Abstract
We used a mouse model to test the hypothesis that the time course and histology of wound healing is altered in hemophilia B. Punch biopsies (3 mm) were placed in the skin of normal mice and mice with hemophilia. The size of the wounds was measured daily until the epidermal defect closed. All wounds closed in mice with hemophilia by 12 days, compared with 10 days in normal animals. Skin from the area of the wound was harvested at different time points and examined histologically. Hemophilic animals developed subcutaneous hematomas; normal animals did not. Macrophage infiltration was significantly delayed in hemophilia B. Unexpectedly, hemophilic mice developed twice as many blood vessels in the healing wounds as controls, and the increased vascularity persisted for at least 2 weeks. The deposition and persistence of ferric iron was also greater in hemophilic mice. We hypothesize that iron plays a role in promoting excess angiogenesis after wounding as it had been proposed to do in hemophilic arthropathy. We have demonstrated that impaired coagulation leads to delayed wound healing with abnormal histology. Our findings have significant implications for treatment of patients with hemophilia, and also highlight the importance of rapidly establishing hemostasis following trauma or surgery.
Introduction
Patients lacking factor VIII (FVIII) (hemophilia A) or FIX (hemophilia B) develop severe, spontaneous bleeding that often fails to respond to local pressure and has a propensity to recur hours later, even if initial hemostasis is achieved. Thrombin formation is markedly impaired1 in hemophilia, so a stable hemostatic plug is not formed. The hemostatic plugs in hemophilic dogs and humans are characterized by defective stabilization of the initial platelet-rich plug in a fibrin meshwork.2,3 The hemostatic plugs have only a thin peripheral rim of fibrin and platelet remnants around a central pool of blood. It has been suggested that patients with hemophilia also have impaired wound healing, although we could find no published work documenting such a defect.
Normal wound healing has been divided into 4 overlapping phases: (1) hemostasis, (2) inflammation, (3) proliferation, and (4) remodeling or resolution. The hemostatic phase commences as soon as vessels are breached during wounding. Platelets adhere to the extracellular matrix and provide primary hemostasis as well as releasing growth factors such as platelet-derived growth factor and transforming growth factor-β. Activated platelets provide the surface on which procoagulant enzymes are activated, leading to the production of thrombin, which acts on fibrinogen to produce a stable fibrin clot. The hemostatic process is important not only because it stops blood loss, but also because it delivers biologically active molecules that influence the subsequent healing and inflammatory responses. Thrombin has potent cytokine- and growth factor–like activities that influence many facets of the healing process.4-9 Thrombin receptor agonists have been shown to speed wound healing in animal models.10-13 Fibrin also plays a critical role in wound healing. It forms the framework on which tissue repair takes place, and fibrin degradation products promote influx of neutrophils14-16 and monocytes/macrophages.17,18 Thus, an adequate hemostatic phase sets the stage for the subsequent phases of wound healing.
The inflammatory phase is characterized by the influx of neutrophils that begins within minutes after injury and peaks within 1 to 2 days. While neutrophils play an important first line of defense against bacterial invasion, a lack of neutrophils does not result in a defect in wound closure, cellularity, or connective tissue formation.19 The neutrophil influx is followed by an influx of monocytes that starts in the first day after injury. After the monocytes move from the blood into the tissues they rapidly mature into macrophages. The macrophages ingest and degrade debris, and also coordinate repair and immune responses through production of an array of cytokines. Macrophages are not essential to closure of the epidermal defect because mice that lack macrophages have normal wound closure times.20 However, fibrogenesis and angiogenesis are strongly influenced by macrophage-derived factors. Macrophages secrete such factors when they are activated by conditions and cytokines within the wound environment. Lymphocytes also infiltrate into the wound site late during the inflammatory phase. Lymphocytes clearly play a role in the immune responses to invading microorganisms, but their role in wound healing is not clear. 21
In skin wounds, the proliferative phase is characterized by epidermal regeneration, angiogenesis, and fibroblast proliferation as the fibrin clot and debris filling the wound site are replaced by granulation tissue. The term “granulation tissue” comes from the fact that the surface of the reparative tissue has a granular appearance. It is very delicate and bleeds easily because it contains large numbers of new vessels but little stabilizing connective tissue. During development of granulation tissue, capillary sprouts invade the wound clot from adjacent vessels and organize into a microvascular network. At the same time, fibroblasts proliferate within the granulation tissue and begin to lay down collagen and other connective tissue components to reinforce the tensile strength of the repaired tissue. Not all of the initial capillary sprouts ultimately develop into functional vessels. As the granulation tissue matures, the overall cellularity of the tissue and the density of blood vessels decreases. A bewildering array of signals and cytokines are potentially involved in orchestrating the process of endothelial sprouting, proliferation, organization into functional vessels, and regression of excess sprouts.
The remodeling or resolution phase can continue for weeks or months as cellular proliferation stops, the inflammatory infiltrate resolves, many of the vessels regress, and the structure of the wound site collagen reorganizes. The bulk of the tissue shrinks and the bulging contour of the wound site returns to near normal or even retracts as the cells are replaced by acellular connective tissue (scar).
Thrombin has a number of activities in vitro that could have a significant impact on different facets of wound healing in vivo. Thus, impaired thrombin generation, as in hemophilia, might lead to a defect in the overall healing rate, or defects in the specific phases of the healing process.
Thrombin is chemotactic and mitogenic for monocytes and macrophages.22-24 In addition, fibrin and its degradation products promote neutrophil and macrophage adhesion and migration.18,25 Based on these findings we hypothesized that macrophage influx and activity would be impaired in hemophilia B (HB).
Thrombin has also been linked to angiogenesis. Thrombin contributes to angiogenesis both by promoting formation of a stable fibrin clot to serve as a matrix for ingrowth of vascular sprouts and by direct receptor-mediated effects on endothelial cells.26-29 Thus, we further hypothesized that angiogenesis will be impaired during wound healing in HB.
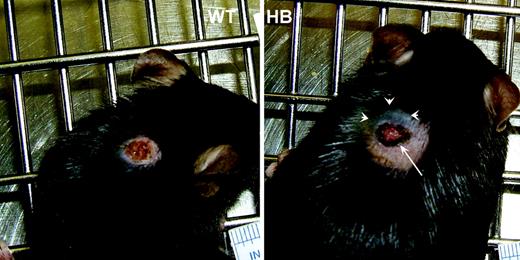
Gross appearance of 3-day-old punch biopsy wounds. Punch biopsy wounds were placed on the backs of wild-type (WT) and hemophilia B (HB) mice as described in “Materials and methods.” These representative photos show that both achieved hemostasis, but the wounds on HB mice tended to be larger and with dark coloration (arrow) due to red blood cells in the clot. The HB mice also developed subcutaneous hemorrhage with discoloration of the overlying skin (arrowheads).
Gross appearance of 3-day-old punch biopsy wounds. Punch biopsy wounds were placed on the backs of wild-type (WT) and hemophilia B (HB) mice as described in “Materials and methods.” These representative photos show that both achieved hemostasis, but the wounds on HB mice tended to be larger and with dark coloration (arrow) due to red blood cells in the clot. The HB mice also developed subcutaneous hemorrhage with discoloration of the overlying skin (arrowheads).
In this work, we tested the general hypothesis that the time course of wound healing is impaired, and also the specific hypotheses that macrophage influx and angiogenesis in the healing wound are impaired in hemophilia B.
Materials and methods
Animal model
Adult wild-type (WT) mice (C57BL/6) and coagulation FIX knock-out (HB) mice were used for these studies.30 The wound-healing studies were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. A single 3-mm biopsy punch wound was placed on the back of each WT and HB mouse as illustrated in Figure 1. Wound size (in 2 dimensions) was recorded daily. Mice were killed and wounded skin collected for histologic evaluation at a range of healing times. We were prepared to treat HB mice that experienced excessive bleeding by administration of a single dose of human coagulation factor IX.31 However, this “rescue” therapy was not needed once we had gained proficiency with the skin biopsy technique.
Microscopic evaluation
Skin was pinned flat in 10% buffered formalin for 6 to 12 hours before wound sites were cut out and processed into paraffin. Specimens were bisected through the center of each wound and embedded with the wound center up. Serial 5-μm sections were cut, placed on Snowcoat X-tra poly-lysine–coated slides (Surgipath, Richmond, IL), and stained with hematoxylin and eosin. Additional sections were stained with Prussian Blue stain for tissue iron and immunohistochemistry. All scoring of special stains was done by a board-certified pathologist (M.H.) who was blinded to the identity of the sections.
Immunohistochemical staining
Slides were placed in a 60°C oven overnight and allowed to cool before dewaxing. Paraffin was removed with three 3-minute immersions in xylene followed by rehydration through graded alcohols. Antigen retrieval was performed by heating to 95°C in DakoCytomation Target Retrieval Solution (Carpinteria, CA) for 10 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 20 minutes. Nonspecific binding was blocked with mouse-on-mouse (MOM) Ig-blocking reagent (Vector Labs, Burlingame, CA), and primary antibodies were diluted in MOM diluent.
Macrophages (F4/80)
Macrophages were identified in tissue sections with rat antibody to mouse F4/80 antigen (Serotec, Raleigh, NC) at a 1:250 dilution, followed by biotinylated anti–rat IgG (Vector Labs) at a 1:250 dilution. Antibody binding was detected with a Dako LSAB2 kit (DakoCytomation) with diaminobenzidine substrate. The slides were counterstained with Dako hematoxylin, dehydrated, and coverslipped with Vectamount permanent mounting medium (Vector Labs, Burlingame, CA).
Macrophage infiltration into the most heavily infiltrated portion of the tissues was graded according to the following scheme: 0, no macrophages detected; 1+, scattered macrophages; 2+, lines of macrophages along tissue planes (ie, macrophages touching on 2 sides); 3+, clusters of macrophages (ie, occasional macrophages touching on 3 or 4 sides); and 4+, sheets of macrophages.
Endothelial cells (CD34)
Endothelial cells were identified by staining for CD34 with a rat monoclonal primary (Serotec) as described in “Macrophages (F4/80)” for F4/80 staining. Vessel profiles were defined as rounded or elongated spaces bounded by CD34-staining endothelial cells. Serial counts of vessel per high-powered field were made from side to side across the wounded area, and averaged for 2 sections per wound.
Fibrin
Skin tissue from WT and HB mice 2 days after wounding was stained for the presence of fibrin using the mouse fibrin-specific antibody 59D8, which was a kind gift from Dr Marschall Runge, University of North Carolina. This antibody has previously been used to detect mouse fibrin.32
Image acquisition and manipulation
Tissue sections were viewed through an Olympus BH-2 microscope (Olympus America, Center Valley, PA) with 2×, 4×, 20×, and 40× Plan Achromat objectives. Digital photographs were taken using a Nikon Coolpix 4500 camera (Melville, NY) using its onboard software. The contrast and color balance of the resulting images were adjusted and images were cropped using Adobe Photoshop software (San Jose, CA).
Results
Initially the biopsy sites looked identical on WT and HB mice. However, over the next 18 hours differences became apparent. The day after wounding there was evidence of external bleeding on many of the HB mice, with variable amounts of blood around the wound and on the cage bedding. Examples of 3-day-old wounds are shown in Figure 1. The dark discoloration on the cranial side of the HB wound (arrowheads) is due to subcutaneous hemorrhage, a finding that was nearly universal in the HB mice.
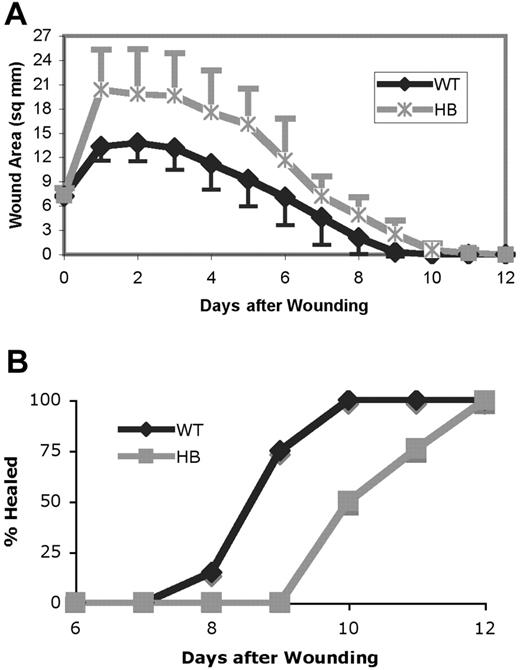
The wound surface areas were calculated from daily measurements and are shown in the top panel of Figure 2. Wounds increased in size in the first day after wounding. However, the wounds on HB mice enlarged to a greater extent than those on WT. As shown in the bottom panel of Figure 2, the wounds on HB mice required a greater overall time for healing than those on the WT mice. The wounds were considered healed when no macroscopically visible epithelial defect or adherent scab was present. The first wounds were healed in WT mice after 8 days, with 100% of the wounds healed by 10 days after wound placement. By contrast, none of the HB wounds were healed at 9 days, and 12 days were required for all wounds to be healed.
Examples of the histologic appearance of healing wounds at selected times from WT mice are shown in Figure 3. On the first day after wounding an intense neutrophil infiltrate filled the clot and adjacent tissues in WT mice. The neutrophilic infiltrate was less intense in HB mice. We did not measure thrombin generation directly in these studies, but stained for fibrin as an indicator of thrombin generation. As expected, immunostaining revealed much more extensive fibrin deposition in the WT than HB wounds (data not shown). Some fibrin was present around the edges of the HB wounds. In both WT and HB wounds the neutrophilic infiltrate was primarily associated with fibrin.
Wound healing is delayed in hemophilia B mice. (A) The size of each wound in 2 dimensions was measured and the wound area in square millimeters calculated. The means ± SDs are plotted. The wound size in HB and WT mice differed significantly at days 1 to 10 (P < .05, indicated by × on the graph). (B) The percentage of wounds that were completely healed. Each point represents data from 18 to 23 mice.
Wound healing is delayed in hemophilia B mice. (A) The size of each wound in 2 dimensions was measured and the wound area in square millimeters calculated. The means ± SDs are plotted. The wound size in HB and WT mice differed significantly at days 1 to 10 (P < .05, indicated by × on the graph). (B) The percentage of wounds that were completely healed. Each point represents data from 18 to 23 mice.
By 3 days after wounding there was greatly increased cellularity of the tissue around the wound site, forming “heaped-up” margins. The “crater” at the biopsy wound site contained an amorphous clot. Epithelial keratinocytes were beginning to migrate in from the edges of the wound. By day 7 most of the WT wounds appeared microscopically to be fully re-epithelialized, though a scab was still adherent to the surface. By 11 days after wounding the normal contour of the skin surface had been restored and the cellularity of the wound site decreased. The wound site could be identified only by the lack of hair follicles (which were excised with the biopsy plug).
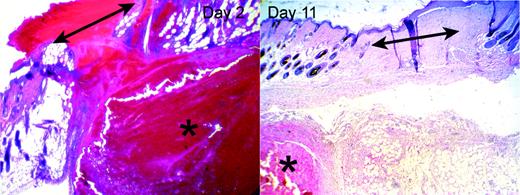
As illustrated in the left panel of Figure 4, the clots covering the wounds in HB mice contained much greater amounts of red blood cells (RBCs) than in the WT mice, whose clots did not contain visible RBCs. In addition, within the 2 days after wounding each HB mouse developed a subcutaneous hematoma near the wound site. The size of the hematoma varied significantly between individuals, and it was not always contiguous with the wound. The hematomas contained large numbers of intact RBCs, few leukocytes, and scattered strands of fibrin. In addition, many HB mice also had small areas of hemorrhage within the granulation tissue and dermal tissue near the wound site.
Epithelial cells proliferated and migrated across the surface of the tissue defect in the HB mice, leading eventually to closure of the epithelial defect. Meanwhile, there was progressive infiltration of the wound site with inflammatory cells and fibroblasts, followed by a decrease in cellularity and restoration of the normal tissue contour by about 11 days. Thus, other than the persistence of subcutaneous hematomas, the wound sites in HB mice were indistinguishable from those in WT mice on hematoxylin and eosin (H&E) sections after healing was completed.
Histology of healing wounds in WT mice. Skin samples were fixed, processed, and stained as described in “Materials and methods.” Representative samples taken 1, 3, 7, and 11 days after wounding are shown. The original magnifications were × 40 (10 × ocular/4 × objective, NA = 0.13).
Histology of healing wounds in WT mice. Skin samples were fixed, processed, and stained as described in “Materials and methods.” Representative samples taken 1, 3, 7, and 11 days after wounding are shown. The original magnifications were × 40 (10 × ocular/4 × objective, NA = 0.13).
In addition to studying the general appearance of the wounds, we used immunohistochemical staining to specifically evaluate additional features of the healing process. After immunostaining, macrophage infiltration was scored as described in “Materials and methods,” and the results are shown in Figure 5. As early as 1 day after wounding in WT animals the macrophage infiltration was beginning in tissues beneath the wound. The infiltrate intensified over the next few days, and was most intense on days 4 to 6. During this period macrophage infiltration involved the entire wound area, including its contained debris and granulation tissue. By day 6 the tissue defect was filled by granulation tissue and covered by epithelium, and smaller numbers of macrophages were scattered diffusely throughout the granulation tissue.
As we had hypothesized, macrophage infiltration in HB mice was markedly delayed compared with WT animals. In addition, the localization of the macrophage infiltrate was different in the WT and HB mice. There was little macrophage infiltration in the granulation tissue filling the wound site in HB mice, but macrophages were instead seen primarily in the tissues deep to the wound and were especially concentrated around the edges of the underlying hematomas. The different pattern of macrophage infiltration suggested that thrombin generation and fibrin deposition at the wound site were the major stimuli for macrophage influx in normal mice, but that these early stimuli were lacking in the HB mice. Instead, the major stimulus for macrophage influx in the HB mice was the presence of blood in the tissues. It was notable that intact RBCs seemed to persist in the tissues of HB mice for many days, suggesting that clearance of debris was impaired in the HB mice. To further explore this possibility, we evaluated the degradation of RBCs and clearance of their hemoglobin content by staining the tissues for ferric iron.
Hematomas adjacent to wounds in HB mice. HB mice developed hematomas (indicated by asterisks) in the subcutaneous tissues in the vicinity of the wound site (indicated by double-headed arrows). The left panel shows an example of a hematoma underlying the site of a 2-day-old wound. The right panel shows a completely healed 11-day wound, with a persistent hematoma in the subcutis. Original magnifications were × 40 (10 × ocular/4 × objective, NA = 0.13).
Hematomas adjacent to wounds in HB mice. HB mice developed hematomas (indicated by asterisks) in the subcutaneous tissues in the vicinity of the wound site (indicated by double-headed arrows). The left panel shows an example of a hematoma underlying the site of a 2-day-old wound. The right panel shows a completely healed 11-day wound, with a persistent hematoma in the subcutis. Original magnifications were × 40 (10 × ocular/4 × objective, NA = 0.13).
We stained wound specimens to assess the deposition and clearance of storage forms of iron in the tissues. The tissue iron stain is based on the reaction of potassium ferrocyanide with iron in the ferric (+3) state to produce an insoluble blue compound. Nearly all iron in hemoglobin is in the ferrous (+2) state. Thus, intact RBCs are not stained, but iron released from degraded RBCs stains blue. The intensity of iron staining was graded as negative (0), weak (1+), positive (2+), or strongly positive (3+) for 2 sites within the tissues: (1) within the area of the biopsy wound; and (2) in the subcutaneous tissues. The results are shown in Figure 6.
At 2 days after wounding none of the WT or HB wounds showed any ferric iron staining, although significant numbers of intact RBCs were obvious. At 4 days the WT wounds were positive for iron in the wound bed, but not in the deeper tissues. This peak of iron levels corresponded with the peak macrophage influx in the WT wounds. However, at 4 days the wounds from HB mice were negative for iron at both tissue sites and still had numerous intact RBCs visible in most specimens. At 6 days the iron staining of the WT wounds was declining in the wound bed and increasing in the deeper tissues, while the HB wounds were now positive in both the wound and deep tissues.
Iron continued to decline in the WT wound beds until by 12 days iron was no longer visible. At this point the iron level was peaking in the deeper tissues. Since we never saw evidence of hemorrhage in the deeper tissues in the WT wounds, we feel that the shift in iron from the wound site to deeper tissues and tissues more distant from the wound site is due to the migration of macrophages out of the wound site carrying with them their load of ingested iron.
Macrophage infiltration into skin wounds in WT and HB mice. Macrophage influx was scored as described in “Materials and methods.” Scores were averaged for 2 sections of 3 to 5 wounds at each time point and the means and SDs are plotted. Points that are significantly different from WT are indicated by “×.”
Macrophage infiltration into skin wounds in WT and HB mice. Macrophage influx was scored as described in “Materials and methods.” Scores were averaged for 2 sections of 3 to 5 wounds at each time point and the means and SDs are plotted. Points that are significantly different from WT are indicated by “×.”
In contrast to the pattern of iron clearance in the WT mice, the wound beds and deeper tissues from HB mice continued to accumulate iron. At 16 days the WT wounds were negative at both sites, while tissues from HB mice remained positive for iron staining.
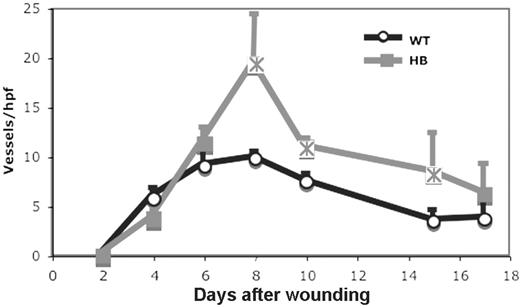
The development of new blood vessels during wound healing was also evaluated by immunohistochemistry. The number of vessels per high power field in fields spanning the wound was counted as described in “Materials and methods” and the results are shown in Figure 7.
The time course of angiogenesis was similar in WT and HB mice. Unexpectedly, the number of vessels in the HB wounds was significantly greater than those seen in WT wounds at days 8 to 15 after wounding. We could not determine in these studies whether the increased number of vessels in HB wounds was due to the production of more vessel sprouts or a failure of the sprouts to regress.
Iron staining in wounded skin from WT and HB mice. The intensity of staining was graded (0-3+) within the wound bed (open symbols) and in the deeper tissues beneath the wound site (closed symbols). The iron staining of WT wounds is shown in panel A and HB wounds in panel B. Each point represents grading from 2 sections of 3 to 4 mice each. Error bars indicate SD.
Iron staining in wounded skin from WT and HB mice. The intensity of staining was graded (0-3+) within the wound bed (open symbols) and in the deeper tissues beneath the wound site (closed symbols). The iron staining of WT wounds is shown in panel A and HB wounds in panel B. Each point represents grading from 2 sections of 3 to 4 mice each. Error bars indicate SD.
Angiogenesis in skin wounds in WT and HB mice. Angiogenesis was assessed by counting vessel profiles across the wound areas as described in “Materials and methods.” The counts are expressed per high-powered (× 40) field. The scores were averaged for 2 sections of 3 to 5 wounds at each time point. Points that are significantly different from WT (P < .05) are indicated by “×.”
Angiogenesis in skin wounds in WT and HB mice. Angiogenesis was assessed by counting vessel profiles across the wound areas as described in “Materials and methods.” The counts are expressed per high-powered (× 40) field. The scores were averaged for 2 sections of 3 to 5 wounds at each time point. Points that are significantly different from WT (P < .05) are indicated by “×.”
Discussion
The absence of FVIII (hemophilia A) or FIX (hemophilia B) results in impaired thrombin generation and defective hemostatic plug formation. The structure of the hemostatic plugs in hemophilic dogs and humans have been described in detail and are characterized by defective stabilization of the initial platelet-rich plug in a fibrin meshwork.2,3 Hemostatic plugs from patients with hemophilia at 2 hours after injury consist of only a thin peripheral layer of fibrin and platelet remnants around a central area of blood with few fibrin fibers. The fibrin formed under hemophilic conditions has an abnormal structure, with increased permeability and decreased mechanical strength.33,34 The hemostatic plugs in patients with hemophilia also tend to be larger than in healthy individuals. The platelets within the plug are less interdigitated and show delayed release of their granules compared with normal hemostatic plugs. The morphology of the hemostatic plugs in hemophilia suggests that FVIII is critical for fibrin formation in the center of the plug, while the small amount of fibrin formed at the periphery of the wound is mediated by the tissue-factor pathway.3 Other studies have suggested that the tissue-factor pathway is activated as an early event during the arrest of bleeding.35
Our current results are completely consistent with those of previous studies in hemophilic dogs and humans. Earlier observations and our present ones can now be viewed in the context of contemporary thought on the mechanism of hemostasis.36 The “extrinsic” or tissue-factor pathway is responsible for initiation of coagulation at sites of injury—both in hemophilic and normal hemostasis. The thrombin generated during this phase of coagulation is insufficient to lead to formation of a stable fibrin clot, but does lead to amplification of the coagulant signal by fully activating platelets and coagulation cofactors. Components of the “intrinsic” pathway are critically important in propagating the subsequent burst of platelet-surface thrombin generation that leads to stabilization of the platelet plug in a fibrin meshwork. The coagulation process is both temporally and spatially nonuniform.37 Thrombin generated via the extrinsic pathway produces fibrin at the edges of the initial platelet plug that are in contact with tissue-factor–bearing cells. Layers of adherent platelets “pave over” the sites of tissue factor expression, providing an insurmountable barrier to the diffusion of activated factors.38 Thus, fibrin formed at sites removed from a tissue-factor source depends on platelet surface thrombin generation, which in turn depends on factors VIIIa and IXa.
In hemophilia, platelet adhesion at a site of injury occurs normally, as does production of FXa and small amounts of thrombin on tissue-factor–bearing cells during initiation of coagulation. However, platelet surface FX activation by FIXa/FVIIIa cannot occur, so platelet surface thrombin generation is inadequate. This results in defective platelet activation, fibrin formation, thrombin activatable fibrinolysis inhibitor (TAFI) activation, and factor XIII activation in the interior of the hemostatic platelet plug.36
It has not been studied previously whether the defective hemostatic process in hemophilia is reflected in impaired wound healing. It seemed likely that impaired thrombin generation might lead to delayed or dysfunctional wound healing, since thrombin affects several parameters that are involved in the healing process. Impaired wound healing was, in fact, recently demonstrated in mice deficient in TAFI.39 Defective TAFI activation occurs if thrombin generation is inadequate, including in hemophilia. Therefore, the lack of FVIII or FIX should have at least as great an effect on wound healing as does the lack of TAFI.
Based on clinical experience and the existing literature, we hypothesized that cutaneous punch biopsy wounds would heal more slowly in mice with HB than in WT mice. This hypothesis was fully supported by our data. We also found that, as expected, macrophage influx into wounds was impaired in HB mice. In spite of the impaired influx of inflammatory cells in HB wounds, we do not think this is the mechanism of delayed wound closure. Macrophages release a range of mediators involved in inflammation and angiogenesis,40 and normally play a role in removing debris from a site of injury. However, macrophages are not necessary for skin wound healing in otherwise normal animals. Mice lacking the PU.1 transcription factor lack macrophages, but repair skin wounds with a time course similar to that in WT littermates.20 However, the growth factor and cytokine profile in the wounds is altered in mice that lack macrophages,41 as is the morphology of the healing response. Thus, we speculate that while some of the morphologic differences in wound healing in WT and HB mice are related to poor thrombin-driven chemotaxis of macrophages, the delay in epithelial closure is likely mediated by different mechanisms.
Epithelial migration and adequacy of cutaneous wound closure was altered in fibrinogen-deficient mice42 as well as in mice deficient in TAFI. These findings suggest that the adequacy and stability of the initial fibrin clot is an important determinant of epidermal and dermal wound closure. Therefore, it seems likely that the delayed wound closure in HB might be due to an inadequate fibrin clot scaffold, possibly exacerbated by increased separation of the wound margins by subcutaneous hemorrhage.
It was interesting that the influx of macrophages was delayed rather than abolished in HB mice. While the early rapid influx of macrophages (1-5 days after wounding) was essentially abolished in HB mice, there was a slower influx of macrophages that peaked at 10 days. This late influx of macrophages occurred after the fibrin clot and necrotic debris had been cleared from the wound area, and as wound closure was nearing completion. Thus, macrophage influx in the HB mice is probably due to signals other than thrombin or fibrin. Most of the macrophages in HB mice were localized in the compressed connective tissue around the hematomas and, to a lesser extent, within the hematomas. Even so, the hematomas persisted for extended periods of time, since they were not resolved more than 2 weeks after wounding. Therefore, we speculate that the presence of iron, cellular debris, or inflammatory mediators in the hematomas serve as stimuli for the delayed macrophage influx.
Iron is usually sequestered by a variety of binding and transport proteins in the tissues and blood for 2 reasons: (1) to prevent iron from participating in electron transfer reactions that can produce highly reactive and injurious free radicals43,44 ; and (2) to prevent iron usage by pathogenic microorganisms. Thus, it is a matter of some importance to have effective mechanisms to metabolize hemoglobin released as a result of hemorrhage or hemolysis and “inactivate” the iron released from it. Free hemoglobin released from RBCs in the blood or tissues binds to haptoglobin. The hemoglobin/haptoglobin complexes, as well as free hemoglobin, binds to a scavenger receptor on macrophages, CD163, which mediates their internalization.45,46 Engaging this receptor up-regulates production of heme oxygenase, which degrades the heme porphyrin rings and releases its iron. The free iron is then primarily bound to ferritin, which “locks” iron in the ferric (+3) oxidation state and limits its participation in electron transfer reactions.
While iron can clearly have directly toxic effects on tissues, it also modulates inflammatory and immune functions. Exposure to iron in vitro enhances macrophage activation and ability to kill microorganisms, as well as production of inflammatory mediators47 and oxidants.48 The release of iron from RBCs may thus be a signal to the immune system that significant injury has occurred and activation of inflammatory and immune responses is desirable. However, binding of hemoglobin/haptoglobin to CD163 promotes production of anti-inflammatory cytokines by macrophages and triggers resolution of the acute inflammatory response.49-51 Thus, iron appears to be intimately involved in orchestrating acute inflammation in response to tissue injury as well as termination of the inflammatory response when it is no longer needed.
Macrophages are the primary means of scavenging hemoglobin and degrading heme. Thus, consistent with the delayed influx of macrophages, conversion of RBC hemoglobin to storage iron was delayed in HB mice. Iron persisted much longer in the tissues of HB mice as well, probably because the HB mice experience more bleeding into the tissues than do the WT mice. At this point we do not know whether there is a defect in macrophage clearance of iron in the HB wounds or whether the persistence of iron is solely due to more hemorrhage at the wound sites. While we never see very large numbers of RBCs in the wound bed during healing, the tendency of granulation tissue to bleed, even in individuals with normal hemostasis, is well recognized. Thus, it is possible that there are repeated small hemorrhages in the granulation tissue in HB mice that continue to deliver iron to the wound site.
We originally hypothesized that angiogenesis would be impaired in HB mice. By contrast, we found angiogenesis to be enhanced in HB mice. Particularly relevant to this finding is the report that “macrophageless” PU.1-null mice have slightly enhanced angiogenesis (30% increase in vessel profiles) during healing. Thus, the abnormality of macrophage influx might contribute to enhanced angiogenesis in HB mice. However, the magnitude of the difference in HB and WT mice is greater than 30%, suggesting that factors other than macrophage-derived cytokines contribute to increased angiogenesis.
We feel that persistence of iron in the tissues likely has a significant impact on angiogenesis and the rate of wound healing. Excess tissue iron has been linked to poor wound healing in chronic venous skin ulcers52 and increased angiogenesis and fibrosis in inflammatory synovitis.53 Free iron promotes inflammation and cellular proliferation54,55 and is thought to play a role in the synovitis and excess angiogenesis observed in patients with hemophilia following repeated hemorrhage into a target joint.56,57 Thus, the defect in macrophage influx, accumulation of tissue iron, and the excess angiogenesis in HB mice may all be linked.
The presence of subcutaneous hematomas was a striking feature of the HB mice. It is possible that the high level of tissue-factor activity in the squamous epithelium58 promotes fibrin clot formation at the skin surface, while the lower level of tissue factor in deeper tissues59 allows (re)bleeding to occur.
The hematomas in the HB mice seemed to resolve remarkably slowly. It strikes us that these hematomas may be analogous to pseudotumor formation in humans with hemophilia. Pseudotumors are large hematomas, often involving deep soft tissues, that resolve very slowly, if at all. Their surgical removal can be difficult and associated with significant bleeding. We speculate that resolution of the hematomas in the HB mice and pseudotumors in humans with hemophilia is exceedingly slow because the signals for macrophage influx and clearance of the hemorrhagic debris are lacking when the extravasated blood fails to clot normally.
In summary, the time course and histology of wound healing is altered in hemophilia B. Specifically, (1) epithelial closure is delayed in HB mice and probably secondary to poor fibrin clot structure; (2) HB mice develop subcutaneous hematomas that resolve slowly; (3) macrophage influx is delayed in HB mice and is likely related to reduced generation of thrombin and fibrin degradation chemotactic agents; (4) tissue iron persists in the wounds and deep tissues of HB mice; and (5) angiogenesis is enhanced in HB mice and may be due to the reduced macrophage influx and increased iron deposition in the tissues.
Prepublished online as Blood First Edition Paper, July 6, 2006; DOI 10.1182/blood-2006-05-020495.
Supported by a research grant from Novo Nordisk A/S (to D.M.M.), the Donald B. Hackel Pathology Fellowship (to A.H.), and by the US Department of Veterans Affairs.
M.H. was responsible for conception and overall design, selecting histochemical techniques, conducting and interpreting microscopic evaluation, taking photomicrographs, analyzing data, and interpreting results, and was primarily responsible for writing the manuscript; A.H. was responsible for processing, embedding, and immunostaining tissues and evaluating their microscopic appearance; A.L. was responsible for implementing and troubleshooting the immunohistochemical techniques; U.H. and H.R.R. were responsible for conception and overall design, interpreting the results, and correlating them with the clinical picture in human hemophilia; and D.M.M. was responsible for conception and overall design, overseeing all aspects of the animal studies, analyzing data, and interpreting the results.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal