Abstract
Lymphocyte adhesion to cells and extracellular matrix (ECM) via integrins plays a pivotal role for the function of the immune system. We show here that endogenous thrombospondin-1 (TSP-1) is a cell-surface ligand for cis interaction of surface receptors in T lymphocytes controlled by integrins and the T-cell antigen receptor (TCR/CD3). Stimulation of CD3 triggers rapid surface expression of TSP-1 in quiescent T cells, whereas activated cells express TSP-1 constitutively. Endogenous TSP-1 is attached to lipoprotein receptor-related protein 1 (LRP1/CD91) and calreticulin (CRT) on the cell surface through its NH2-terminal domain. Adhesion via integrins to ICAM-1 or ECM components up-regulates TSP turnover dramatically from a low level in nonadherent cells, whereas CD3 stimulation inhibits TSP turnover through interference with CD91/CRT-mediated internalization. Integrin-associated protein (IAP/CD47) is essential for TSP turnover and adhesion through interaction with the C-terminal domain of TSP-1 in response to triggering signals delivered at the NH2-terminal. These results indicate that endogenous TSP-1 connects separate cell-surface receptors functionally and regulates T-cell adhesion.
Introduction
Is cell adhesion controlled solely by interactions between cell-surface receptors and their ligands on other cells or within the extracellular matrix (ECM) and by intracellular signal transduction mechanisms, or do interactions between molecules within the same plasma membrane play any role? We have been investigating the possible existence of such cis receptor communication in lymphocytes. Lymphocytes carry out immune surveillance throughout the organism by their unique capacity to periodically exit from the bloodstream and migrate in tissues. To maintain this constant repositioning and migratory behavior, lymphocytes interact extensively with endothelial cells and ECM components, and therefore adhesive interactions are of crucial importance for the function of the immune system.1,2 Our hypothesis was that thrombospondin-1 (TSP-1), owing to its large size and capacity to interact with multiple surface receptors, may act as an endogenous cell-surface ligand within the same plasma membrane and with a possible functional importance for adhesion of T lymphocytes. TSP-1 is a 180-kDa homotrimeric multidomain ECM glycoprotein. It binds several receptors on the cell surface, including heparin sulfate proteoglycans, calreticulin (CRT), CD36, integrin-associated protein (IAP/CD47), lipoprotein receptor-related protein 1 (LRP1/CD91), as well as α3β1, α4β1, and αvβ3 integrins.3-6 CRT is a widely expressed 50-kDa calcium-binding protein7 implicated in a diverse number of functions,8-13 associated with CD91 on the cell surface.4,14 CD91 is a large cell-surface receptor expressed in a variety of tissues and is a member of the low-density lipoprotein (LDL) receptor family that also includes the LDL receptor, the VLDL receptor, megalin, and APOER2.15,16 CD91 mediates the cellular internalization and degradation of TSP-1.17
Integrins are a family of heterodimeric transmembrane cell-adhesion receptors that mediate vital lymphocyte functions including adhesion and motility.18 In lymphocytes, integrin-mediated interactions with ligands on cells and within the ECM induce cell adhesion, spreading, and locomotion and are crucial for extravasation and antigen recognition.1,19-24 However, the mechanisms that regulate integrin-dependent adhesion to cells and ECM have not been definitely established, and the relationship between lymphocyte adhesion and migration is not understood.
The T-cell antigen receptor, the TCR/CD3 complex, plays a pivotal role for the capacity of lymphocytes to respond to antigens and develop immune responses but also for T-cell adhesion to cells and ECM components. Thus, the integrin receptors on unstimulated T cells do not mediate efficient adhesion to counterreceptors on cells or ECM ligands. Stimulation of the TCR/CD3 complex, however, results within minutes in increased integrin-dependent adhesion that does not require increased integrin expression on the T-cell surface, and antigen receptor engagement delivers a stop signal to migrating T cells.25-28 The TCR/CD3 signaling to β integrins seems to require phosphoinositide-3 kinase (PI3-k)–dependent recruitment of tyrosine kinases regulating actin polymerization and enhancement of β1-integrin avidity through changes in integrin conformation.29,30
T-cell adhesion to other cells and ECM components probably requires precise coordination with the capacity of constant migration and with signals delivered through antigen recognition. Therefore, mechanisms coordinating the effects of environmental interactions on vital cell-surface receptors, such as integrins and TCR/CD3, with the function of other cell-surface receptors would be advantageous from a regulatory point of view. Here we show that endogenous T-cell TSP-1 is the hub of such a mechanism operating at the level of the cell-surface membrane. The starting point for the present investigation was our recent finding that T cells produce TSP-1,31 taken together with a new observation described here that TSP-negative or weakly positive T cells fresh from the blood can be induced to express cell-surface TSP within minutes through CD3 stimulation, provided the cells are in contact with an ECM substrate. The findings we report here identify TSP-1 as an endogenous ligand for communication between integrins, CRT/CD91, and CD47 and demonstrate that the turnover of T-cell TSP-1 is regulated by adhesion via integrins and stimulation of TCR/CD3.
Materials and methods
Chemicals and antibodies
Human plasma fibronectin was purified as described.32 Bovine serum albumin (BSA), brefeldin A, and AG490 were purchased from Sigma (St Louis, MO). Poly-L-lysine (molecular mass 5300) was purchased from Miles-Yeda (Rehovoth, Israel). UO126 was from Promega (Madison, WI) and GM6001 was from Chemicon (Temecula, CA). IL-2, IL-4, and the chemokine SDF-1α were from Genzyme Diagnostics (Cambridge, MA). Receptor-associated protein (RAP) was obtained from Oxford Biomedical Research (Oxford, MI) and ICAM-1 was from R&D Systems, Europe (Abingdon, United Kingdom). Anti-CD29 (clone Lia 1/2, IgG1), anti-CD3 (clone SK 7, IgG1), and anti-CD62L (clone dreg56) were obtained from Becton Dickinson (Mountain View, CA). Anti-CD49 (clone HP2/1) was from Immunotech (Marseille, France). Dynabeads were from Dynal AS (Oslo, Norway). Mouse IgG and anti-CD45 (clone T29-33) were from Dako A/G (Glostrup, Denmark). Goat antimouse antibody was from Dacopatts A/S (Glostrup, Denmark). Anti–TSP-1 clone MBC 200.1 (also called TSP–Ab-9, IgG1), clone A6.1 (also called TSP–Ab-4, IgG1), and clone C 6.7 (also called TSP–Ab-3, IgG1) were from NEO-MARKERS (Fremont, CA). Anti-CD91 (clone A2MRα2) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CRT (clone FMC75) was from Biosite (Täby, Sweden). Anti–tissue inhibitor of metalloproteinases-1 (anti–TIMP-1; clone 7-6C1) was from Oncogene (Cambridge, MA). Anti-CD47 (clone Bric 126 and clone C1km1) was from Chemicon, and anti-CD47 (Clone B6H12.2) was from NEO-MARKERS. Antifibronectin (clone IST 1, IgG1) was obtained from Sera Lab (Loughborough, United Kingdom). Biotinylated peroxidase and avidin were from Vector Laboratories (Burlingame, CA). The peptides RWI ESK HKS DFG KFV LSS (the TSP-1 binding site in CRT, called peptide CRT19-36 in this article), ELT GAA RKG SGR RLV KGP D (hep1), and RSK AST LGE RDL KPS AAV G (hep2, control peptide 1) were synthesized by the Biomolecular Resource Facility (University of Lund, Sweden), and RSK AGT LGE RDL KPG ARV G (scrambled hep1 peptide, control peptide 2), KRFYVVMWKK (4N1K), KVFRWKYVMK (scrambled 4N1K), and KRFYGGMWKK (mutated 4N1K, control peptide 3) by Tripep (Novum Research Park, Huddinge, Sweden).
Cells
The cell lines CCRF HSB2, Molt-4, P30, and Peer were purchased from American Type Culture Collection (Rockville, MD). The birch (Bet v 1)–specific T-cell clone AF24 was kindly provided by Dr J. van Neerven (ALK A/S, Horsholm, Denmark). This cell line was regularly stimulated with anti-CD3 or specific antigen as previously described.33 Human peripheral-blood T cells (PBTs) were purified as previously described.31 Blood T cells were stimulated with anti-CD3 or specific antigen and cultured in the presence of IL-2 and IL-4 for 3 to 5 days before the experiments. The birch (Bet v 1)–specific T-cell clone AF24 was stimulated with anti-CD3 or specific antigen and cultured in the presence of IL-2 and IL-4 for 5 to 12 days before the experiments. All cells were cultured in RPMI 1640 (Gibco, Paisley, Scotland) supplemented with 2 mM L-glutamine, 0.16% sodium bicarbonate, 10 000 U/mL benzylpenicillin, 10 000 μg/mL streptomycin, and 10% FCS or in serum-free AIM-V medium (Gibco). Peptides, brefeldin A, RAP, and monoclonal antibodies when applied to study the influence on antigen expression or adhesion were added immediately before the experiments.
Real-time PCR
The methodology of RNA extraction, cDNA synthesis, and real-time quantitative polymerase chain reaction (PCR) has been described previously.34 Primer and probe sequences for the transcripts were as follows: TSP-1 forward (F), 5′-TGC ACT GAG TGT CAC TGT CAG AA-3′; TSP-1 reverse (R), CAT TGG AGC AGG GCA TGA T; TSP-1 probe, FAM-TA CCA TCT GCA AAA AGG TGT CCT GCC C-TAMRA; CRT-F, CAC GGA GAC TCA GAA TAC AAC ATC AT; CRT-R, TCA TCC TTG CAA CGG ATG TC; CRT probe, FAM-CA AGG GCA AGA ACG TGC TGA TCA ACA A-TAMRA; LRP1-F, TGA CGA GGC CCC TGA GAT T; LRP1-R, CAG GCA GTT ATG CTC GTT TGG; LRP1 probe, FAM-CA CAG AGT AAG GCC CAG CGA TGC C-TAMRA. The PCR reaction was performed and analyzed on the ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA).
Quantitative immunocytochemistry
The expression of various antigens by adherent T cells was analyzed using immunocytochemistry after staining with monoclonal antibodies (mAbs). Glass slides were coated with poly-L-lysine (10 μg/mL) or fibronectin (10 μg/mL) at 4°C overnight. T cells were attached to these slides in PBS or AIM-V medium at 37°C for 15 minutes unless otherwise stated and subsequently fixed in 2% paraformaldehyde at 4°C for 20 minutes, after which time the TSP-1 expression was detected with the mAbs and a complex of biotinylated peroxidase and avidin (Vector Laboratories). For detection of intracellular antigens, cells were fixed in 2% paraformaldehyde in EBSS buffer and permeabilized by washing in EBSS buffer containing 0.1% saponin, then the cells were reacted with mAbs in washing buffer containing saponin. The cells were examined in a Nikon Eclipse E1000M microscope (Nikon, Melville, NY), Nikon Plan Apo 40×/0.95 NA DICM lenses, a Nikon DXM1200F digital camera, and a Nikon automatic camera tamer (ACT-1), version 2.63 acquisition software. The intensity of the immunocytochemical staining was quantified using the image processing and analysis program ImageJ (http://rsb.info.nih.gov/ij/) and analyzed by histogram of the distribution of gray values in each image or by measurements for each particle displayed in the images.
Biotinylation and immunoprecipitation
The surface membrane of intact lymphocytes was labeled with D-biotinyle-aminocaproic acid-N-hydroxysuccinimide ester (biotin-7-NHS) as described by the manufacturer (Roche Molecular Biochemical, Indianapolis, IN). Immunoprecipitation was carried out as described previously.31 The proteins were separated on 6% or 4% to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels35 and transferred to the Hybond enhanced chemiluminescence (ECL) membrane (Amersham, Arlington Heights, IL) and detected using the BM chemiluminescence's blotting kit (Roche).
Cell adhesion and migration
To study cell adhesion, plastic Petri dishes (90 mm; Heger A/S, Oslo, Norway) or glass slides were coated with collagen type IV (10 μg/mL), BSA (10 μg/mL), or fibronectin (10 μg/mL) and were extensively washed before use. The cells (10 000/position) in AIM-V medium were incubated on the substrates in a humidified CO2 incubator at 37°C for 15 or 30 minutes. Cells were fixed in 2.5% cold glutaraldehyde (GTA) for 10 minutes, and unbound cells were removed by gentle aspiration. The number of adherent cells per microscopic field (× 20 objective) was counted. For cell-migration studies, 3-dimensional (3D) collagen type 1 was used and prepared as described.31,36 Antibodies, RAP, and peptides were present with the cells in adhesion and migration experiments. Cells (1.0 × 106) in AIM-V medium were added to each well and allowed to migrate for different times. The cells were fixed in 2.5% GTA and washed twice with PBS. Cell morphology and cell migration were routinely, unless otherwise stated, evaluated in 7 fixed positions in each well and at 10-μm intervals throughout the gel, and the cells were identified as described previously.31
Involvement of CD47 in integrin-dependent T-cell adhesion. (A) The influence of 4N1K and a scrambled control peptide, at a concentration of 50 μM, on adhesion of activated blood T cells (90% of the adherent cells were CD3+) to fibronectin (20 μg/mL) and ICAM-1 (2 μg/mL). (B) 4N1K (50 μM) inhibits the enhancement of T-cell adhesion induced by anti-CD3 in T cells fresh from the blood. (C) Monoclonal antibodies to the CD47-binding C-terminal domain of TSP-1 (Ab-3) and to CD47 (B6H12), at a concentration of 2 μg/mL, inhibit spreading/pseudopodia formation in AF24 T cells on fibronectin (10 μg/mL). It is evident that in the presence of Ab-3 a high percentage of the cells were either not spread or showed a peripheral zone of flattened cytoplasm without pseudopodia. One representative experiment of 3 to 4 independent experiments is shown. Error bars: Statistics: Mann-Whitney U test (C).
Involvement of CD47 in integrin-dependent T-cell adhesion. (A) The influence of 4N1K and a scrambled control peptide, at a concentration of 50 μM, on adhesion of activated blood T cells (90% of the adherent cells were CD3+) to fibronectin (20 μg/mL) and ICAM-1 (2 μg/mL). (B) 4N1K (50 μM) inhibits the enhancement of T-cell adhesion induced by anti-CD3 in T cells fresh from the blood. (C) Monoclonal antibodies to the CD47-binding C-terminal domain of TSP-1 (Ab-3) and to CD47 (B6H12), at a concentration of 2 μg/mL, inhibit spreading/pseudopodia formation in AF24 T cells on fibronectin (10 μg/mL). It is evident that in the presence of Ab-3 a high percentage of the cells were either not spread or showed a peripheral zone of flattened cytoplasm without pseudopodia. One representative experiment of 3 to 4 independent experiments is shown. Error bars: Statistics: Mann-Whitney U test (C).
Statistical analysis
The results are shown as mean ± SD unless otherwise specified. The significance of the data was evaluated by Student t test unless otherwise specified and P value less than .05 was judged significant.
Results
T lymphocytes express CRT and TSP-1 on the surface
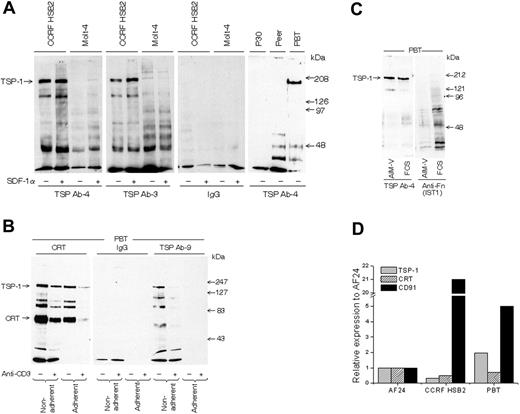
All experiments, with one exception (Figure 1B), were performed with in vitro–activated blood T cells (PBTs) because they show a high turnover of cell-surface TSP-1. To study the validity of findings in blood T cells, a birch-specific T-cell clone (AF24) also characterized by high TSP turnover was used. The lymphocytes were cultured in serum-free AIM-V medium to exclude any interference from exogenous proteins. Figure 2A demonstrates that T-cell lines and activated “normal” blood T cells express TSP-1 on their surface, as demonstrated by the detection of a major 180-kDa band by SDS-PAGE after immunoprecipitation of biotinylated cells with 2 different monoclonal anti–TSP-1 antibodies. The low molecular weight components immunoprecipitated with TSP antibodies probably represent TSP fragments or other TSP-associated components. SDF-1α augmented this TSP-1 band in the T-cell line CCRFHSB2, which confirms and extends our previous findings in blood T cells.31 It can further be seen in Figure 2B that immunoprecipitation of cell-surface CRT coprecipitated cell-surface TSP-1 (confirmed by Western blotting) and that an additional anti–TSP-1 antibody (Ab-9) also detected cell-surface TSP-1, as indicated by a major 180-kDa band. Western immunoblotting of whole-cell lysate confirmed the presence of TSP-1 (Figure 2C). T cells expressed mRNA for TSP-1, CD91, and CRT (Figure 2D). Figure 2B also demonstrates that the anti–TSP-1 antibody Ab-9, as well as the antibodies to CRT and CD91, showed weaker reactivity after adhesion, pointing to the possibility that TSP-1/CRT/CD91 disappeared during adhesion. Cross-linking of anti-CD3 on the lymphocyte surface also decreased the expression of CRT and TSP-1, and cross-linking seemed to enhance the effect of adhesion on the disappearance of CRT and TSP-1. First, it is important that anti-CD3 presented in this way did not increase TSP-1 (Figure 2B) or CD91 (not shown), since anti-CD3 antibodies attached to a surface augmented CD91 expression in AF24 and triggered TSP-1 expression in T cells fresh from the blood (Figure 3A-B). Second, the modulation of TSP-1 by cross-linking points to a possible association with CD3.
T lymphocytes express TSP-1 and CRT on the cell surface. (A) TSP-1 expression in T-cell lines (CCRF HSB2, Molt-4, P30, and Peer) and normal peripheral blood T cells (PBTs; 93% CD3+) in suspension as detected by gel analysis (6% SDS-PAGE) of immunoprecipitated material from biotinylated cells using 2 different monoclonal antibodies to TSP-1. The cell lines were cultured with and without SDF-1α for 30 minutes before biotinylation. (B) SDS-PAGE gels (6%) showing CRT and TSP-1 in normal T cells (96% CD3+) allowed to adhere to a fibronectin-coated (10 μg/mL) surface for 15 minutes before biotinylation and immunoprecipitation. The cells had been incubated with or without anti-CD3 and a cross-linking anti-immunoglobulin for 30 minutes before the adhesion. (C) Detection of TSP-1 and fibronectin after blotting of SDS-PAGE–separated material from T cells in suspension cultured in RPMI with 10% FCS or in AIM-V, respectively. (D) Relative expression of mRNA for CRT, TSP-1, and CD91 in T cells determined by real-time PCR. CCRF HSB2, AF24, and PBTs show significant mRNA expression for all 3 proteins. One representative experiment of 2 to 8 independent experiments is shown.
T lymphocytes express TSP-1 and CRT on the cell surface. (A) TSP-1 expression in T-cell lines (CCRF HSB2, Molt-4, P30, and Peer) and normal peripheral blood T cells (PBTs; 93% CD3+) in suspension as detected by gel analysis (6% SDS-PAGE) of immunoprecipitated material from biotinylated cells using 2 different monoclonal antibodies to TSP-1. The cell lines were cultured with and without SDF-1α for 30 minutes before biotinylation. (B) SDS-PAGE gels (6%) showing CRT and TSP-1 in normal T cells (96% CD3+) allowed to adhere to a fibronectin-coated (10 μg/mL) surface for 15 minutes before biotinylation and immunoprecipitation. The cells had been incubated with or without anti-CD3 and a cross-linking anti-immunoglobulin for 30 minutes before the adhesion. (C) Detection of TSP-1 and fibronectin after blotting of SDS-PAGE–separated material from T cells in suspension cultured in RPMI with 10% FCS or in AIM-V, respectively. (D) Relative expression of mRNA for CRT, TSP-1, and CD91 in T cells determined by real-time PCR. CCRF HSB2, AF24, and PBTs show significant mRNA expression for all 3 proteins. One representative experiment of 2 to 8 independent experiments is shown.
TCR/CD3 determines T-cell expression of CD91 and TSP-1. (A) Anti-CD3 Abs (0.125 μg/mL) coated on fibronectin or poly-L-lysine substrata augment CD91 expression on the surface of AF24 cells as demonstrated by quantitative immunocytochemistry and immunoprecipitation of biotinylated cells. (B) Anti-CD3 antibodies (0.125μg/mL) postcoated on a fibronectin-coated surface trigger cell-surface TSP-1 in freshly purified blood lymphocytes. (C) Anti-CD3 antibodies (0.125 μg/mL) postcoated on a fibronectin-coated surface inhibit TSP turnover as shown by abrogation of the inhibitory effect of brefeldin A (10 μg/mL) on TSP expression. For immunocytochemistry, the cells were fixed in PFA after 15 minutes and the expression of TSP-1 and CD91 was determined by quantitative immunocytochemistry. For immunoprecipitation, the adherent cells were biotinylated and subsequently immunoprecipitated and separated on a 4% to 20% SDS-PAGE gel. One representative experiment of 3 to 7 independent experiments is shown. Error bars: SEM. Statistics: Mann-Whitney U test (B-C). Bars: 20 μm.
TCR/CD3 determines T-cell expression of CD91 and TSP-1. (A) Anti-CD3 Abs (0.125 μg/mL) coated on fibronectin or poly-L-lysine substrata augment CD91 expression on the surface of AF24 cells as demonstrated by quantitative immunocytochemistry and immunoprecipitation of biotinylated cells. (B) Anti-CD3 antibodies (0.125μg/mL) postcoated on a fibronectin-coated surface trigger cell-surface TSP-1 in freshly purified blood lymphocytes. (C) Anti-CD3 antibodies (0.125 μg/mL) postcoated on a fibronectin-coated surface inhibit TSP turnover as shown by abrogation of the inhibitory effect of brefeldin A (10 μg/mL) on TSP expression. For immunocytochemistry, the cells were fixed in PFA after 15 minutes and the expression of TSP-1 and CD91 was determined by quantitative immunocytochemistry. For immunoprecipitation, the adherent cells were biotinylated and subsequently immunoprecipitated and separated on a 4% to 20% SDS-PAGE gel. One representative experiment of 3 to 7 independent experiments is shown. Error bars: SEM. Statistics: Mann-Whitney U test (B-C). Bars: 20 μm.
In conclusion, T cells at early stages of development as well as mature T cells, represented by TALL cell lines and blood T cells from healthy individuals, respectively, express cell-surface TSP-1, and TSP-1 is associated with CRT on the cell surface. However, the magnitude of the expression and the behavior of this TSP seem to differ markedly between different cell types. For example, the TALL line Peer has intracellular TSP-1 but relatively little TSP-1 on the surface, and TALL lines generally exhibit negligible turnover of cell-surface TSP-1 in comparison with mature T cells. These differences in TSP expression related to developmental stage merit further investigation.
RAP and CRT displace cell-surface TSP-1
To examine the behavior and fate of TSP-1 during β1-integrin–mediated adhesion, quantitative immunocytochemistry of cells fixed in situ was applied to prevent antibody-induced effects on antigen expression and turnover during the detection procedure. The immunocytochemistry experiment in Figure 4 confirmed that TSP-1 is expressed on the surface of T cells. This detection of TSP-1 was cell surface specific, since it did not reveal TIMP-1 although TIMP-1 was present intracellularly (Figure 4A).
TSP-1 and CRT are associated with CD91 on the cell surface in nonlymphoid cells.4,14 To investigate whether CRT and TSP-1 are associated with CD91 also in T cells, we took advantage of the fact that RAP is an inhibitor of ligand binding of many LDL receptor family members including CD91.37 RAP decreased cell-surface TSP-1 significantly and rendered the T cells strongly surface positive for CD91 (Figure 4B). Exogenous CRT also displaced TSP-1 and exposed CD91 (Figure 4C). The displacement of TSP-1 by RAP and CRT probably means that TSP-1/CRT complexes are associated with CD91 on the T-cell surface. Interestingly, RAP was recently shown to reduce cell-surface CRT in macrophages, supporting a competitive effect of RAP and CRT on CD91 binding sites.38
Integrin ligation up-regulates the turnover of TSP-1 via CD47
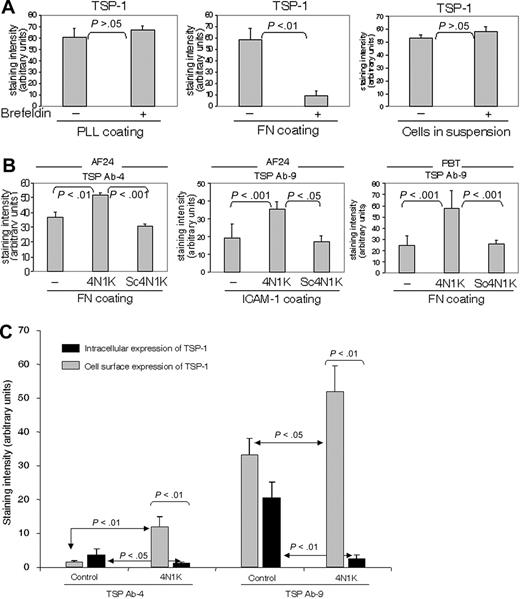
The turnover of cell-surface TSP-1, as reflected by the effect on TSP-1 expression of brefeldin A, an inhibitor of intracellular transport and secretion, was markedly higher when the T cells were on fibronectin and collagen type IV (not shown) than on poly-l-lysine and in suspension (Figure 5A). A high TSP turnover was also noted in cells on ICAM-1 (not shown; however, see Figure 5B concerning the effect of ICAM-1 on TSP expression).
To investigate possible mechanisms of the substrate-dependent turnover of TSP-1, we examined whether 4N1K, the CD47-binding sequence in TSP-1,39 influenced TSP expression. The 4N1K was found to augment the cell-surface expression of TSP-1 on fibronectin and ICAM-1 significantly, whereas a scrambled control peptide did not affect TSP expression (Figure 5B). This strongly indicates that interaction between TSP-1 and CD47 determines the turnover of TSP-1. In an experiment performed to investigate the influence of 4N1K on the fate of TSP-1, this peptide was found to increase the cell-surface expression of TSP-1 and simultaneously reduce the amount of intracellular TSP-1, indicating that CD47 contributes to TSP internalization (Figure 5C). These effects of a peptide inhibiting the interaction between TSP-1 and CD47 indicate a critical role for CD47 in the high turnover of endogenous TSP-1 on fibronectin and ICAM-1.
TCR/CD3, CD91, and TSP-1
Insoluble anti-CD3 antibodies caused a rapid increase of cell-surface CD91 expression independent of the substratum on which the cells were adhered (Figure 3A). SDS-PAGE of immunoprecipitated biotinylated material suggested that this CD91 was associated with multiple other molecules or was degraded, which is interesting in light of the finding that the extracellular α-chain of CD91 is highly susceptible to proteolysis by membrane-type matrix metalloproteinases.40 In activated T cells, this effect of anti-CD3 antibodies was specific for CD91, since there was no comparable augmentation of the expression of TSP-1. However, in T cells fresh from the blood, which generally have relatively little or no TSP-1 on their surface, insoluble anti-CD3 rendered the cells surface positive for TSP-1 within minutes if they were simultaneously allowed to bind to fibronectin (Figure 3B) or ICAM-1 (results not shown). This appearance of TSP-1 was abrogated by brefeldin A, indicating that stimulation of CD3 triggered an intracellular source of TSP-1. Antibodies to CD4 and CD8 did not influence the expression of CD91 or TSP-1, indicating that the effect of anti-CD3 was specific (results not shown). Figure 3C shows that the turnover of cell-surface TSP-1 was reduced substantially after stimulation of CD3 with antibodies. The results in Figure 3 thus demonstrate that CD3 has major regulatory effects on CD91 expression, on TSP turnover, and on TSP expression in quiescent cells. A reasonable conclusion is that the reduced turnover of TSP-1 induced by CD3 stimulation reflects a primary effect on CD91, leading to inhibition of TSP-1 internalization.
Behavior and fate of cell-surface TSP-1 in T lymphocytes. (A) Detection of TSP-1 (Ab4) and TIMP-1 in nonpermeabilized and per-meabilized AF24 cells after 15 minutes on fibronectin (FN) and poly-L-lysine (PLL). It is evident that the control molecule TIMP-1 is not expressed on the cell surface. Arrowheads point to cells. (B-C) RAP (B) and CRT (C) displace TSP-1 and expose CD91. AF24 cells were incubated with RAP (50 μg/mL) or CRT (1 μg/mL) during adhesion to fibronectin, and the cell-surface expression of TSP-1 was determined after 15 minutes. One representative experiment of 3 to 8 independent experiments is shown. Error bars indicate SEM. Statistics: Mann-Whitney U test. Bars: 10 μm.
Behavior and fate of cell-surface TSP-1 in T lymphocytes. (A) Detection of TSP-1 (Ab4) and TIMP-1 in nonpermeabilized and per-meabilized AF24 cells after 15 minutes on fibronectin (FN) and poly-L-lysine (PLL). It is evident that the control molecule TIMP-1 is not expressed on the cell surface. Arrowheads point to cells. (B-C) RAP (B) and CRT (C) displace TSP-1 and expose CD91. AF24 cells were incubated with RAP (50 μg/mL) or CRT (1 μg/mL) during adhesion to fibronectin, and the cell-surface expression of TSP-1 was determined after 15 minutes. One representative experiment of 3 to 8 independent experiments is shown. Error bars indicate SEM. Statistics: Mann-Whitney U test. Bars: 10 μm.
Involvement of CD47 in T-cell adhesion
The CD47 binding site in TSP-1 (4N1K) inhibited and in some experiments virtually abrogated T-cell adhesion on ICAM-1 and fibronectin, whereas a scrambled control peptide did not affect adhesion (Figure 1A). Furthermore, 4N1K inhibited T-cell adhesion induced by anti-CD3 in T cells fresh from the blood (Figure 1B). Both AF24 and activated blood T cells attached to ICAM-1 using αLβ2 and to fibronectin using α4β1 and α5β1, whereas AF24 attached to collagen type IV by α1β1 and α2β1 (not shown). A monoclonal antibody (Ab-3) to the C-terminal CD47-binding domain of TSP-1 decreased the number of spread cells, and many cells had a spherical morphology. In addition, however, a portion of the cells developed a characteristic nonpolarized flattened morphology without pseudopodia (Figure 1C). Another anti–TSP-1 antibody (Ab-4) also decreased the number of adherent cells. A monoclonal antibody to CD47 (B6H12.2) also decreased spreading, whereas antibodies to the collagen-binding domain of TSP-1, TIMP-1 (Figure 1C), and fibronectin (not shown) did not affect the spreading process. The inhibitory effect of anti–TSP-1 antibodies on adhesion, spreading, and pseudopodia formation together with the fact that 4N1K inhibits both adhesion and spreading indicate that adhesion per se as well as pseudopodia formation and polarization of the T cell is dependent on interaction of endogenous TSP-1 with CD47.
Peptide binding to the NH2-terminal domain of TSP-1 enhances adhesion through CD47
The CRT binding site in TSP-1 has been localized to the NH2-terminal domain of TSP-1 and characterized.41 While the whole CRT molecule displaced TSP-1 from the cell surface effectively, the TSP-1 binding site in CRT, which resides within residues 19-36 (hence termed CRT19-36),41 rather increased TSP-1 expression initially (0-15 minutes). CRT19-36 markedly enhanced integrin-dependent adhesion and cytoplasmic spreading of T cells on fibronectin and collagen IV, whereas control peptides did not augment adhesion (Figure 6A). Noteworthy, 4N1K prevented the increased adhesion induced by CRT19-36 (Figure 6B). A monoclonal anti-CD29 antibody inhibited T-cell adhesion induced by CRT19-36, showing that the augmented adhesion was β1-integrin dependent (Figure 6C). The stimulation of T-cell adhesion by CRT19-36 and the inhibition of this effect by 4N1K strongly support the conclusion that a triggering signal at the NH2-terminal domain of endogenous TSP-1 induces T-cell adhesion through its C-terminal via CD47. In addition, this result suggests that integrin binding to separate recognition sites within the TSP-1 molecule3 may also trigger an adhesion signal through CD47 (Figure 1).
Integrin ligation up-regulates the turnover of TSP-1 via CD47. (A) Comparison of the turnover of TSP-1 (Ab4) in PBTs on different substrata and in suspension (91% of the adherent cells on PLL and fibronectin were CD3+). The cells were incubated for 30 minutes in the absence or presence of brefeldin A (10 μg/mL). (B) 4N1K, a peptide corresponding to the C-terminal CD47-binding domain of TSP-1, at a concentration of 50 μM inhibits the turnover of cell-surface TSP-1 in T cells on fibronectin (10 μg/mL) and ICAM-1 (2 μg/mL), whereas a scrambled control peptide, Sc4N1K (50 μM), does not affect TSP turnover. Lymphocytes were allowed to adhere to fibronectin for 30 minutes in the presence or absence of peptides before fixation (94% of the PBTs were CD3+). (C) 4N1K augments cell-surface TSP-1 and reduces intracellular TSP-1, indicating that binding to CD47 induces internalization of cell-surface TSP-1. AF24 cells were allowed to adhere to glass slides coated with fibronectin in the absence or presence of 4N1K or control peptide for 15 minutes before fixation. TSP-1 expression was measured using quantitative immunocytochemistry. One representative experiment of 3 to 4 independent experiments is shown. Error bars: Statistics: Mann-Whitney U test (C).
Integrin ligation up-regulates the turnover of TSP-1 via CD47. (A) Comparison of the turnover of TSP-1 (Ab4) in PBTs on different substrata and in suspension (91% of the adherent cells on PLL and fibronectin were CD3+). The cells were incubated for 30 minutes in the absence or presence of brefeldin A (10 μg/mL). (B) 4N1K, a peptide corresponding to the C-terminal CD47-binding domain of TSP-1, at a concentration of 50 μM inhibits the turnover of cell-surface TSP-1 in T cells on fibronectin (10 μg/mL) and ICAM-1 (2 μg/mL), whereas a scrambled control peptide, Sc4N1K (50 μM), does not affect TSP turnover. Lymphocytes were allowed to adhere to fibronectin for 30 minutes in the presence or absence of peptides before fixation (94% of the PBTs were CD3+). (C) 4N1K augments cell-surface TSP-1 and reduces intracellular TSP-1, indicating that binding to CD47 induces internalization of cell-surface TSP-1. AF24 cells were allowed to adhere to glass slides coated with fibronectin in the absence or presence of 4N1K or control peptide for 15 minutes before fixation. TSP-1 expression was measured using quantitative immunocytochemistry. One representative experiment of 3 to 4 independent experiments is shown. Error bars: Statistics: Mann-Whitney U test (C).
Discussion
These analyses unveil a mechanism for cis interaction of receptors within the same plasma membrane and identify endogenous TSP-1 as a key molecule in this mechanism. Anchorage to substrata via β1 and β2 integrins up-regulates the turnover of cell-surface TSP-1, whereas ligation of the TCR/CD3 complex inhibits TSP turnover through inhibition of CD91-dependent internalization. Stimulation of CD3 induces rapid cell-surface expression of TSP-1 in T cells fresh from the blood. The present results further show that cell adhesion via β1 integrins is dependent on endogenous TSP-1 and CD47. That CD47 is a receptor for regulation of adhesion and spreading is consistent with earlier findings by other investigators using exogenous stimulators of CD47.39,42,43 However, our results indicate that cell adhesion is regulated by CD47 and an endogenous stimulating ligand within the same plasma membrane. This type of autocrine mechanism may have immense functional significance by enabling receptor communication and control of function at the level where multiple environmental interactions and recognition phenomena take place.
TSP turnover seems to be coupled to the functional activity of the cell as shown by the fact that T cells in suspension have a relatively low TSP turnover, whereas cells in contact with ECM components and ICAM-1 up-regulate TSP turnover. The high turnover of T-cell TSP-1 is consistent with an important functional role during adhesive interactions. The fact that T lymphocytes can rapidly up-regulate the turnover of cell-surface TSP-1 via integrins may play a critical role for their capacity to undergo transition from a passive free-floating state in the blood to an active tissue state characterized by frequent adhesive interactions and migration. Noteworthy, CD3 stimulation down-regulates TSP turnover and in this way increases the duration of contacts between TSP-1 and its cell-surface receptors. Given that T-cell TSP-1 is preferentially involved in adhesion and motility, TCR/CD3-induced inhibition of TSP turnover may provide a means for the cell to give preference to antigen-dependent proliferative responses competing with integrin-dependent motility. We favor an alternative concept according to which integrins and TCR/CD3 are synergetic regulators of T-cell functions through TSP-1. Accordingly, integrin-mediated up-regulation of TSP production and CD3-stimulated focusing of TSP molecules onto cell-surface receptors may collaborate and amplify specific functional effects of TSP-1, as suggested by the marked CD3-induced potentiation of TSP-1 expression in T cells fresh from the blood. The expression of T-cell TSP-1 is further influenced by SDF-1α, suggesting that the ligand function of TSP-1 also encompasses chemokine effects.
TSP turnover is critically dependent on interaction with CD47. CD47, also known as integrin-associated protein, is a widely expressed multispan transmembrane protein, which is physically and functionally associated with αvβ3, αIIbβ3, α2β1, and α4β1.39,42 CD47 is an integral membrane protein that is required for granulocyte and T-cell recruitment to sites of infection.44,45 CD47 may also function as a costimulator to regulate T-cell survival, activation, and differentiation of T-helper 1 (Th1) and Th2 effector cells.46,47 Our data reveal that integrin ligation and CD47 determine TSP turnover via endogenous TSP-1. A signal through integrin-binding sites in TSP-1 is thus transduced to CD47 via the C-terminal domain of the TSP molecule. Furthermore, we were able to demonstrate that a peptide interaction with the NH2-terminal domain of TSP-1 exerts a regulatory effect on T-cell adhesion through the C-terminal domain of TSP-1 and CD47.
Triggering of CD47-dependent T-cell adhesion through the N-terminal domain of TSP-1. (A) Stimulation of adhesion and spreading of AF24 T cells on collagen type IV and fibronectin by CRT19-36, at a concentration of 50 μM. Flattened cells (phase dark) with pseudopodia dominate among cells treated with CRT19-36, whereas spherical cells (phase bright) dominate in controls (hep2). The top right panels show enhancement of TSP polarization in cells with CRT19-36. (B) 4N1K inhibits T-cell adhesion induced by CRT19-36; both peptides used at a concentration of 50 μM. (C) An anti–β1-integrin antibody (CD29, 4 μg/mL) inhibits adhesion and spreading of AF24 T cells on fibronectin (10 μg/mL) induced by CRT19-36 as determined after 15 minutes. A control peptide, hep1, did not stimulate adhesion. One representative of 3 to 9 independent experiments is shown. Error bars indicate SEM.
Triggering of CD47-dependent T-cell adhesion through the N-terminal domain of TSP-1. (A) Stimulation of adhesion and spreading of AF24 T cells on collagen type IV and fibronectin by CRT19-36, at a concentration of 50 μM. Flattened cells (phase dark) with pseudopodia dominate among cells treated with CRT19-36, whereas spherical cells (phase bright) dominate in controls (hep2). The top right panels show enhancement of TSP polarization in cells with CRT19-36. (B) 4N1K inhibits T-cell adhesion induced by CRT19-36; both peptides used at a concentration of 50 μM. (C) An anti–β1-integrin antibody (CD29, 4 μg/mL) inhibits adhesion and spreading of AF24 T cells on fibronectin (10 μg/mL) induced by CRT19-36 as determined after 15 minutes. A control peptide, hep1, did not stimulate adhesion. One representative of 3 to 9 independent experiments is shown. Error bars indicate SEM.
CD91 has been identified as an internalization receptor for exogenous TSP-148 and the present results indicate that CD91 also mediates internalization of endogenous TSP-1. The finding that interference with the ligand-binding properties of CD91 using RAP and CRT displaced TSP-1 is consistent with recent findings in another cell system38 and supports an association of TSP-1 to CRT/CD91. It is likely that TSP-1 in nonadherent cells is attached to CRT/CD91 and to β1 and β2 integrins and interacts with CD47 as a consequence of integrin ligation. In the mature immune system, lymphocytes are programmed for continuous migration, and therefore studies of lymphocyte adhesion with special reference to the function of circulating mature lymphocytes require test cells with adequate migration capacity. Noteworthy, there is a correlation between high turnover of TSP-1 and capacity to migrate into 3D substrata. Activated blood T cells as well as the T-cell clone AF24 have a pronounced capacity to migrate into 3D collagen and Matrigel, penetrating 500 to 600 μm in a few hours, whereas TALL cell lines migrate 20 μm or less during the same period.31,49
Cell-surface receptors such as integrins and TCR/CD3 are independent elements within the plasma membrane thought to communicate with other receptors through intracellular signal transduction.29,30 Our results suggest an additional direct communication between cell-surface receptors via an endogenous ligand. Each chain of the trimolecular TSP-1 may bind to CD47, integrins, and CRT/CD91, although the cross-linking capacity is probably restricted by the flexibility of the TSP molecule. TSP-1 may cross-link and assemble these components and in this way facilitate functional interactions between vital cell-surface receptors. Therefore, endogenous TSP-1 may enable the T cell to integrate adhesive and motile stimuli from endothelial cells and ECM components with antigen recognition.
How can the lymphocyte up-regulate the turnover of cell-surface TSP-1 and promote adhesion? The augmented turnover probably reflects an increased integrin-dependent transport of intracellular TSP-1 to the cell surface accompanied by internalization. TSP-1 probably supports adhesion by cross-linking CRT/CD91 and integrin molecules to CD47, implying that complexes of CRT/CD91, integrins, TSP-1, and CD47 may play a critical role, perhaps as sites for coordination of signaling between cell-surface receptors and cytoskeletal components. Interestingly, CRT has been shown to be important for cell adhesion and also CD91 has been suggested to participate in adhesion.8,12,50 It also seems reasonable to assume that “controlled” lymphocyte migration is adhesion dependent. Thus, a rather plausible relationship between adhesion and migration, based on the fact that the turnover of TSP-1 is controlled by adhesion, is that the high TSP turnover is directed by the requirements of a rapidly migrating cell. In T cells without capacity to up-regulate TSP turnover in response to adhesive contacts with ICAM-1 or ECM components, TSP-1 may be a limiting factor for adhesion as well as for locomotion. This may have important implications for lymphocyte recirculation and homing.
Model of T-cell TSP-1 and its interactions with cell-surface receptors. TSP-1 is associated to CRT/CD91 and/or integrin at the NH2-terminal domain before adhesion, as indicated by small circles. Adhesive interactions with ECM components or ICAM-1 via integrins trigger interaction of the C-terminal domain of TSP-1 with CD47 (small circle) and up-regulate TSP turnover. Ligation of TCR/CD3 inhibits internalization of TSP-1, CRT, and CD91 and focuses TSP-1 onto its cell-surface receptors.
Model of T-cell TSP-1 and its interactions with cell-surface receptors. TSP-1 is associated to CRT/CD91 and/or integrin at the NH2-terminal domain before adhesion, as indicated by small circles. Adhesive interactions with ECM components or ICAM-1 via integrins trigger interaction of the C-terminal domain of TSP-1 with CD47 (small circle) and up-regulate TSP turnover. Ligation of TCR/CD3 inhibits internalization of TSP-1, CRT, and CD91 and focuses TSP-1 onto its cell-surface receptors.
Figure 7 presents a model of interactions of T-cell TSP-1 with other cell-surface components. We postulate that TSP-1 is important for the capacity of T cells to migrate between separate compartments of the organism through regulation of integrin-dependent adhesion. This is consistent with the findings that the turnover of TSP-1 is up-regulated by the integrin ligands fibronectin and ICAM-1, which are present on endothelial cells and within the ECM, and that TSP turnover depends on interactions with CD47. In nonadherent cells, TSP-1 is attached to CD91/CRT and integrins. Integrin ligation is proposed to catalyze binding of TSP-1 to CD47 and the formation of a molecular lattice comprising CRT/CD91, integrin, and CD47. It is thus likely that TSP-1 on T cells in suspension has a limited association to its putative receptors, for example only to CRT/CD91 and integrin, and that integrin ligation brings TSP-1 into contact with CD47. These collaborative interactions of TSP-1, through its C- and NH2-terminal domains, with CD47, CRT/CD91, and integrins are postulated to support adhesion. The CD3/TCR complex, when ligated, inhibits the turnover of T-cell TSP-1 through up-regulation of the cell-surface expression of CD91 or down-regulation of its capacity to internalize. Quiescent T cells fresh from the blood have relatively little cell-surface TSP-1, and stimulation of TCR/CD3 triggers rapid expression of TSP-1 through inhibition of turnover, thus enhancing their adhesion to integrin ligands through TSP-1–CD47 interaction.
Lymphocyte migration and adhesion to cells and ECM components via integrins are functional properties responsible for lymphocyte infiltration of different organs and organ damage during the course of immunologic diseases of autoimmune and allergic origin as well as during rejection of transplants. The interactions between CRT/CD91, TSP-1, and CD47 and the control of these interactions by integrins and TCR/CD3 provide potential targets for interference with lymphocyte infiltration and prevention of tissue damage in various inflammatory conditions caused by the immune system. Thus, it may be possible to tailor treatment to combat T-cell infiltration through T-cell TSP-1 in several ways. The identification of a putative mechanism for integrated control of interactions between surface receptors involved in adhesion, migration, growth, and survival is also of great interest with respect to the understanding of the function of the immune system.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-04-016832.
Supported by Swedish Cancer Foundation project 1940.
S.S.L. and Z.L. performed research and analyzed data, M.U. performed research, and K.-G.S. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal