Abstract
Palifermin, a recombinant human keratinocyte growth factor, was tested for potential benefits on acute graft-versus-host disease (GVHD) and hematopoietic recovery in allogeneic hematopoietic stem cell transplantation (HSCT) recipients. This randomized, double-blind, placebo-controlled, dose-escalation study assessed the safety and tolerability of palifermin (n = 69) as compared with placebo (n = 31) in patients conditioned with cyclophosphamide and fractionated total-body irradiation (Cy/TBI) or busulfan and cyclophosphamide (Bu/Cy) and given methotrexate along with a calcineurin inhibitor (cyclosporine A, tacrolimus) for GVHD prophylaxis. All patients received 3 doses before conditioning and either 3 (cohort 1), 6 (cohort 2), or 9 (cohort 3) doses after HSCT. Palifermin doses were 40 μg/kg per day (cohort 1 only) or 60 μg/kg per day (all cohorts). Six patients (placebo = 2, palifermin = 4) experienced a total of 11 dose-limiting toxicities (most often skin, respiratory, or oral mucositis). The most common adverse events included edema, infection, skin pain, or rash. Times to neutrophil and platelet engraftment were similar. No significant differences in acute GVHD incidence or severity, survival, or day 100 relapse rates were observed between groups. Palifermin was associated with reduced incidence and mean severity of mucositis in patients conditioned with Cy/TBI but not Bu/Cy. We conclude that palifermin was generally safe in allogeneic HSCTs but had no significant effect on engraftment, acute GVHD, or survival in this trial.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a standard treatment option for a variety of hematologic malignancies. A major advantage of allo-HSCT is the potential for therapeutic benefits from graft-versus-leukemia (GVL) effects, which are mediated by donor T and natural killer cells and thought to eradicate host malignancy, reduce the incidence of relapse, and prolong survival.1,2 Unfortunately, GVL effects are closely associated with acute graft-versus-host disease (aGVHD), the major limiting toxicity of allogeneic transplantation. During aGVHD, the skin, gastrointestinal tract, and liver are damaged by both cellular and inflammatory cytokine effectors.3 Depletion of T cells from the graft effectively prevents aGVHD, but it also limits GVL effects and increases the rate of graft failure.4-7 An alternative approach to the prevention of aGVHD is to retain mature T cells in the stem cell graft but to disrupt the amplification of inflammatory cytokine effectors and thereby protect organs from aGVHD injury.3,8 The prevention of gastrointestinal tract injury is considered critical in minimizing subsequent systemic aGVHD.8
Keratinocyte growth factor (KGF) was first described as a growth factor for epithelial cells and has demonstrated protection against chemotherapeutic or radiation injury as well as aiding the healing process of various epithelia.9,10 Palifermin (Kepivance), a recombinant human KGF (rHuKGF), specifically stimulates the growth and antiapoptotic potential of epithelial cells expressing the KGF receptor without directly affecting nonepithelial cells lacking this receptor. Palifermin has been found to markedly reduce chemotherapy- and radiation-induced injury to the mucosal lining of the oral cavity and the lower gastrointestinal tract in a variety of animal models.11-15 In particular, palifermin induces cell growth, differentiation, and thickening of the epithelial tissues (squamous epithelium of the oral cavity and glandular tissue of the intestinal column), thereby helping to provide cytoprotective effects throughout the gastrointestinal epithelia.
The therapeutic potential of palifermin in preventing and ameliorating the effects of gastrointestinal injury as a result of aGVHD after high-dose chemoradiotherapy has been demonstrated in mouse transplantation models.16,17 Palifermin pretreatment of mice for 3 days prior to conditioning reduced the histologic damage as a result of aGVHD and improved long-term survival. Additional administration of palifermin after HSCT did not significantly improve survival.17 In a second aGVHD animal model, palifermin administration from day –3 before HSCT to day 7 after HSCT significantly reduced aGVHD mortality and the severity of clinical aGVHD.16 In this model, when mice received lethal doses of P815 leukemic cells at the time of transplantation, palifermin was associated with significantly improved leukemia-free survival compared with T-cell–depleted controls (45% versus 0%, P < .01), implying preservation of the GVL effects of the allograft. In preclinical allogeneic transplantation models, palifermin also reduced serum lipopolysaccharide and tumor necrosis factor α levels, factors augmenting the severity of experimental aGVHD.16,18 In other murine models, palifermin increased alloengraftment in sublethally irradiated recipients of allogeneic T-cell–depleted bone marrow (BM).19 Thus, in rodents, palifermin has beneficial effects on GVHD and alloengraftment without the apparent loss of GVL activity.
Clinically, palifermin has been shown to reduce the incidence and duration of severe oral mucositis in patients with hematologic malignancies undergoing myelotoxic therapy and autologous HSCT.20 The present phase 1/2 study was undertaken at the University of Minnesota and the University of Michigan to determine the safety and effect on acute GVHD of palifermin administered before conditioning and after allo-HSCT.
Patients, materials, and methods
Patient characteristics
Eligible patients were between 3 and 65 years of age; had a diagnosis of a hematologic malignancy (including myelodysplastic syndromes); had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; and were eligible for an allo-HSCT after conditioning therapy that consisted of either cyclophosphamide and total-body irradiation (Cy/TBI) (used at the University of Minnesota) or busulfan and cyclophosphamide (Bu/Cy) (used at the University of Michigan). Patients needed a 6 of 6 human leukocyte antigen–matched sibling donor who would provide donor BM or filgrastim-stimulated peripheral blood progenitor cells.
Patients were ineligible if they had previously received allo-HSCT; were to receive a T-cell–depleted donor graft; or had an active chronic skin disease, preexistent inflammatory bowel disease, uncontrolled (antibioticresistant) bacterial infection, hepatitis, or HIV infection. All patients provided informed consent, and the protocol was approved by the institutional review boards of each participating institution.
Study schema. (a) Cy 60 mg/kg per day, TBI total dose 13.2 Gy, fractionated as 165 cGy twice daily for 4 days; (b) busulfan 1 mg/kg per dose given 4 times daily, Cy 60 mg/kg per day; (c) GVHD prophylaxis: cyclosporine A (in the Cy/TBI group) or cyclosporine A or tacrolimus (in the Bu/Cy group) starting day –3 in combination with methotrexate 15 mg/m2, IV bolus on day +1 and methotrexate 10 mg/m2, IV bolus on days 3, 6, and 11; (d) filgrastim 5 μg/kg per day from 24 hours after transplantation until neutrophil recovery (absolute neutrophil count [ANC], 1 × 109/L for 3 consecutive days, or 10 × 109/L for 1 day, whichever occurred first). K40 indicates palifermin 40 μg/kg per day; K60, palifermin 60 μg/kg per day; P, placebo; Cy, cyclophosphamide; Bu, busulfan; RT, radiotherapy.
Study schema. (a) Cy 60 mg/kg per day, TBI total dose 13.2 Gy, fractionated as 165 cGy twice daily for 4 days; (b) busulfan 1 mg/kg per dose given 4 times daily, Cy 60 mg/kg per day; (c) GVHD prophylaxis: cyclosporine A (in the Cy/TBI group) or cyclosporine A or tacrolimus (in the Bu/Cy group) starting day –3 in combination with methotrexate 15 mg/m2, IV bolus on day +1 and methotrexate 10 mg/m2, IV bolus on days 3, 6, and 11; (d) filgrastim 5 μg/kg per day from 24 hours after transplantation until neutrophil recovery (absolute neutrophil count [ANC], 1 × 109/L for 3 consecutive days, or 10 × 109/L for 1 day, whichever occurred first). K40 indicates palifermin 40 μg/kg per day; K60, palifermin 60 μg/kg per day; P, placebo; Cy, cyclophosphamide; Bu, busulfan; RT, radiotherapy.
Study design and end points
This randomized, double-blind, placebo-controlled, dose-escalation trial was conducted at 2 study centers (University of Minnesota and University of Michigan). Three cohorts were sequentially enrolled with individual doses and schedules as shown in Figure 1. Randomization was structured to achieve balance between the placebo and palifermin groups within each study site and in each cohort with stratification based on conditioning regimen and/or patient age. In cohort 1, 6 patients (3 per conditioning regimen) were randomly assigned to placebo and palifermin at either 40 or 60 μg/kg per day. In cohorts 2 and 3, randomization was 1:2 placebo and palifermin at 60 μg/kg per day with at least 12 patients receiving palifermin in cohort 2 and 24 patients receiving palifermin in cohort 3. All patients received placebo or palifermin on days –11 to –9 and days 0, 1, and 2. Patients in cohort 2 received additional study drug on days 7 to 9 (totaling 6 doses), and those in cohort 3 received additional study drug on days 7 to 9 and 14 to 16 (totaling 9 doses). All randomly assigned patients were followed during the study period of 100 days for engraftment, aGVHD, and survival; and for 30 days for dose-limiting toxicities (DLTs) and adverse experiences. No patient was lost to follow-up. Dose escalation between each cohort was allowed only after data review by the safety monitoring committee and only if less than 33% of the patients randomly assigned to palifermin in cohort 1 or 2 experienced a DLT. A DLT was defined as any unexpected, nonhematologic, National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 to 4 adverse event considered possibly, probably, or definitely related to the study drug. Specific events scored as DLTs were grade 3 to 4 adverse events associated with significant morbidity; severe skin, gut, or liver toxicities occurring before day 30 with histologic confirmation as aGVHD; grade 4 mucositis compromising the airway or associated with significant bleeding; and grade 4 rash requiring treatment. Patients who discontinued the study drug because of an adverse event or who died before day 30 were replaced to obtain a complete assessment of the safety parameters. Patients who discontinued because of a DLT were not replaced because the incidence of DLT was a primary study end point for dose escalation.
The primary objective of this study was to determine the safety and tolerability of palifermin. Secondary objectives were to determine overall survival, the incidence and severity of aGVHD, the incidence of transplantation-related toxicity, and the time to marrow engraftment as well as to evaluate the incidence, severity, and duration of oral and lower gastrointestinal tract mucositis. Analysis was planned to examine potential interactions between palifermin and conditioning regimens.
Adverse events were assessed daily during the study period and graded according to the World Health Organization (WHO) and CTC toxicities scale. Graft-versus-host-disease was graded weekly during the first 2 months after transplantation, then every other week to day 100 by specific observers according to consensus criteria.21 Severity of GVHD was determined clinically (physical examination and laboratory serum values), with biopsies of affected organs when available. Oral mucositis was assessed 3 times a week during hospitalization, by designated observers, using the WHO toxicity scale.
Conditioning therapy and allogeneic GVHD prophylaxis
The Cy/TBI conditioning regimen included Cy 60 mg/kg/d on days –7 and –6, and TBI 165 cGy twice daily on days –4 to –1; Bu/Cy included oral Bu 1 mg/kg per dose given 4 times daily on days –7 to –4 and Cy 60 mg/kg per day on days –3 and –2. Infusion of hematopoietic cells from the allogeneic donor was performed on day 0, with study drug administration beginning at least 2 hours after the final graft infusion.
Prophylaxis against aGVHD consisted of methotrexate (15 mg/m2, IV bolus on day +1; and methotrexate 10 mg/m2, IV bolus on days 3, 6, and 11) and cyclosporine A (University of Minnesota) or tacrolimus (University of Michigan) starting at day –3. In the absence of aGVHD, tacrolimus or cyclosporin A was tapered by 10% per week beginning on day 60. In some patients, as is currently clinical practice, day 11 methotrexate was not administered according to the status of the patient but by physician discretion.
Filgrastim was administered at 5 μg/kg per day from 24 hours after transplantation until neutrophil recovery (absolute neutrophil count [ANC] ≥ 1 × 109/L for 3 consecutive days, or ≥ 10 × 109/L for 1 day, whichever occurred first).
Statistical methods
Patients who discontinued study drug for reasons other than an event of DLT were replaced and were not considered in escalating the dose for the next cohort (phase 1 portion of the study). Although data were checked before dose escalation, the 2 centers continued to randomly assign patients at the current dose. Data for all patients randomly assigned and who received a transplant were used in all other analyses (intent-to-treat). Placebo groups from the 3 cohorts were combined, whereas patients who received palifermin were analyzed for each cohort individually and combined. Safety parameters, including DLTs, were tabulated by body system, severity, and investigator-determined relation to study drug. Comparisons of patients who received palifermin to those who received placebo included analysis by grade of adverse event and preparative regimen as well as the incidence, duration, and severity of aGVHD and mucositis. Treatment group differences in frequencies were tested by the Cochran-Mantel-Haenszel method, and means of severity grades were tested by the t test, all stratified by center.
Survival curves were calculated according to the Kaplan-Meier method. Time to relapse, aGVHD, and neutrophil and platelet engraftments were estimated by cumulative incidence, treating nonevent deaths as a competing risk. Point estimates and confidence intervals for study parameters were provided, and treatment groups were compared by the log-rank test. No correction for multiple analyses or comparisons was planned or performed.
Results
Patient demographic and baseline characteristics
One hundred patients were enrolled in this study, 31 patients were randomly assigned to receive placebo and 69 to receive palifermin. Baseline demographic and disease characteristics were balanced between the placebo and palifermin groups, and enrollment was balanced between the centers (Table 1). Patient characteristics were similar in both groups and between test sites.
Baseline demographic and disease characteristics
. | . | Palifermin . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cohort 1 . | . | Cohort 2 . | Cohort 3 . | . | . | ||||
. | Placebo . | 40 μg/kg or 240 μg/kg total dose . | 60 μg/kg or 360 μg/kg total dose . | 60 μg/kg or 540 μg/kg total dose . | 60 μg/kg or 720 μg/kg total dose . | All patients . | P* . | ||||
| Randomized, no. | 31 | 8 | 10 | 14 | 37 | 69 | |||||
| Center, no. (%) | |||||||||||
| Michigan | 14 (45) | 3 (37) | 4 (40) | 7 (50) | 18 (49) | 32 (46) | .91 | ||||
| Minnesota | 17 (55) | 5 (63) | 6 (60) | 7 (50) | 19 (51) | 37 (54) | |||||
| Sex, no. (%) | |||||||||||
| Male | 18 (58) | 6 (75) | 6 (60) | 10 (71) | 18 (49) | 40 (58) | .99 | ||||
| Female | 13 (42) | 2 (25) | 4 (40) | 4 (29) | 19 (51) | 29 (42) | |||||
| Median age, y (range) | 46 (7-63) | 44 (33-64) | 43.5 (24-55) | 48.5 (22-60) | 47 (7-65) | 46 (7-65) | .58 | ||||
| Median weight, kg (range) | 78 (25-159) | 73 (64-96) | 76 (50-119) | 92 (48-124) | 82 (26-114) | 81 (26-124) | .74 | ||||
| Disease, no. (%) | |||||||||||
| AII | 1 (3) | 0 | 1 (10) | 3 (21) | 4 (11) | 8 (12) | .08 | ||||
| AML | 12 (39) | 4 (50) | 3 (30) | 6 (42) | 11 (30) | 24 (35) | |||||
| CML | 8 (26) | 2 (25) | 2 (20) | 1 (7) | 2 (5) | 7 (10) | |||||
| MDS | 6 (19) | 0 | 0 | 1 (7) | 5 (14) | 6 (9) | |||||
| NHL | 1 (3) | 1 (13) | 0 | 3 (21) | 9 (24) | 13 (19) | |||||
| Hodgkin | 0 | 0 | 1 (10) | 0 | 0 | 1 (1) | |||||
| Other malignancies | 3 (10) | 1 (13) | 3 (30) | 0 | 6 (17) | 10 (14) | |||||
. | . | Palifermin . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cohort 1 . | . | Cohort 2 . | Cohort 3 . | . | . | ||||
. | Placebo . | 40 μg/kg or 240 μg/kg total dose . | 60 μg/kg or 360 μg/kg total dose . | 60 μg/kg or 540 μg/kg total dose . | 60 μg/kg or 720 μg/kg total dose . | All patients . | P* . | ||||
| Randomized, no. | 31 | 8 | 10 | 14 | 37 | 69 | |||||
| Center, no. (%) | |||||||||||
| Michigan | 14 (45) | 3 (37) | 4 (40) | 7 (50) | 18 (49) | 32 (46) | .91 | ||||
| Minnesota | 17 (55) | 5 (63) | 6 (60) | 7 (50) | 19 (51) | 37 (54) | |||||
| Sex, no. (%) | |||||||||||
| Male | 18 (58) | 6 (75) | 6 (60) | 10 (71) | 18 (49) | 40 (58) | .99 | ||||
| Female | 13 (42) | 2 (25) | 4 (40) | 4 (29) | 19 (51) | 29 (42) | |||||
| Median age, y (range) | 46 (7-63) | 44 (33-64) | 43.5 (24-55) | 48.5 (22-60) | 47 (7-65) | 46 (7-65) | .58 | ||||
| Median weight, kg (range) | 78 (25-159) | 73 (64-96) | 76 (50-119) | 92 (48-124) | 82 (26-114) | 81 (26-124) | .74 | ||||
| Disease, no. (%) | |||||||||||
| AII | 1 (3) | 0 | 1 (10) | 3 (21) | 4 (11) | 8 (12) | .08 | ||||
| AML | 12 (39) | 4 (50) | 3 (30) | 6 (42) | 11 (30) | 24 (35) | |||||
| CML | 8 (26) | 2 (25) | 2 (20) | 1 (7) | 2 (5) | 7 (10) | |||||
| MDS | 6 (19) | 0 | 0 | 1 (7) | 5 (14) | 6 (9) | |||||
| NHL | 1 (3) | 1 (13) | 0 | 3 (21) | 9 (24) | 13 (19) | |||||
| Hodgkin | 0 | 0 | 1 (10) | 0 | 0 | 1 (1) | |||||
| Other malignancies | 3 (10) | 1 (13) | 3 (30) | 0 | 6 (17) | 10 (14) | |||||
AML indicates acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma.
P values for significance test represent all palifermin versus placebo.
Most patients received the scheduled number of palifermin doses in each dose cohort: 5 (87%) of 6 received 40 μg/kg per day and 6 (100%) of 6 received 6 doses of 60 μg/kg per day in cohort 1; 11 (79%) of 14 received 9 doses of 60 μg/kg per day in cohort 2; and 24 (65%) of 35 received 12 doses of 60 μg/kg per day in cohort 3.
Twenty patients did not receive all study doses (Table 2). Seventeen patients discontinued the study drug and were replaced to allow a full assessment of safety. Discontinuations were due to adverse events (n = 8), transplantation delay (n = 4), patient refusal (n = 1), disease progression before HSCT (n = 1), palifermin overdose (n = 1), and death before day 30 (n = 2) (Table 2; data not shown). Three patients with DLTs were discontinued and not replaced. The most frequent reason for discontinuation was erythematous skin reactions, sometimes associated with severe pain in the hands and feet.
Discontinuations for patients receiving placebo or palifermin
. | . | Palifermin . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cohort 1 . | . | Cohort 2 . | Cohort 3 . | . | ||||
. | Placebo . | 40 μg/kg or 240 μg/kg total dose . | 60 μg/kg or 360 μg/kg total dose . | 60 μg/kg or 540 μg/kg total dose . | 60 μg/kg or 720 μg/kg total dose . | All . | ||||
| Patients randomly assigned, no. | 31 | 8 | 10 | 14 | 37 | 69 | ||||
| Patients discontinued and replaced, no. (%) | 1 (3) | 1 (13) | 0 | 2 (14) | 14 (38) | 17 (25) | ||||
| Patients with DLT who discontinued and were not replaced, no. (%) | 1 (3) | 0 | 0 | 2 (14) | 1 (3) | 3 (4) | ||||
| Deaths of patients who received a transplant, no. (%) | 5 (16) | 2 (25)* | 1 (10) | 3 (21) | 6 (17)* | 12 (18) | ||||
. | . | Palifermin . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Cohort 1 . | . | Cohort 2 . | Cohort 3 . | . | ||||
. | Placebo . | 40 μg/kg or 240 μg/kg total dose . | 60 μg/kg or 360 μg/kg total dose . | 60 μg/kg or 540 μg/kg total dose . | 60 μg/kg or 720 μg/kg total dose . | All . | ||||
| Patients randomly assigned, no. | 31 | 8 | 10 | 14 | 37 | 69 | ||||
| Patients discontinued and replaced, no. (%) | 1 (3) | 1 (13) | 0 | 2 (14) | 14 (38) | 17 (25) | ||||
| Patients with DLT who discontinued and were not replaced, no. (%) | 1 (3) | 0 | 0 | 2 (14) | 1 (3) | 3 (4) | ||||
| Deaths of patients who received a transplant, no. (%) | 5 (16) | 2 (25)* | 1 (10) | 3 (21) | 6 (17)* | 12 (18) | ||||
One patient was replaced.
Safety
Six patients experienced a total of 11 DLTs, 2 in the placebo group and 4 in the palifermin groups. In the palifermin groups, all DLTs occurred in patients who received 8 or more palifermin doses (Table 2; data not shown). The 11 DLTs included 4 respiratory events (2 placebo, 2 palifermin), and 2 incidences of grade 4 oral mucositis (1 placebo, 1 palifermin). The remaining 5 DLTs (3 skin reactions, 1 cardiac event, and 1 hepatic enzyme elevation) all occurred in the palifermin group.
Most adverse events occurred with similar frequencies in the placebo and palifermin groups. Adverse events that occurred with an incidence at least 10% greater in the palifermin group than placebo group included edema (palifermin, 78%; placebo, 65%), infection (palifermin, 11%; placebo, 0%), local pain (palifermin, 88%; placebo, 77%), and skin reactions (palifermin, 94%; placebo, 68%) (Table 3). Only the incidence of skin reactions differed significantly (P < .01) between the groups. Grade 3 to 4 adverse events occurring more frequently in the palifermin group than in the placebo group included skin reactions (22% versus 13%), diarrhea (10% versus 2%), local pain (10% versus 2%), and cardiac events (12% versus 6%). None of these grade 3 to 4 adverse events were significantly different between the groups.
Adverse events occurring with an incidence of 10% or more higher in patients treated with palifermin compared with placebo
Adverse event . | Placebo, % . | Palifermin, % . | P . |
|---|---|---|---|
| Edema | 65 | 78 | .15 |
| Infection | 0 | 11 | .17 |
| Local pain | 77 | 88 | .07 |
| Skin rash | 68 | 94 | <.01 |
Adverse event . | Placebo, % . | Palifermin, % . | P . |
|---|---|---|---|
| Edema | 65 | 78 | .15 |
| Infection | 0 | 11 | .17 |
| Local pain | 77 | 88 | .07 |
| Skin rash | 68 | 94 | <.01 |
Eighteen patients (9 patients at each study site) died before day 100. Two of the 18 patients died before day 30: 1 patient from severe mucositis, elevated bilirubin, hypoxia, pulmonary edema, renal failure, hepatic dysfunction; and a second patient during conditioning because of progressive leukemic leukocytosis and intracranial hemorrhage.
Regimen-related toxicity
On the basis of the pharmacology and clinical experience with palifermin, the incidences of mucositis and diarrhea were of special interest, as were elevations in serum amylase and lipase (adverse events previously observed with palifermin administration). Although the overall difference in the mean severity of oral mucositis was significantly lower in patients receiving palifermin (2.8 versus 2.3, P = .01), subgroup analysis by transplant site showed that the major difference occurred at the Minnesota site, where the incidence of severe oral mucositis (WHO grade 3 to 4) was reduced from 100% to 81% (P = .05) and the mean severity grade from 3.1 to 2.4 (P < .05). At the University of Michigan, where patients received a less mucotoxic Bu/Cy conditioning regimen, mucositis was less severe, and patients who received placebo and palifermin had similar rates of WHO grade 3 to 4 mucositis (50% versus 44%) and mean severity. (2.5 versus 2.0).
No significant differences were seen in the mean grades of diarrhea between patients in the placebo (1.0) and palifermin (1.3) groups. The proportions of patients experiencing grade 3 to 4 elevations from baseline of serum amylase or lipase levels were similar for those receiving placebo (10%) and those receiving palifermin (7%).
Methotrexate dosing
Completion of methotrexate dosing did not differ between treatment groups or in different dosing cohorts and was similar at each center (not shown). Similar numbers of patients in the placebo or palifermin group in cohort 1 received all 4 scheduled doses of methotrexate (67% versus 76%, P = .66). In cohorts 2 and 3, (68% of placebo patients and 71% of palifermin patients received all scheduled methotrexate treatments (P = .82). The maximum grade of oral mucositis in patients who received placebo or palifermin did not differ whether the patient received methotrexate on day 11 or had the dose withheld on day 11 (Table 4). Therefore, there was no apparent confounding of methotrexate administration with either the observed severity of mucositis or the incidence and severity of aGVHD.
Effect of palifermin and methotrexate on oral mucositis severity
. | Maximum grade of oral mucositis, no. (%) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 . | 1 . | 2 . | 3 . | 4 . | P* . | ||||
| Palifermin | .42 | |||||||||
| With methotrexate on day 11 | 4 (8) | 4 (8) | 8 (16) | 33 (67) | 0 (0) | |||||
| Without methotrexate on day 11 | 1 (6) | 3 (19) | 2 (13) | 10 (63) | 0 (0) | |||||
| Placebo | ||||||||||
| With methotrexate on day 11 | 0 (0) | 0 (0) | 7 (27) | 18 (69) | 1 (4) | |||||
| Without methotrexate on day 11 | 0 (0) | 0 (0) | 0 (0) | 4 (80) | 1 (20) | |||||
. | Maximum grade of oral mucositis, no. (%) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 . | 1 . | 2 . | 3 . | 4 . | P* . | ||||
| Palifermin | .42 | |||||||||
| With methotrexate on day 11 | 4 (8) | 4 (8) | 8 (16) | 33 (67) | 0 (0) | |||||
| Without methotrexate on day 11 | 1 (6) | 3 (19) | 2 (13) | 10 (63) | 0 (0) | |||||
| Placebo | ||||||||||
| With methotrexate on day 11 | 0 (0) | 0 (0) | 7 (27) | 18 (69) | 1 (4) | |||||
| Without methotrexate on day 11 | 0 (0) | 0 (0) | 0 (0) | 4 (80) | 1 (20) | |||||
P value is comparison of grades 0 to 2 versus 3 to 4 in groups with or without day 11 methotrexate by palifermin versus placebo.
GVHD
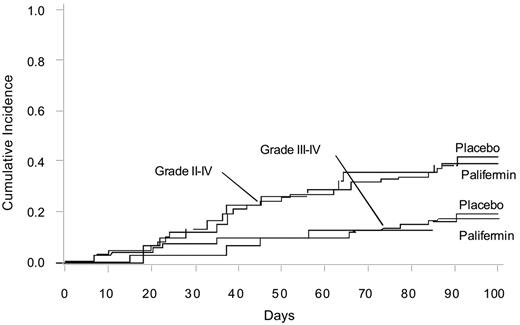
No significant difference was seen in the incidence of grades 2 to 4 or grade 3 to 4 aGVHD on day 100 after allogeneic HSCT in patients receiving palifermin (Figure 2). No difference was noted in the severity of the maximum grade of aGVHD or maximum stage in the skin, liver, or gastrointestinal tract (data not shown). The administration of day 11 methotrexate did not have a significant effect on the incidence of grades 2 to 4 or grade 3 to 4 aGVHD in the placebo or palifermin groups (P > .75; not shown).
Hematopoietic recovery
Time-to-engraftment values for ANC and platelet counts were similar for the placebo and palifermin treatment groups (data not shown). Graft failure did not occur.
Survival and relapse
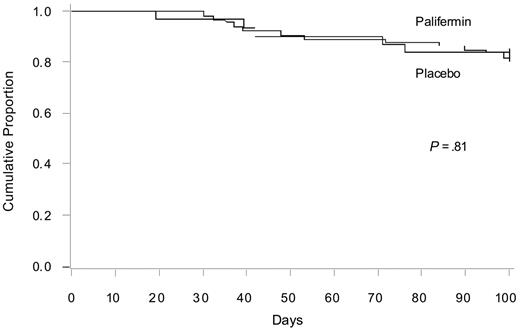
Survival rates on day 100 were similar overall and between centers, and no significant differences were observed between the 84% (95% confidence interval, 71%-97%) survival in the patients receiving placebo and the 82% (95% confidence interval, 73%-91%) survival in the patients receiving palifermin (Figure 3). Ten patients experienced a disease relapse before study day 100: 4 from the placebo group, 1 each from the 40- and 60-μg/kg groups in cohort 1, and 2 each from cohorts 2 and 3. No significant differences in the rate of relapse were seen between the placebo and palifermin groups (not shown).
Discussion
This was the first randomized study to assess the safety, tolerability, and aGVHD effects of palifermin in patients receiving allogeneic HSCT. Because palifermin could conceivably intensify GVHD through its effects on epithelial cells that are GVHD targets, one important result of this study was that palifermin administration had no significant effect on aGVHD incidence and severity. Palifermin administration did correlate with less severe oral mucositis in the more mucotoxic Cy/TBI radiation-containing conditioning regimen, whereas the grade of diarrhea was not significantly altered by palifermin administration. Day 100 survival and relapse rates and time to neutrophil and platelet recovery were not affected by palifermin administration. Patients treated with palifermin had a higher incidence of DLTs and study withdrawals due to adverse events (most commonly skin reactions and pain in the hands and feet). Adverse events were similar in the palifermin and placebo groups with the exception of a significantly higher incidence of skin pain in the palifermin-treated group. Thus, palifermin given at these doses and schedules along with myeloablative conditioning regimens lessened mucositis severity, albeit only in patients conditioned with Cy/TBI, but it had no significant effect on aGVHD incidence or severity, hematopoietic recovery, relapse, or day 100 survival rates.
Cumulative incidence of aGVHD grades 2 to 4 and 3 to 4 for placebo and palifermin therapy.
Cumulative incidence of aGVHD grades 2 to 4 and 3 to 4 for placebo and palifermin therapy.
Cumulative survival according to the Kaplan-Meier method for placebo and palifermin.
Cumulative survival according to the Kaplan-Meier method for placebo and palifermin.
Three distinct preclinical models have indicated that the administration of palifermin reduces the incidence and severity of aGVHD in the allo-HSCT setting.16,17,19 Chemoradiotherapy (Cy/TBI) conditioning was tested in only one model.17 Palifermin given for 3 doses prior to chemoradiotherapy resulted in an approximate 20% survival rate at 5.5 weeks after BMT versus 0% in controls. In other studies, controls died by 5.5 weeks after BMT, whereas palifermin given before TBI conditioning resulted in 19% of recipients surviving for approximately 10.5 weeks after BMT.17 Histologic analysis revealed a protective effect on GVHD target organs which was more evident early (day 5) than later (day 14) after BMT. In other rodent studies, we have shown that beneficial effects of rHuKGF given before conditioning therapy and BMT on GVHD target organ injury or repair can be overcome in part by adding Cy to the TBI conditioning regimen.22,23 In studies with TBI-only conditioning, GVHD lethality was not uniform (19% survival in controls), and under those conditions, palifermin before and after BMT treatment (days –3 to +7) led to a high (78%) survival rate.16 In another TBI-only conditioning study, survival with palifermin was related to the incidence and rapidity of GVHD-induced deaths.17 Thus, in rodents, palifermin protection from lethality and organ tissue injury varies with the conditioning regimen (Cy/TBI versus TBI) and intensity. In rodents given palifermin before a Cy/TBI conditioning regimen, only a low proportion of mice were able to survive the GVHD lethality process and those mice were not GVHD free.17 We interpret the rodent data to indicate that palifermin can modify GVHD but that the magnitude of this effect in chemoradiotherapy-conditioned recipients is insufficient to rescue the majority of mice from lethality or to fully prevent GVHD-associated tissue injury under the conditions tested.
On the basis of the rodent data demonstrating partial effects on GVHD lethality and GVHD-induced tissue injury along with the known mucositis protective effects in patients receiving autologous HSCT and peritransplantation palifermin, this clinical study in patients who receive an allo-HSCT was designed to explore the phase 1/2 testing of extended duration of palifermin administration with the goal of both reducing conditioning regimen injury to the gastrointestinal as well as aiding in the prevention or repair of GVHD injury via effects on GVHD target organs. Despite the failure of palifermin to prevent aGVHD in this study, it is possible that palifermin could have aGVHD protective effects using other conditioning regimens, palifermin schedules, or other allograft settings, including populations with greater risks of aGVHD, such as those receiving unrelated donor grafts.
Similar to its effects in patients receiving autologous HSCT,20 palifermin was associated with a reduced incidence and severity of oral mucositis in patients receiving a more substantial mucotoxic regimen of high-dose chemotherapy with fractionated TBI but not the less mucotoxic regimen, Bu/Cy. Because the current study was not designed with mucositis as a primary end point and because only 54 patients received the more mucotoxic chemoradiotherapy-conditioning regimen, additional studies are needed to confirm palifermin's ability to reduce the incidence and duration of severe oral mucositis and its related sequelae in patients conditioned with Cy/TBI and to rigorously monitor narcotic and hyperalimentation use, days of hospitalization, and patient-reported mucositis outcome in the allo-HSCT setting using a variety of conditioning regimens.20,24
In this study, the administration of methotrexate could have modified the effects of palifermin. Palifermin causes epithelial cell hyperplasia25 and thus could increase epithelial cell injury in patients given methotrexate. Alternatively, palifermin could reduce oral epithelial cell injury by building up the oral mucosa to better withstand methotrexate damage. We observed no interaction between palifermin and methotrexate in the severity of mucositis or tolerance of all 4 scheduled doses of methotrexate.
Adverse reactions did not differ from the placebo and palifermin groups except for more frequent skin pain. Skin reactions in patients receiving palifermin have also been seen in the auto-HSCT setting. Spielberger et al20 reported that 6 doses of palifermin were associated with reversible, usually mild to moderate, skin events, such as rash, pruritus, erythema, and edema. Six patients withdrew from the study during the third cycle of palifermin administration because of dysesthesias in the feet, palms, or lips. The episodic and repeated palifermin administration (injection for 3 days, rest for 4 days) may have enhanced patients' discomfort with these dysesthesias, which resolved promptly after discontinuation of palifermin. The biologic basis for these symptoms is unknown.
In considering the potential benefits of palifermin in the allo-HSCT setting, it is important to mention that palifermin given prior to TBI or Cy/TBI in rodents improved thymopoiesis and peripheral immune reconstitution, likely via effects on thymic epithelial cells.26-28 In a nonhuman primate model of myeloablatation followed by autologous HSCT, the peritransplantation administration of palifermin increased thymopoiesis and peripheral immune system recovery, indicating that the beneficial immune system effects of palifermin are not restricted to small animal models.29 Therefore, palifermin also may have uses in allo-HSCT to speed thymopoiesis and immune system recovery. We do not have data that address the question as to whether palifermin can accelerate immune recovery in patients who receive an allo-HSCT because the end points of this phase 1/2 study did not include assessment of thymic function in these patients; this matter will require future studies. In designing such trials, it is important to note that GVHD itself induces substantial thymic injury in myeloablated rodents28 and patients.30,31 Therefore, palifermin may be useful in reducing GVHD-induced thymic injury in patients receiving T-replete grafts or in accelerating thymopoiesis and immune system recovery in patients receiving rigorously T-cell–depleted grafts who are prone especially to opportunistic infections until the donor immune system is reestablished.31
In conclusion, we found no significant effect of palifermin on the incidence and severity of aGVHD. Mucositis was less severe in patients receiving a mucotoxic chemoradiotherapy but not a less mucotoxic chemotherapy-only regimen. No effects on hematologic recovery, day 100 survival, or relapse rates were evident. Further blinded prospective studies will be required to identify favorable effects of palifermin after allotransplantation, especially in patients receiving other types of allogeneic donor grafts and conditioning regimens and for the purpose of rigorously identifying the potential beneficial effects of palifermin on both mucositis and immune deficiency and their sequelae.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-04-017780.
Supported by National Institutes of Health grants R01 HL073794, NIH P01 CA03952, and 2P30CA046592; Food and Drug Administration grant FRD 020201; and Amgen.
B.R.B. designed the study, performed research, analyzed data, and wrote the paper; D.J.W. and S.S. performed research, analyzed data, and participated in writing the paper; T.D. and A.G. analyzed data and participated in writing the paper; and J.L.M.F. designed the study, performed research, analyzed data, and participated in writing the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
J.L.M.F. is the recipient of a Doris Duke Distinguished Clinical Scientist award.

![Figure 1. Study schema. (a) Cy 60 mg/kg per day, TBI total dose 13.2 Gy, fractionated as 165 cGy twice daily for 4 days; (b) busulfan 1 mg/kg per dose given 4 times daily, Cy 60 mg/kg per day; (c) GVHD prophylaxis: cyclosporine A (in the Cy/TBI group) or cyclosporine A or tacrolimus (in the Bu/Cy group) starting day –3 in combination with methotrexate 15 mg/m2, IV bolus on day +1 and methotrexate 10 mg/m2, IV bolus on days 3, 6, and 11; (d) filgrastim 5 μg/kg per day from 24 hours after transplantation until neutrophil recovery (absolute neutrophil count [ANC], 1 × 109/L for 3 consecutive days, or 10 × 109/L for 1 day, whichever occurred first). K40 indicates palifermin 40 μg/kg per day; K60, palifermin 60 μg/kg per day; P, placebo; Cy, cyclophosphamide; Bu, busulfan; RT, radiotherapy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/9/10.1182_blood-2006-04-017780/4/m_zh80210603190001.jpeg?Expires=1769296783&Signature=FyYY1403K0oCp3uSa4pbnekQHcbK-3y2BANJp2IHje8mkOk8ugZlMOQ3iFQHQFHjpdZomT5tX~SJjbwOVGC~Qa4o2kGVhtXxKmuD~OqXj3OjSFU2SGIPee32ZE-EWeg-Gjgn8V5fIYzLA9~HHtucj9yk0NQ89r6Ju3ht4YjpjCONibCVkH64xhBM0~h21bgYZaBK-la3Gsb0o4WK54l1YcRSg3HL8Kgq17j-0vvp99AtYIpJehbxK6j2o8k4kcr3Rs7z2LgSqgvywPiX7Q2SLGJANHsQqRvNg0VrsjA3q88DljeNK-nW4oX28HqFAvRh35ar28Hw7wTc1y94zO44yA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal