV-1,23,30-32 throughout various organs. p24+ macrophage-like cells were detected in these organs after R5-tropic HIV-1JRCSF infection. These data may help explain the susceptibility of hNOG mice to both R5- and X4-tropic HIV strains and also shed light on the active replenishment of these target cells in mice.
SCID mouse systems have been actively used in the evaluation of anti–HIV-1 drugs.9,11,21 In most cases, HIV-1 detection levels reach a peak within a month after inoculation and level off, accompanied by CD4+ T-cell depletion.3,12,13 Although suitable for short-term experiments, it is also true that these models require large numbers of mice because of large variations in infection efficiency. In contrast, very stable infections were noted in our hNOG mice that were inoculated with a high dose of HIVs. They did not show rapid CD4/CD8 decrease in spite of high levels of viremia persisting for more than 40 days. Efficient hematopoiesis and thymopoiesis of human cells probably compensated for the loss of CD4+ T cells, allowing for persistent infection. This capacity of the hNOG mouse system makes it attractive as a model for the long-term evaluation of anti–HIV-1 drugs. In addition to destroying mature blood cells, altered hematopoiesis in BM and the thymus has also been reported to be responsible for immunodeficiency in patients with AIDS.33,34 To study hematopoietic abnormalities in HIV-1 infection, both SCID-hu (Thy/Liv) mice8,35,36 and SIV- or SHIV-infected macaque models20,37-39 have been used. The current hNOG mouse system, in which human cells are efficiently reproduced from stem cells and then settled into hematopoietic organs, offers a promising model for the study of events that occur after infection not only with R5-tropic HIV-1 but also with X4-tropic HIV-1. Indeed, the BM of hNOG mice infected with R5-tropic HIV-1 exhibited exceptionally elevated levels of HIV-DNA copies. On the other hand, the thymus of X4-tropic HIV-1MNp–infected hNOG mice yielded large numbers of HIV-DNA copies, which seemed to correlate with the predominant expression of CXCR4 on the thymocytes. Thus, further observation is essential to address whether AIDS symptoms such as considerable CD4+ T-cell depletion and hematopoietic abnormalities eventually occur in these mice.
It is noteworthy that human antibodies against both HIV-1 Env gp120 and Gag p24 antigens were detected in mice no. 136-3, no. 157-3, and no. 157-4 after exposure to high titers of HIV-1, suggesting that hNOG mice have the ability to respond to HIV-1 antigens. This encourages us to develop antibody-based HIV vaccine candidates, although additional modifications are required for the stable induction of immune responses. Importantly, since the seroconverted mice showed high viremia and high numbers of proviral DNA copies in the spleen, BM, and thymus, abundant viral production may stimulate human B-cell responses against HIV-1 and generate specific antibodies. These mice showed little or no detectable human IgG against HIV-1, as determined by Western blot analysis (data not shown), suggesting that very low levels of class-switching occurred in these mice, though further study is required.
In addition to the humoral immune responses, the induction of primary T-cell responses is critical for the study of HIV-specific immune responses and pathogenesis, as well as for vaccine development. Although we did not demonstrate the T-cell ability to respond to virus antigens, human T cells from the spleen proliferated when stimulated with anti–human CD3 antibodies (data not shown), indicating that the human T cells in the NOG mice that received transplants of hematopoietic stem cells are capable of responding to T-cell receptor–mediated signals and are expected to be able to elicit primary antigen-specific immune responses against foreign antigens. To address whether the specific T-cell responses may be induced will be one of the important studies.
In conclusion, the NOG mice that received transplants of human hematopoietic stem cells successfully achieved systemic and persistent infection with both R5-tropic and X4-tropic HIV-1, and generated humoral immune responses against HIV-1. These capacities of the hNOG mouse model may be very attractive for the study of HIV pathogenesis and humoral immune responses induced by HIV vaccine candidates.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest statement: The authors declare no competing financial interests.
Contributions: S.W., K.T., N.S., M.H., and N.Y. designed the study; S.W., K.T., S.O., S.H., M.Y., Y.S., M.Z.D., and Z.Y. carried out the research; M.I. contributed live mice; S.W., K.T., and T.M. analyzed the data; N.S., M.H., and N.Y. controlled the data; S.W. wrote the paper; and all authors checked the final version of the manuscript.
Acknowledgments
We thank Yuetsu Tanaka of the University of Ryukyus, Tetsutaro Sata of NIID, and Shuzo Matsushita of Kumamoto University for their kind provision of mAbs to HIV-1, as well as Yukoku Tamaoka of Saiseikai Central Hospital, Toshio Akashi of Kumakiri Obstetric and Gynecologic Clinic, and Hideo Mugishima of Nihon University School of Medicine for their provision of umbilical cord blood. We also would like to express our gratitude to Ken Watanabe and Hideko Ogata of Tokyo Medical and Dental University for their skillful technical support.
This work was supported by grants from Research on Health Sciences focusing on Drug Innovation, the Japan Health Sciences Foundation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal